The Potent and Selective Histamine H3 Receptor Antagonist E169 Counteracts Cognitive Deficits and Mitigates Disturbances in the PI3K/AKT/GSK-3β Signaling Pathway in MK801-Induced Amnesia in Mice
Abstract
1. Introduction
2. Results
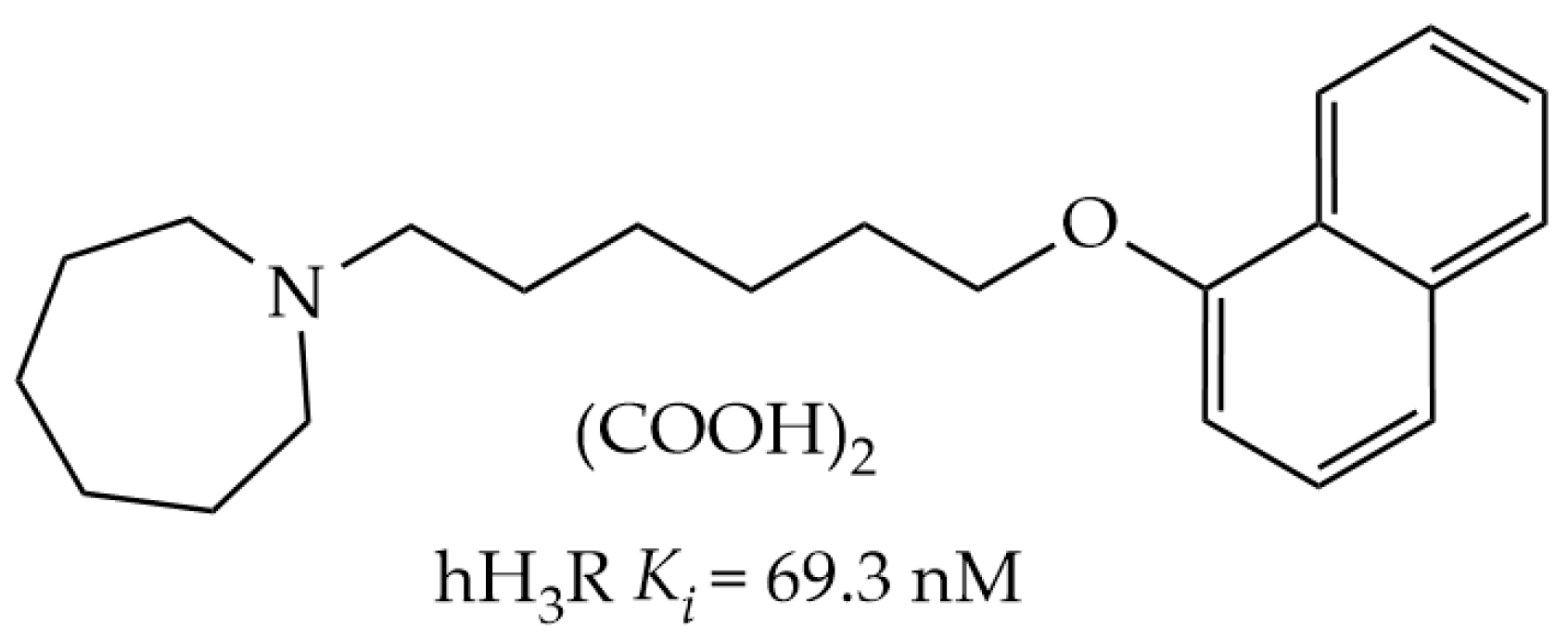
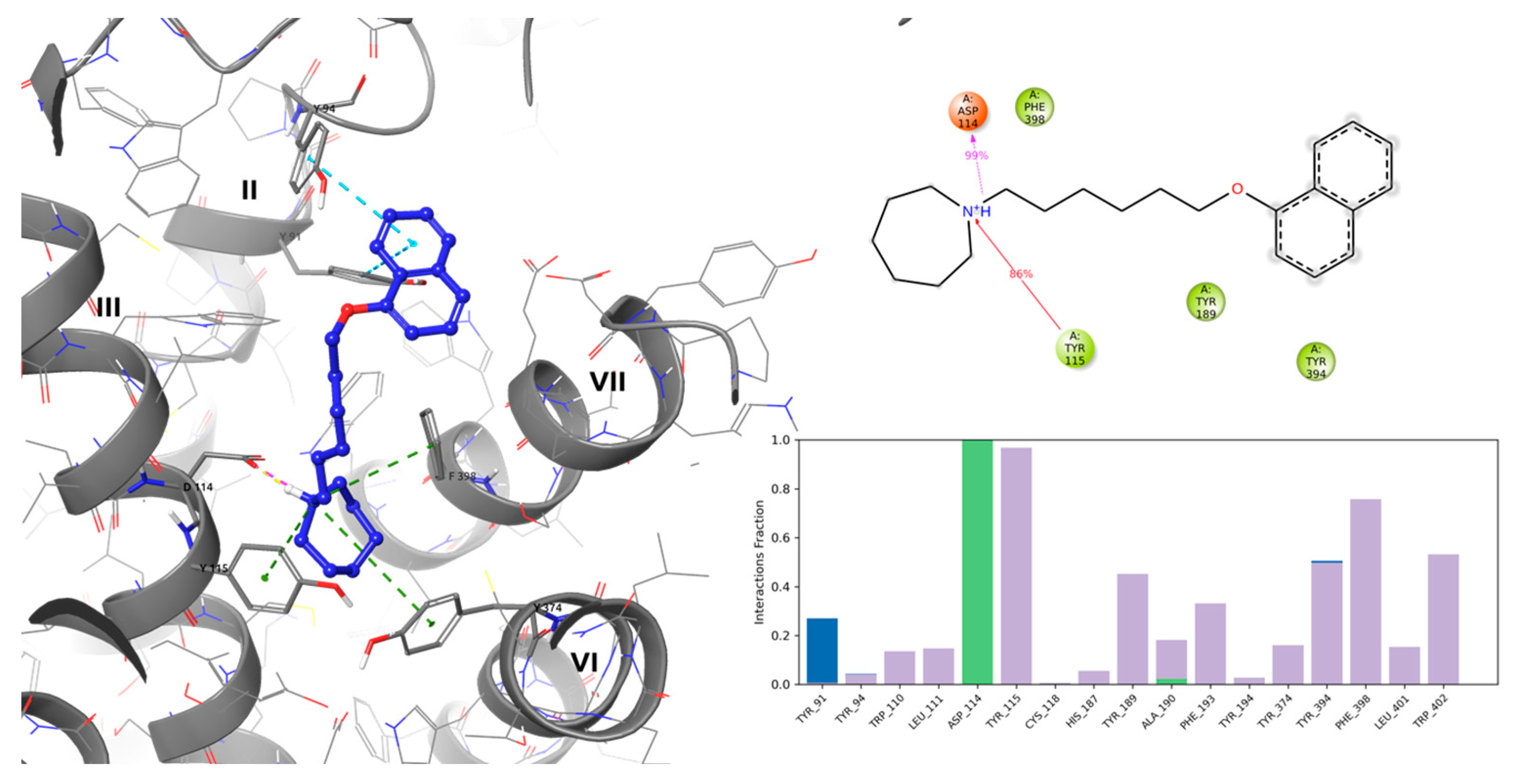
2.1. In Silico Evaluation of E169
2.1.1. In Silico ADMET and Drug-Likeness Properties
2.1.2. In Silico Docking Studies
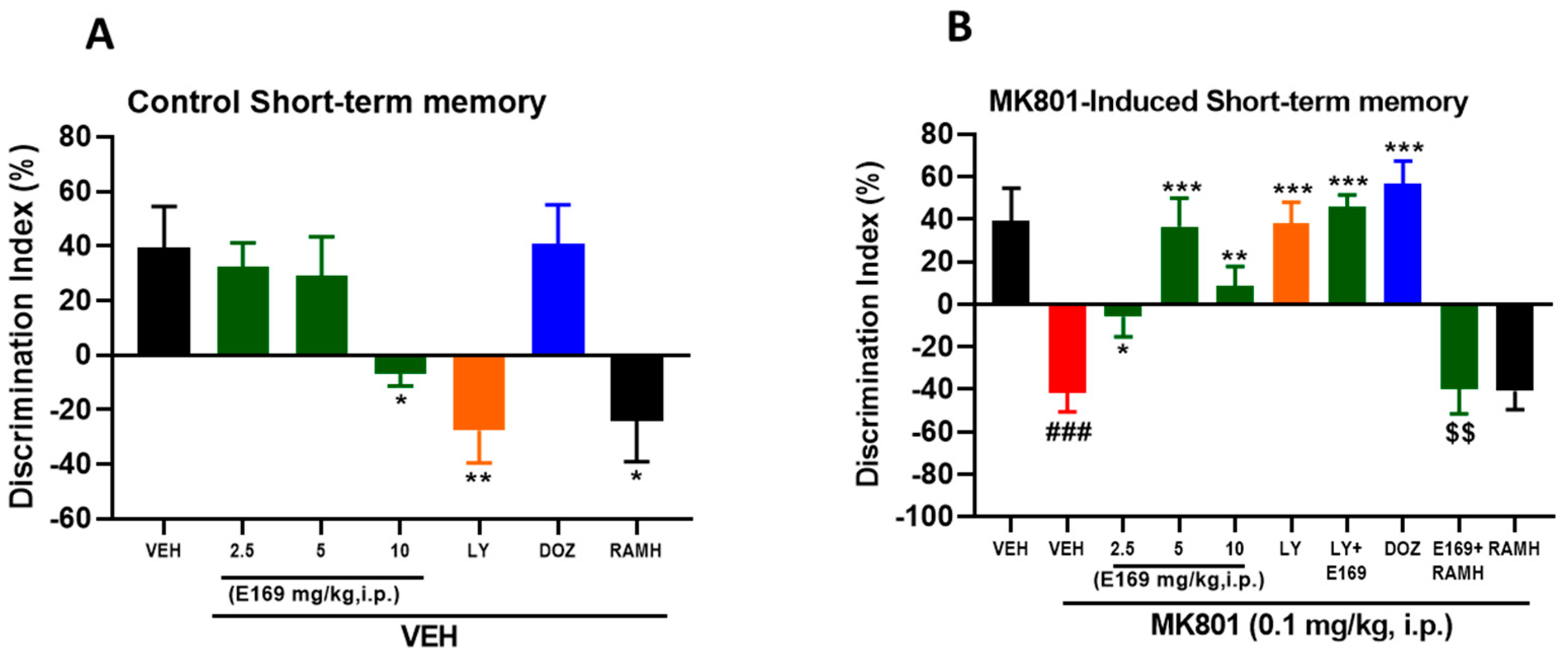
2.2. E169 Ameliorated MK801-Induced Short-Term Memory Deficits in C57BL/6J Mice
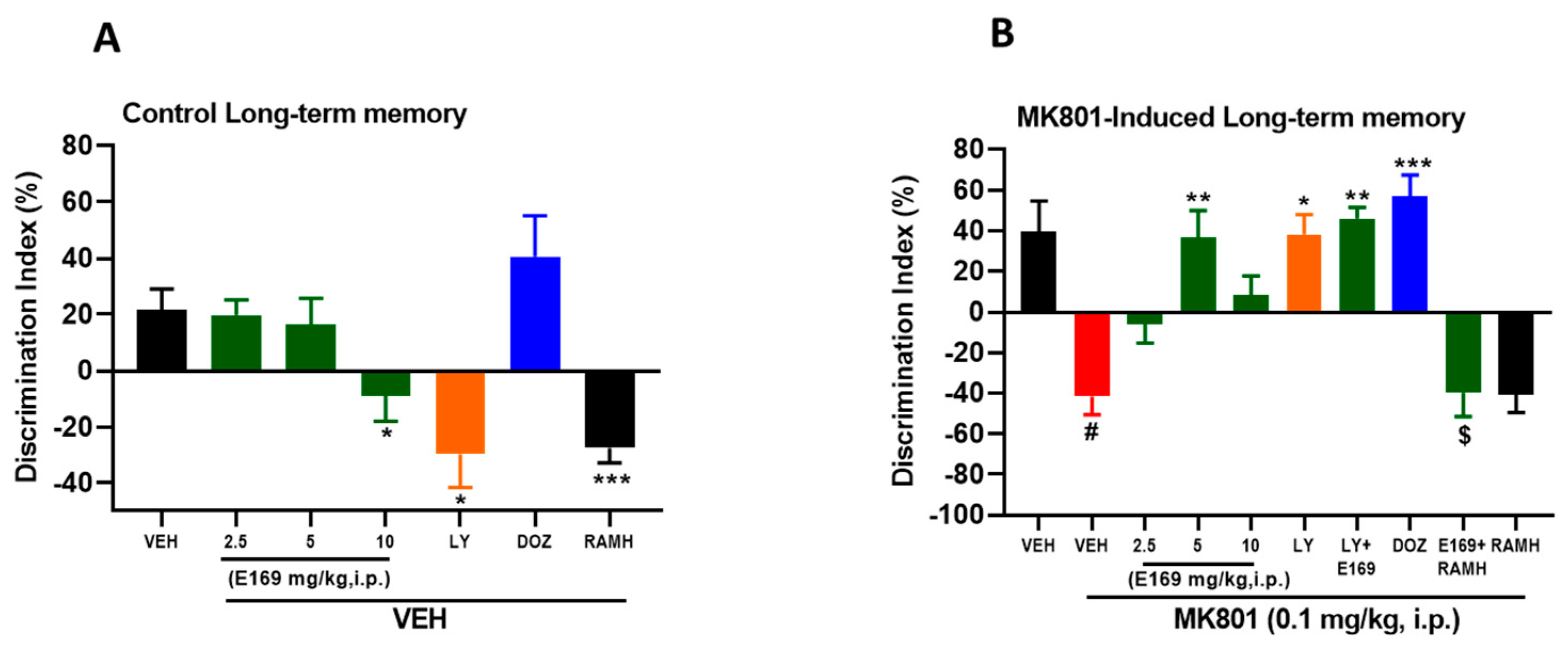
2.3. E169 Ameliorated MK801-Induced Long-Term Memory Deficits in C57BL/6J Mice
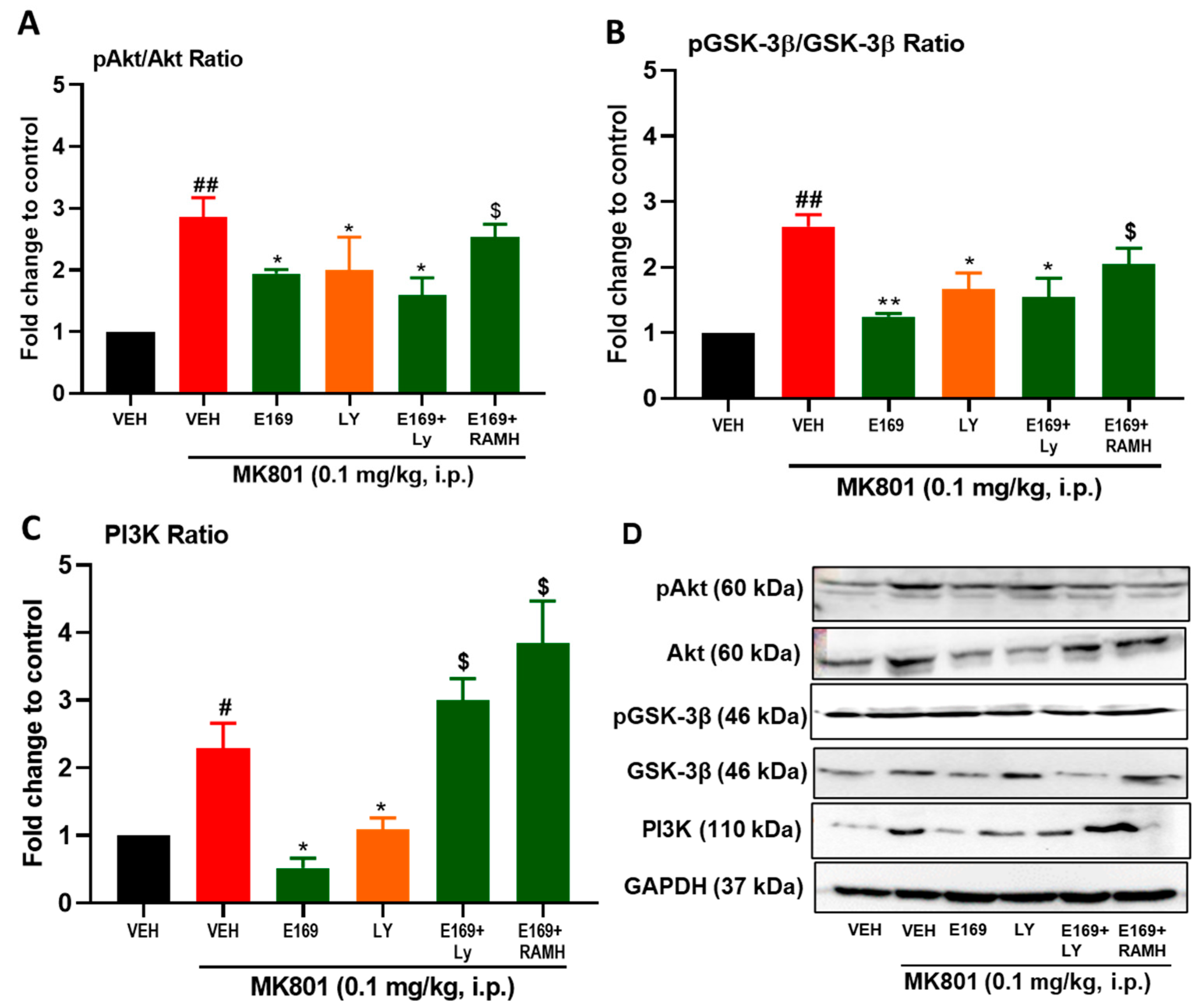
2.4. E169 Mitigated the Level of Disturbance in Expression of PI3K, Akt, and GSK-3β Proteins in the Hippocampus of Treated Mice
2.5. Effects of E169 on Disturbed Anxiety-Like Levels and Locomotor Activity of Tested Mice
2.5.1. Results for Control Mice Assessed on STM and LTM in OFT
2.5.2. Results for Mice with MK801-Induced Amnesia on STM and LTM in OFT (Amnesic Mice)
3. Discussion
4. Materials and Methods
4.1. In Silico Calculations
4.1.1. ADMET Calculations
4.1.2. Molecular Docking Studies
4.2. Animals
4.3. Drugs
4.4. Reagents and Antibodies
4.5. Behavioral Tests
4.5.1. Novel Object Recognition Test (NORT)
- -
- MK801 (0.1 mg/kg i.p.) was injected 15 min before (T1) and (T2) sessions;
- -
- E169 (2.5–10 mg/kg i.p.) was injected 60 min before (T1) and (T2) sessions;
- -
- LY294002 (LY; 7.5 mg/kg, i.p.) was injected 75 min before (T1) and (T2) sessions;
- -
- RAMH (10 mg/kg, i.p.) was injected 30 min before (T1) and (T2) sessions;
- -
- VEH (DMSO) was injected at all these time points, 75, 60, 30, 15 min, before (T1) and (T2).
4.5.2. Open Field Test (OFT)
4.6. Biochemical Assessment
4.6.1. Brain Collection and Tissue Processing
4.6.2. Western Blotting
4.7. Statistical Analyses
5. Conclusions
6. Study Limitations and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Medhurst, A.; Roberts, J.; Lee, J.; Chen, C.-H.; Brown, S.; Roman, S.; Lai, M. Characterization of Histamine H3 Receptors in Alzheimer’s Disease Brain and Amyloid over-Expressing TASTPM Mice. Br. J. Pharmacol. 2009, 157, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Medhurst, A.D.; Atkins, A.R.; Beresford, I.J.; Brackenborough, K.; Briggs, M.A.; Calver, A.R.; Cilia, J.; Cluderay, J.E.; Crook, B.; Davis, J.B.; et al. GSK189254, a Novel H3 Receptor Antagonist That Binds to Histamine H3 Receptors in Alzheimer’s Disease Brain and Improves Cognitive Performance in Preclinical Models. J. Pharm. Exp. 2007, 321, 1032–1045. [Google Scholar] [CrossRef] [PubMed]
- Lopes da Silva, S.; Vellas, B.; Elemans, S.; Luchsinger, J.; Kamphuis, P.; Yaffe, K.; Sijben, J.; Groenendijk, M.; Stijnen, T. Plasma Nutrient Status of Patients with Alzheimer’s Disease: Systematic Review and Meta-Analysis. Alzheimer’s Dement. 2014, 10, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Doraiswamy, P.M. Non-Cholinergic Strategies for Treating and Preventing Alzheimer’s Disease. CNS Drugs 2002, 16, 811–824. [Google Scholar] [CrossRef]
- Cavalli, A.; Bolognesi, M.L.; Minarini, A.; Rosini, M.; Tumiatti, V.; Recanatini, M.; Melchiorre, C. Multi-Target-Directed Ligands to Combat Neurodegenerative Diseases. J. Med. Chem. 2008, 51, 347–372. [Google Scholar] [CrossRef] [PubMed]
- Alachkar, A.; Łażewska, D.; Kieć-Kononowicz, K.; Sadek, B. The Histamine H3 Receptor Antagonist E159 Reverses Memory Deficits Induced by Dizocilpine in Passive Avoidance and Novel Object Recognition Paradigm in Rats. Front. Pharm. 2017, 8, 709. [Google Scholar] [CrossRef] [PubMed]
- Sadek, B.; Stark, H. Cherry-Picked Ligands at Histamine Receptor Subtypes. Neuropharmacology 2016, 106, 56–73. [Google Scholar] [CrossRef]
- Sadek, B.; Saad, A.; Sadeq, A.; Jalal, F.; Stark, H. Histamine H3 Receptor as a Potential Target for Cognitive Symptoms in Neuropsychiatric Diseases. Behav. Brain Res. 2016, 312, 415–430. [Google Scholar] [CrossRef]
- Wake, H.; Moorhouse, A.J.; Miyamoto, A.; Nabekura, J. Microglia: Actively Surveying and Shaping Neuronal Circuit Structure and Function. Trends Neurosci. 2013, 36, 209–217. [Google Scholar] [CrossRef]
- Eissa, N.; Awad, M.A.; Thomas, S.D.; Venkatachalam, K.; Jayaprakash, P.; Zhong, S.; Stark, H.; Sadek, B. Simultaneous Antagonism at H3R/D2R/D3R Reduces Autism-like Self-Grooming and Aggressive Behaviors by Mitigating MAPK Activation in Mice. Int. J. Mol. Sci. 2022, 24, 526. [Google Scholar] [CrossRef]
- Obulesu, M.; Jhansilakshmi, M. Neuroinflammation in Alzheimer’s Disease: An Understanding of Physiology and Pathology. Int. J. Neurosci. 2014, 124, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Bagyinszky, E.; Giau, V.V.; Shim, K.; Suk, K.; An, S.S.A.; Kim, S. Role of Inflammatory Molecules in the Alzheimer’s Disease Progression and Diagnosis. J. Neurol. Sci. 2017, 376, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Tournier, B.B.; Tsartsalis, S.; Ceyzériat, K.; Garibotto, V.; Millet, P. In Vivo TSPO Signal and Neuroinflammation in Alzheimer’s Disease. Cells 2020, 9, 1941. [Google Scholar] [CrossRef]
- O’Neill, L.A.J.; Hardie, D.G. Metabolism of Inflammation Limited by AMPK and Pseudo-Starvation. Nature 2013, 493, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Ge, F.; Wang, F.; Yan, X.; Li, Z.; Wang, X. Association of BAFF with PI3K/Akt/MTOR Signaling in Lupus Nephritis. Mol. Med. Rep. 2017, 16, 5793–5798. [Google Scholar] [CrossRef] [PubMed]
- Łażewska, D.; Kaleta, M.; Hagenow, S.; Mogilski, S.; Latacz, G.; Karcz, T.; Lubelska, A.; Honkisz, E.; Handzlik, J.; Reiner, D.; et al. Novel Naphthyloxy Derivatives - Potent Histamine H3 Receptor Ligands. Synthesis and Pharmacological Evaluation. Bioorg. Med. Chem. 2018, 26, 2573–2585. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Yang, L.; Liu, Z.; Lou, S.; Mei, S.; Li, M.; Chen, Z.; Zhang, H. Structural Basis for Recognition of Antihistamine Drug by Human Histamine Receptor. Nat. Commun. 2022, 13, 6105. [Google Scholar] [CrossRef]
- Levoin, N.; Calmels, T.; Poupardin-Olivier, O.; Labeeuw, O.; Danvy, D.; Robert, P.; Berrebi-Bertrand, I.; Ganellin, C.R.; Schunack, W.; Stark, H.; et al. Refined Docking as a Valuable Tool for Lead Optimization: Application to Histamine H3 Receptor Antagonists. Arch. Pharm. 2008, 341, 610–623. [Google Scholar] [CrossRef]
- Sadek, B.; Saad, A.; Subramanian, D.; Shafiullah, M.; Łażewska, D.; Kieć-Kononowiczc, K. Anticonvulsant and Procognitive Properties of the Non-Imidazole Histamine H3 Receptor Antagonist DL77 in Male Adult Rats. Neuropharmacology 2016, 106, 46–55. [Google Scholar] [CrossRef]
- Winters, B.D.; Saksida, L.M.; Bussey, T.J. Object Recognition Memory: Neurobiological Mechanisms of Encoding, Consolidation and Retrieval. Neurosci. Biobehav. Rev. 2008, 32, 1055–1070. [Google Scholar] [CrossRef]
- Giovannini, M.G.; Bartolini, L.; Bacciottini, L.; Greco, L.; Blandina, P. Effects of Histamine H3 Receptor Agonists and Antagonists on Cognitive Performance and Scopolamine-Induced Amnesia. Behav. Brain Res. 1999, 104, 147–155. [Google Scholar] [CrossRef]
- Ligneau, X.; Perrin, D.; Landais, L.; Camelin, J.-C.; Calmels, T.P.G.; Berrebi-Bertrand, I.; Lecomte, J.-M.; Parmentier, R.; Anaclet, C.; Lin, J.-S.; et al. BF2.649 [1-{3-[3-(4-Chlorophenyl)Propoxy]Propyl}piperidine, Hydrochloride], a Nonimidazole Inverse Agonist/Antagonist at the Human Histamine H3 Receptor: Preclinical Pharmacology. J. Pharm. Exp. 2007, 320, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Giannoni, P.; Medhurst, A.D.; Passani, M.B.; Giovannini, M.G.; Ballini, C.; Corte, L.D.; Blandina, P. Regional Differential Effects of the Novel Histamine H3 Receptor Antagonist 6-[(3-Cyclobutyl-2,3,4,5-Tetrahydro-1H-3-Benzazepin-7-Yl)Oxy]-N-Methyl-3-Pyridinecarboxamide Hydrochloride (GSK189254) on Histamine Release in the Central Nervous System of Freely Moving Rats. J. Pharm. Exp. 2010, 332, 164–172. [Google Scholar] [CrossRef]
- Griebel, G.; Pichat, P.; Pruniaux, M.-P.; Beeské, S.; Lopez-Grancha, M.; Genet, E.; Terranova, J.-P.; Castro, A.; Sánchez, J.A.; Black, M.; et al. SAR110894, a Potent Histamine H₃-Receptor Antagonist, Displays Procognitive Effects in Rodents. Pharm. Biochem. Behav. 2012, 102, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Blandina, P.; Giorgetti, M.; Bartolini, L.; Cecchi, M.; Timmerman, H.; Leurs, R.; Pepeu, G.; Giovannini, M.G. Inhibition of Cortical Acetylcholine Release and Cognitive Performance by Histamine H3 Receptor Activation in Rats. Br. J. Pharm. 1996, 119, 1656–1664. [Google Scholar] [CrossRef] [PubMed]
- Witkin, J.M.; Nelson, D.L. Selective Histamine H3 Receptor Antagonists for Treatment of Cognitive Deficiencies and Other Disorders of the Central Nervous System. Pharmacol. Ther. 2004, 103, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Pascoli, V.; Boer-Saccomani, C.; Hermant, J.-F. H3 Receptor Antagonists Reverse Delay-Dependent Deficits in Novel Object Discrimination by Enhancing Retrieval. Psychopharmacology 2009, 202, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Dewachter, I.; Ris, L.; Jaworski, T.; Seymour, C.M.; Kremer, A.; Borghgraef, P.; De Vijver, H.; Godaux, E.; Van Leuven, F. GSK3beta, a Centre-Staged Kinase in Neuropsychiatric Disorders, Modulates Long Term Memory by Inhibitory Phosphorylation at Serine-9. Neurobiol. Dis. 2009, 35, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, S.F.; Pfankuch, T.; Ohtsu, H.; Raber, J. Anxiety and Cognition in Female Histidine Decarboxylase Knockout (Hdc(-/-)) Mice. Behav. Brain Res. 2006, 168, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Svenningsson, P.; Tzavara, E.T.; Carruthers, R.; Rachleff, I.; Wattler, S.; Nehls, M.; McKinzie, D.L.; Fienberg, A.A.; Nomikos, G.G.; Greengard, P. Diverse Psychotomimetics Act through a Common Signaling Pathway. Science 2003, 302, 1412–1415. [Google Scholar] [CrossRef]
- Bitner, R.S.; Markosyan, S.; Nikkel, A.L.; Brioni, J.D. In-Vivo Histamine H3 Receptor Antagonism Activates Cellular Signaling Suggestive of Symptomatic and Disease Modifying Efficacy in Alzheimer’s Disease. Neuropharmacology 2011, 60, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Passani, M.B.; Blandina, P. Histamine Receptors in the CNS as Targets for Therapeutic Intervention. Trends Pharmacol. Sci. 2011, 32, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Eissa, N.; Khan, N.; Ojha, S.K.; Łazewska, D.; Kieć-Kononowicz, K.; Sadek, B. The Histamine H3 Receptor Antagonist DL77 Ameliorates MK801-Induced Memory Deficits in Rats. Front. Neurosci. 2018, 12. [Google Scholar] [CrossRef] [PubMed]
- Watts, K.S.; Dalal, P.; Murphy, R.B.; Sherman, W.; Friesner, R.A.; Shelley, J.C. ConfGen: A Conformational Search Method for Efficient Generation of Bioactive Conformers. J. Chem. Inf. Model. 2010, 50, 534–546. [Google Scholar] [CrossRef]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shelley, M.; Perry, J.K.; et al. Glide: A New Approach for Rapid, Accurate Docking and Scoring. 1. Method and Assessment of Docking Accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Bowers, K.; Chow, E.; Xu, H.; Dror, R.; Eastwood, M.; Gregersen, B.; Klepeis, J.; Kolossvary, I.; Moraes, M.; Sacerdoti, F.; et al. Scalable Algorithms for Molecular Dynamics Simulations on Commodity Clusters. In Proceedings of the ACM/IEEE SC 2006 Conference on Supercomputing (SC’06), Tampa, FL, USA, 11–17 November 2006; ACM Press: New York, NY, USA, 2006; p. 84, ISBN 978-0-7695-2700-0. [Google Scholar]
- Lomize, M.A.; Lomize, A.L.; Pogozheva, I.D.; Mosberg, H.I. OPM: Orientations of Proteins in Membranes Database. Bioinformatics 2006, 22, 623–625. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of Simple Potential Functions for Simulating Liquid Water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Ennaceur, A.; Delacour, J. A New One-Trial Test for Neurobiological Studies of Memory in Rats. 1: Behavioral Data. Behav. Brain Res. 1988, 31, 47–59. [Google Scholar] [CrossRef]
- Prut, L.; Belzung, C. The Open Field as a Paradigm to Measure the Effects of Drugs on Anxiety-like Behaviors: A Review. Eur. J. Pharm. 2003, 463, 3–33. [Google Scholar] [CrossRef]
- Bahi, A.; Schwed, J.S.; Walter, M.; Stark, H.; Sadek, B. Anxiolytic and Antidepressant-like Activities of the Novel and Potent Non-Imidazole Histamine H3 Receptor Antagonist ST-1283. Drug Des. Devel. 2014, 8, 627–637. [Google Scholar] [CrossRef]
- Sadek, B.; Khan, N.; Darras, F.H.; Pockes, S.; Decker, M. The Dual-Acting AChE Inhibitor and H3 Receptor Antagonist UW-MD-72 Reverses Amnesia Induced by Scopolamine or Dizocilpine in Passive Avoidance Paradigm in Rats. Physiol. Behav. 2016, 165, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Eissa, N.; Azimullah, S.; Jayaprakash, P.; Jayaraj, R.L.; Reiner, D.; Ojha, S.K.; Beiram, R.; Stark, H.; Łażewska, D.; Kieć-Kononowicz, K.; et al. The Dual-Active Histamine H3 Receptor Antagonist and Acetylcholine Esterase Inhibitor E100 Alleviates Autistic-Like Behaviors and Oxidative Stress in Valproic Acid Induced Autism in Mice. Int. J. Mol. Sci. 2020, 21, 3996. [Google Scholar] [CrossRef] [PubMed]
- Walsh, R.N.; Cummins, R.A. The Open-Field Test: A Critical Review. Psychol. Bull 1976, 83, 482–504. [Google Scholar] [CrossRef] [PubMed]
- Eissa, N.; Venkatachalam, K.; Jayaprakash, P.; Falkenstein, M.; Dubiel, M.; Frank, A.; Reiner-Link, D.; Stark, H.; Sadek, B. The Multi-Targeting Ligand ST-2223 with Histamine H3 Receptor and Dopamine D2/D3 Receptor Antagonist Properties Mitigates Autism-Like Repetitive Behaviors and Brain Oxidative Stress in Mice. Int. J. Mol. Sci. 2021, 22, 1947. [Google Scholar] [CrossRef]
- Venkatachalam, K.; Eissa, N.; Awad, M.A.; Jayaprakash, P.; Zhong, S.; Stölting, F.; Stark, H.; Sadek, B. The Histamine H3R and Dopamine D2R/D3R Antagonist ST-713 Ameliorates Autism-like Behavioral Features in BTBR T+tf/J Mice by Multiple Actions. Biomed. Pharm. 2021, 138, 111517. [Google Scholar] [CrossRef]
- Squillace, M.; Dodero, L.; Federici, M.; Migliarini, S.; Errico, F.; Napolitano, F.; Krashia, P.; Di Maio, A.; Galbusera, A.; Bifone, A.; et al. Dysfunctional Dopaminergic Neurotransmission in Asocial BTBR Mice. Transl. Psychiatry 2014, 4, e427. [Google Scholar] [CrossRef]

| Group | Time Spent in Center (s) | Time Spent in Periphery (s) | Total Distance Travelled (cm) | |||
|---|---|---|---|---|---|---|
| A. Control | STM | LTM | STM | LTM | STM | LTM |
| VEH | 123.86 ± 0.37 | 156.91 ± 4.54 | 176.14 ± 1.41 | 143.09 ± 18.62 | 3304.84 ± 206.76 | 2884.67 ± 172.10 |
| E169 (2.5 mg) | 118.35 ± 5.58 | 152.59 ± 4.20 | 181.65 ± 11.26 | 147.41 ± 7.50 | 3140.35 ± 257.18 | 2670 ± 47.31 |
| E169 (5 mg) | 146.51 ± 5.45 | 149.71 ± 7.50 | 153.49 ± 11.64 | 150.29 ± 10.87 | 3093.34 ± 215.06 | 2487.24 ± 110.40 |
| E169 (10 mg) | 150.30 ± 7.78 | 159.21 ± 10.08 | 149.70 ± 12.72 | 140.79 ± 4.41 | 3145.25 ± 165.51 | 2570.80 ± 83.06 |
| LY294002 | 75.22 ± 7.44 *** | 109.27 ± 6.48 *** | 224.78 ± 9.33 *** | 190.73 ± 10.41 ** | 3106.14 ± 180.92 | 2745.22 ± 145.24 |
| RAMH | 90.22 ± 10.65 ** | 112.56 ± 7.68 *** | 209.78 ± 11.80 * | 187.44 ± 14.90 * | 3002.52 ± 309.43 | 2686.43 ± 81.74 |
| B. MK801 | STM | LTM | STM | LTM | STM | LTM |
| VEH | 97.70 ± 3.03 #### | 121.12 ± 6.07 ## | 202.3 ± 10.98 # | 178.88 ± 4.56 # | 2701 ± 285.58 | 2531.66 ± 42.18 |
| E169 (2.5 mg) | 110.95 ± 9.33 | 131.47 ± 6.64 | 189.05 ± 16.68 | 168.53 ± 9.564 | 2854.33 ± 71.75 | 2648.32 ± 104.28 |
| E169 (5 mg) | 135.54 ± 9.42 ** | 154.08 ± 6.63 * | 164.46 ± 6.33 * | 145.92 ± 10.67 * | 2801.36 ± 91.97 | 2705.98 ± 90.67 |
| E169 (10 mg) | 122.98 ± 8.42 * | 133.45 ± 5.75 | 177.02 ± 5.77 * | 166.55 ± 11.73 | 2794.12 ± 65.99 | 2629.48 ± 60.76 |
| LY294002 | 138.58 ± 5.36 **** | 148.29 ± 6.58 * | 161.42 ± 6.84 * | 151.71 ± 4.44 * | 2956.03 ± 37.58 | 2722.14 ± 133.25 |
| E169 + RAMH | 111.49 ± 4.74 $ | 118.18 ± 2.36 $ | 188.51 ± 4.24 $$ | 181.82 ± 6.55 $ | 2807.93 ± 93.09 | 2744.54 ± 81.64 |
| E169 + LY294002 | 152.52 ± 8.80 * | 148.62 ± 8.27 * | 147.48 ± 10.02 * | 151.38 ± 12.79 * | 2797.74 ± 105.52 | 2700.49 ± 104.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdalla, S.; Eissa, N.; Jayaprakash, P.; Beiram, R.; Kuder, K.J.; Łażewska, D.; Kieć-Kononowicz, K.; Sadek, B. The Potent and Selective Histamine H3 Receptor Antagonist E169 Counteracts Cognitive Deficits and Mitigates Disturbances in the PI3K/AKT/GSK-3β Signaling Pathway in MK801-Induced Amnesia in Mice. Int. J. Mol. Sci. 2023, 24, 12719. https://doi.org/10.3390/ijms241612719
Abdalla S, Eissa N, Jayaprakash P, Beiram R, Kuder KJ, Łażewska D, Kieć-Kononowicz K, Sadek B. The Potent and Selective Histamine H3 Receptor Antagonist E169 Counteracts Cognitive Deficits and Mitigates Disturbances in the PI3K/AKT/GSK-3β Signaling Pathway in MK801-Induced Amnesia in Mice. International Journal of Molecular Sciences. 2023; 24(16):12719. https://doi.org/10.3390/ijms241612719
Chicago/Turabian StyleAbdalla, Sabna, Nermin Eissa, Petrilla Jayaprakash, Rami Beiram, Kamil J. Kuder, Dorota Łażewska, Katarzyna Kieć-Kononowicz, and Bassem Sadek. 2023. "The Potent and Selective Histamine H3 Receptor Antagonist E169 Counteracts Cognitive Deficits and Mitigates Disturbances in the PI3K/AKT/GSK-3β Signaling Pathway in MK801-Induced Amnesia in Mice" International Journal of Molecular Sciences 24, no. 16: 12719. https://doi.org/10.3390/ijms241612719
APA StyleAbdalla, S., Eissa, N., Jayaprakash, P., Beiram, R., Kuder, K. J., Łażewska, D., Kieć-Kononowicz, K., & Sadek, B. (2023). The Potent and Selective Histamine H3 Receptor Antagonist E169 Counteracts Cognitive Deficits and Mitigates Disturbances in the PI3K/AKT/GSK-3β Signaling Pathway in MK801-Induced Amnesia in Mice. International Journal of Molecular Sciences, 24(16), 12719. https://doi.org/10.3390/ijms241612719







