Implications of Physical Exercise on Episodic Memory and Anxiety: The Role of the Serotonergic System
Abstract
1. Introduction
2. Episodic Memory
3. Anxiety
4. Episodic Memory and Anxiety
5. Exercise and Episodic Memory
5.1. Clinical Studies about Exercise and Episodic Memory
5.2. Pre-Clinical Studies about Exercise and Episodic Memory
6. Exercise and Anxiety
6.1. Pre-Clinical Studies on Exercise and Anxiety
6.2. Clinical Studies about Exercise and Anxiety
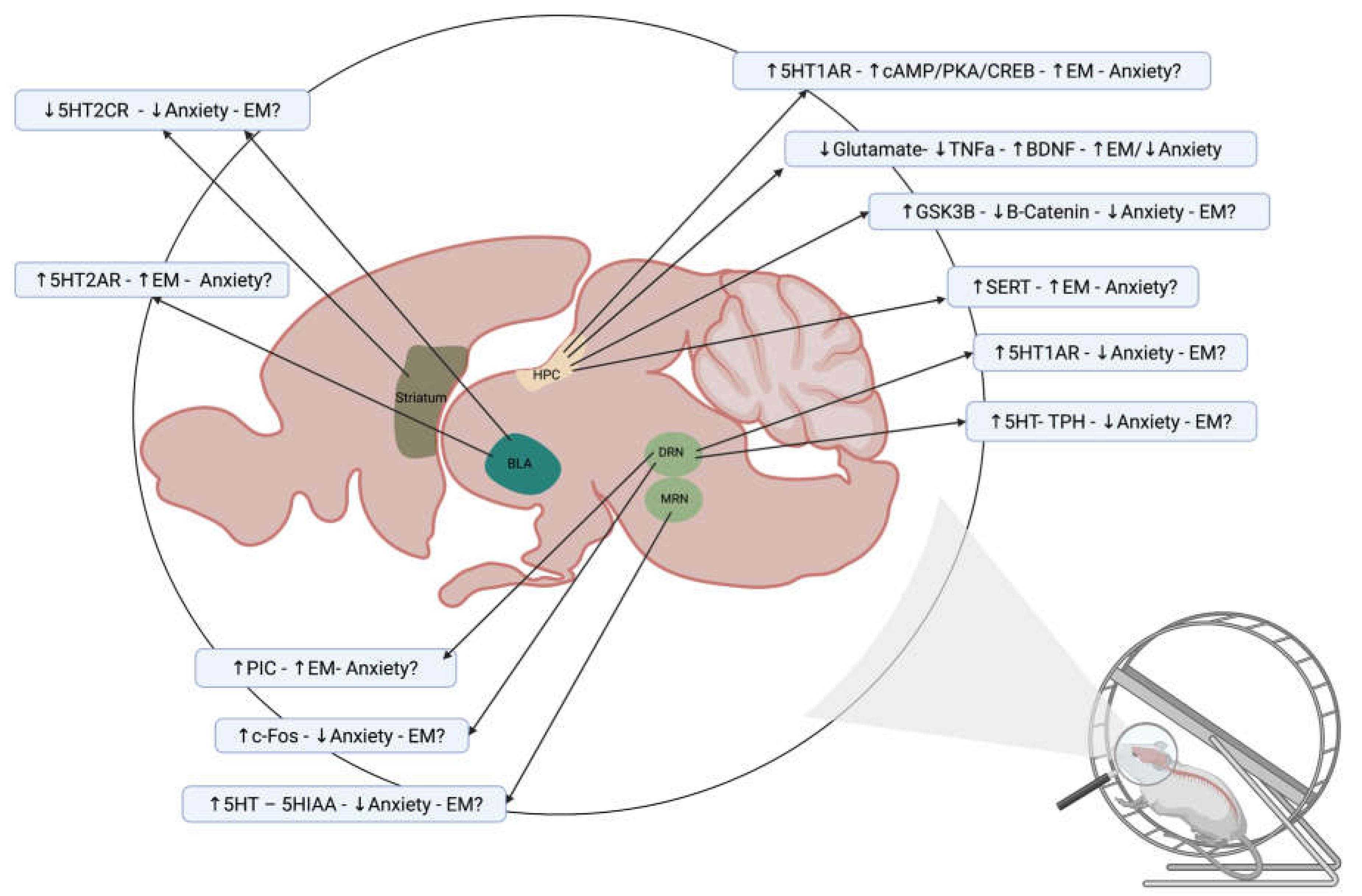
7. The Role of the Serotoninergic System on the Effects of Exercise on Episodic Memory and Anxiety
| Type of Study | Type of Exercise | Effect on Episodic Memory | References | Effect on Anxiety | References |
|---|---|---|---|---|---|
| Preclinical | Aerobic | Improvement | [100,102,103,105,106,107,117,122,133,134] | Anxiolytic | [101,104,106,112,113,117,121,123,124,133,134] |
| Resistance | Improvement | [64] | N.D. | ||
| Clinical | Aerobic | Improvement | [67,68,69,72,73,74,75,76,77,78,79,80,81,83,87,88,89,92,93,94,96,97,98] | Anxiolytic | [125] |
| Resistance | Improvement | [71,84,91,93,96,97,98] | Anxiolytic | [127] |
| Type of Exercise | Effect on Memory by the 5-HT System | References | Effect on Anxiety by the 5-HT System | References |
|---|---|---|---|---|
| Aerobic | Improvement | [29] | Anxiolytic | [137,138,143,144,147,151,153,155,156,158] |
| Resistance | N.D. | N.D. |
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miller, R.M.; Marriott, D.; Trotter, J.; Hammond, T.; Lyman, D.; Call, T.; Walker, B.; Christensen, N.; Haynie, D.; Badura, Z.; et al. Running Exercise Mitigates the Negative Consequences of Chronic Stress on Dorsal Hippocampal Long-Term Potentiation in Male Mice. Neurobiol. Learn. Mem. 2018, 149, 28–38. [Google Scholar] [CrossRef]
- Sun, Q.; Townsend, M.K.; Okereke, O.I.; Franco, O.H.; Hu, F.B.; Grodstein, F. Physical Activity at Midlife in Relation to Successful Survival in Women at Age 70 Years or Older. Arch. Intern. Med. 2010, 170, 194–201. [Google Scholar] [CrossRef]
- Janssen, I.; Leblanc, A.G. Systematic Review of the Health Benefits of Physical Activity and Fitness in School-Aged Children and Youth. Int. J. Behav. Nutr. Phys. Act. 2010, 7, 40. [Google Scholar] [CrossRef]
- World Health Organization. Global Status Report on Physical Activity 2022; World Health Organization: Geneva, Switzerland, 2022; ISBN 978-92-4-005915-3. [Google Scholar]
- Santos, A.C.; Willumsen, J.; Meheus, F.; Ilbawi, A.; Bull, F.C. The Cost of Inaction on Physical Inactivity to Public Health-Care Systems: A Population-Attributable Fraction Analysis. Lancet Glob. Health 2023, 11, e32–e39. [Google Scholar] [CrossRef]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M.; Diabetes Prevention Program Research Group. Reduction in the Incidence of Type 2 Diabetes with Lifestyle Intervention or Metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [CrossRef]
- Lee, D.-C.; Pate, R.R.; Lavie, C.J.; Sui, X.; Church, T.S.; Blair, S.N. Leisure-Time Running Reduces All-Cause and Cardiovascular Mortality Risk. J. Am. Coll. Cardiol. 2014, 64, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Blair, S.N.; Morris, J.N. Healthy Hearts—And the Universal Benefits of Being Physically Active: Physical Activity and Health. Ann. Epidemiol. 2009, 19, 253–256. [Google Scholar] [CrossRef]
- Berchicci, M.; Lucci, G.; Di Russo, F. Benefits of Physical Exercise on the Aging Brain: The Role of the Prefrontal Cortex. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 1337–1341. [Google Scholar] [CrossRef]
- Langlois, F.; Vu, T.T.M.; Chassé, K.; Dupuis, G.; Kergoat, M.-J.; Bherer, L. Benefits of Physical Exercise Training on Cognition and Quality of Life in Frail Older Adults. J. Gerontol. B Psychol. Sci. Soc. Sci. 2013, 68, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Tomporowski, P.D.; Davis, C.L.; Miller, P.H.; Naglieri, J.A. Exercise and Children’s Intelligence, Cognition, and Academic Achievement. Educ. Psychol. Rev. 2008, 20, 111–131. [Google Scholar] [CrossRef] [PubMed]
- Stroth, S.; Kubesch, S.; Dieterle, K.; Ruchsow, M.; Heim, R.; Kiefer, M. Physical Fitness, but Not Acute Exercise Modulates Event-Related Potential Indices for Executive Control in Healthy Adolescents. Brain Res. 2009, 1269, 114–124. [Google Scholar] [CrossRef]
- Aghjayan, S.L.; Bournias, T.; Kang, C.; Zhou, X.; Stillman, C.M.; Donofry, S.D.; Kamarck, T.W.; Marsland, A.L.; Voss, M.W.; Fraundorf, S.H.; et al. Aerobic Exercise Improves Episodic Memory in Late Adulthood: A Systematic Review and Meta-Analysis. Commun. Med. 2022, 2, 15. [Google Scholar] [CrossRef]
- Vreugdenhil, A.; Cannell, J.; Davies, A.; Razay, G. A Community-Based Exercise Programme to Improve Functional Ability in People with Alzheimer’s Disease: A Randomized Controlled Trial. Scand. J. Caring Sci. 2012, 26, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Liang, J.; Xu, Y.; Wang, Y. Effects of Physical Activity and Exercise on the Cognitive Function of Patients with Alzheimer Disease: A Meta-Analysis. BMC Geriatr. 2019, 19, 181. [Google Scholar] [CrossRef] [PubMed]
- David, F.J.; Robichaud, J.A.; Leurgans, S.E.; Poon, C.; Kohrt, W.M.; Goldman, J.G.; Comella, C.L.; Vaillancourt, D.E.; Corcos, D.M. Exercise Improves Cognition in Parkinson’s Disease: The PRET-PD Randomized, Clinical Trial. Mov. Disord. 2015, 30, 1657–1663. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, S.; Sherrington, C.; Tiedemann, A. Exercise Augmentation Compared with Usual Care for Post-Traumatic Stress Disorder: A Randomized Controlled Trial. Acta Psychiatr. Scand. 2015, 131, 350–359. [Google Scholar] [CrossRef]
- Sylvia, L.G.; Friedman, E.S.; Kocsis, J.H.; Bernstein, E.E.; Brody, B.D.; Kinrys, G.; Kemp, D.E.; Shelton, R.C.; McElroy, S.L.; Bobo, W.V.; et al. Association of Exercise with Quality of Life and Mood Symptoms in a Comparative Effectiveness Study of Bipolar Disorder. J. Affect. Disord. 2013, 151, 722–727. [Google Scholar] [CrossRef]
- Dauwan, M.; Begemann, M.J.H.; Heringa, S.M.; Sommer, I.E. Exercise Improves Clinical Symptoms, Quality of Life, Global Functioning, and Depression in Schizophrenia: A Systematic Review and Meta-Analysis. Schizophr. Bull. 2016, 42, 588–599. [Google Scholar] [CrossRef]
- Acil, A.A.; Dogan, S.; Dogan, O. The Effects of Physical Exercises to Mental State and Quality of Life in Patients with Schizophrenia. J. Psychiatr. Ment. Health Nurs. 2008, 15, 808–815. [Google Scholar] [CrossRef]
- Airaksinen, E.; Larsson, M.; Forsell, Y. Neuropsychological Functions in Anxiety Disorders in Population-Based Samples: Evidence of Episodic Memory Dysfunction. J. Psychiatr. Res. 2005, 39, 207–214. [Google Scholar] [CrossRef]
- Deckersbach, T.; Savage, C.R.; Reilly-Harrington, N.; Clark, L.; Sachs, G.; Rauch, S.L. Episodic Memory Impairment in Bipolar Disorder and Obsessive–Compulsive Disorder: The Role of Memory Strategies. Bipolar Disord. 2004, 6, 233–244. [Google Scholar] [CrossRef]
- Bearden, C.E.; Glahn, D.C.; Monkul, E.S.; Barrett, J.; Najt, P.; Villarreal, V.; Soares, J.C. Patterns of Memory Impairment in Bipolar Disorder and Unipolar Major Depression. Psychiatry Res. 2006, 142, 139–150. [Google Scholar] [CrossRef]
- Sinoff, G.; Werner, P. Anxiety Disorder and Accompanying Subjective Memory Loss in the Elderly as a Predictor of Future Cognitive Decline. Int. J. Geriatr. Psychiatry 2003, 18, 951–959. [Google Scholar] [CrossRef]
- Contreras-Osorio, F.; Ramirez-Campillo, R.; Cerda-Vega, E.; Campos-Jara, R.; Martínez-Salazar, C.; Reigal, R.E.; Hernández-Mendo, A.; Carneiro, L.; Campos-Jara, C. Effects of Physical Exercise on Executive Function in Adults with Depression: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public. Health 2022, 19, 15270. [Google Scholar] [CrossRef] [PubMed]
- Lago, T.R.; Hsiung, A.; Leitner, B.P.; Duckworth, C.J.; Balderston, N.L.; Chen, K.Y.; Grillon, C.; Ernst, M. Exercise Modulates the Interaction between Cognition and Anxiety in Humans. Cogn. Emot. 2019, 33, 863–870. [Google Scholar] [CrossRef]
- Griffin, É.W.; Mullally, S.; Foley, C.; Warmington, S.A.; O’Mara, S.M.; Kelly, Á.M. Aerobic Exercise Improves Hippocampal Function and Increases BDNF in the Serum of Young Adult Males. Physiol. Behav. 2011, 104, 934–941. [Google Scholar] [CrossRef]
- Seifert, T.; Brassard, P.; Wissenberg, M.; Rasmussen, P.; Nordby, P.; Stallknecht, B.; Adser, H.; Jakobsen, A.H.; Pilegaard, H.; Nielsen, H.B.; et al. Endurance Training Enhances BDNF Release from the Human Brain. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2010, 298, R372–R377. [Google Scholar] [CrossRef] [PubMed]
- Pietrelli, A.; Matković, L.; Vacotto, M.; Lopez-Costa, J.J.; Basso, N.; Brusco, A. Aerobic Exercise Upregulates the BDNF-Serotonin Systems and Improves the Cognitive Function in Rats. Neurobiol. Learn. Mem. 2018, 155, 528–542. [Google Scholar] [CrossRef]
- Straube, B. An Overview of the Neuro-Cognitive Processes Involved in the Encoding, Consolidation, and Retrieval of True and False Memories. Behav. Brain Funct. 2012, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Atance, C.M.; O’Neill, D.K. Episodic Future Thinking. Trends Cogn. Sci. 2001, 5, 533–539. [Google Scholar] [CrossRef]
- Szpunar, K.K.; Watson, J.M.; McDermott, K.B. Neural Substrates of Envisioning the Future. Proc. Natl. Acad. Sci. USA 2007, 104, 642–647. [Google Scholar] [CrossRef]
- Dickerson, S.S.; Kemeny, M.E. Acute Stressors and Cortisol Responses: A Theoretical Integration and Synthesis of Laboratory Research. Psychol. Bull. 2004, 130, 355–391. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.A.; Fortin, N.J. The Evolution of Episodic Memory. Proc. Natl. Acad. Sci. USA 2013, 110, 10379–10386. [Google Scholar] [CrossRef]
- Dickerson, B.C.; Sperling, R.A. Functional Abnormalities of the Medial Temporal Lobe Memory System in Mild Cognitive Impairment and Alzheimer’s Disease: Insights from Functional MRI Studies. Neuropsychologia 2008, 46, 1624–1635. [Google Scholar] [CrossRef] [PubMed]
- Lippa, C.F.; Dickson, D.W. Hippocampal Sclerosis Dementia: Expanding the Phenotypes of Frontotemporal Dementias? Neurology 2004, 63, 414–415. [Google Scholar] [CrossRef]
- Drevets, W.C.; Price, J.L.; Furey, M.L. Brain Structural and Functional Abnormalities in Mood Disorders: Implications for Neurocircuitry Models of Depression. Brain Struct. Funct. 2008, 213, 93–118. [Google Scholar] [CrossRef]
- Shin, L.M.; Shin, P.S.; Heckers, S.; Krangel, T.S.; Macklin, M.L.; Orr, S.P.; Lasko, N.; Segal, E.; Makris, N.; Richert, K.; et al. Hippocampal Function in Posttraumatic Stress Disorder. Hippocampus 2004, 14, 292–300. [Google Scholar] [CrossRef]
- Fung, A.W.T.; Lee, J.S.W.; Lee, A.T.C.; Lam, L.C.W. Anxiety Symptoms Predicted Decline in Episodic Memory in Cognitively Healthy Older Adults: A 3-Year Prospective Study. Int. J. Geriatr. Psychiatry 2018, 33, 748–754. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association, DSM-5 Task Force. Diagnostic and Statistical Manual of Mental Disorders: DSM-5TM, 5th ed.; American Psychiatric Publishing, Inc.: Arlington, VA, USA, 2013; p. xliv, 947. ISBN 978-0-89042-554-1. [Google Scholar]
- Belzung, C.; Philippot, P. Anxiety from a Phylogenetic Perspective: Is There a Qualitative Difference between Human and Animal Anxiety? Neural Plast. 2007, 2007, 59676. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; Chiu, W.T.; Demler, O.; Merikangas, K.R.; Walters, E.E. Prevalence, Severity, and Comorbidity of 12-Month DSM-IV Disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 2005, 62, 617–627. [Google Scholar] [CrossRef]
- World Health Organization. Depression and Other Common Mental Disorders; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Jameson, J.P.; Blank, M.B. Diagnosis and Treatment of Depression and Anxiety in Rural and Nonrural Primary Care: National Survey Results. Psychiatr. Serv. Wash. DC 2010, 61, 624–627. [Google Scholar] [CrossRef]
- Weisberg, R.B.; Dyck, I.; Culpepper, L.; Keller, M.B. Psychiatric Treatment in Primary Care Patients with Anxiety Disorders: A Comparison of Care Received from Primary Care Providers and Psychiatrists. Am. J. Psychiatry 2007, 164, 276–282. [Google Scholar] [CrossRef]
- Davidson, J.R. First-Line Pharmacotherapy Approaches for Generalized Anxiety Disorder. J. Clin. Psychiatry 2009, 70 (Suppl. S2), 25–31. [Google Scholar] [CrossRef] [PubMed]
- Huffman, J.C.; Alpert, J.E. An Approach to the Psychopharmacologic Care of Patients: Antidepressants, Antipsychotics, Anxiolytics, Mood Stabilizers, and Natural Remedies. Med. Clin. N. Am. 2010, 94, 1141–1160. [Google Scholar] [CrossRef]
- Bouton, M.E.; Mineka, S.; Barlow, D.H. A Modern Learning Theory Perspective on the Etiology of Panic Disorder. Psychol. Rev. 2001, 108, 4–32. [Google Scholar] [CrossRef] [PubMed]
- Mathews, A.; MacLeod, C. Cognitive Vulnerability to Emotional Disorders. Annu. Rev. Clin. Psychol. 2005, 1, 167–195. [Google Scholar] [CrossRef]
- Miranda, R.; Mennin, D.S. Depression, Generalized Anxiety Disorder, and Certainty in Pessimistic Predictions about the Future. Cogn. Ther. Res. 2007, 31, 71–82. [Google Scholar] [CrossRef]
- Ladouceur, R.; Gosselin, P.; Dugas, M.J. Experimental Manipulation of Intolerance of Uncertainty: A Study of a Theoretical Model of Worry. Behav. Res. Ther. 2000, 38, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Q.; Szpunar, K.K.; Godovich, S.A.; Schacter, D.L.; Hofmann, S.G. Episodic Future Thinking in Generalized Anxiety Disorder. J. Anxiety Disord. 2015, 36, 1–8. [Google Scholar] [CrossRef]
- Payne, J.D.; Jackson, E.D.; Hoscheidt, S.; Ryan, L.; Jacobs, W.J.; Nadel, L. Stress Administered Prior to Encoding Impairs Neutral but Enhances Emotional Long-Term Episodic Memories. Learn. Mem. 2007, 14, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Noël, A.; Quinette, P.; Guillery-Girard, B.; Dayan, J.; Piolino, P.; Marquis, S.; de la Sayette, V.; Viader, F.; Desgranges, B.; Eustache, F. Psychopathological Factors, Memory Disorders and Transient Global Amnesia. Br. J. Psychiatry J. Ment. Sci. 2008, 193, 145–151. [Google Scholar] [CrossRef]
- Lachman, M.E.; Agrigoroaei, S. Low Perceived Control as a Risk Factor for Episodic Memory: The Mediational Role of Anxiety and Task Interference. Mem. Cognit. 2012, 40, 287–296. [Google Scholar] [CrossRef]
- Moscovitch, D.A.; Gavric, D.L.; Merrifield, C.; Bielak, T.; Moscovitch, M. Retrieval Properties of Negative vs. Positive Mental Images and Autobiographical Memories in Social Anxiety: Outcomes with a New Measure. Behav. Res. Ther. 2011, 49, 505–517. [Google Scholar] [CrossRef]
- Cansino, S.; Torres-Trejo, F.; Estrada-Manilla, C.; Hernández-Ramos, E.; Martínez-Galindo, J.G.; Gómez-Fernández, T.; Ayala-Hernández, M.; Ramírez-González, M.D.; Ruiz-Velasco, S. Mediators of Episodic Memory Decay across the Adult Life Span. Sci. Rep. 2018, 8, 2610. [Google Scholar] [CrossRef]
- Pajkossy, P.; Racsmány, M. Beneficial Effect of Trait Worry on Episodic Retrieval: A Link Overshadowed by Trait Anxiety. Personal. Individ. Differ. 2014, 60, S52. [Google Scholar] [CrossRef]
- Pajkossy, P.; Keresztes, A.; Racsmány, M. The Interplay of Trait Worry and Trait Anxiety in Determining Episodic Retrieval: The Role of Cognitive Control. Q. J. Exp. Psychol. 2017, 70, 2234–2250. [Google Scholar] [CrossRef] [PubMed]
- Hitchcock, C.; Werner-Seidler, A.; Blackwell, S.E.; Dalgleish, T. Autobiographical Episodic Memory-Based Training for the Treatment of Mood, Anxiety and Stress-Related Disorders: A Systematic Review and Meta-Analysis. Clin. Psychol. Rev. 2017, 52, 92–107. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.G.; Madore, K.P.; Schacter, D.L. Worrying about the Future: An Episodic Specificity Induction Impacts Problem Solving, Reappraisal, and Well-Being. J. Exp. Psychol. Gen. 2016, 145, 402–418. [Google Scholar] [CrossRef] [PubMed]
- Cassilhas, R.C.; Tufik, S.; De Mello, M.T. Physical Exercise, Neuroplasticity, Spatial Learning and Memory. Cell. Mol. Life Sci. 2016, 73, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Voss, M.W.; Soto, C.; Yoo, S.; Sodoma, M.; Vivar, C.; Van Praag, H. Exercise and Hippocampal Memory Systems. Trends Cogn. Sci. 2019, 23, 318–333. [Google Scholar] [CrossRef]
- Cassilhas, R.C.; Viana, V.A.R.; Grassmann, V.; Santos, R.T.; Santos, R.F.; Tufik, S.; Mello, M.T. The Impact of Resistance Exercise on the Cognitive Function of the Elderly. Med. Sci. Sports Exerc. 2007, 39, 1401–1407. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Kassow, M.; Kulka, A.; Gunter, T.C.; Rothermich, K.; Kotz, S.A. Exercising during Learning Improves Vocabulary Acquisition: Behavioral and ERP Evidence. Neurosci. Lett. 2010, 482, 40–44. [Google Scholar] [CrossRef]
- Salas, C.R.; Minakata, K.; Kelemen, W.L. Walking before Study Enhances Free Recall but Not Judgement-of-Learning Magnitude. J. Cogn. Psychol. 2011, 23, 507–513. [Google Scholar] [CrossRef]
- Kattenstroth, J.-C.; Kalisch, T.; Holt, S.; Tegenthoff, M.; Dinse, H.R. Six Months of Dance Intervention Enhances Postural, Sensorimotor, and Cognitive Performance in Elderly without Affecting Cardio-Respiratory Functions. Front. Aging Neurosci. 2013, 5, 5. [Google Scholar] [CrossRef]
- Johnson, L.; Loprinzi, P.D. The Effects of Acute Exercise on Episodic Memory Function among Young University Students: Moderation Considerations by Biological Sex. Health Promot. Perspect. 2019, 9, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, P.D.; McRaney, K.; De Luca, K.; McDonald, A. Exercise and Episodic Specificity Induction on Episodic Memory Function. Medicina 2019, 55, 422. [Google Scholar] [CrossRef]
- Barha, C.K.; Davis, J.C.; Falck, R.S.; Nagamatsu, L.S.; Liu-Ambrose, T. Sex Differences in Exercise Efficacy to Improve Cognition: A Systematic Review and Meta-Analysis of Randomized Controlled Trials in Older Humans. Front. Neuroendocrinol. 2017, 46, 71–85. [Google Scholar] [CrossRef]
- Loprinzi, P.D.; Green, D.; Wages, S.; Cheke, L.G.; Jones, T. Experimental Effects of Acute High-Intensity Resistance Exercise on Episodic Memory Function: Consideration for Post-Exercise Recovery Period. J. Lifestyle Med. 2020, 10, 7–20. [Google Scholar] [CrossRef]
- Labban, J.D.; Etnier, J.L. Effects of Acute Exercise on Long-Term Memory. Res. Q. Exerc. Sport 2011, 82, 712–721. [Google Scholar] [CrossRef]
- Etnier, J.L.; Wideman, L.; Labban, J.D.; Piepmeier, A.T.; Pendleton, D.M.; Dvorak, K.K.; Becofsky, K. The Effects of Acute Exercise on Memory and Brain-Derived Neurotrophic Factor (BDNF). J. Sport Exerc. Psychol. 2016, 38, 331–340. [Google Scholar] [CrossRef]
- Labban, J.D.; Etnier, J.L. The Effect of Acute Exercise on Encoding and Consolidation of Long-Term Memory. J. Sport Exerc. Psychol. 2018, 40, 336–342. [Google Scholar] [CrossRef]
- Yanes, D.; Loprinzi, P. Experimental Effects of Acute Exercise on Iconic Memory, Short-Term Episodic, and Long-Term Episodic Memory. J. Clin. Med. 2018, 7, 146. [Google Scholar] [CrossRef] [PubMed]
- Sng, E.; Frith, E.; Loprinzi, P.D. Experimental Effects of Acute Exercise on Episodic Memory Acquisition: Decomposition of Multi-Trial Gains and Losses. Physiol. Behav. 2018, 186, 82–84. [Google Scholar] [CrossRef]
- Siddiqui, A.; Loprinzi, P. Experimental Investigation of the Time Course Effects of Acute Exercise on False Episodic Memory. J. Clin. Med. 2018, 7, 157. [Google Scholar] [CrossRef] [PubMed]
- Dilley, E.K.; Zou, L.; Loprinzi, P.D. The Effects of Acute Exercise Intensity on Episodic and False Memory among Young Adult College Students. Health Promot. Perspect. 2019, 9, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, P.D.; Roig, M.; Tomporowski, P.D.; Javadi, A.-H.; Kelemen, W.L. Effects of Acute Exercise on Memory: Considerations of Exercise Intensity, Post-Exercise Recovery Period and Aerobic Endurance. Mem. Cognit. 2023, 51, 1011–1026. [Google Scholar] [CrossRef]
- Suwabe, K.; Hyodo, K.; Byun, K.; Ochi, G.; Yassa, M.A.; Soya, H. Acute Moderate Exercise Improves Mnemonic Discrimination in Young Adults. Hippocampus 2017, 27, 229–234. [Google Scholar] [CrossRef]
- Suwabe, K.; Byun, K.; Hyodo, K.; Reagh, Z.M.; Roberts, J.M.; Matsushita, A.; Saotome, K.; Ochi, G.; Fukuie, T.; Suzuki, K.; et al. Rapid Stimulation of Human Dentate Gyrus Function with Acute Mild Exercise. Proc. Natl. Acad. Sci. USA 2018, 115, 10487–10492. [Google Scholar] [CrossRef]
- Loprinzi, P.D.; Edwards, M.K.; Frith, E. Potential Avenues for Exercise to Activate Episodic Memory-related Pathways: A Narrative Review. Eur. J. Neurosci. 2017, 46, 2067–2077. [Google Scholar] [CrossRef]
- Haynes, J.T.; Frith, E.; Sng, E.; Loprinzi, P.D. Experimental Effects of Acute Exercise on Episodic Memory Function: Considerations for the Timing of Exercise. Psychol. Rep. 2019, 122, 1744–1754. [Google Scholar] [CrossRef]
- Weinberg, L.; Hasni, A.; Shinohara, M.; Duarte, A. A Single Bout of Resistance Exercise Can Enhance Episodic Memory Performance. Acta Psychol. 2014, 153, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Amico, G.; Braun, T.; Schaefer, S. Can Acute Resistance Exercise Facilitate Episodic Memory Encoding? Curr. Psychol. 2023, 42, 10910–10923. [Google Scholar] [CrossRef]
- Loprinzi, P.D.; Loenneke, J.P.; Storm, B.C. Effects of Acute Aerobic and Resistance Exercise on Episodic Memory Function. Q. J. Exp. Psychol. 2021, 74, 1264–1283. [Google Scholar] [CrossRef] [PubMed]
- Sng, E.; Frith, E.; Loprinzi, P.D. Temporal Effects of Acute Walking Exercise on Learning and Memory Function. Am. J. Health Promot. 2018, 32, 1518–1525. [Google Scholar] [CrossRef]
- Frith, E.; Sng, E.; Loprinzi, P.D. Randomized Controlled Trial Evaluating the Temporal Effects of High-Intensity Exercise on Learning, Short-Term and Long-Term Memory, and Prospective Memory. Eur. J. Neurosci. 2017, 46, 2557–2564. [Google Scholar] [CrossRef] [PubMed]
- Hötting, K.; Reich, B.; Holzschneider, K.; Kauschke, K.; Schmidt, T.; Reer, R.; Braumann, K.-M.; Röder, B. Differential Cognitive Effects of Cycling versus Stretching/Coordination Training in Middle-Aged Adults. Health Psychol. 2012, 31, 145–155. [Google Scholar] [CrossRef]
- Iuliano, E.; Fiorilli, G.; Aquino, G.; Di Costanzo, A.; Calcagno, G.; Di Cagno, A. Twelve-Week Exercise Influences Memory Complaint but Not Memory Performance in Older Adults: A Randomized Controlled Study. J. Aging Phys. Act. 2017, 25, 612–620. [Google Scholar] [CrossRef]
- Best, J.R.; Chiu, B.K.; Liang Hsu, C.; Nagamatsu, L.S.; Liu-Ambrose, T. Long-Term Effects of Resistance Exercise Training on Cognition and Brain Volume in Older Women: Results from a Randomized Controlled Trial. J. Int. Neuropsychol. Soc. 2015, 21, 745–756. [Google Scholar] [CrossRef]
- Jonasson, L.S.; Nyberg, L.; Kramer, A.F.; Lundquist, A.; Riklund, K.; Boraxbekk, C.-J. Aerobic Exercise Intervention, Cognitive Performance, and Brain Structure: Results from the Physical Influences on Brain in Aging (PHIBRA) Study. Front. Aging Neurosci. 2017, 8, 336. [Google Scholar] [CrossRef]
- Komulainen, P.; Kivipelto, M.; Lakka, T.A.; Savonen, K.; Hassinen, M.; Kiviniemi, V.; Hänninen, T.; Rauramaa, R. Exercise, Fitness and Cognition—A Randomised Controlled Trial in Older Individuals: The DR’s EXTRA Study. Eur. Geriatr. Med. 2010, 1, 266–272. [Google Scholar] [CrossRef]
- Hayes, S.M.; Alosco, M.L.; Hayes, J.P.; Cadden, M.; Peterson, K.M.; Allsup, K.; Forman, D.E.; Sperling, R.A.; Verfaellie, M. Physical Activity Is Positively Associated with Episodic Memory in Aging. J. Int. Neuropsychol. Soc. 2015, 21, 780–790. [Google Scholar] [CrossRef]
- Dougherty, R.J.; Schultz, S.A.; Boots, E.A.; Ellingson, L.D.; Meyer, J.D.; Van Riper, S.; Stegner, A.J.; Edwards, D.F.; Oh, J.M.; Einerson, J.; et al. Relationships between Cardiorespiratory Fitness, Hippocampal Volume, and Episodic Memory in a Population at Risk for Alzheimer’s Disease. Brain Behav. 2017, 7, e00625. [Google Scholar] [CrossRef]
- Nouchi, R.; Taki, Y.; Takeuchi, H.; Hashizume, H.; Nozawa, T.; Sekiguchi, A.; Nouchi, H.; Kawashima, R. Beneficial Effects of Short-Term Combination Exercise Training on Diverse Cognitive Functions in Healthy Older People: Study Protocol for a Randomized Controlled Trial. Trials 2012, 13, 200. [Google Scholar] [CrossRef]
- Bossers, W.J.R.; Van Der Woude, L.H.V.; Boersma, F.; Hortobágyi, T.; Scherder, E.J.A.; Van Heuvelen, M.J.G. A 9-Week Aerobic and Strength Training Program Improves Cognitive and Motor Function in Patients with Dementia: A Randomized, Controlled Trial. Am. J. Geriatr. Psychiatry 2015, 23, 1106–1116. [Google Scholar] [CrossRef]
- Suzuki, T.; Shimada, H.; Makizako, H.; Doi, T.; Yoshida, D.; Ito, K.; Shimokata, H.; Washimi, Y.; Endo, H.; Kato, T. A Randomized Controlled Trial of Multicomponent Exercise in Older Adults with Mild Cognitive Impairment. PLoS ONE 2013, 8, e61483. [Google Scholar] [CrossRef]
- Vogel-Ciernia, A.; Wood, M.A. Examining Object Location and Object Recognition Memory in Mice. Curr. Protoc. Neurosci. 2014, 69, 8–31. [Google Scholar] [CrossRef]
- Gobeske, K.T.; Das, S.; Bonaguidi, M.A.; Weiss, C.; Radulovic, J.; Disterhoft, J.F.; Kessler, J.A. BMP Signaling Mediates Effects of Exercise on Hippocampal Neurogenesis and Cognition in Mice. PLoS ONE 2009, 4, e7506. [Google Scholar] [CrossRef]
- Schoenfeld, T.J.; Rada, P.; Pieruzzini, P.R.; Hsueh, B.; Gould, E. Physical Exercise Prevents Stress-Induced Activation of Granule Neurons and Enhances Local Inhibitory Mechanisms in the Dentate Gyrus. J. Neurosci. 2013, 33, 7770–7777. [Google Scholar] [CrossRef]
- Lafenetre, P. Exercise Can Rescue Recognition Memory Impairment in a Model with Reduced Adult Hippocampal Neurogenesis. Front. Behav. Neurosci. 2009, 3, 790. [Google Scholar] [CrossRef]
- Sun, B.-F.; Wang, Q.-Q.; Yu, Z.-J.; Yu, Y.; Xiao, C.-L.; Kang, C.-S.; Ge, G.; Linghu, Y.; Zhu, J.-D.; Li, Y.-M.; et al. Exercise Prevents Memory Impairment Induced by Arsenic Exposure in Mice: Implication of Hippocampal BDNF and CREB. PLoS ONE 2015, 10, e0137810. [Google Scholar] [CrossRef]
- Zheng, J.; Jiang, Y.-Y.; Xu, L.-C.; Ma, L.-Y.; Liu, F.-Y.; Cui, S.; Cai, J.; Liao, F.-F.; Wan, Y.; Yi, M. Adult Hippocampal Neurogenesis along the Dorsoventral Axis Contributes Differentially to Environmental Enrichment Combined with Voluntary Exercise in Alleviating Chronic Inflammatory Pain in Mice. J. Neurosci. 2017, 37, 4145–4157. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Drudis, L.; Amorós-Aguilar, L.; Torras-Garcia, M.; Serra-Elias, B.; Costa-Miserachs, D.; Portell-Cortés, I.; Coll-Andreu, M. Delayed Voluntary Physical Exercise Restores “When” and “Where” Object Recognition Memory after Traumatic Brain Injury. Behav. Brain Res. 2021, 400, 113048. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-F.; Huang, T.-Y.; Kuo, Y.-M.; Yu, L.; Chen, H.; Jen, C.J. Early Postinjury Exercise Reverses Memory Deficits and Retards the Progression of Closed-Head Injury in Mice: Early Postinjury Exercise Improves Memory Deficits and Brain Damage. J. Physiol. 2013, 591, 985–1000. [Google Scholar] [CrossRef] [PubMed]
- Ten Brinke, L.F.; Bolandzadeh, N.; Nagamatsu, L.S.; Hsu, C.L.; Davis, J.C.; Miran-Khan, K.; Liu-Ambrose, T. Aerobic Exercise Increases Hippocampal Volume in Older Women with Probable Mild Cognitive Impairment: A 6-Month Randomised Controlled Trial. Br. J. Sports Med. 2015, 49, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Dinoff, A.; Herrmann, N.; Swardfager, W.; Liu, C.S.; Sherman, C.; Chan, S.; Lanctôt, K.L. The Effect of Exercise Training on Resting Concentrations of Peripheral Brain-Derived Neurotrophic Factor (BDNF): A Meta-Analysis. PLoS ONE 2016, 11, e0163037. [Google Scholar] [CrossRef] [PubMed]
- Cassilhas, R.C.; Lee, K.S.; Fernandes, J.; Oliveira, M.G.M.; Tufik, S.; Meeusen, R.; De Mello, M.T. Spatial Memory Is Improved by Aerobic and Resistance Exercise through Divergent Molecular Mechanisms. Neuroscience 2012, 202, 309–317. [Google Scholar] [CrossRef]
- Stubbs, B.; Vancampfort, D.; Rosenbaum, S.; Firth, J.; Cosco, T.; Veronese, N.; Salum, G.A.; Schuch, F.B. An Examination of the Anxiolytic Effects of Exercise for People with Anxiety and Stress-Related Disorders: A Meta-Analysis. Psychiatry Res. 2017, 249, 102–108. [Google Scholar] [CrossRef]
- Wipfli, B.M.; Rethorst, C.D.; Landers, D.M. The Anxiolytic Effects of Exercise: A Meta-Analysis of Randomized Trials and Dose-Response Analysis. J. Sport Exerc. Psychol. 2008, 30, 392–410. [Google Scholar] [CrossRef]
- Duman, C.H.; Schlesinger, L.; Russell, D.S.; Duman, R.S. Voluntary Exercise Produces Antidepressant and Anxiolytic Behavioral Effects in Mice. Brain Res. 2008, 1199, 148–158. [Google Scholar] [CrossRef]
- Salam, J.; Fox, J.; Detroy, E.; Guignon, M.; Wohl, D.; Falls, W. Voluntary Exercise in C57 Mice Is Anxiolytic across Several Measures of Anxiety. Behav. Brain Res. 2009, 197, 31–40. [Google Scholar] [CrossRef]
- Hillman, C.H.; Erickson, K.I.; Kramer, A.F. Be Smart, Exercise Your Heart: Exercise Effects on Brain and Cognition. Nat. Rev. Neurosci. 2008, 9, 58–65. [Google Scholar] [CrossRef]
- Salmon, P. Effects of Physical Exercise on Anxiety, Depression, and Sensitivity to Stress: A Unifying Theory. Clin. Psychol. Rev. 2001, 21, 33–61. [Google Scholar] [CrossRef]
- Ahmadalipour, A.; Rashidy-Pour, A. Effects of Treadmill Running Exercise during the Adolescent Period of Life on Behavioral Deficits in Juvenile Rats Induced by Prenatal Morphine Exposure. Physiol. Behav. 2015, 139, 26–33. [Google Scholar] [CrossRef]
- Bashiri, H.; Enayati, M.; Bashiri, A.; Salari, A.-A. Swimming Exercise Improves Cognitive and Behavioral Disorders in Male NMRI Mice with Sporadic Alzheimer-like Disease. Physiol. Behav. 2020, 223, 113003. [Google Scholar] [CrossRef]
- Sadeghi, M.; Peeri, M.; Hosseini, M.-J. Adolescent Voluntary Exercise Attenuated Hippocampal Innate Immunity Responses and Depressive-like Behaviors Following Maternal Separation Stress in Male Rats. Physiol. Behav. 2016, 163, 177–183. [Google Scholar] [CrossRef]
- Dahl, R.E. Adolescent Brain Development: A Period of Vulnerabilities and Opportunities. Keynote Address. Ann. N. Y. Acad. Sci. 2004, 1021, 1–22. [Google Scholar] [CrossRef]
- Spear, L.P. Adolescent Brain Development and Animal Models. Ann. N. Y. Acad. Sci. 2004, 1021, 23–26. [Google Scholar] [CrossRef]
- Patki, G.; Li, L.; Allam, F.; Solanki, N.; Dao, A.T.; Alkadhi, K.; Salim, S. Moderate Treadmill Exercise Rescues Anxiety and Depression-like Behavior as Well as Memory Impairment in a Rat Model of Posttraumatic Stress Disorder. Physiol. Behav. 2014, 130, 47–53. [Google Scholar] [CrossRef]
- Castilla-Ortega, E.; Rosell-Valle, C.; Pedraza, C.; Rodríguez de Fonseca, F.; Estivill-Torrús, G.; Santín, L.J. Voluntary Exercise Followed by Chronic Stress Strikingly Increases Mature Adult-Born Hippocampal Neurons and Prevents Stress-Induced Deficits in “what-When-Where” Memory. Neurobiol. Learn. Mem. 2014, 109, 62–73. [Google Scholar] [CrossRef]
- Cunha, M.P.; Oliveira, Á.; Pazini, F.L.; Machado, D.G.; Bettio, L.E.B.; Budni, J.; Aguiar, A.S.; Martins, D.F.; Santos, A.R.S.; Rodrigues, A.L.S. The Antidepressant-like Effect of Physical Activity on a Voluntary Running Wheel. Med. Sci. Sports Exerc. 2013, 45, 851–859. [Google Scholar] [CrossRef]
- Pietrelli, A.; Lopez-Costa, J.; Goñi, R.; Brusco, A.; Basso, N. Aerobic Exercise Prevents Age-Dependent Cognitive Decline and Reduces Anxiety-Related Behaviors in Middle-Aged and Old Rats. Neuroscience 2012, 202, 252–266. [Google Scholar] [CrossRef] [PubMed]
- Merom, D.; Phongsavan, P.; Wagner, R.; Chey, T.; Marnane, C.; Steel, Z.; Silove, D.; Bauman, A. Promoting Walking as an Adjunct Intervention to Group Cognitive Behavioral Therapy for Anxiety Disorders--a Pilot Group Randomized Trial. J. Anxiety Disord. 2008, 22, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Kazeminia, M.; Salari, N.; Vaisi-Raygani, A.; Jalali, R.; Abdi, A.; Mohammadi, M.; Daneshkhah, A.; Hosseinian-Far, M.; Shohaimi, S. The Effect of Exercise on Anxiety in the Elderly Worldwide: A Systematic Review and Meta-Analysis. Health Qual. Life Outcomes 2020, 18, 363. [Google Scholar] [CrossRef] [PubMed]
- Gordon, B.R.; McDowell, C.P.; Lyons, M.; Herring, M.P. Resistance Exercise Training for Anxiety and Worry Symptoms among Young Adults: A Randomized Controlled Trial. Sci. Rep. 2020, 10, 17548. [Google Scholar] [CrossRef]
- Schuch, F.B.; Vancampfort, D. Physical Activity, Exercise, and Mental Disorders: It Is Time to Move On. Trends Psychiatry Psychother. 2021, 43, 177–184. [Google Scholar] [CrossRef]
- Loprinzi, P.; Ponce, P.; Frith, E. Hypothesized Mechanisms through Which Acute Exercise Influences Episodic Memory. Physiol. Int. 2018, 105, 285–297. [Google Scholar] [CrossRef]
- Loprinzi, P. The Role of Astrocytes on the Effects of Exercise on Episodic Memory Function. Physiol. Int. 2019, 106, 21–28. [Google Scholar] [CrossRef]
- Loprinzi, P.D.; Zou, L.; Li, H. The Endocannabinoid System as a Potential Mechanism through Which Exercise Influences Episodic Memory Function. Brain Sci. 2019, 9, 112. [Google Scholar] [CrossRef]
- Loprinzi, P.D.; Moore, D.; Loenneke, J.P. Does Aerobic and Resistance Exercise Influence Episodic Memory through Unique Mechanisms? Brain Sci. 2020, 10, 913. [Google Scholar] [CrossRef]
- Hopkins, M.E.; Bucci, D.J. BDNF Expression in Perirhinal Cortex Is Associated with Exercise-Induced Improvement in Object Recognition Memory. Neurobiol. Learn. Mem. 2010, 94, 278–284. [Google Scholar] [CrossRef]
- da Silva, R.; de Morais, A.; de Melo, J.; Macedo, P.; Costa, L.; Batista-de-Oliveira Hornsby, M. Neonatal Exercise Prevents Anxiety-Related Behavior and Improves Episodic Memory in Adult but Not in Aged Rats. FASEB J. 2015, 29, 840.14. [Google Scholar] [CrossRef]
- Murphy, D.L.; Fox, M.A.; Timpano, K.R.; Moya, P.R.; Ren-Patterson, R.; Andrews, A.M.; Holmes, A.; Lesch, K.-P.; Wendland, J.R. How the Serotonin Story Is Being Rewritten by New Gene-Based Discoveries Principally Related to SLC6A4, the Serotonin Transporter Gene, Which Functions to Influence All Cellular Serotonin Systems. Neuropharmacology 2008, 55, 932–960. [Google Scholar] [CrossRef]
- Murphy, D.L.; Moya, P.R.; Fox, M.A.; Rubenstein, L.M.; Wendland, J.R.; Timpano, K.R. Anxiety and Affective Disorder Comorbidity Related to Serotonin and Other Neurotransmitter Systems: Obsessive–Compulsive Disorder as an Example of Overlapping Clinical and Genetic Heterogeneity. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20120435. [Google Scholar] [CrossRef]
- Otsuka, T.; Nishii, A.; Amemiya, S.; Kubota, N.; Nishijima, T.; Kita, I. Effects of Acute Treadmill Running at Different Intensities on Activities of Serotonin and Corticotropin-Releasing Factor Neurons, and Anxiety- and Depressive-like Behaviors in Rats. Behav. Brain Res. 2016, 298, 44–51. [Google Scholar] [CrossRef]
- Wang, L.R.; Kim, S.-H.; Baek, S.-S. Effects of Treadmill Exercise on the Anxiety-like Behavior through Modulation of GSK3β/β-Catenin Signaling in the Maternal Separation Rat Pup. J. Exerc. Rehabil. 2019, 15, 206–212. [Google Scholar] [CrossRef]
- Li, X.; Zhu, W.; Roh, M.-S.; Friedman, A.B.; Rosborough, K.; Jope, R.S. In Vivo Regulation of Glycogen Synthase Kinase-3beta (GSK3beta) by Serotonergic Activity in Mouse Brain. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2004, 29, 1426–1431. [Google Scholar] [CrossRef]
- Omata, N.; Chiu, C.-T.; Moya, P.R.; Leng, Y.; Wang, Z.; Hunsberger, J.G.; Leeds, P.; Chuang, D.-M. Lentivirally Mediated GSK-3β Silencing in the Hippocampal Dentate Gyrus Induces Antidepressant-like Effects in Stressed Mice. Int. J. Neuropsychopharmacol. 2011, 14, 711–717. [Google Scholar] [CrossRef]
- Latapy, C.; Rioux, V.; Guitton, M.J.; Beaulieu, J.-M. Selective Deletion of Forebrain Glycogen Synthase Kinase 3β Reveals a Central Role in Serotonin-Sensitive Anxiety and Social Behaviour. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2012, 367, 2460–2474. [Google Scholar] [CrossRef]
- Gould, T.D.; Chen, G.; Manji, H.K. In Vivo Evidence in the Brain for Lithium Inhibition of Glycogen Synthase Kinase-3. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2004, 29, 32–38. [Google Scholar] [CrossRef]
- Park, S.-S.; Park, H.-S.; Kim, T.-W.; Lee, S.-J. Effects of Swimming Exercise on Social Isolation-Induced Memory Impairment and Apoptosis in Old Rats. J. Exerc. Rehabil. 2020, 16, 234–241. [Google Scholar] [CrossRef]
- Seo, J.-H.; Kim, T.-W.; Kim, C.-J.; Sung, Y.-H.; Lee, S.-J. Treadmill Exercise during Pregnancy Ameliorates Post-Traumatic Stress Disorder-Induced Anxiety-like Responses in Maternal Rats. Mol. Med. Rep. 2013, 7, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Ge, R.; Dai, Y. Three-Week Treadmill Exercise Enhances Persistent Inward Currents, Facilitates Dendritic Plasticity, and Upregulates the Excitability of Dorsal Raphe Serotonin Neurons in EPet-EYFP Mice. Front. Cell. Neurosci. 2020, 14, 575626. [Google Scholar] [CrossRef] [PubMed]
- Calizo, L.H.; Akanwa, A.; Ma, X.; Pan, Y.; Lemos, J.C.; Craige, C.; Heemstra, L.A.; Beck, S.G. Raphe Serotonin Neurons Are Not Homogenous: Electrophysiological, Morphological and Neurochemical Evidence. Neuropharmacology 2011, 61, 524–543. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, B.N.; Strong, P.V.; Loughridge, A.B.; Day, H.E.W.; Clark, P.J.; Mika, A.; Hellwinkel, J.E.; Spence, K.G.; Fleshner, M. 5-HT2C Receptors in the Basolateral Amygdala and Dorsal Striatum Are a Novel Target for the Anxiolytic and Antidepressant Effects of Exercise. PLoS ONE 2012, 7, e46118. [Google Scholar] [CrossRef]
- Abe, K.; Fujimoto, T.; Akaishi, T.; Misawa, M. Stimulation of Basolateral Amygdaloid Serotonin 5-HT2C Receptors Promotes the Induction of Long-Term Potentiation in the Dentate Gyrus of Anesthetized Rats. Neurosci. Lett. 2009, 451, 65–68. [Google Scholar] [CrossRef]
- Pitsikas, N.; Sakellaridis, N. The 5-HT2C Receptor Antagonist RO 60-0491 Counteracts Rats’ Retention Deficits in a Recognition Memory Task. Brain Res. 2005, 1054, 200–202. [Google Scholar] [CrossRef]
- Khaliq, S.; Haider, S.; Saleem, S.; Memon, Z.; Haleem, D.J. Influence of Serotonergic 5-HT2C Receptor Antagonist Mesulergine in the Reversal of Memory Deficits Induced by MCPP. J. Coll. Physicians Surg. Pak. 2012, 22, 75–79. [Google Scholar]
- Leem, Y.-H.; Jang, J.-H.; Park, J.-S.; Kim, H.-S. Exercise Exerts an Anxiolytic Effect against Repeated Restraint Stress through 5-HT2A-Mediated Suppression of the Adenosine A2A Receptor in the Basolateral Amygdala. Psychoneuroendocrinology 2019, 108, 182–189. [Google Scholar] [CrossRef]
- Zhang, G.; Ásgeirsdóttir, H.N.; Cohen, S.J.; Munchow, A.H.; Barrera, M.P.; Stackman, R.W. Stimulation of Serotonin 2A Receptors Facilitates Consolidation and Extinction of Fear Memory in C57BL/6J Mice. Neuropharmacology 2013, 64, 403–413. [Google Scholar] [CrossRef]
- Motaghinejad, O.; Motaghinejad, M.; Motevalian, M.; Rahimi-Sharbaf, F.; Beiranvand, T. The Effect of Maternal Forced Exercise on Offspring Pain Perception, Motor Activity and Anxiety Disorder: The Role of 5-HT2 and D2 Receptors and CREB Gene Expression. J. Exerc. Rehabil. 2017, 13, 514–525. [Google Scholar] [CrossRef]
- Van Wel, J.H.P.; Kuypers, K.P.C.; Theunissen, E.L.; Bosker, W.M.; Bakker, K.; Ramaekers, J.G. Blockade of 5-HT2 Receptor Selectively Prevents MDMA-Induced Verbal Memory Impairment. Neuropsychopharmacology 2011, 36, 1932–1939. [Google Scholar] [CrossRef]
- Kim, M.H.; Leem, Y.H. Chronic Exercise Improves Repeated Restraint Stress-Induced Anxiety and Depression through 5HT1A Receptor and CAMP Signaling in Hippocampus. J. Exerc. Nutr. Biochem. 2014, 18, 97–104. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, T.W.; Park, H.K.; Yoon, S.; You, A.H.; Moon, E.J.; Shin, D.H.; Cho, H. Postnatal Treadmill Exercise Alleviates Prenatal Stress-Induced Anxiety in Offspring Rats by Enhancing Cell Proliferation through 5-Hydroxytryptamine 1A Receptor Activation. Int. Neurourol. J. 2016, 20, S57–S64. [Google Scholar] [CrossRef]
- Huang, M.; Kwon, S.; Rajagopal, L.; He, W.; Meltzer, H.Y. 5-HT1A Parital Agonism and 5-HT7 Antagonism Restore Episodic Memory in Subchronic Phencyclidine-Treated Mice: Role of Brain Glutamate, Dopamine, Acetylcholine and GABA. Psychopharmacology 2018, 235, 2795–2808. [Google Scholar] [CrossRef]
- Matsunaga, D.; Nakagawa, H.; Ishiwata, T. Difference in the Brain Serotonin and Its Metabolite Level and Anxiety-like Behavior between Forced and Voluntary Exercise Conditions in Rats. Neurosci. Lett. 2021, 744, 135556. [Google Scholar] [CrossRef]
- Laugeray, A.; Launay, J.-M.; Callebert, J.; Surget, A.; Belzung, C.; Barone, P.R. Evidence for a Key Role of the Peripheral Kynurenine Pathway in the Modulation of Anxiety- and Depression-like Behaviours in Mice: Focus on Individual Differences. Pharmacol. Biochem. Behav. 2011, 98, 161–168. [Google Scholar] [CrossRef]
- Lim, A.; Harijanto, C.; Vogrin, S.; Guillemin, G.; Duque, G. Does Exercise Influence Kynurenine/Tryptophan Metabolism and Psychological Outcomes in Persons With Age-Related Diseases? A Systematic Review. Int. J. Tryptophan Res. IJTR 2021, 14, 1178646921991119. [Google Scholar] [CrossRef]
- Agudelo, L.Z.; Femenía, T.; Orhan, F.; Porsmyr-Palmertz, M.; Goiny, M.; Martinez-Redondo, V.; Correia, J.C.; Izadi, M.; Bhat, M.; Schuppe-Koistinen, I.; et al. Skeletal Muscle PGC-1α1 Modulates Kynurenine Metabolism and Mediates Resilience to Stress-Induced Depression. Cell 2014, 159, 33–45. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Illesca-Matus, R.; Ardiles, N.M.; Munoz, F.; Moya, P.R. Implications of Physical Exercise on Episodic Memory and Anxiety: The Role of the Serotonergic System. Int. J. Mol. Sci. 2023, 24, 11372. https://doi.org/10.3390/ijms241411372
Illesca-Matus R, Ardiles NM, Munoz F, Moya PR. Implications of Physical Exercise on Episodic Memory and Anxiety: The Role of the Serotonergic System. International Journal of Molecular Sciences. 2023; 24(14):11372. https://doi.org/10.3390/ijms241411372
Chicago/Turabian StyleIllesca-Matus, Ricardo, Nicolás M. Ardiles, Felipe Munoz, and Pablo R. Moya. 2023. "Implications of Physical Exercise on Episodic Memory and Anxiety: The Role of the Serotonergic System" International Journal of Molecular Sciences 24, no. 14: 11372. https://doi.org/10.3390/ijms241411372
APA StyleIllesca-Matus, R., Ardiles, N. M., Munoz, F., & Moya, P. R. (2023). Implications of Physical Exercise on Episodic Memory and Anxiety: The Role of the Serotonergic System. International Journal of Molecular Sciences, 24(14), 11372. https://doi.org/10.3390/ijms241411372





