Hybrids of Sterically Hindered Phenols and Diaryl Ureas: Synthesis, Switch from Antioxidant Activity to ROS Generation and Induction of Apoptosis
Abstract
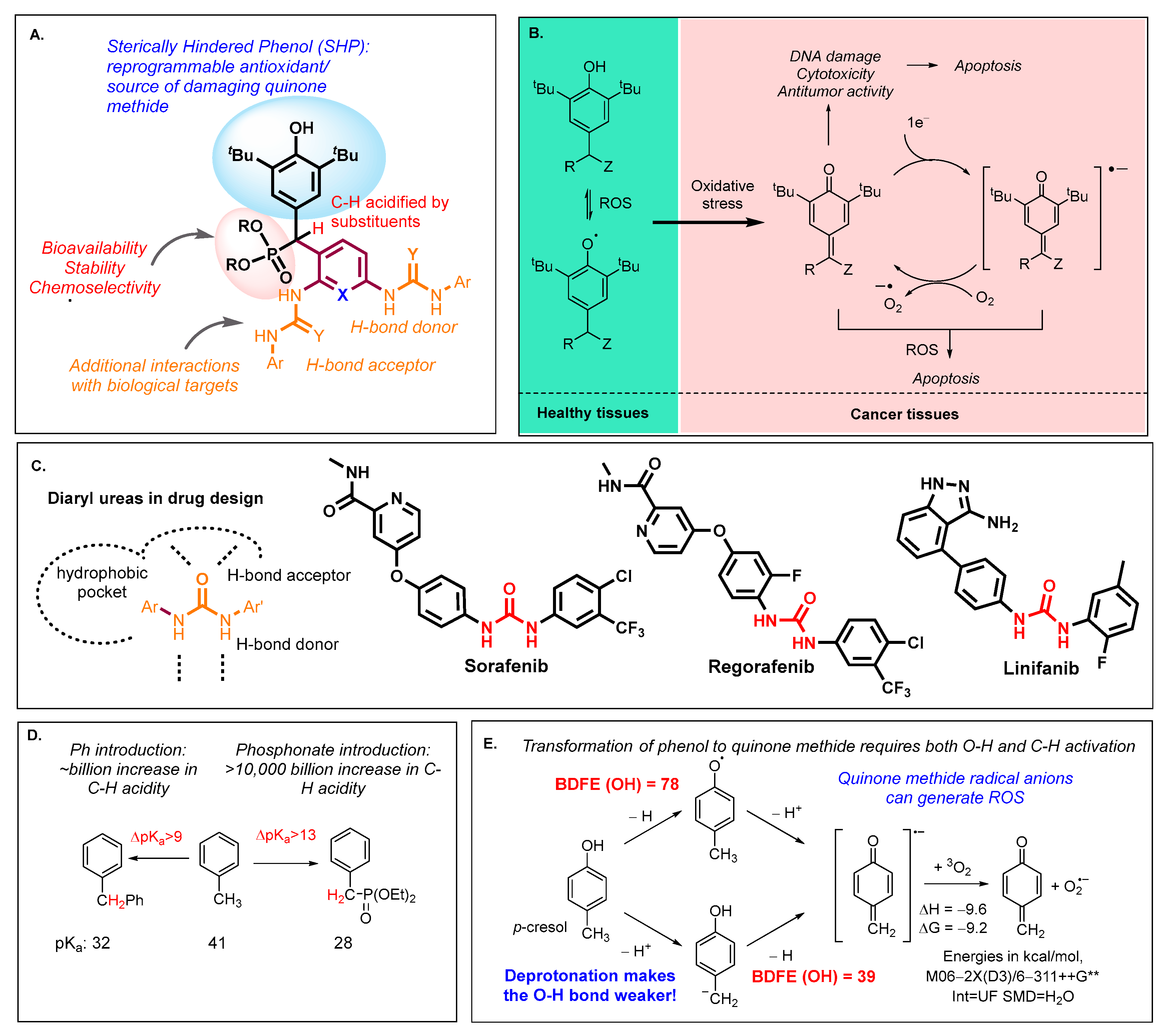
1. Introduction
- (a)
- Regulation of the efficiency of quinone methide generation in vivo by introducing electron-withdrawing phosphoryl groups at the benzylic position of SHPs;
- (b)
- Modification of compounds with diaryl urea fragments known to interfere with glycolysis pathways, the important energy source for cancer metabolism.
2. Results
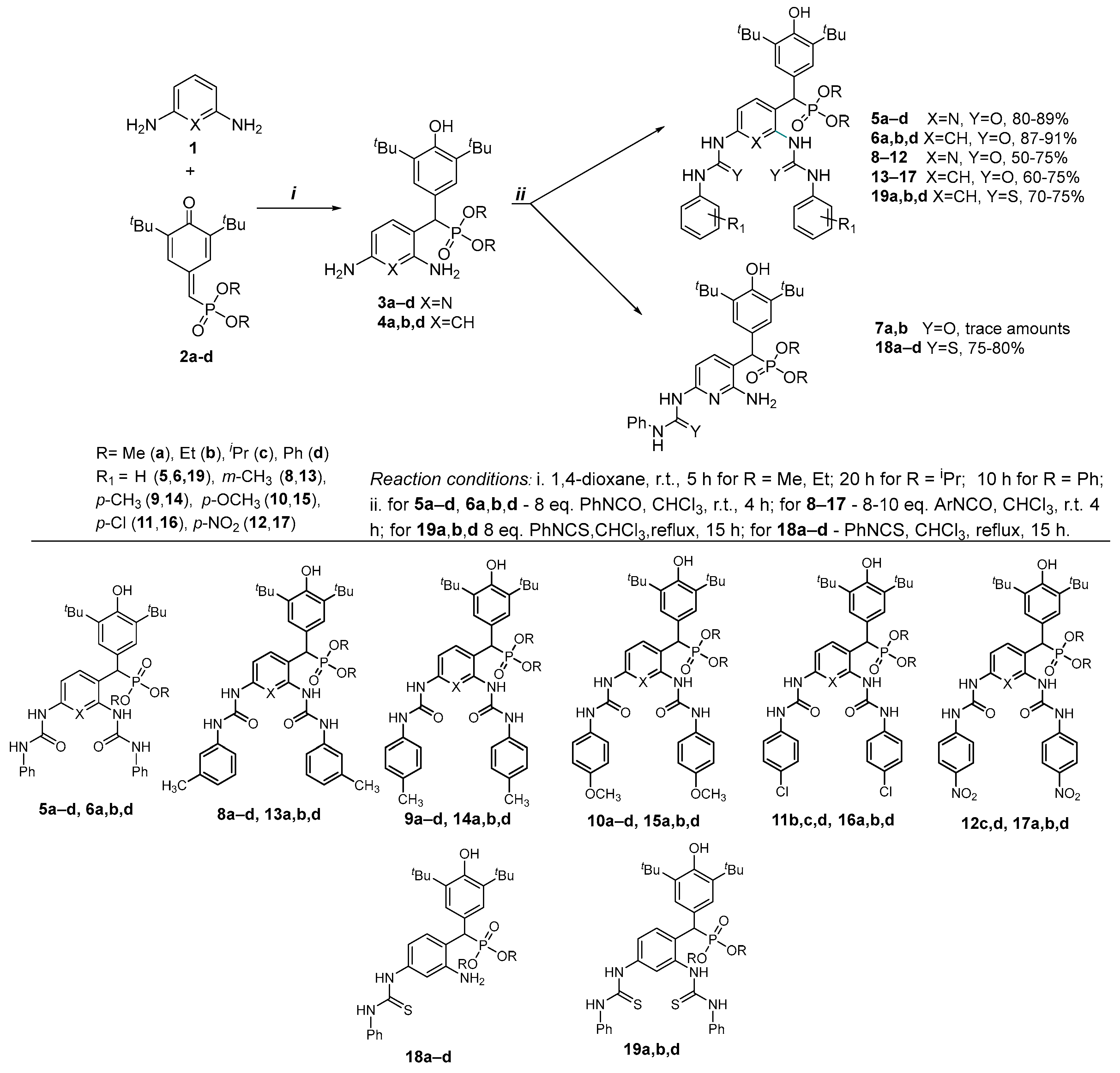
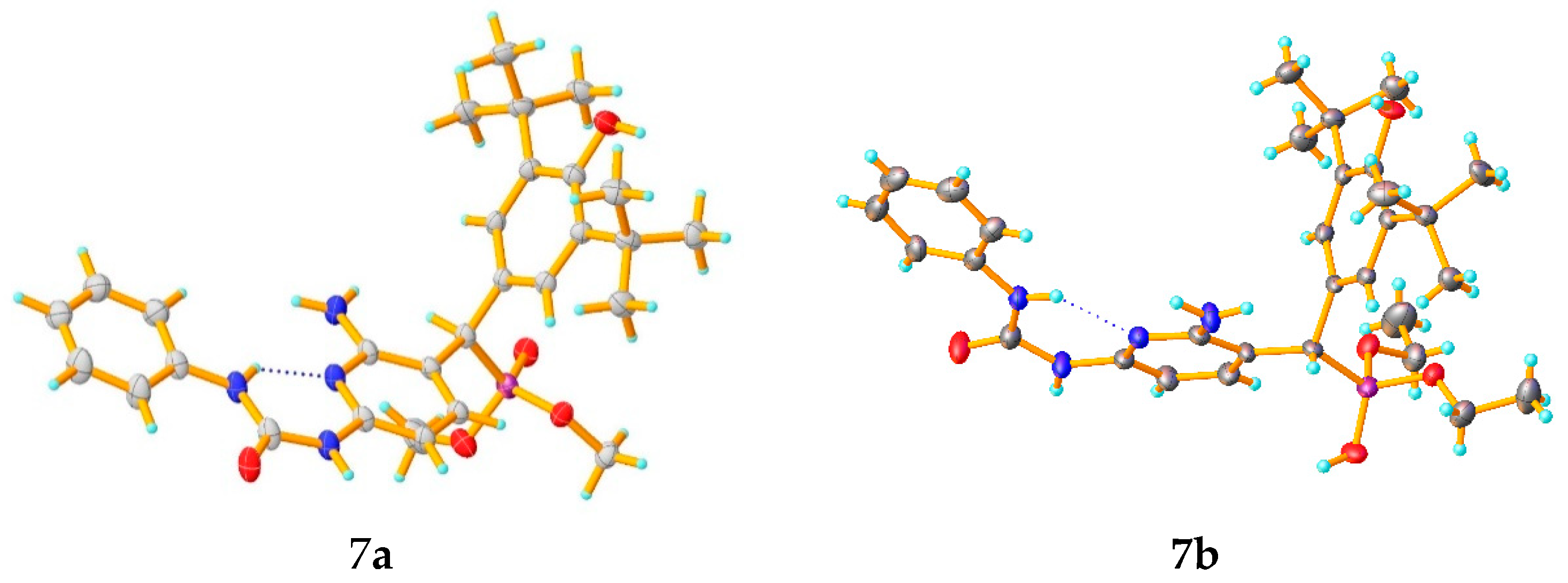
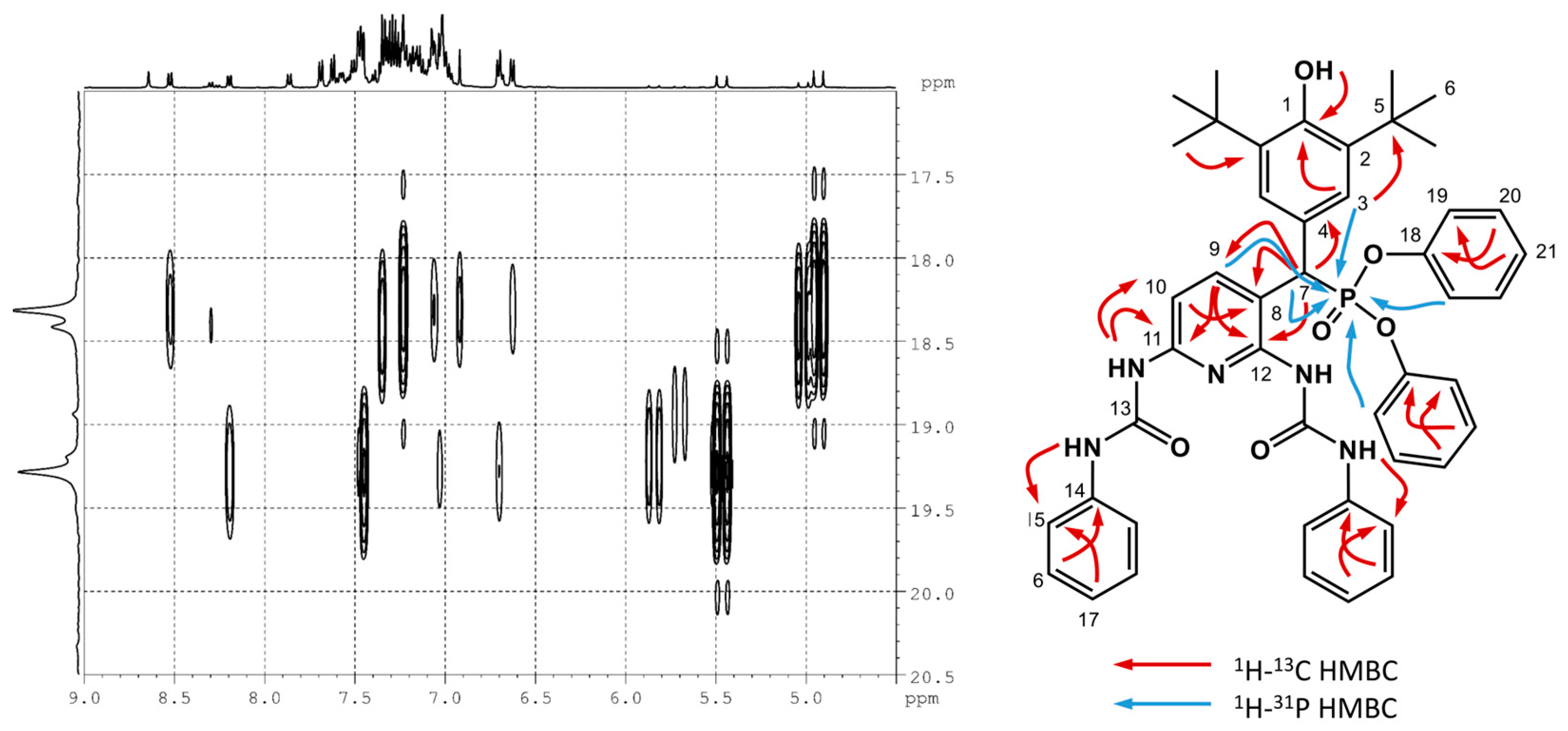
2.1. Chemistry
2.2. Biological Evaluation
2.2.1. Cytotoxic Profile
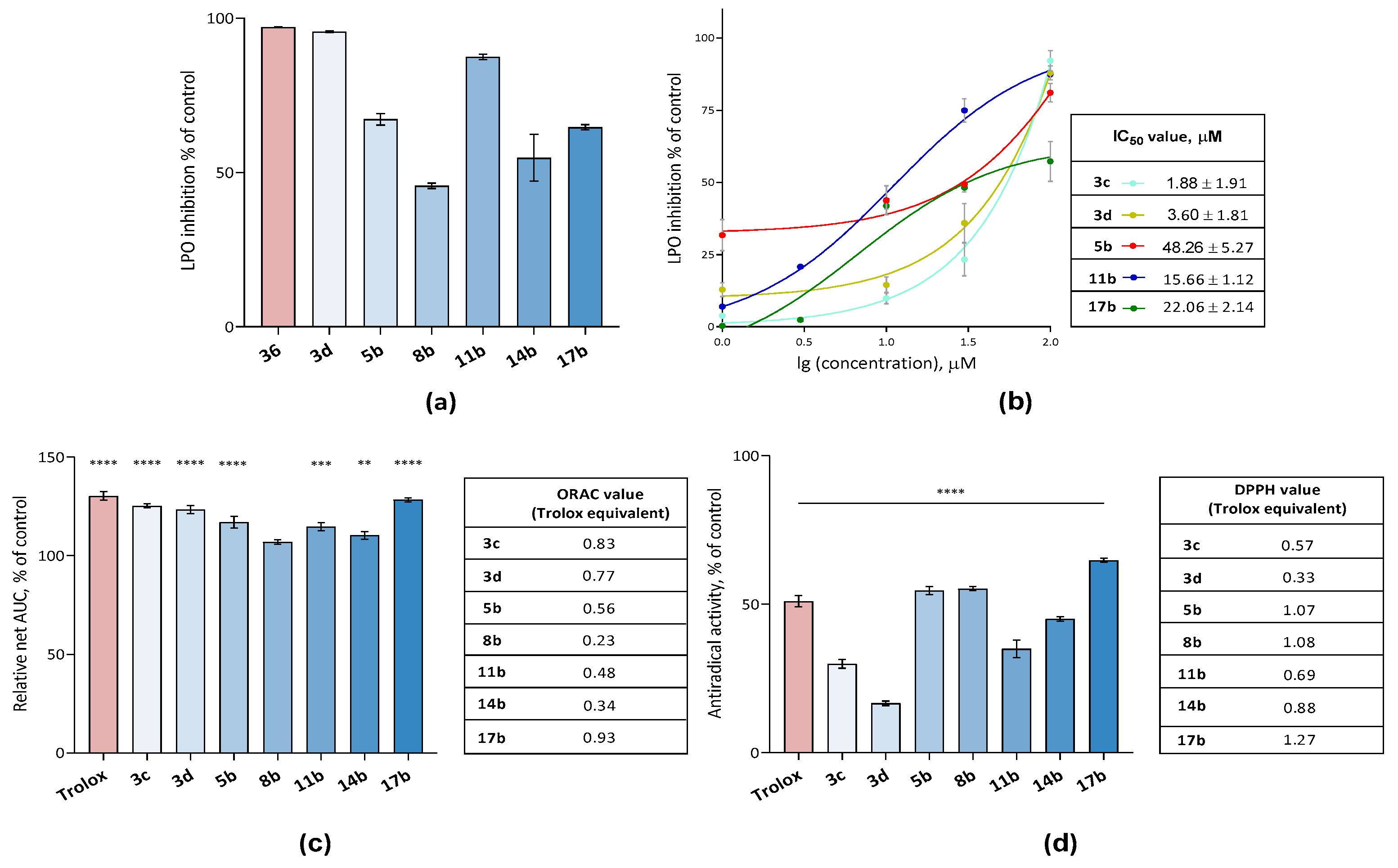
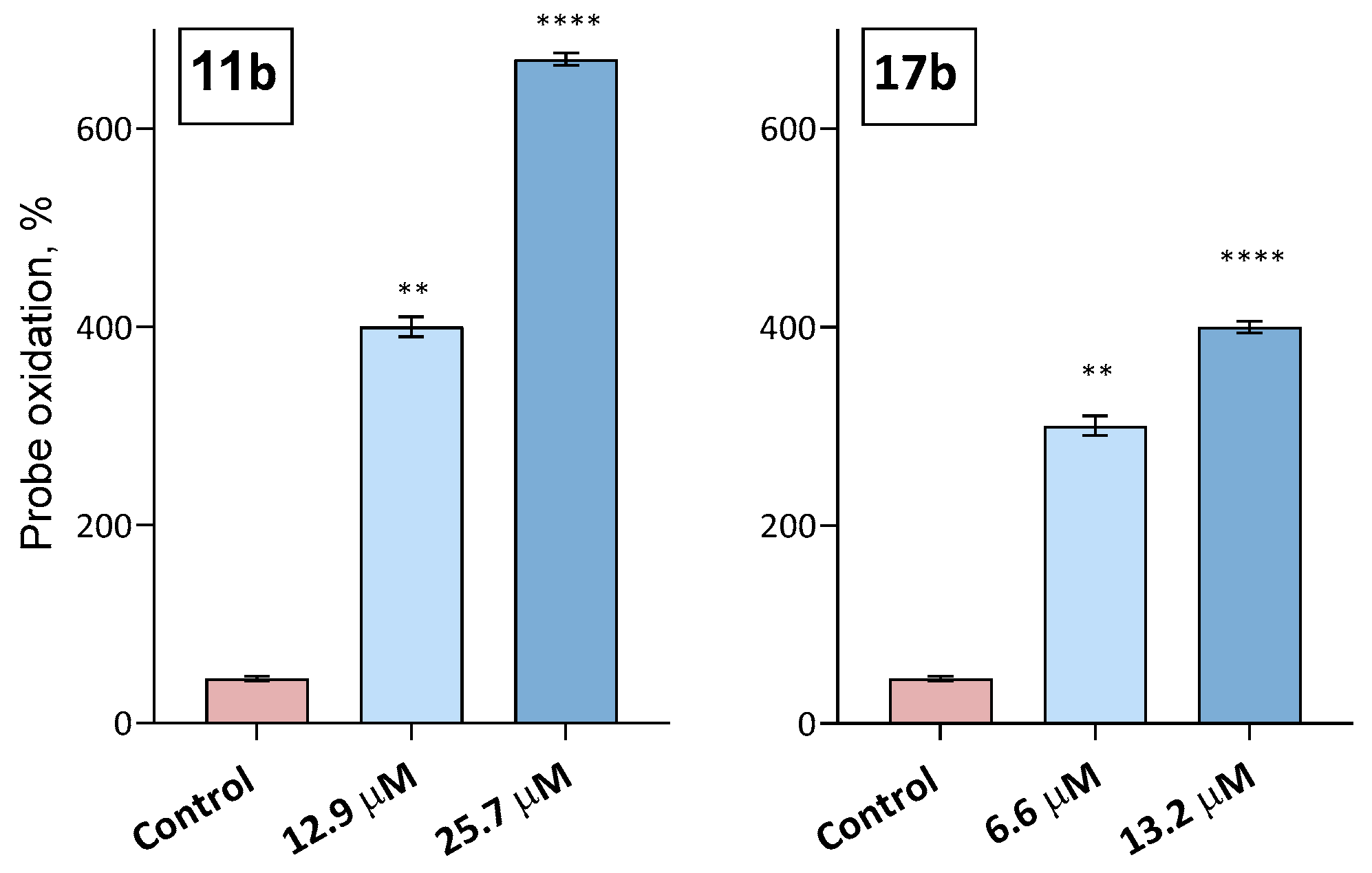
2.2.2. Analysis of the Antioxidant Potential of Compounds: Switch from Antioxidants to Oxidants
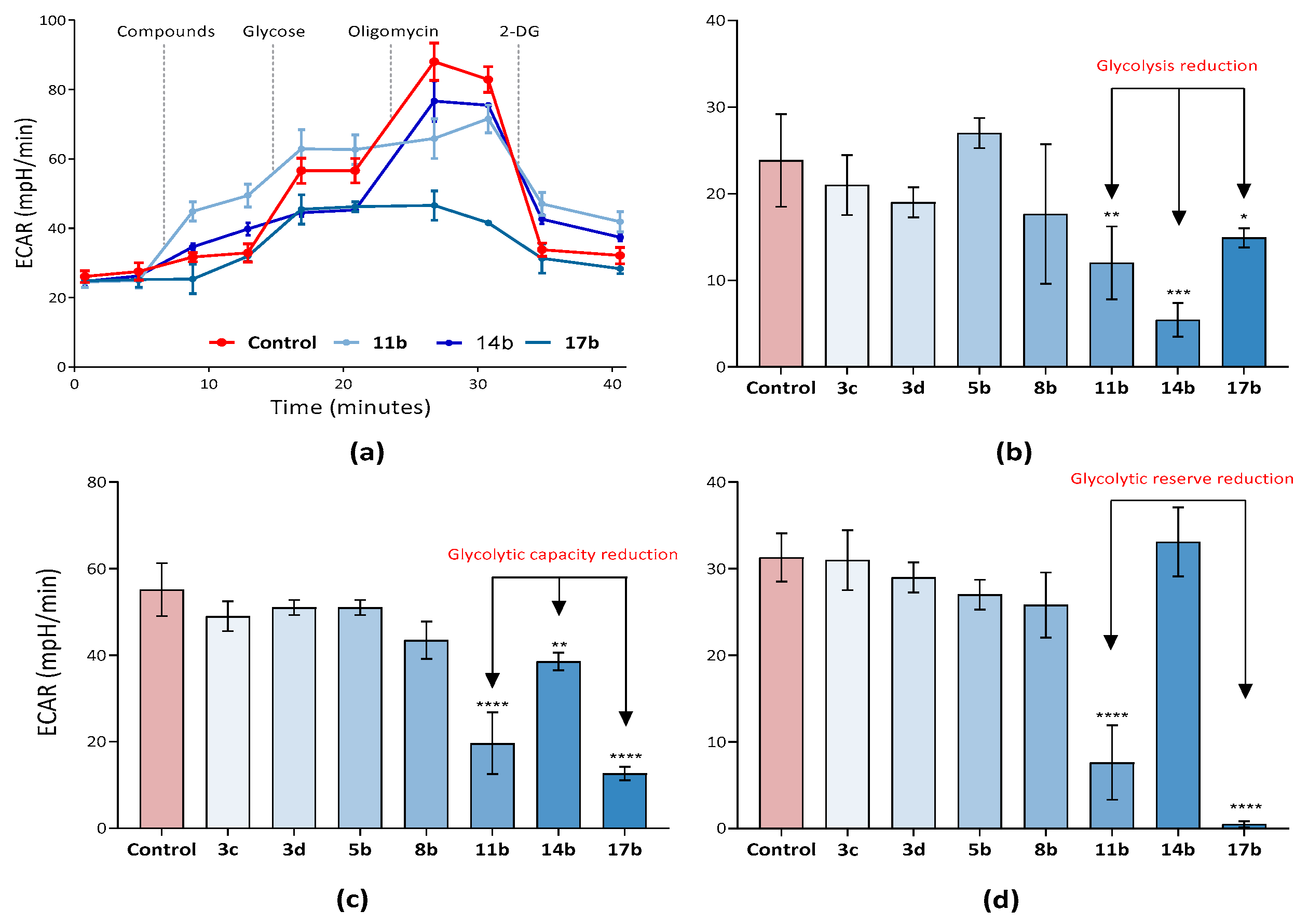
2.2.3. Analysis of the Effect of Synthesized Compounds on Tumor Cell Metabolism—Glycolysis and the Activity of the Key Allosteric Enzymes of This Process
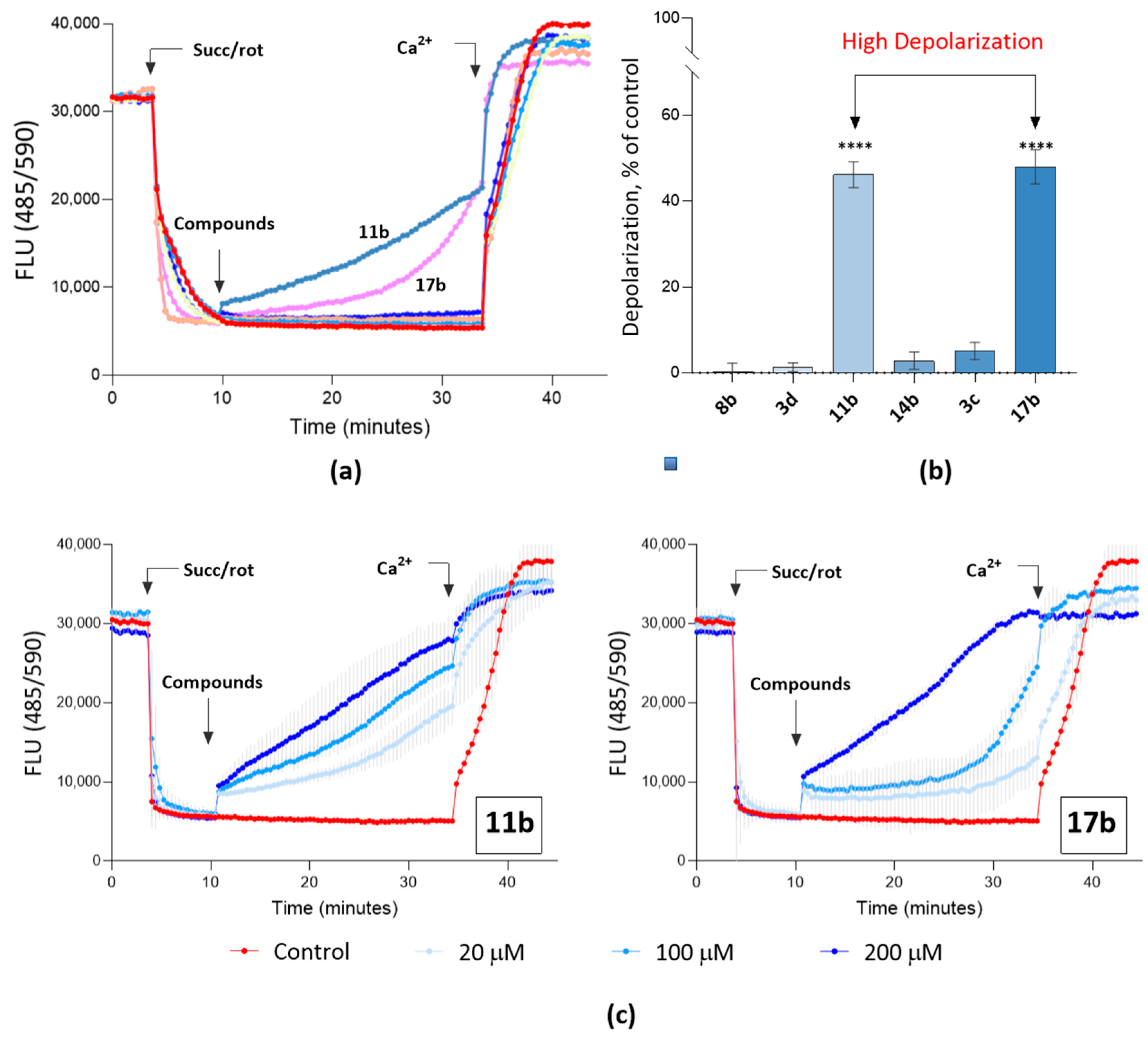
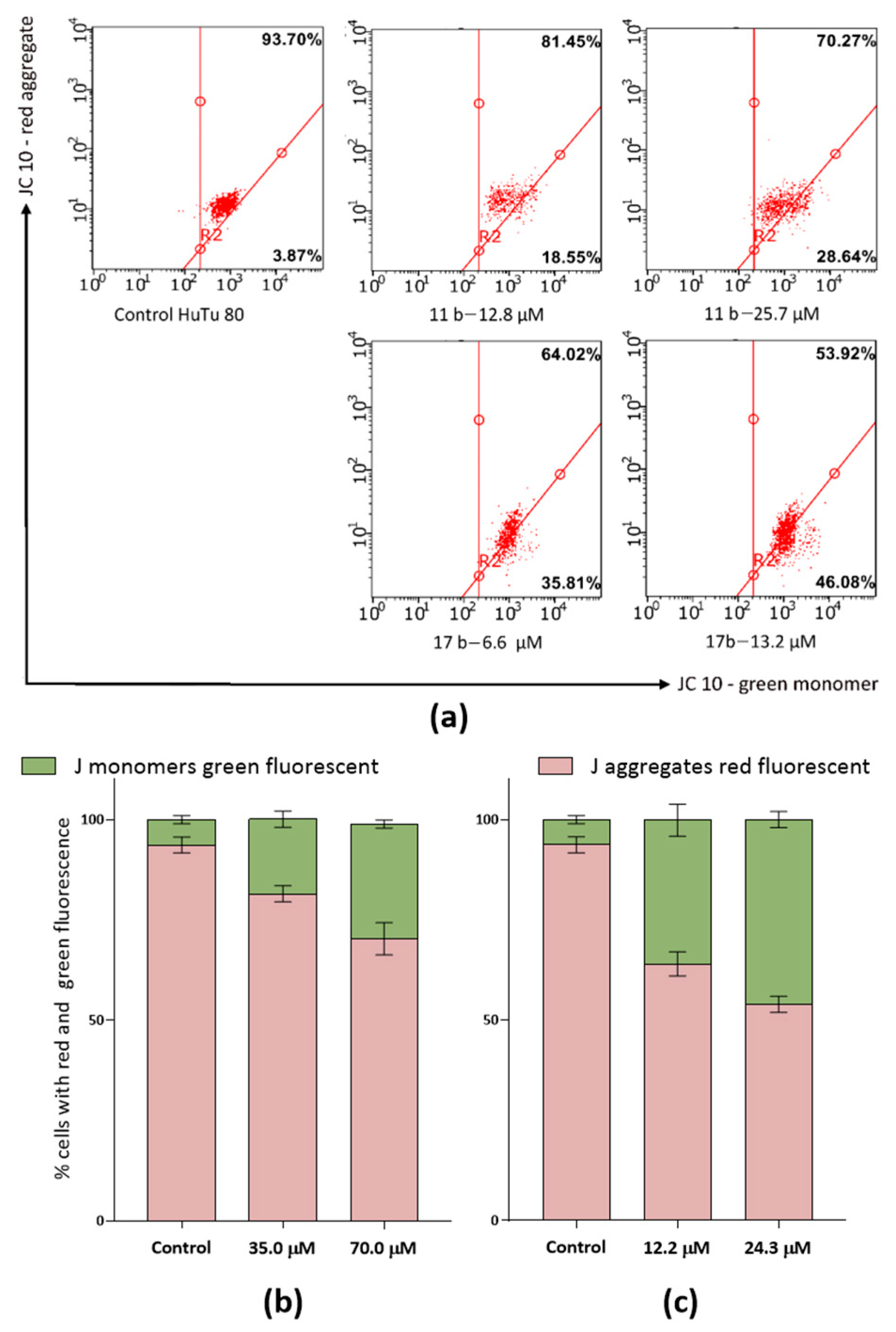
2.2.4. Study of the Effect of Compounds on Mitochondrial Characteristics
2.2.5. Pro-Apoptotic Effect of Compounds 11b and 17b
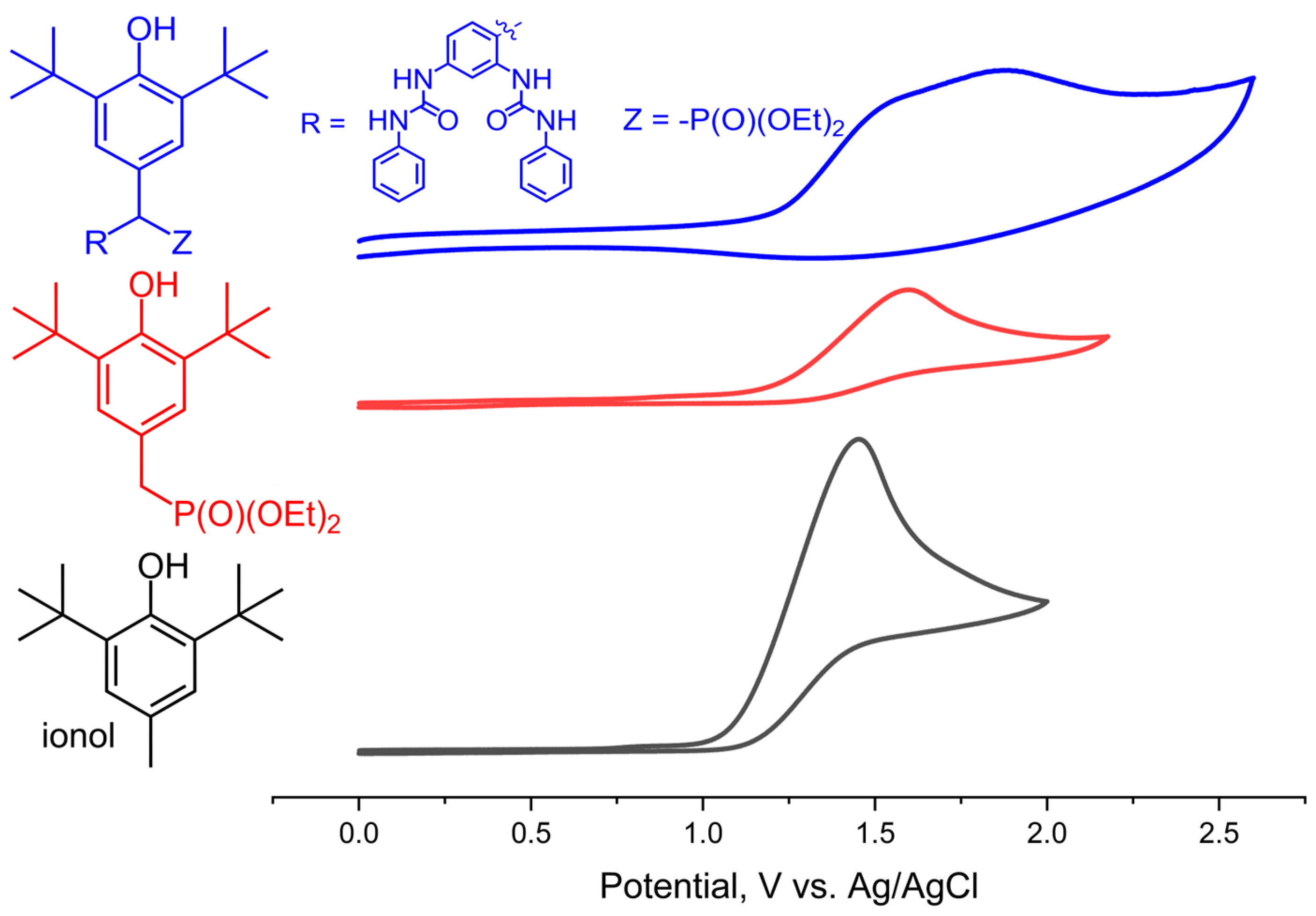
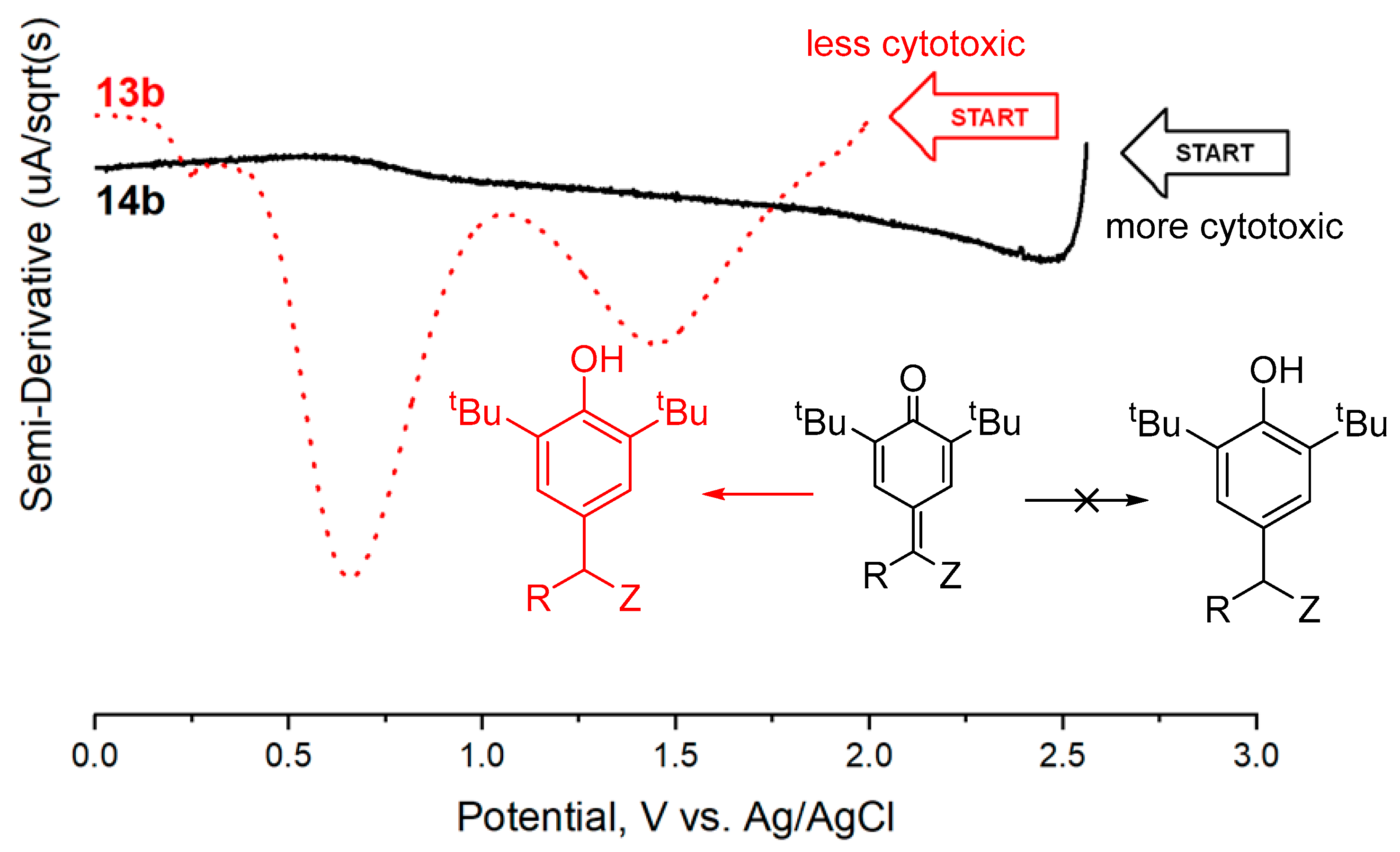
2.3. Electrochemical Measurements
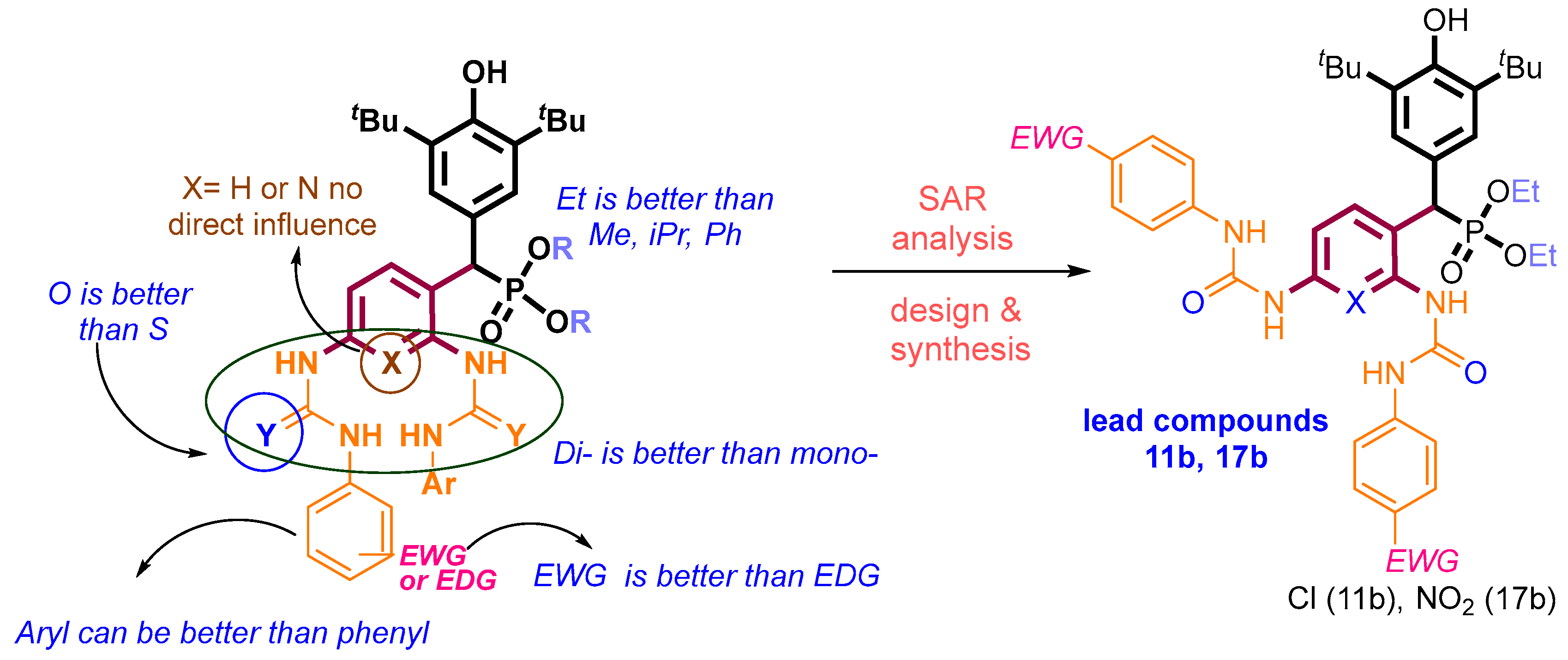
3. Discussion
4. Materials and Methods
4.1. Materials
Chemicals
4.2. General Procedures for Compound Identification
4.3. Biology
4.3.1. Cells and Materials
4.3.2. Cytotoxicity Assay
4.3.3. Flow Cytometry Assay
4.3.4. Assessment of Lipid Peroxidation (LPO)
4.3.5. Membrane Potential of Isolated Mitochondria
4.3.6. Glycolysis Flux Assay
4.3.7. Statistical Analysis
4.4. Electrochemical Measurements
4.5. Molecular Docking
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does It Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef]
- Panieri, E.; Santoro, M.M. Ros Homeostasis and Metabolism: A Dangerous Liason in Cancer Cells. Cell Death Dis. 2016, 7, e2253. [Google Scholar] [CrossRef]
- Czowski, B.J.; Romero-Moreno, R.; Trull, K.J.; White, K.A. Cancer and PH Dynamics: Transcriptional Regulation, Proteostasis, and the Need for New Molecular Tools. Cancers 2020, 12, 2760. [Google Scholar] [PubMed]
- Yang, W.Y.; Breiner, B.; Kovalenko, S.V.; Ben, C.; Singh, M.; LeGrand, S.N.; Sang, Q.X.A.; Strouse, G.F.; Copland, J.A.; Alabugin, I.V. C-Lysine Conjugates: PH-Controlled Light-Activated Reagents for Efficient Double-Stranded DNA Cleavage with Implications for Cancer Therapy. J. Am. Chem. Soc. 2009, 131, 11458–11470. [Google Scholar] [CrossRef]
- Pérez-Herrero, E.; Fernández-Medarde, A. The Reversed Intra- and Extracellular PH in Tumors as a Unified Strategy to Chemotherapeutic Delivery Using Targeted Nanocarriers. Acta Pharm. Sin. B 2021, 11, 2243–2264. [Google Scholar] [PubMed]
- Breiner, B.; Kaya, K.; Roy, S.; Yang, W.Y.; Alabugin, I.V. Hybrids of Amino Acids and Acetylenic DNA-Photocleavers: Optimising Efficiency and Selectivity for Cancer Phototherapy. Org. Biomol. Chem. 2012, 10, 3974–3987. [Google Scholar]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in Cancer Therapy: The Bright Side of the Moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, B.R.; Heleno, S.A.; Oliveira, M.B.P.P.; Barros, L.; Ferreira, I.C.F.R. Phenolic Compounds: Current Industrial Applications, Limitations and Future Challenges. Food Funct. 2021, 12, 14–29. [Google Scholar]
- Singh, J.; Sahil, M.; Ray, S.; Dcosta, C.; Panjikar, S.; Krishnamoorthy, G.; Mondal, J.; Anand, R. Phenol Sensing in Nature Modulated via a Conformational Switch Governed by Dynamic Allostery. J. Biol. Chem. 2022, 298, 102399. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jin, P.; Guan, Y.; Luo, M.; Wang, Y.; He, B.; Li, B.; He, K.; Cao, J.; Huang, C.; et al. Exploiting Polyphenol-Mediated Redox Reorientation in Cancer Therapy. Pharmaceuticals 2022, 15, 1540. [Google Scholar] [CrossRef]
- Shatokhin, S.S.; Tuskaev, V.A.; Gagieva, S.C.; Markova, A.A.; Pozdnyakov, D.I.; Denisov, G.L.; Melnikova, E.K.; Bulychev, B.M.; Oganesyan, E.T. Synthesis, Cytotoxicity and Antioxidant Activity of New 1,3-Dimethyl-8-(Chromon-3-Yl)-Xanthine Derivatives Containing 2,6-Di-Tert-Butylphenol Fragments. New J. Chem. 2022, 46, 621–631. [Google Scholar] [CrossRef]
- Burger, A.M.; Kaur, G.; Alley, M.C.; Supko, J.G.; Malspeis, L.; Grever, M.R.; Sausville, E.A. Tyrphostin AG17, [(3,5-Di-Tert-Butyl-4-Hydroxybenzylidene)-Malononitrile], Inhibits Cell Growth by Disrupting Mitochondria. Cancer Res. 1995, 55, 2794–2799. [Google Scholar]
- Kotieva, I.M.; Dodokhova, M.A.; Safronenko, A.V.; Alkhuseyn-Kulyaginova, M.S.; Milaeva, E.R.; Nikitin, E.A.; Shpakovsky, D.B.; Kotieva, E.M.; Kotieva, V.M.; Starostin, S.I. Antitumor Effectiveness of Combination Therapy with Platinum and Hybrid Organotin Compound on the Lewis Epidermoid Carcinoma Model with Metronomic Administration. J. Clin. Oncol. 2022, 40, e15080. [Google Scholar] [CrossRef]
- Jiang, X.; Hu, C.; Ferchen, K.; Nie, J.; Cui, X.; Chen, C.H.; Cheng, L.; Zuo, Z.; Seibel, W.; He, C.; et al. Targeted Inhibition of STAT/TET1 Axis as a Therapeutic Strategy for Acute Myeloid Leukemia. Nat. Commun. 2017, 8, 2099. [Google Scholar] [CrossRef]
- Edwards, C.M.; Mueller, G.; Roelofs, A.J.; Chantry, A.; Perry, M.; Russell, R.G.G.; Van Camp, B.; Guyon-Gellin, Y.; Niesor, E.J.; Bentzen, C.L.; et al. ApomineTM, an Inhibitor of HMG-CoA-Reductase, Promotes Apoptosis of Myeloma Cells in Vitro and Is Associated with a Modulation of Myeloma in Vivo. Int. J. Cancer 2007, 120, 1657–1663. [Google Scholar] [CrossRef] [PubMed]
- Gnanaguru, G.; Mackey, A.; Choi, E.Y.; Arta, A.; Rossato, F.A.; Gero, T.W.; Urquhart, A.J.; Scott, D.A.; D’Amore, P.A.; Ng, Y.S.E. Discovery of Sterically-Hindered Phenol Compounds with Potent Cytoprotective Activities against Ox-LDL–Induced Retinal Pigment Epithelial Cell Death as a Potential Pharmacotherapy. Free Radic. Biol. Med. 2022, 178, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Kupfer, R.; Dwyer-Nield, L.D.; Malkinson, A.M.; Thompson, J.A. Lung Toxicity and Tumor Promotion by Hydroxylated Derivatives of 2,6-Di-Tert-Butyl-4-Methylphenol (BHT) and 2-Tert-Butyl-4-Methyl-6-Iso-Propylphenol: Correlation with Quinone Methide Reactivity. Chem. Res. Toxicol. 2002, 15, 1106–1112. [Google Scholar] [CrossRef]
- Bolton, J.L. Quinone Methide Bioactivation Pathway: Contribution to Toxicity and/or Cytoprotection? Curr. Org. Chem. 2014, 18, 61–69. [Google Scholar] [CrossRef]
- Lalitha Naishima, N.; Faizan, S.; Raju, R.M.; Satya, A.; Sruthi, V.L.; Ng, V.; Kumar Sharma, G.; Vasanth, K.S.; Kumar Shivaraju, V.; Ramu, R.; et al. Design, Synthesis, Analysis, Evaluation of Cytotoxicity Against MCF-7 Breast Cancer Cells, 3D QSAR Studies and EGFR, HER2 Inhibition Studies on Novel Biginelli 1,4-Dihydropyrimidines. J. Mol. Struct. 2023, 1277, 84–92. [Google Scholar] [CrossRef]
- Neidlinger, A.; Förster, C.; Heinze, K. Conformational Switching of Multi-Responsive Ferrocenyl-Phenol Conjugates. European J. Org. Chem. 2016, 2016, 4852–4864. [Google Scholar] [CrossRef]
- Klopčič, I.; Dolenc, M.S. Chemicals and Drugs Forming Reactive Quinone and Quinone Imine Metabolites. Chem. Res. Toxicol. 2019, 32, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, W.; Moe, B.; Wang, H.; Li, X.F. Chemical and Toxicological Characterization of Halobenzoquinones, an Emerging Class of Disinfection Byproducts. Chem. Res. Toxicol. 2015, 28, 306–318. [Google Scholar] [CrossRef]
- Wani, T.H.; Surendran, S.; Jana, A.; Chakrabarty, A.; Chowdhury, G. Quinone-Based Antitumor Agent Sepantronium Bromide (YM155) Causes Oxygen-Independent Redox-Activated Oxidative DNA Damage. Chem. Res. Toxicol. 2018, 31, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.M.; Bordwell, F.G. Equilibrium Acidities and Homolytic Bond Dissociation Energies of the Acidic Carbon-Hydrogen Bonds in P-Substituted Triphenylphosphonium Cations. J. Am. Chem. Soc. 1994, 116, 968–972. [Google Scholar] [CrossRef]
- Chugunova, E.; Gibadullina, E.; Matylitsky, K.; Bazarbayev, B.; Neganova, M.; Volcho, K.; Rogachev, A.; Akylbekov, N.; Nguyen, H.B.T.; Voloshina, A.; et al. Diverse Biological Activity of Benzofuroxan/Sterically Hindered Phenols Hybrids. Pharmaceuticals 2023, 16, 499. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Huang, R.; Gou, S.; Wang, Z.; Wang, H. Anticancer Platinum (IV) Prodrugs Containing Monoaminophosphonate Ester as a Targeting Group Inhibit Matrix Metalloproteinases and Reverse Multidrug Resistance. Bioconjugate Chem. 2017, 28, 1305–1323. [Google Scholar] [CrossRef]
- Heidel, K.M.; Dowd, C.S. Phosphonate Prodrugs: An Overview and Recent Advances. Future Med. Chem. 2019, 11, 1625–1643. [Google Scholar] [CrossRef]
- Hochdörffer, K.; Abu Ajaj, K.; Schäfer-Obodozie, C.; Kratz, F. Development of Novel Bisphosphonate Prodrugs of Doxorubicin for Targeting Bone Metastases That Are Cleaved PH Dependently or by Cathepsin B: Synthesis, Cleavage Properties, and Binding Properties to Hydroxyapatite as Well as Bone Matrix. J. Med. Chem. 2012, 55, 7502–7515. [Google Scholar] [CrossRef]
- Listro, R.; Rossino, G.; Piaggi, F.; Sonekan, F.F.; Rossi, D.; Linciano, P.; Collina, S. Urea-Based Anticancer Agents. Exploring 100-Years of Research with an Eye to the Future. Front. Chem. 2022, 10, 995351. [Google Scholar] [CrossRef]
- Wu, Y.-C.; Ren, X.-Y.; Rao, G.-W. Research Progress of Diphenyl Urea Derivatives as Anticancer Agents and Synthetic Methodologies. Mini Rev. Org. Chem. 2018, 16, 617–630. [Google Scholar] [CrossRef]
- Machulkin, A.E.; Shafikov, R.R.; Uspenskaya, A.A.; Petrov, S.A.; Ber, A.P.; Skvortsov, D.A.; Nimenko, E.A.; Zyk, N.U.; Smirnova, G.B.; Pokrovsky, V.S.; et al. Synthesis and Biological Evaluation of PSMA Ligands with Aromatic Residues and Fluorescent Conjugates Based on Them. J. Med. Chem. 2021, 64, 4532–4552. [Google Scholar] [CrossRef]
- Petrov, S.A.; Zyk, N.Y.; Machulkin, A.E.; Beloglazkina, E.K.; Majouga, A.G. PSMA-Targeted Low-Molecular Double Conjugates for Diagnostics and Therapy. Eur. J. Med. Chem. 2021, 225, 113752. [Google Scholar] [CrossRef]
- Garuti, L.; Roberti, M.; Bottegoni, G.; Ferraro, M. Diaryl Urea: A Privileged Structure in Anticancer Agents. Curr. Med. Chem. 2016, 23, 1528–1548. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Waymouth, R.M. Urea Anions: Simple, Fast, and Selective Catalysts for Ring-Opening Polymerizations. J. Am. Chem. Soc. 2017, 139, 1645–1652. [Google Scholar] [CrossRef] [PubMed]
- Gazizov, A.S.; Smolobochkin, A.V.; Rizbayeva, T.S.; Vatsadze, S.Z.; Burilov, A.R.; Sinyashin, O.G.; Alabugin, I.V. “Stereoelectronic Deprotection of Nitrogen”: Recovering Nucleophilicity with a Conformational Change. J. Org. Chem. 2023, 88, 6868–6877. [Google Scholar] [CrossRef]
- Catalano, A.; Iacopetta, D.; Sinicropi, M.S.; Franchini, C. Diarylureas as Antitumor Agents. Appl. Sci. 2021, 11, 374. [Google Scholar] [CrossRef]
- Gibadullina, E.; Nguyen, T.T.; Strelnik, A.; Sapunova, A.; Voloshina, A.; Sudakov, I.; Vyshtakalyuk, A.; Voronina, J.; Pudovik, M.; Burilov, A. New 2,6-Diaminopyridines Containing a Sterically Hindered Benzylphosphonate Moiety in the Aromatic Core as Potential Antioxidant and Anti-Cancer Drugs. Eur. J. Med. Chem. 2019, 184, 111735. [Google Scholar] [CrossRef]
- Klippel, S.; Jakubikova, J.; Delmore, J.; Ooi, M.; Mcmillin, D.; Kastritis, E.; Laubach, J.; Richardson, P.G.; Anderson, K.C.; Mitsiades, C.S. Methyljasmonate Displays in Vitro and in Vivo Activity against Multiple Myeloma Cells. Br. J. Haematol. 2012, 159, 340–351. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, F.; Nan, Y.; Qu, L.; Na, W.; Jia, C.; Chen, X. PKM2 Inhibitor Shikonin Overcomes the Cisplatin Resistance in Bladder Cancer by Inducing Necroptosis. Int. J. Biol. Sci. 2018, 14, 1883–1891. [Google Scholar] [CrossRef]
- Guo, M.; Wang, Z.; Wang, Z.; Zhang, L. Enhanced Electrochemical Determination of Quercetin in a Choline Chloride-Based Ionic Liquid. Anal. Methods 2023, 15, 1378–1386. [Google Scholar] [CrossRef]
- Simić, A.; Manojlović, D.; Šegan, D.; Todorović, M. Electrochemical Behavior and Antioxidant and Prooxidant Activity of Natural Phenolics. Molecules 2007, 12, 2327–2340. [Google Scholar] [CrossRef] [PubMed]
- Akyeva, A.Y.; Kansuzyan, A.V.; Vukich, K.S.; Kuhn, L.; Saverina, E.A.; Minyaev, M.E.; Pechennikov, V.M.; Egorov, M.P.; Alabugin, I.V.; Vorobyev, S.V.; et al. Remote Stereoelectronic Effects in Pyrrolidone- and Caprolactam-Substituted Phenols: Discrepancies in Antioxidant Properties Evaluated by Electrochemical Oxidation and H-Atom Transfer Reactivity. J. Org. Chem. 2022, 87, 5371–5384. [Google Scholar] [CrossRef] [PubMed]
- Sestili, P.; Diamantini, G.; Bedini, A.; Cerioni, L.; Tommasini, I.; Tarzia, G.; Cantoni, O. Plant-Derived Phenolic Compounds Prevent the DNA Single-Strand Breakage and Cytotoxicity Induced by Tert-Butylhydroperoxide via an Iron-Chelating Mechanism. Biochem. J. 2002, 364, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Van Hung, P. Phenolic Compounds of Cereals and Their Antioxidant Capacity. Crit. Rev. Food Sci. Nutr. 2016, 56, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Uchida, M.; Nakajin, S.; Toyoshima, S.; Shinoda, M. Antioxidative Effect of Sesamol and Related Compounds on Lipid Peroxidation. Biol. Pharm. Bull. 1996, 19, 623–626. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Socrier, L.; Quéro, A.; Verdu, M.; Song, Y.; Molinié, R.; Mathiron, D.; Pilard, S.; Mesnard, F.; Morandat, S. Flax Phenolic Compounds as Inhibitors of Lipid Oxidation: Elucidation of Their Mechanisms of Action. Food Chem. 2019, 274, 651–658. [Google Scholar] [CrossRef]
- Jomová, K.; Hudecova, L.; Lauro, P.; Simunkova, M.; Alwasel, S.H.; Alhazza, I.M.; Valko, M. A Switch between Antioxidant and Prooxidant Properties of the Phenolic Compounds Myricetin, Morin, 3′,4′-Dihydroxyflavone, Taxifolin and 4-Hydroxy-Coumarin in the Presence of Copper(II) Ions: A Spectroscopic, Absorption Titration and DNA Damage Study. Molecules 2019, 24, 4335. [Google Scholar] [CrossRef]
- Amić, A.; Marković, Z.; Dimitrić Marković, J.M.; Milenković, D.; Stepanić, V. Antioxidative Potential of Ferulic Acid Phenoxyl Radical. Phytochemistry 2020, 170, 112218. [Google Scholar] [CrossRef]
- Boedtkjer, E.; Pedersen, S.F. The Acidic Tumor Microenvironment as a Driver of Cancer. Annu. Rev. Physiol. 2020, 82, 103–126. [Google Scholar] [CrossRef]
- Cassim, S.; Pouyssegur, J. Tumor Microenvironment: A Metabolic Player That Shapes the Immune Response. Int. J. Mol. Sci. 2019, 21, 157. [Google Scholar] [CrossRef]
- Torti, S.V.; Manz, D.H.; Paul, B.T.; Blanchette-Farra, N.; Torti, F.M. Iron and Cancer. Annu. Rev. Nutr. 2018, 38, 97–125. [Google Scholar] [CrossRef]
- Morales, M.; Xue, X. Targeting Iron Metabolism in Cancer Therapy. Theranostics 2021, 11, 8412–8429. [Google Scholar] [CrossRef]
- York, E.; McNaughton, D.A.; Roseblade, A.; Cranfield, C.G.; Gale, P.A.; Rawling, T. Structure–Activity Relationship and Mechanistic Studies of Bisaryl Urea Anticancer Agents Indicate Mitochondrial Uncoupling by a Fatty Acid-Activated Mechanism. ACS Chem. Biol. 2022, 17, 2065–2073. [Google Scholar] [CrossRef] [PubMed]
- Gibadullina, E.M.; Shaekhov, T.R.; Badrtdinov, A.K.; Burilov, A.R. α-Phosphorylated 2,6-Di-Tert-Butyl-4-Methylidene-2,5-Cyclohexadienones in the Reactions with Meta-Phenylenediamine. Russ. Chem. Bull. 2014, 63, 1455–1456. [Google Scholar] [CrossRef]
- Präbst, K.; Engelhardt, H.; Ringgeler, S.; Hübner, H. Basic Colorimetric Proliferation Assays: MTT, WST, and Resazurin. In Cell Viability Assays: Methods and Protocols; Gilbert Daniel, F., Friedrich, O., Eds.; Springer: New York, NY, USA, 2017; pp. 1–17. ISBN 978-1-4939-6960-9. [Google Scholar]
- Neganova, M.; Semakov, A.; Aleksandrova, Y.; Yandulova, E.; Pukhov, S.; Anikina, L.; Klochkov, S. N-Alkylation of Anthracycline Antibiotics by Natural Sesquiterpene Lactones as a Way to Obtain Antitumor Agents with Reduced Side Effects. Biomedicines 2021, 9, 547. [Google Scholar] [CrossRef] [PubMed]
- Akerman, K.E.; Wikström, M.K. Safranine as a Probe of the Mitochondrial Membrane Potential. FEBS Lett. 1976, 68, 191–197. [Google Scholar] [CrossRef]
- AAT Bioquest, Inc. IC50 Calculator. 2023. Available online: https://www.aatbio.com/tools/ic50-calculator (accessed on 25 June 2022).
- Schrödinger, LLC. Schrödinger Release 2023-1: Maestro; Schrödinger Inc.: New York, NY, USA, 2021. [Google Scholar]
- Madhavi Sastry, G.; Adzhigirey, M.; Day, T.; Annabhimoju, R.; Sherman, W. Protein and Ligand Preparation: Parameters, Protocols, and Influence on Virtual Screening Enrichments. J. Comput. Aided Mol. Des. 2013, 27, 221–234. [Google Scholar] [CrossRef]
- Schrödinger, LLC. Schrödinger Release 2023-1: Protein Preparation Wizard, Epik, Impact; Schrödinger Inc.: New York, NY, USA, 2021. [Google Scholar]
- Jacobson, M.P.; Pincus, D.L.; Rapp, C.S.; Day, T.J.F.; Honig, B.; Shaw, D.E.; Friesner, R.A. A Hierarchical Approach to All-Atom Protein Loop Prediction. Proteins Struct. Funct. Genet. 2004, 55, 351–367. [Google Scholar] [CrossRef]
- Jacobson, M.P.; Friesner, R.A.; Xiang, Z.; Honig, B. On the Role of the Crystal Environment in Determining Protein Side-Chain Conformations. J. Mol. Biol. 2002, 320, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger, LLC. Schrödinger Release 2023-1: Prime; Schrödinger Inc.: New York, NY, USA, 2021. [Google Scholar]
- Harder, E.; Damm, W.; Maple, J.; Wu, C.; Reboul, M.; Xiang, J.Y.; Wang, L.; Lupyan, D.; Dahlgren, M.K.; Knight, J.L.; et al. OPLS3: A Force Field Providing Broad Coverage of Drug-like Small Molecules and Proteins. J. Chem. Theory Comput. 2016, 12, 281–296. [Google Scholar] [CrossRef]
- Schrödinger, LLC. Schrödinger Release 2023-1: LigPrep; Schrödinger Inc.: New York, NY, USA, 2021. [Google Scholar]
- Farid, R.; Day, T.; Friesner, R.A.; Pearlstein, R.A. New Insights about HERG Blockade Obtained from Protein Modeling, Potential Energy Mapping, and Docking Studies. Bioorg Med. Chem. 2006, 14, 3160–3173. [Google Scholar] [CrossRef] [PubMed]
- Sherman, W.; Day, T.; Jacobson, M.P.; Friesner, R.A.; Farid, R. Novel Procedure for Modeling Ligand/Receptor Induced Fit Effects. J. Med. Chem. 2006, 49, 534–553. [Google Scholar] [CrossRef] [PubMed]
- Sherman, W.; Beard, H.S.; Farid, R. Use of an Induced Fit Receptor Structure in Virtual Screening. Chem. Biol. Drug Des. 2006, 67, 83–84. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger, LLC. Schrödinger Release 2023-1: Induced Fit Docking Protocol; Schrödinger Inc.: New York, NY, USA, 2021. [Google Scholar]
- Schrödinger, LLC. Schrödinger Release 2023-1: Glide; Schrödinger Inc.: New York, NY, USA, 2021. [Google Scholar]
- Sheldrick, G.M. SHELXTL v.6.12, Structure Determination Software Suite. Bruker AXS: Madison, WI, USA, 2000. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian Inc.: Wallingford, CT, USA, 2016.
- Zhao, Y.; Truhlar, D.G. Exploring the Limit of Accuracy of the Global Hybrid Meta Density Functional for Main-Group Thermochemistry, Kinetics, and Noncovalent Interactions. J. Chem. Theory Comput. 2008, 4, 1849–1868. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. Construction of a Generalized Gradient Approximation by Restoring the Density-Gradient Expansion and Enforcing a Tight Lieb-Oxford Bound. J. Chem. Phys. 2008, 128. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the Damping Function in Dispersion Corrected Density Functional Theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Schwabe, T.; Grimme, S. Double-Hybrid Density Functionals with Long-Range Dispersion Corrections: Higher Accuracy and Extended Applicability. Phys. Chem. Chem. Phys. 2007, 9, 3397–3406. [Google Scholar] [CrossRef]

| Compounds | IC50, µM | ||||
|---|---|---|---|---|---|
| Cancer Cell Lines | Normal Cell Lines | ||||
| *M-HeLa | MCF-7 | HuTu 80 | SH-SY5Y | Chang Liver | |
| 3a | 16.1 ± 1.3 | 31.0 ± 2.6 | >100 | >100 | >100 |
| 3b | 17.1 ± 1.3 | 34.2 ± 3.0 | 86.6 ± 6.8 | 77.0 ± 1.9 | >100 |
| 3c | 38.0 ± 2.8 | 21.8 ± 1.7 (SI~5) | >100 | 30.4 ± 0.7 (SI~3) | 100.5 ± 2.7 |
| 3d | 7.4 ± 0.7 (SI~7) | 16.4 ± 1.7 (SI~3) | 63.2 ± 5.6 | 18.8 ± 0.2 | 52.3 ± 3.5 |
| 5b | 62.5 ± 5.2 | 36.3 ± 2.8 | 20.0 ± 1.7 | 17.9 ± 1.9 | 31.4 ± 2.4 |
| 8b | 62.04 ± 2.2 | 35.4 ± 2.8 | 8.0 ± 0.6 | 41.0 ± 0.3 | 13.0 ± 1.2 |
| 9d | 78.9 ± 6.2 | 76.6 ± 6.2 | 50.0 ± 4.0 | >100 | >100 |
| 10b | >100 | 82.1 ± 6.5 | 45.0 ± 3.6 | >100 | 81.2 ± 6.4 |
| 11b | 70.0 ± 5.5 | 63.1 ± 4.9 | 25.7 ± 2.1 (SI~3) | 46.4 ± 0.92 | 99.4 ± 7.8 |
| 13a | 75.0 ± 6.0 | 77.4 ± 6.3 | 75.1 ± 6.1 | >100 | 92.0 ± 7.2 |
| 13b | 96.3 ± 7.5 | 57.7 ± 4.6 | 40.1 ± 3.1 | 86.3 ± 4.0 | 68.2 ± 5.4 |
| 14b | 14.1 ± 1.2 | 13.7 ± 1.2 | 15.7 ± 1.3 | 27.0 ± 1.1 | 21.0 ± 1.8 |
| 15b | >100 | 96.3 ± 7.6 | 58.7 ± 4.6 | >100 | 96.0 ± 7.6 |
| 16b | 100.3 ± 8.6 | >100 | 61.0 ± 4.8 | >100 | >100 |
| 17a | 60.0 ± 4.7 | 73.6 ± 5.8 | 41.0 ± 3.1 | >100 | 86.0 ± 6.6 |
| 17b | 24.3 ± 1.9 (SI~3) | 51.0 ± 4.2 | 13.2 ± 1.1 (SI~6) | 54.8 ± 2.1 | 74.0 ± 5.9 |
| 18c | >100 | 44.0 ± 3.3 | 53.0 ± 4.1 | >100 | 45.0 ± 3.6 |
| Tamoxifen | 28.0 ± 2.5 | 25.0 ± 2.2 | - | - | 46.2 ± 3.5 |
| SF | 25.0 ± 1.9 | 27.5 ± 2.3 | 6.2 ± 0.5 | - | 21.7 ± 1.7 |
| Compounds | Docking Score | Compounds | Docking Score |
|---|---|---|---|
| 11bR | −7.1 | 11bS | −7.8 |
| 17bR | −7.5 | 17bS | −7.1 |
| Reference | −7.0 | Reference | −7.0 |
| Compounds | Docking Score | Compounds | Docking Score |
|---|---|---|---|
| 11bR | −13.5 | 11bS | −13.4 |
| 17bR | −13.7 | 17bS | - |
| Reference | −10.9 | Reference | −10.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gibadullina, E.; Neganova, M.; Aleksandrova, Y.; Nguyen, H.B.T.; Voloshina, A.; Khrizanforov, M.; Nguyen, T.T.; Vinyukova, E.; Volcho, K.; Tsypyshev, D.; et al. Hybrids of Sterically Hindered Phenols and Diaryl Ureas: Synthesis, Switch from Antioxidant Activity to ROS Generation and Induction of Apoptosis. Int. J. Mol. Sci. 2023, 24, 12637. https://doi.org/10.3390/ijms241612637
Gibadullina E, Neganova M, Aleksandrova Y, Nguyen HBT, Voloshina A, Khrizanforov M, Nguyen TT, Vinyukova E, Volcho K, Tsypyshev D, et al. Hybrids of Sterically Hindered Phenols and Diaryl Ureas: Synthesis, Switch from Antioxidant Activity to ROS Generation and Induction of Apoptosis. International Journal of Molecular Sciences. 2023; 24(16):12637. https://doi.org/10.3390/ijms241612637
Chicago/Turabian StyleGibadullina, Elmira, Margarita Neganova, Yulia Aleksandrova, Hoang Bao Tran Nguyen, Alexandra Voloshina, Mikhail Khrizanforov, Thi Thu Nguyen, Ekaterina Vinyukova, Konstantin Volcho, Dmitry Tsypyshev, and et al. 2023. "Hybrids of Sterically Hindered Phenols and Diaryl Ureas: Synthesis, Switch from Antioxidant Activity to ROS Generation and Induction of Apoptosis" International Journal of Molecular Sciences 24, no. 16: 12637. https://doi.org/10.3390/ijms241612637
APA StyleGibadullina, E., Neganova, M., Aleksandrova, Y., Nguyen, H. B. T., Voloshina, A., Khrizanforov, M., Nguyen, T. T., Vinyukova, E., Volcho, K., Tsypyshev, D., Lyubina, A., Amerhanova, S., Strelnik, A., Voronina, J., Islamov, D., Zhapparbergenov, R., Appazov, N., Chabuka, B., Christopher, K., ... Alabugin, I. (2023). Hybrids of Sterically Hindered Phenols and Diaryl Ureas: Synthesis, Switch from Antioxidant Activity to ROS Generation and Induction of Apoptosis. International Journal of Molecular Sciences, 24(16), 12637. https://doi.org/10.3390/ijms241612637










