Endothelial Damage, Neutrophil Extracellular Traps and Platelet Activation in COVID-19 vs. Community-Acquired Pneumonia: A Case–Control Study
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
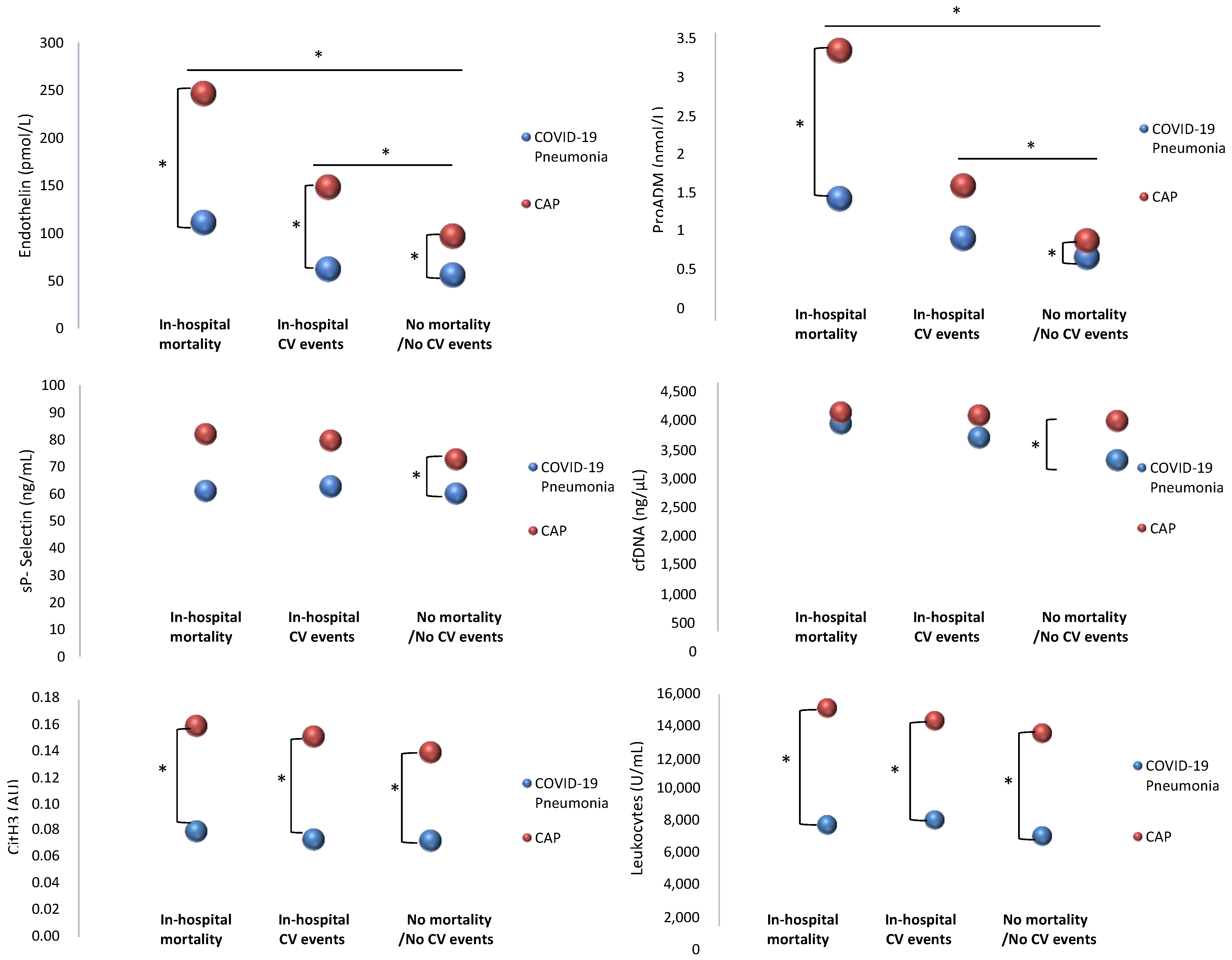
2.2. Clinical Outcomes: In-Hospital and 1-Year Follow-Up Complications
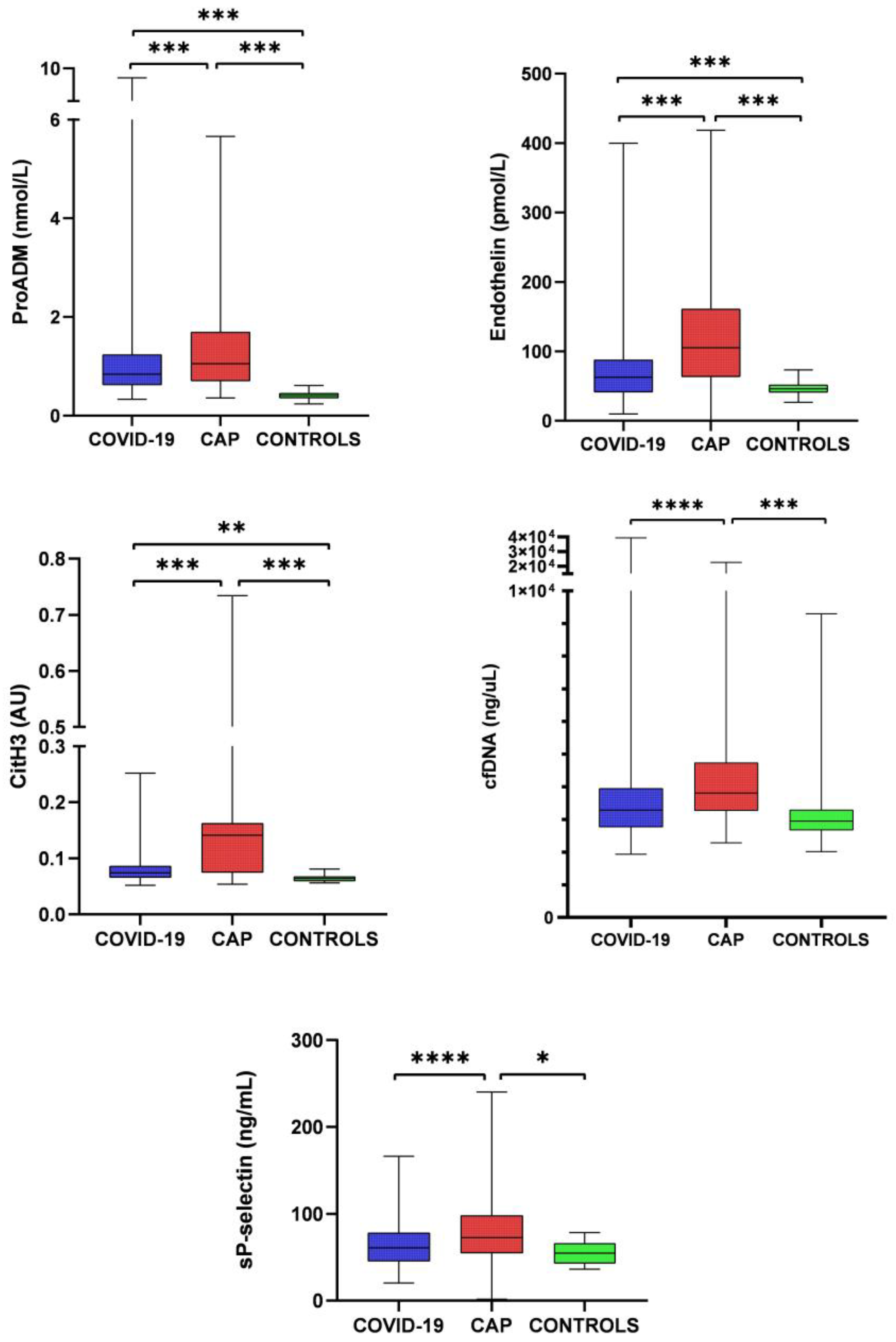
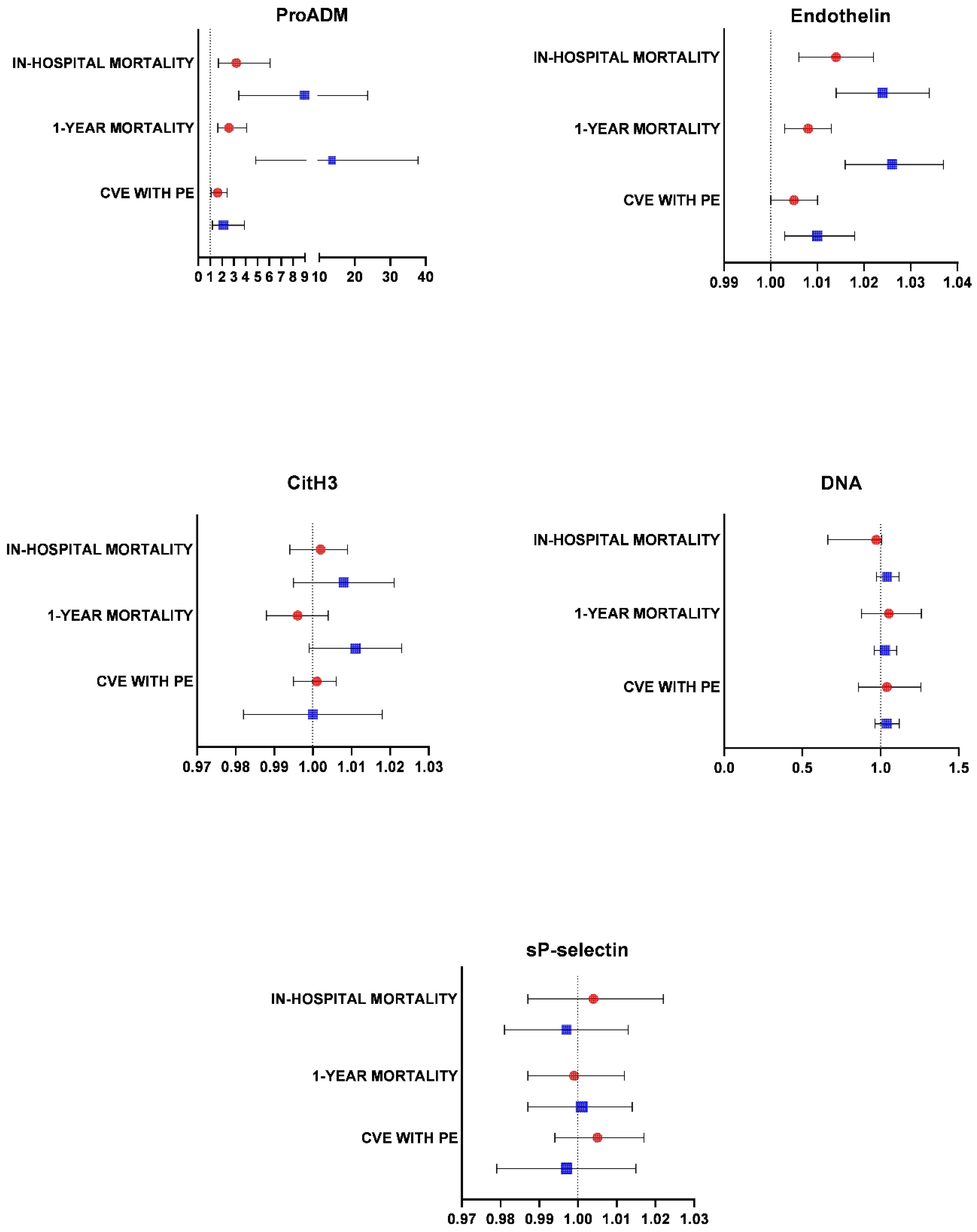
2.3. Endothelial Damage, NETosis, Platelet Activation, and Inflammatory and Immunological Markers
3. Discussion
4. Materials and Methods
4.1. Study Design and Participation
4.2. Blood Samples
4.3. Neutrophil Extracellular Traps
4.4. Platelet Activation
4.5. Endothelial Damage
4.6. Clinical Outcomes: Definitions
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; Cohen, S.L.; et al. Presenting Characteristics, Comorbidities, and Outcomes among 5700 Patients Hospitalized with COVID-19 in the New York City Area. JAMA—J. Am. Med. Assoc. 2020, 323, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- Osuchowski, M.F.; Winkler, M.S.; Skirecki, T.; Cajander, S.; Shankar-Hari, M.; Lachmann, G.; Monneret, G.; Venet, F.; Bauer, M.; Brunkhorst, F.M.; et al. The COVID-19 Puzzle: Deciphering Pathophysiology and Phenotypes of a New Disease Entity. Lancet Respir. Med. 2021, 9, 622–642. [Google Scholar] [CrossRef] [PubMed]
- Saffarzadeh, M.; Juenemann, C.; Queisser, M.A.; Lochnit, G.; Barreto, G.; Galuska, S.P.; Lohmeyer, J.; Preissner, K.T. Neutrophil Extracellular Traps Directly Induce Epithelial and Endothelial Cell Death: A Predominant Role of Histones. PLoS ONE 2012, 7, e32366. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, E.E.; Andrews, R.K. Neutrophil Extracellular Traps (NETs) and Infection-Related Vascular Dysfunction. Blood Rev. 2012, 26, 255–259. [Google Scholar] [CrossRef]
- Wichmann, D.; Sperhake, J.P.; Lütgehetmann, M.; Steurer, S.; Edler, C.; Heinemann, A.; Heinrich, F.; Mushumba, H.; Kniep, I.; Schröder, A.S.; et al. Autopsy Findings and Venous Thromboembolism in Patients with COVID-19: A Prospective Cohort Study. Ann. Intern. Med. 2020, 173, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Cangemi, R.; Calvieri, C.; Falcone, M.; Cipollone, F.; Ceccarelli, G.; Pignatelli, P.; D’Ardes, D.; Pirro, M.; Alessandri, F.; Lichtner, M.; et al. Comparison of Thrombotic Events and Mortality in Patients with Community-Acquired Pneumonia and COVID-19: A Multicenter Observational Study. Thromb. Haemost. 2022, 122, 257–266. [Google Scholar] [CrossRef]
- Canzano, P.; Brambilla, M.; Porro, B.; Cosentino, N.; Tortorici, E.; Vicini, S.; Poggio, P.; Cascella, A.; Pengo, M.F.; Veglia, F.; et al. Platelet and Endothelial Activation as Potential Mechanisms Behind the Thrombotic Complications of COVID-19 Patients. JACC Basic to Transl. Sci. 2021, 6, 202–218. [Google Scholar] [CrossRef]
- Brinkmann, V. Neutrophil Extracellular Traps Kill Bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Papayannopoulos, V. Neutrophil Extracellular Traps in Immunity and Disease. Nat. Rev. Immunol. 2018, 18, 134–147. [Google Scholar] [CrossRef]
- Nel, J.G.; Theron, A.J.; Durandt, C.; Tintinger, G.R.; Pool, R.; Mitchell, T.J.; Feldman, C.; Anderson, R. Pneumolysin Activates Neutrophil Extracellular Trap Formation. Clin. Exp. Immunol. 2016, 184, 358–367. [Google Scholar] [CrossRef][Green Version]
- Koupenova, M.; Clancy, L.; Corkrey, H.A.; Freedman, J.E. Circulating Platelets as Mediators of Immunity, Inflammation, and Thrombosis. Circ. Res. 2018, 122, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.J.; Radic, M. Neutrophil Extracellular Traps (NETs): Double-Edged Swords of Innate Immunity. J. Immunol. 2012, 189, 2689–2695. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, F.; Giaglis, S.; Hahn, S.; Blum, C.A.; Baumgartner, C.; Kutz, A.; van Breda, S.V.; Mueller, B.; Schuetz, P.; Christ-Crain, M.; et al. Markers of Neutrophil Extracellular Traps Predict Adverse Outcome in Community-Acquired Pneumonia: Secondary Analysis of a Randomised Controlled Trial. Eur. Respir. J. 2018, 51, 1701389. [Google Scholar] [CrossRef] [PubMed]
- Petito, E.; Falcinelli, E.; Paliani, U.; Cesari, E.; Vaudo, G.; Sebastiano, M.; Cerotto, V.; Guglielmini, G.; Gori, F.; Malvestiti, M.; et al. Association of Neutrophil Activation, More Than Platelet Activation, with Thrombotic Complications in Coronavirus Disease 2019. J. Infect. Dis. 2021, 223, 933–994. [Google Scholar] [CrossRef]
- Kinnare, N.; Hook, J.S.; Patel, P.A.; Monson, N.L.; Moreland, J.G. Neutrophil Extracellular Trap Formation Potential Correlates with Lung Disease Severity in COVID-19 Patients. Inflammation 2022, 45, 800–811. [Google Scholar] [CrossRef]
- Zhou, Y.; Guo, S.; He, Y.; Zuo, Q.; Liu, D.; Xiao, M.; Fan, J.; Li, X. COVID-19 Is Distinct From SARS-CoV-2-Negative Community-Acquired Pneumonia. Front. Cell. Infect. Microbiol. 2020, 10, 322. [Google Scholar] [CrossRef]
- Zhang, Z. Propensity Score Method: A Non-Parametric Technique to Reduce Model Dependence. Ann. Transl. Med. 2017, 5, 7. [Google Scholar] [CrossRef]
- Ebihara, T.; Matsumoto, H.; Matsubara, T.; Togami, Y.; Nakao, S.; Matsuura, H.; Kojima, T.; Sugihara, F.; Okuzaki, D.; Hirata, H.; et al. Cytokine Elevation in Severe COVID-19 from Longitudinal Proteomics Analysis: Comparison With Sepsis. Front. Immunol. 2022, 12, 798338. [Google Scholar] [CrossRef]
- Palma Medina, L.M.; Babačić, H.; Dzidic, M.; Parke, Å.; Garcia, M.; Maleki, K.T.; Unge, C.; Lourda, M.; Kvedaraite, E.; Chen, P.; et al. Targeted Plasma Proteomics Reveals Signatures Discriminating COVID-19 from Sepsis with Pneumonia. Respir. Res. 2023, 24, 1–19. [Google Scholar] [CrossRef]
- Zafer, M.M.; El-Mahallawy, H.A.; Ashour, H.M. Severe COVID-19 and Sepsis: Immune Pathogenesis and Laboratory Markers. Microorganisms 2021, 9, 159. [Google Scholar] [CrossRef]
- Aid, M.; Busman-Sahay, K.; Vidal, S.J.; Maliga, Z.; Bondoc, S.; Starke, C.; Terry, M.; Jacobson, C.A.; Wrijil, L.; Ducat, S.; et al. Vascular Disease and Thrombosis in SARS-CoV-2-Infected Rhesus Macaques. Cell 2020, 183, 1354–1366.e13. [Google Scholar] [CrossRef] [PubMed]
- Melero, I.; Villalba-Esparza, M.; Recalde-Zamacona, B.; Jiménez-Sánchez, D.; Teijeira, Á.; Argueta, A.; García-Tobar, L.; Álvarez-Gigli, L.; Sainz, C.; Garcia-Ros, D.; et al. Neutrophil Extracellular Traps, Local IL-8 Expression, and Cytotoxic T-Lymphocyte Response in the Lungs of Patients With Fatal COVID-19. Chest 2022, 162, 1006–1016. [Google Scholar] [CrossRef] [PubMed]
- Dömer, D.; Walther, T.; Möller, S.; Behnen, M.; Laskay, T. Neutrophil Extracellular Traps Activate Proinflammatory Functions of Human Neutrophils. Front. Immunol. 2021, 12, 636954. [Google Scholar] [CrossRef] [PubMed]
- Hudock, K.M.; Collins, M.S.; Imbrogno, M.; Snowball, J.; Kramer, E.L.; Brewington, J.J.; Gollomp, K.; McCarthy, C.; Ostmann, A.J.; Kopras, E.J.; et al. Neutrophil Extracellular Traps Activate IL-8 and IL-1 Expression in Human Bronchial Epithelia. Am. J. Physiol.—Lung Cell. Mol. Physiol. 2020, 319, L137–L147. [Google Scholar] [CrossRef]
- Menéndez, R.; Méndez, R.; Aldás, I.; Reyes, S.; Gonzalez-Jimenez, P.; España, P.P.; Almirall, J.; Alonso, R.; Suescun, M.; Martinez-Dolz, L.; et al. Community-Acquired Pneumonia Patients at-Risk for Early and Long-Term Cardiovascular Events Are Identified by Cardiac Biomarkers. Chest 2019, 156, 1080–1091. [Google Scholar] [CrossRef] [PubMed]
- Méndez, R.; González-Jiménez, P.; Latorre, A.; Piqueras, M.; Bouzas, L.; Yépez, K.; Ferrando, A.; Zaldívar-Olmeda, E.; Moscardó, A.; Alonso, R.; et al. Acute and Sustained Increase in Endothelial Biomarkers in COVID-19. Thorax 2021, 77, 400–403. [Google Scholar] [CrossRef]
- Yang, K.Y.; Liu, K.T.; Chen, Y.C.; Chen, C.S.; Lee, Y.C.; Perng, R.P.; Feng, J.Y. Plasma Soluble Vascular Endothelial Growth Factor Receptor-1 Levels Predict Outcomes of Pneumonia-Related Septic Shock Patients: A Prospective Observational Study. Crit. Care 2011, 15, R11. [Google Scholar] [CrossRef]
- Gutbier, B.; Neuhauß, A.-K.; Reppe, K.; Ehrler, C.; Santel, A.; Kaufmann, J.; Scholz, M.; Weissmann, N.; Morawietz, L.; Mitchell, T.J.; et al. Prognostic and Pathogenic Role of Angiopoietin-1 and -2 in Pneumonia. Am. J. Respir. Crit. Care Med. 2018, 198, 220–231. [Google Scholar] [CrossRef]
- Bonaventura, A.; Vecchié, A.; Dagna, L.; Martinod, K.; Dixon, D.L.; Van Tassell, B.W.; Dentali, F.; Montecucco, F.; Massberg, S.; Levi, M.; et al. Endothelial Dysfunction and Immunothrombosis as Key Pathogenic Mechanisms in COVID-19. Nat. Rev. Immunol. 2021, 21, 319–332. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial Cell Infection and Endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Cangemi, R.; Casciaro, M.; Rossi, E.; Calvieri, C.; Bucci, T.; Calabrese, C.M.; Taliani, G.; Falcone, M.; Palange, P.; Bertazzoni, G.; et al. Platelet Activation Is Associated with Myocardial Infarction in Patients with Pneumonia. J. Am. Coll. Cardiol. 2014, 64, 1917–1925. [Google Scholar] [CrossRef] [PubMed]
- Manne, B.K.; Denorme, F.; Middleton, E.; Portier, I.; Jesse, W. Platelet Gene Expression and Function in COVID-19 Patients. Blood 2020, 136, 1317–1329. [Google Scholar] [CrossRef] [PubMed]
- Bhatraju, P.K.; Morrell, E.D.; Zelnick, L.; Sathe, N.A.; Chai, X.Y.; Sakr, S.S.; Sahi, S.K.; Sader, A.; Lum, D.M.; Liu, T.; et al. Comparison of Host Endothelial, Epithelial and Inflammatory Response in ICU Patients with and without COVID-19: A Prospective Observational Cohort Study. Crit. Care 2021, 25, 148. [Google Scholar] [CrossRef]
- Hokama, L.T.; Veiga, A.D.M.; Menezes, M.C.S.; Sardinha Pinto, A.A.; de Lima, T.M.; Ariga, S.K.K.; Barbeiro, H.V.; Barbeiro, D.F.; de Lucena Moreira, C.; Stanzani, G.; et al. Endothelial Injury in COVID-19 and Septic Patients. Microvasc. Res. 2022, 140, 104303. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Rivera, C.; Zhang, Y.; Dobbs, K.; Markowitz, T.E.; Dalgard, C.L.; Oler, A.J.; Claybaugh, D.R.; Draper, D.; Truong, M.; Delmonte, O.M.; et al. Multicenter Analysis of Neutrophil Extracellular Trap Dysregulation in Adult and Pediatric COVID-19. JCI Insight 2022, 7, e160332. [Google Scholar] [CrossRef] [PubMed]
- Karsli, E.; Sabirli, R.; Altintas, E.; Canacik, O.; Sabirli, G.T.; Kaymaz, B.; Kurt, Ö.; Koseler, A. Soluble P-Selectin as a Potential Diagnostic and Prognostic Biomarker for COVID-19 Disease: A Case-Control Study. Life Sci. 2021, 277, 119634. [Google Scholar] [CrossRef]
- Lee, C.C.E.; Ali, K.; Connell, D.; Mordi, I.R.; George, J.; Lang, E.M.; Lang, C.C. COVID-19-Associated Cardiovascular Complications. Diseases 2021, 9, 47. [Google Scholar] [CrossRef]
- Long, B.; Brady, W.J.; Koyfman, A.; Gottlieb, M. Cardiovascular Complications in COVID-19. Am. J. Emerg. Med. 2020, 38, 1504–1507. [Google Scholar] [CrossRef]
- Aldás, I.; Menéndez, R.; Méndez, R.; España, P.P.; Almirall, J.; Boderías, L.; Rajas, O.; Zalacaín, R.; Vendrell, M.; Mir, I.; et al. Eventos Cardiovasculares Tempranos y Tardíos En Pacientes Ingresados Por Neumonía Adquirida En La Comunidad. Arch. Bronconeumol. 2020, 56, 551–558. [Google Scholar] [CrossRef]
- Nappi, F.; Bellomo, F.; Singh, S.S.A. Insights into the Role of Neutrophils and Neutrophil Extracellular Traps in Causing Cardiovascular Complications in Patients with COVID-19: A Systematic Review. J. Clin. Med. 2022, 11, 2460. [Google Scholar] [CrossRef]
- Knight, S.R.; Ho, A.; Pius, R.; Buchan, I.; Carson, G.; Drake, T.M.; Dunning, J.; Fairfield, C.J.; Gamble, C.; Green, C.A.; et al. Risk Stratification of Patients Admitted to Hospital with Covid-19 Using the ISARIC WHO Clinical Characterisation Protocol: Development and Validation of the 4C Mortality Score. BMJ 2020, 370, m3339. [Google Scholar] [CrossRef] [PubMed]
- Menéndez, R.; Torres, A.; Aspa, J.; Capelastegui, A.; Prat, C.; Rodríguez de Castro, F. Sociedad Española de Neumología y Cirugía Torácica [Community Acquired Pneumonia. New Guidelines of the Spanish Society of Chest Diseases and Thoracic Surgery (SEPAR)]. Arch. Bronconeumol. 2010, 46, 543–558. [Google Scholar] [CrossRef] [PubMed]
- Prina, E.; Ranzani, O.T.; Torres, A. Community-Acquired Pneumonia. Lancet 2015, 386, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, M.I.; Faverio, P.; Anzueto, A. Long-Term Prognosis in Community-Acquired Pneumonia. Curr. Opin. Infect. Dis. 2013, 26, 151–158. [Google Scholar] [CrossRef]
- Huang, D.T.; Angus, D.C.; Kellum, J.A.; Pugh, N.A.; Weissfeld, L.A.; Struck, J.; Delude, R.L.; Rosengart, M.R.; Yealy, D.M. Midregional Proadrenomedullin as a Prognostic Tool in Community-Acquired Pneumonia. Chest 2009, 136, 823–831. [Google Scholar] [CrossRef]
- Bello, S.; Lasierra, A.B.; Mincholé, E.; Fandos, S.; Ruiz, M.A.; Vera, E.; de Pablo, F.; Ferrer, M.; Menendez, R.; Torres, A. Prognostic Power of Proadrenomedullin in Community-Acquired Pneumonia Is Independent of Aetiology. Eur. Respir. J. 2012, 39, 1144–1155. [Google Scholar] [CrossRef]
- Menéndez, R.; Méndez, R.; Almansa, R.; Ortega, A.; Alonso, R.; Suescun, M.; Ferrando, A.; Feced, L.; Bermejo-Martin, J.F. Simultaneous Depression of Immunological Synapse and Endothelial Injury Is Associated with Organ Dysfunction in Community-Acquired Pneumonia. J. Clin. Med. 2019, 8, 1404. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in COVID-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Montrucchio, G.; Sales, G.; Rumbolo, F.; Palmesino, F.; Fanelli, V.; Urbino, R.; Filippini, C.; Mengozzi, G.; Brazzi, L. Effectiveness of Mid-Regional Proadrenomedullin (MR-ProADM) as Prognostic Marker in COVID-19 Critically Ill Patients: An Observational Prospective Study. PLoS ONE 2021, 16, e0246771. [Google Scholar] [CrossRef]
- Sozio, E.; Moore, N.A.; Fabris, M.; Ripoli, A.; Rumbolo, F.; Minieri, M.; Boverio, R.; Rodríguez Mulero, M.D.; Lainez-Martinez, S.; Martínez Martínez, M.; et al. Identification of COVID-19 Patients at Risk of Hospital Admission and Mortality: A European Multicentre Retrospective Analysis of Mid-Regional pro-Adrenomedullin. Respir. Res. 2022, 23, 221. [Google Scholar] [CrossRef]
- Mangioni, D.; Oggioni, M.; Chatenoud, L.; Liparoti, A.; Uceda Renteria, S.; Alagna, L.; Biscarini, S.; Bolis, M.; Di Modugno, A.; Mussa, M.; et al. Prognostic Value of Mid-Region Proadrenomedullin and In Vitro Interferon Gamma Production for In-Hospital Mortality in Patients with COVID-19 Pneumonia and Respiratory Failure: An Observational Prospective Study. Viruses 2022, 14, 1683. [Google Scholar] [CrossRef] [PubMed]
- Morimont, L.; Dechamps, M.; David, C.; Bouvy, C.; Gillot, C.; Haguet, H.; Favresse, J.; Ronvaux, L.; Candiracci, J.; Herzog, M.; et al. NETosis and Nucleosome Biomarkers in Septic Shock and Critical COVID-19 Patients: An Observational Study. Biomolecules 2022, 12, 1038. [Google Scholar] [CrossRef]
- Uranga, A.; Quintana, J.M.; Aguirre, U.; Artaraz, A.; Diez, R.; Pascual, S.; Ballaz, A.; España, P.P. Predicting 1-Year Mortality after Hospitalization for Community-Acquired Pneumonia. PLoS ONE 2018, 13, e0192750. [Google Scholar] [CrossRef]
- Bruns, A.H.W.; Oosterheert, J.J.; Cucciolillo, M.C.; El Moussaoui, R.; Groenwold, R.H.H.; Prins, J.M.; Hoepelman, A.I.M. Cause-Specific Long-Term Mortality Rates in Patients Recovered from Community-Acquired Pneumonia as Compared with the General Dutch Population. Clin. Microbiol. Infect. 2011, 17, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Yende, S.; D’Angelo, G.; Kellum, J.A.; Weissfeld, L.; Fine, J.; Welch, R.D.; Kong, L.; Carter, M.; Angus, D.C. GenIMS Investigators Inflammatory Markers at Hospital Discharge Predict Subsequent Mortality after Pneumonia and Sepsis. Am. J. Respir. Crit. Care Med. 2008, 177, 1242–1247. [Google Scholar] [CrossRef] [PubMed]
- González, J.; Zuil, M.; Benítez, I.D.; de Gonzalo-Calvo, D.; Aguilar, M.; Santisteve, S.; Vaca, R.; Minguez, O.; Seck, F.; Torres, G.; et al. One Year Overview and Follow-Up in a Post-COVID Consultation of Critically Ill Patients. Front. Med. 2022, 9, 897990. [Google Scholar] [CrossRef]
- Dechamps, M.; De Poortere, J.; Octave, M.; Ginion, A.; Robaux, V.; Pirotton, L.; Bodart, J.; Gruson, D.; Van Dievoet, M.A.; Douxfils, J.; et al. Dexamethasone Modulates the Cytokine Response but Not COVID-19-Induced Coagulopathy in Critically Ill. Int. J. Mol. Sci. 2023, 24, 7278. [Google Scholar] [CrossRef]
- Wong, S.L.; Demers, M.; Martinod, K.; Gallant, M.; Wang, Y.; Goldfine, A.B.; Kahn, C.R.; Wagner, D.D. Diabetes Primes Neutrophils to Undergo NETosis Which Severely Impairs Wound Healing. Nat. Med. 2015, 21, 815. [Google Scholar] [CrossRef]
- González-Jiménez, P.; Méndez, R.; Latorre, A.; Piqueras, M.; Balaguer-Cartagena, M.N.; Moscardó, A.; Alonso, R.; Hervás, D.; Reyes, S.; Menéndez, R. Neutrophil Extracellular Traps and Platelet Activation for Identifying Severe Episodes and Clinical Trajectories in COVID-19. Int. J. Mol. Sci. 2023, 24, 6690. [Google Scholar] [CrossRef]
- Vallés, J.; Lago, A.; Santos, M.T.; Latorre, A.M.; Tembl, J.I.; Salom, J.B.; Nieves, C.; Moscardó, A. Neutrophil Extracellular Traps Are Increased in Patients With Acute Ischemic Stroke: Prognostic Significance. Thromb. Haemost. 2017, 117, 1919–1929. [Google Scholar] [CrossRef]
- Méndez, R.; Moscardó, A.; Latorre, A.; Feced, L.; González-Jiménez, P.; Piró, A.; Alcaraz-Serrano, V.; Scioscia, G.; Amaro, R.; Torres, A.; et al. Soluble P-Selectin in Acute Exacerbations and Stable Bronchiectasis in Adults. Ann. Am. Thorac. Soc. 2019, 16, 1587–1591. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.E.; Imai, K.; King, G.; Stuart, E.A. MatchIt: Nonparametric Preprocessing for Parametric Causal Inference. J. Stat. Softw. 2011, 42, 1–28. [Google Scholar] [CrossRef]
| COVID-19 Pneumonia (n = 179) | CAP (n = 179) | |
|---|---|---|
| Age, years, median (1st quartile, 3rd quartile) | 65 (53, 79) | 70 (56, 79) |
| Male sex, no. (%) | 105 (58.7) | 108 (60.3) |
| Current or former smokers, no. (%) | 48 (26.8) | 141 (78.8) |
| Coexisting conditions, no. (%) | ||
| Hypertension | 78 (43.6) | 86 (48.0) |
| Diabetes | 45 (25.1) | 47 (26.7) |
| Dyslipidemia | 57 (31.8) | 71 (39.7) |
| Overweight * | 102 (57.0) | 69 (38.6) |
| COPD | 4 (2.2) | 42 (23.5) |
| Asthma | 8 (4.5) | 24 (13.4) |
| Chronic heart disease | 23 (12.9) | 61 (34.1) |
| Chronic renal disease | 27 (15.1) | 26 (14.5) |
| Neurological disease | 27 (15.1) | 21 (11.7) |
| SpO2/FiO2 at admission (1st quartile, 3rd quartile) | 452.4 (435.7, 457.1) | 442.9 (419.1, 457.1) |
| Radiological data at admission | ||
| Bilateral infiltrates, no. (%) | 111 (62.0) | 33 (18.4) |
| Maximum respiratory support, no. (%) | ||
| No respiratory support | 79 (44.1) | 66 (36.9) |
| O2 nasal cannula | 18 (10.1) | 78 (43.6) |
| O2 venturi/reservoir mask | 56 (31.3) | 22 (12.3) |
| HFNC | 9 (5.0) | 2 (1.1) |
| CPAP/NIMV | 2 (1.1) | 7 (3.9) |
| MV | 15 (8.4) | 4 (2.2) |
| Etiology, no. (%) | NA | 102 (57) |
| Bacterial | NA | 88 (49.2) |
| Streptococcus pneumoniae | NA | 38 (21.2) |
| Atypical | NA | 36 (20.1) |
| Viral | NA | 25 (14) |
| Influenza | NA | 19 (10.6) |
| Mixed (bacterial and viral) | NA | 11 (6.1) |
| COVID-19 Pneumonia (n = 179) | CAP (n = 179) | p-Value | |
|---|---|---|---|
| In-hospital outcomes | |||
| Mortality, no. (%) | 26 (14.5) | 7 (3.9) | 0.001 |
| Cardiac complications, no. (%) | 11 (6.2) | 18 (10.1) | 0.176 |
| Acute coronary syndrome, no. (%) | 3 (1.7) | 0 (0.0) | 0.082 |
| Arrhythmia, no. (%) | 5 (2.8) | 9 (5.0) | 0.276 |
| Congestive heart failure, no. (%) | 4 (2.2) | 11 (6.2) | 0.065 |
| Pulmonary embolism, no. (%) | 9 (5.0) | 0 (0.0) | 0.002 |
| Stroke, no. (%) | 0 (0.0) | 2 (1.1) | 0.156 |
| Total 1-year outcomes | |||
| Mortality, no. (%) | 33 (18.4) | 20 (11.2) | 0.053 |
| Cardiac complications, no. (%) | 14 (7.8) | 35 (19.6) | 0.001 |
| Acute coronary syndrome, no. (%) | 3 (1.7) | 5 (2.8) | 0.475 |
| Arrhythmia, no. (%) | 6 (3.4) | 18 (10.1) | 0.011 |
| Congestive heart failure, no. (%) | 6 (3.4) | 21 (11.7) | 0.003 |
| Pulmonary embolism, no. (%) | 9 (5.0) | 0 (0.0) | 0.002 |
| Stroke, no. (%) | 0 (0.0) | 5 (2.8) | 0.024 |
| COVID-19 Pneumonia (n = 153) | CAP (n = 172) | p-Value | |
|---|---|---|---|
| 1-year follow-up mortality, no. (%) | 7 (4.6) | 13 (7.6) | 0.001 |
| 1-year cardiovascular complications, no. (%) | 3 (2.0) | 23 (13.4) | 0.003 |
| 1-year acute coronary syndrome, no. (%) | 0 (0.0) | 5 (2.9) | 0.082 |
| 1-year arrhythmia, no. (%) | 1 (0.7) | 13 (7.6) | 0.005 |
| 1-year congestive heart failure, no. (%) | 2 (1.3) | 14 (8.1) | 0.009 |
| 1-year pulmonary embolism, no. (%) | 0 (0.0) | 0 (0.0) | NA |
| 1-year stroke, no. (%) | 0 (0.0) | 3 (1.7) | 0.287 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Jiménez, P.; Méndez, R.; Latorre, A.; Mengot, N.; Piqueras, M.; Reyes, S.; Moscardó, A.; Alonso, R.; Amara-Elori, I.; Menéndez, R. Endothelial Damage, Neutrophil Extracellular Traps and Platelet Activation in COVID-19 vs. Community-Acquired Pneumonia: A Case–Control Study. Int. J. Mol. Sci. 2023, 24, 13194. https://doi.org/10.3390/ijms241713194
González-Jiménez P, Méndez R, Latorre A, Mengot N, Piqueras M, Reyes S, Moscardó A, Alonso R, Amara-Elori I, Menéndez R. Endothelial Damage, Neutrophil Extracellular Traps and Platelet Activation in COVID-19 vs. Community-Acquired Pneumonia: A Case–Control Study. International Journal of Molecular Sciences. 2023; 24(17):13194. https://doi.org/10.3390/ijms241713194
Chicago/Turabian StyleGonzález-Jiménez, Paula, Raúl Méndez, Ana Latorre, Noé Mengot, Mónica Piqueras, Soledad Reyes, Antonio Moscardó, Ricardo Alonso, Isabel Amara-Elori, and Rosario Menéndez. 2023. "Endothelial Damage, Neutrophil Extracellular Traps and Platelet Activation in COVID-19 vs. Community-Acquired Pneumonia: A Case–Control Study" International Journal of Molecular Sciences 24, no. 17: 13194. https://doi.org/10.3390/ijms241713194
APA StyleGonzález-Jiménez, P., Méndez, R., Latorre, A., Mengot, N., Piqueras, M., Reyes, S., Moscardó, A., Alonso, R., Amara-Elori, I., & Menéndez, R. (2023). Endothelial Damage, Neutrophil Extracellular Traps and Platelet Activation in COVID-19 vs. Community-Acquired Pneumonia: A Case–Control Study. International Journal of Molecular Sciences, 24(17), 13194. https://doi.org/10.3390/ijms241713194







