Localized Increased Permeability of Blood–Brain Barrier for Antibody Conjugates in the Cuprizone Model of Demyelination
Abstract
1. Introduction
2. Results
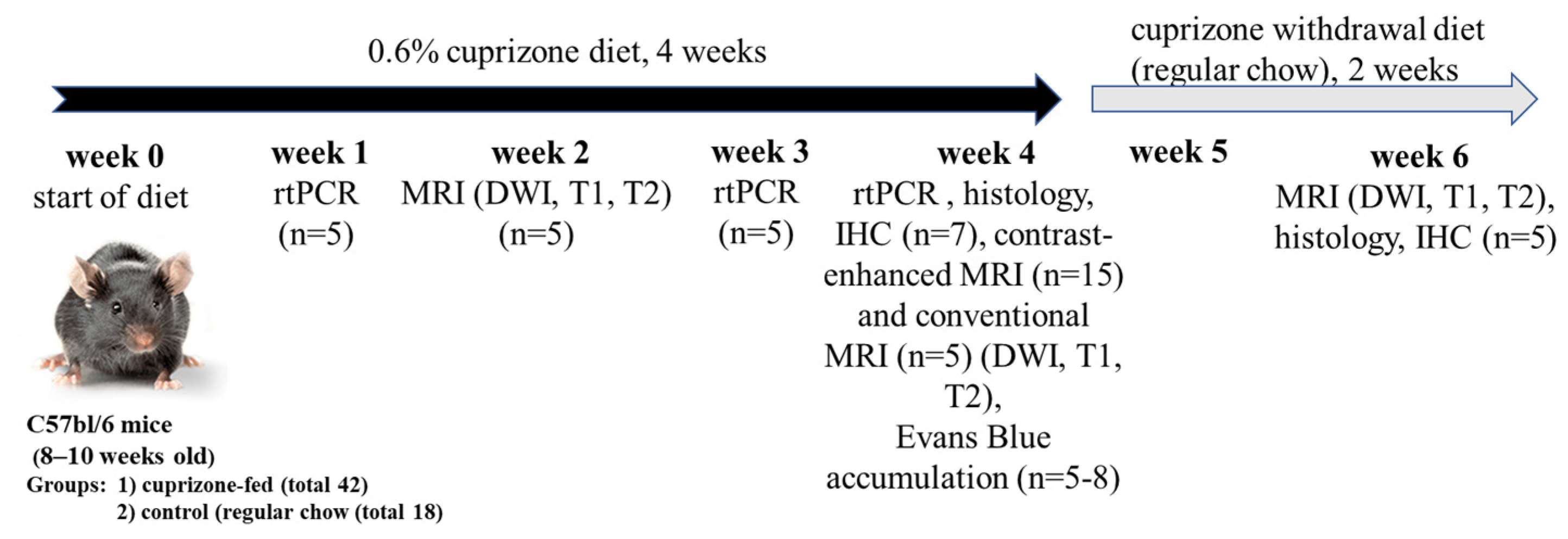
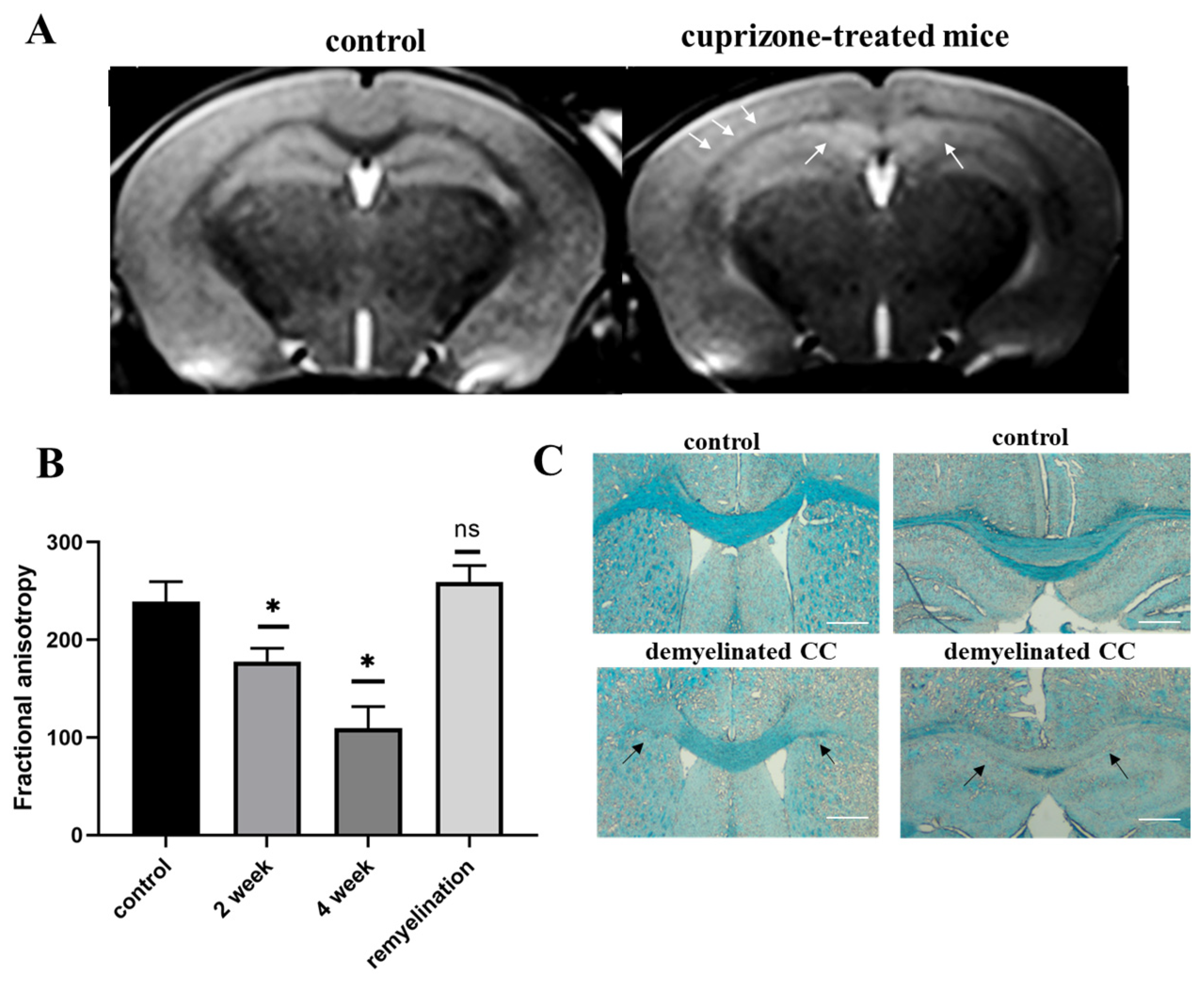
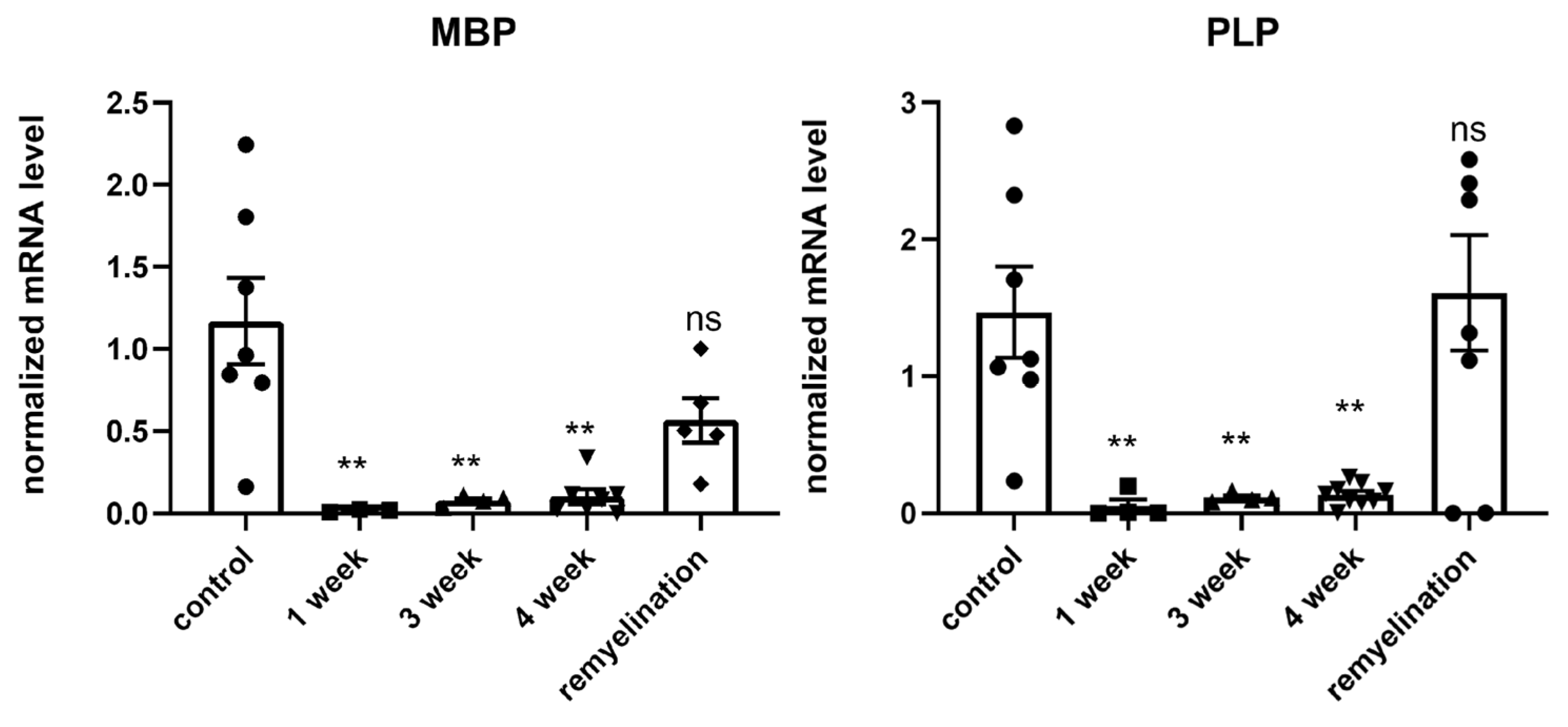
2.1. Validation of the Cuprizone Model of Demyelination in Mice
2.2. Detection of Evans Blue Dye in the Brain of Cuprizone-Treated Mice
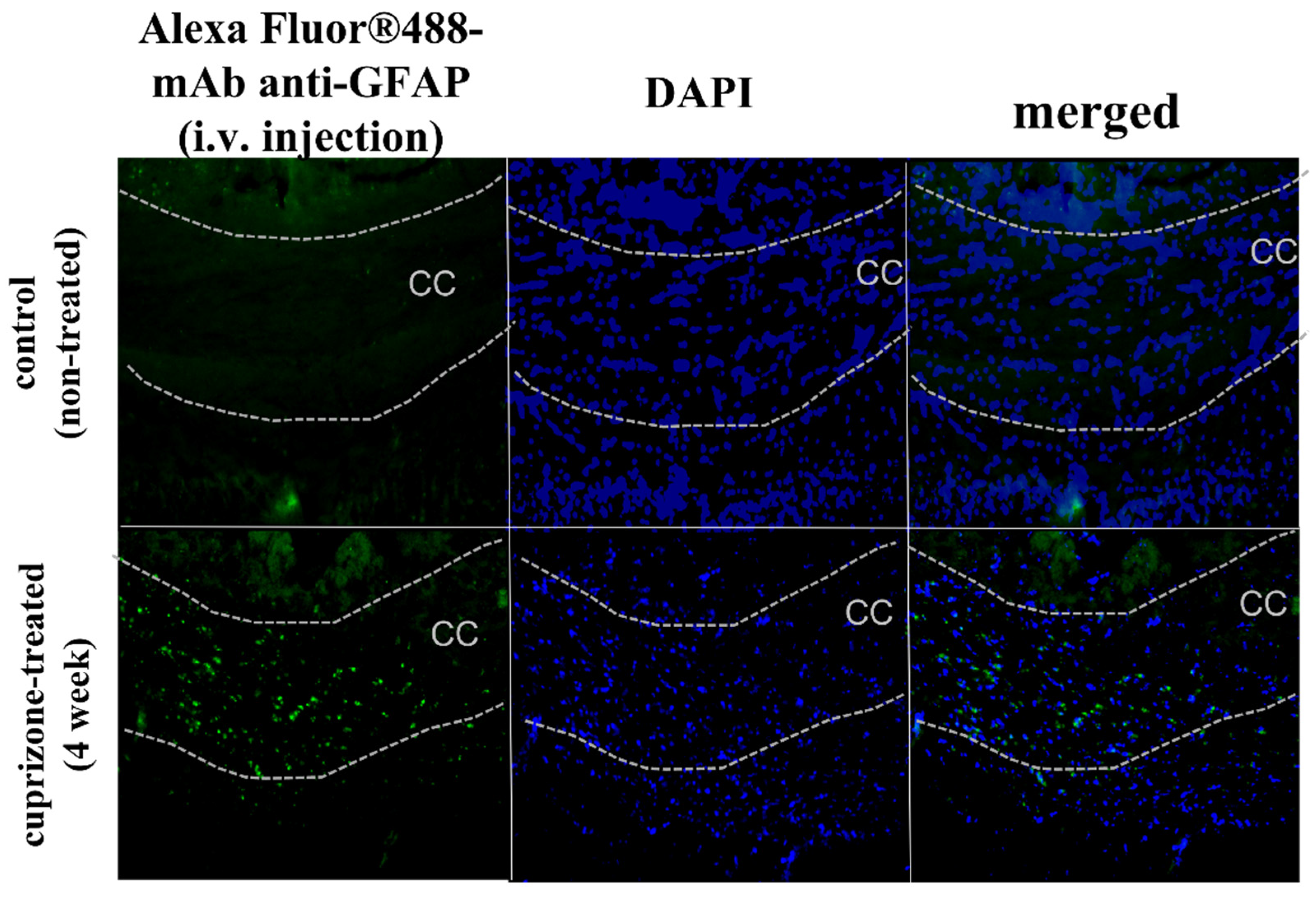
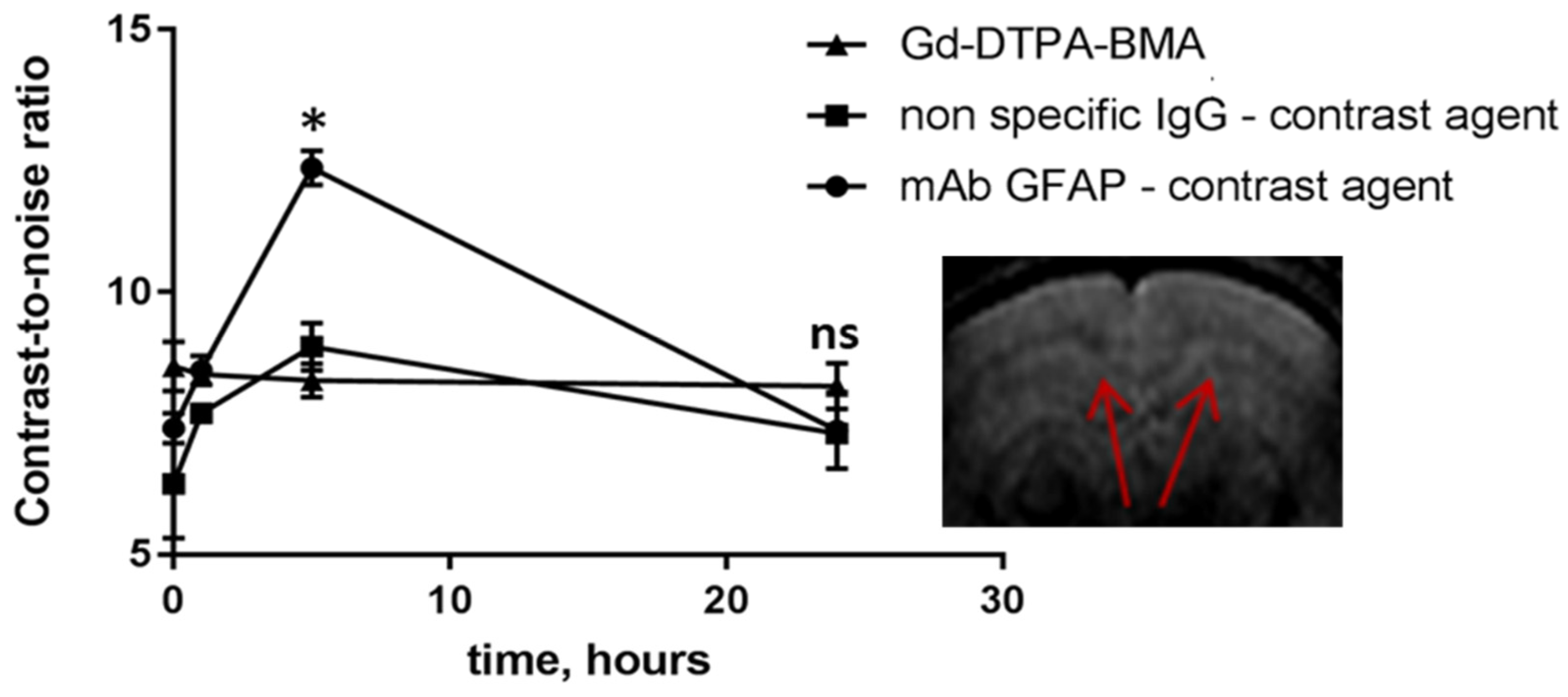
2.3. Accumulation of Antibody Conjugates in the Brains of Cuprizone-Treated Mice
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Experimental Design and Modeling of Cuprizone-Induced Demyelination
4.3. rt-PCR Analysis
4.4. Luxol Fast Blue Staining
4.5. Immunohistochemistry of GFAP and VEGFR2
4.6. Magnetic Resonance Imaging (MRI)
4.7. Analysis of Evans Blue Accumulation
4.8. Accumulation of Antibody Conjugates
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Osorio-Querejeta, I.; Sáenz-Cuesta, M.; Muñoz-Culla, M.; Otaegui, D. Models for Studying Myelination, Demyelination and Remyelination. Neuromol. Med. 2017, 19, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, G.K.; Morell, P. The Neurotoxicant, Cuprizone, as a Model to Study Demyelination and Remyelination in the Central Nervous System. Brain Pathol. 2001, 11, 107–116. [Google Scholar] [CrossRef] [PubMed]
- McMahon, E.J.; Suzuki, K.; Matsushima, G.K. Peripheral Macrophage Recruitment in Cuprizone-Induced CNS Demyelination despite an Intact Blood-Brain Barrier. J. Neuroimmunol. 2002, 130, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Gudi, V.; Gingele, S.; Skripuletz, T.; Stangel, M. Glial Response during Cuprizone-Induced de- and Remyelination in the CNS: Lessons Learned. Front. Cell. Neurosci. 2014, 8, 73. [Google Scholar] [CrossRef]
- Remington, L.T.; Babcock, A.A.; Zehntner, S.P.; Owens, T. Microglial Recruitment, Activation, and Proliferation in Response to Primary Demyelination. Am. J. Pathol. 2007, 170, 1713–1724. [Google Scholar] [CrossRef]
- Torkildsen; Brunborg, L.A.; Myhr, K.M.; Bø, L. The Cuprizone Model for Demyelination. Acta Neurol. Scand. 2008, 117, 72–76. [Google Scholar] [CrossRef]
- Bakker, D.A.; Ludwin, S.K. Blood-Brain Barrier Permeability during Cuprizone-Induced Demyelination. Implications for the Pathogenesis of Immune-Mediated Demyelinating Diseases. J. Neurol. Sci. 1987, 78, 125–137. [Google Scholar] [CrossRef]
- Suzuki, K.; Kikkawa, Y. Status Spongiosus of CNS and Hepatic Changes Induced by Cuprizone (Biscyclohexanone Oxalyldihydrazone). Am. J. Pathol. 1969, 54, 307–325. [Google Scholar]
- Kondo, A.; Nakano, T.; Suzuki, K. Blood-Brain Barrier Permeability to Horseradish Peroxidase in Twitcher and Cuprizone-Intoxicated Mice. Brain Res. 1987, 425, 186–190. [Google Scholar] [CrossRef]
- Hiremath, M.M.; Saito, Y.; Knapp, G.W.; Ting, J.P.Y.; Suzuki, K.; Matsushima, G.K. Microglial/Macrophage Accumulation during Cuprizone-Induced Demyelination in C57BL/6 Mice. J. Neuroimmunol. 1998, 92, 38–49. [Google Scholar] [CrossRef]
- Hedayatpour, A.; Ragerdi, I.; Pasbakhsh, P.; Kafami, L.; Atlasi, N.; Mahabadi, V.P.; Ghasemi, S.; Mahmoudi, R. Promotion of Remyelination by Adipose Mesenchymal Stem Cell Transplantation in a Cuprizone Model of Multiple Sclerosis. Cell J. 2013, 15, 142–151. [Google Scholar]
- Sen, M.K.; Mahns, D.A.; Coorssen, J.R.; Shortland, P.J. The Roles of Microglia and Astrocytes in Phagocytosis and Myelination: Insights from the Cuprizone Model of Multiple Sclerosis. Glia 2022, 70, 1215–1250. [Google Scholar] [CrossRef]
- Almuslehi, M.S.M.; Sen, M.K.; Shortland, P.J.; Mahns, D.A.; Coorssen, J.R. CD8 T-Cell Recruitment Into the Central Nervous System of Cuprizone-Fed Mice: Relevance to Modeling the Etiology of Multiple Sclerosis. Front. Cell. Neurosci. 2020, 14, 43. [Google Scholar] [CrossRef]
- Sen, M.K.; Almuslehi, M.S.M.; Shortland, P.J.; Mahns, D.A.; Coorssen, J.R. Proteomics of Multiple Sclerosis: Inherent Issues in Defining the Pathoetiology and Identifying (Early) Biomarkers. Int. J. Mol. Sci. 2021, 22, 7377. [Google Scholar] [CrossRef]
- Tejedor, L.S.; Wostradowski, T.; Gingele, S.; Skripuletz, T.; Gudi, V.; Stangel, M. The Effect of Stereotactic Injections on Demyelination and Remyelination: A Study in the Cuprizone Model. J. Mol. Neurosci. 2017, 61, 479–488. [Google Scholar] [CrossRef]
- Shelestak, J.; Singhal, N.; Frankle, L.; Tomor, R.; Sternbach, S.; McDonough, J.; Freeman, E.; Clements, R. Increased Blood-Brain Barrier Hyperpermeability Coincides with Mast Cell Activation Early under Cuprizone Administration. PLoS ONE 2020, 15, e0234001. [Google Scholar] [CrossRef]
- Berghoff, S.A.; Düking, T.; Spieth, L.; Winchenbach, J.; Stumpf, S.K.; Gerndt, N.; Kusch, K.; Ruhwedel, T.; Möbius, W.; Saher, G. Blood-Brain Barrier Hyperpermeability Precedes Demyelination in the Cuprizone Model. Acta Neuropathol. Commun. 2017, 5, 94. [Google Scholar] [CrossRef]
- Zirngibl, M.; Assinck, P.; Sizov, A.; Caprariello, A.V.; Plemel, J.R. Oligodendrocyte Death and Myelin Loss in the Cuprizone Model: An Updated Overview of the Intrinsic and Extrinsic Causes of Cuprizone Demyelination. Mol. Neurodegener. 2022, 17, 34. [Google Scholar] [CrossRef]
- Abakumova, T.; Abakumov, M.; Shein, S.; Chelushkin, P.; Bychkov, D.; Mukhin, V.; Yusubalieva, G.; Grinenko, N.; Kabanov, A.; Nukolova, N.; et al. Connexin 43-Targeted T1 Contrast Agent for MRI Diagnosis of Glioma. Contrast Media Mol. Imaging 2016, 11, 15–23. [Google Scholar] [CrossRef]
- Shein, S.A.; Kuznetsov, I.I.; Abakumova, T.O.; Chelushkin, P.S.; Melnikov, P.A.; Korchagina, A.A.; Bychkov, D.A.; Seregina, I.F.; Bolshov, M.A.; Kabanov, A.V.; et al. VEGF- and VEGFR2-Targeted Liposomes for Cisplatin Delivery to Glioma Cells. Mol. Pharm. 2016, 13, 3712–3723. [Google Scholar] [CrossRef]
- Abakumov, M.A.; Nukolova, N.V.; Sokolsky-Papkov, M.; Shein, S.A.; Sandalova, T.O.; Vishwasrao, H.M.; Grinenko, N.F.; Gubsky, I.L.; Abakumov, A.M.; Kabanov, A.V.; et al. VEGF-Targeted Magnetic Nanoparticles for MRI Visualization of Brain Tumor. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Abakumova, T.O.; Kuz’kina, A.A.; Zharova, M.V.; Pozdeeva, D.A.; Gubskii, I.L.; Shepeleva, I.I.; Antonova, O.M.; Nukolova, N.V.; Kekelidze, Z.I.; Chekhonin, V.P. Cuprizone Model as a Tool for Preclinical Studies of the Efficacy of Multiple Sclerosis Diagnosis and Therapy. Bull. Exp. Biol. Med. 2015, 159, 111–115. [Google Scholar] [CrossRef]
- Morell, P.; Barrett, C.V.; Mason, J.L.; Toews, A.D.; Hostettler, J.D.; Knapp, G.W.; Matsushima, G.K. Gene Expression in Brain during Cuprizone-Induced Demyelination and Remyelination. Mol. Cell. Neurosci. 1998, 12, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Jurevics, H.; Largent, C.; Hostettler, J.; Sammond, D.W.; Matsushima, G.K.; Kleindienst, A.; Toews, A.D.; Morell, P. Alterations in Metabolism and Gene Expression in Brain Regions during Cuprizone-Induced Demyelination and Remyelination. J. Neurochem. 2002, 82, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Rodriguez, M.d.l.A.; Gingele, S.; Schröder, L.J.; Möllenkamp, T.; Stangel, M.; Skripuletz, T.; Gudi, V. Astroglial and Oligodendroglial Markers in the Cuprizone Animal Model for De- and Remyelination. Histochem. Cell Biol. 2022, 158, 15–38. [Google Scholar] [CrossRef]
- Skripuletz, T.; Hackstette, D.; Bauer, K.; Gudi, V.; Pul, R.; Voss, E.; Berger, K.; Kipp, M.; Baumgärtner, W.; Stangel, M. Astrocytes Regulate Myelin Clearance through Recruitment of Microglia during Cuprizone-Induced Demyelination. Brain 2013, 136, 147–167. [Google Scholar] [CrossRef]
- Hibbits, N.; Yoshino, J.; Le, T.Q.; Armstrong, R.C. Astrogliosis during Acute and Chronic Cuprizone Demyelination and Implications for Remyelination. ASN Neuro 2012, 4, 393–408. [Google Scholar] [CrossRef]
- Birukova, A.A.; Lee, S.; Starosta, V.; Wu, T.; Ho, T.; Kim, J.; Berliner, J.A.; Birukov, K.G. A Role for VEGFR2 Activation in Endothelial Responses Caused by Barrier Disruptive OxPAPC Concentrations. PLoS ONE 2012, 7, e30957. [Google Scholar] [CrossRef]
- Nag, S.; Manias, J.; Eubanks, J.H.; Stewart, D.J. Increased Expression of Vascular Endothelial Growth Factor-D Following Brain Injury. Int. J. Mol. Sci. 2019, 20, 1594. [Google Scholar] [CrossRef]
- Ryu, H.W.; Lim, W.; Jo, D.; Kim, S.; Park, J.T.; Min, J.J.; Hyun, H.; Kim, H.S. Low-Dose Evans Blue Dye for near-Infrared Fluorescence Imaging in Photothrombotic Stroke Model. Int. J. Med. Sci. 2018, 15, 696–702. [Google Scholar] [CrossRef]
- Wick, M.J.; Harral, J.W.; Loomis, Z.L.; Dempsey, E.C. An Optimized Evans Blue Protocol to Assess Vascular Leak in the Mouse. J. Vis. Exp. 2018, 2018, e57037. [Google Scholar] [CrossRef]
- Readnower, R.D.; Chavko, M.; Adeeb, S.; Conroy, M.D.; Pauly, J.R.; McCarron, R.M.; Sullivan, P.G. Increase in Blood-Brain Barrier Permeability, Oxidative Stress, and Activated Microglia in a Rat Model of Blast-Induced Traumatic Brain Injury. J. Neurosci. Res. 2010, 88, 3530–3539. [Google Scholar] [CrossRef]
- Keep, R.F.; Zhou, N.; Xiang, J.; Andjelkovic, A.V.; Hua, Y.; Xi, G. Vascular Disruption and Blood-Brain Barrier Dysfunction in Intracerebral Hemorrhage. Fluids Barriers CNS 2014, 11, 18. [Google Scholar] [CrossRef]
- Manaenko, A.; Chen, H.; Kammer, J.; Zhang, J.H.; Tang, J. Comparison Evans Blue Injection Routes: Intravenous vs. Intraperitoneal, for Measurement of Blood-Brain Barrier in a Mice Hemorrhage Model. J. Neurosci. Methods 2011, 195, 206–210. [Google Scholar] [CrossRef]
- Saunders, N.R.; Dziegielewska, K.M.; Møllgård, K.; Habgood, M.D. Markers for Blood-Brain Barrier Integrity: How Appropriate Is Evans Blue in the Twenty-First Century and What Are the Alternatives? Front. Neurosci. 2015, 9, 385. [Google Scholar] [CrossRef]
- Marchi, N.; Cavaglia, M.; Fazio, V.; Bhudia, S.; Hallene, K.; Janigro, D. Peripheral Markers of Blood-Brain Barrier Damage. Clin. Chim. Acta 2004, 342, 1–12. [Google Scholar] [CrossRef]
- Marzan, D.E.; Brügger-Verdon, V.; West, B.L.; Liddelow, S.; Samanta, J.; Salzer, J.L. Activated Microglia Drive Demyelination via CSF1R Signaling. Glia 2021, 69, 1583–1604. [Google Scholar] [CrossRef]
- Fallier-Becker, P.; Bonzheim, I.; Pfeiffer, F. Cuprizone Feeding Induces Swollen Astrocyte Endfeet. Pflugers Arch. Eur. J. Physiol. 2022, 474, 1275–1283. [Google Scholar] [CrossRef]
- Koch, S.; Claesson-Welsh, L. Signal Transduction by Vascular Endothelial Growth Factor Receptors. Cold Spring Harb. Perspect. Med. 2012, 2, a006502. [Google Scholar] [CrossRef]
- Holmes, K.; Roberts, O.L.; Thomas, A.M.; Cross, M.J. Vascular Endothelial Growth Factor Receptor-2: Structure, Function, Intracellular Signalling and Therapeutic Inhibition. Cell. Signal. 2007, 19, 2003–2012. [Google Scholar] [CrossRef]
- Kuczynski, E.A.; Patten, S.G.; Coomber, B.L. VEGFR2 Expression and TGF-β Signaling in Initial and Recurrent High-Grade Human Glioma. Oncology 2011, 81, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Korchagina, A.A.; Shein, S.A.; Leopol’D, A.V.; Volgina, N.E.; Gurina, O.I.; Lazarenko, I.P.; Antonova, O.M.; Baklaushev, V.P.; Chekhonin, V.P. Generation of Recombinant Extracellular Fragment of Vascular Endothelial Growth Factor Receptor 2 and Specific Monoclonal Antibodies to This Receptor. Bull. Exp. Biol. Med. 2014, 156, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Yusubalieva, G.M.; Baklaushev, V.P.; Gurina, O.I.; Tsitrin, E.B.; Chekhonin, V.P. Immunochemical Analysis of Glial Fibrillary Acidic Protein as a Tool to Assess Astroglial Reaction in Experimental C6 Glioma. Bull. Exp. Biol. Med. 2010, 149, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Burke, D. A Modification for the Combined Staining of Cells and Fibers in the Nervous System. Am. J. Med. Technol. 1968, 34, 667–670. [Google Scholar]
- Invitrogen Alexa Fluor ® 488 Microscale Protein Labeling Kit (A30006). Protocol. 2006, pp. 1–6. Available online: https://www.thermofisher.com/document-connect/document-connect.html?url=https://assets.thermofisher.com/TFS-Assets%2FLSG%2Fmanuals%2Fmp30006.pdf (accessed on 26 June 2023).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abakumova, T.; Kuzkina, A.; Koshkin, P.; Pozdeeva, D.; Abakumov, M.; Melnikov, P.; Ionova, K.; Gubskii, I.; Gurina, O.; Nukolova, N.; et al. Localized Increased Permeability of Blood–Brain Barrier for Antibody Conjugates in the Cuprizone Model of Demyelination. Int. J. Mol. Sci. 2023, 24, 12688. https://doi.org/10.3390/ijms241612688
Abakumova T, Kuzkina A, Koshkin P, Pozdeeva D, Abakumov M, Melnikov P, Ionova K, Gubskii I, Gurina O, Nukolova N, et al. Localized Increased Permeability of Blood–Brain Barrier for Antibody Conjugates in the Cuprizone Model of Demyelination. International Journal of Molecular Sciences. 2023; 24(16):12688. https://doi.org/10.3390/ijms241612688
Chicago/Turabian StyleAbakumova, Tatiana, Anastasia Kuzkina, Philipp Koshkin, Daria Pozdeeva, Maxim Abakumov, Pavel Melnikov, Klavdia Ionova, Ilia Gubskii, Olga Gurina, Natalia Nukolova, and et al. 2023. "Localized Increased Permeability of Blood–Brain Barrier for Antibody Conjugates in the Cuprizone Model of Demyelination" International Journal of Molecular Sciences 24, no. 16: 12688. https://doi.org/10.3390/ijms241612688
APA StyleAbakumova, T., Kuzkina, A., Koshkin, P., Pozdeeva, D., Abakumov, M., Melnikov, P., Ionova, K., Gubskii, I., Gurina, O., Nukolova, N., & Chekhonin, V. (2023). Localized Increased Permeability of Blood–Brain Barrier for Antibody Conjugates in the Cuprizone Model of Demyelination. International Journal of Molecular Sciences, 24(16), 12688. https://doi.org/10.3390/ijms241612688






