Canonical Transient Receptor Potential Channel 3 Contributes to Cerebral Blood Flow Changes Associated with Cortical Spreading Depression in Mice
Abstract
1. Introduction
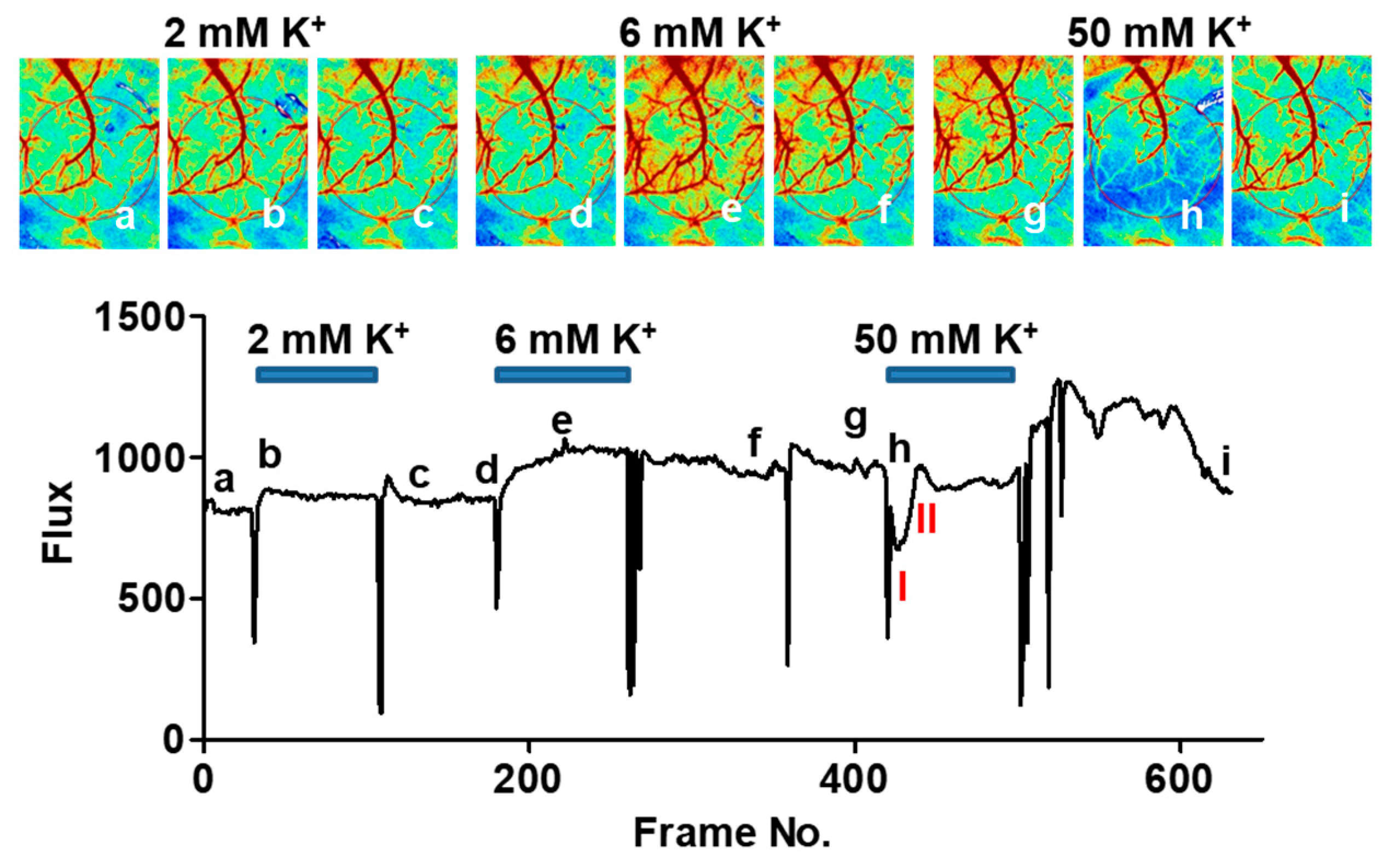
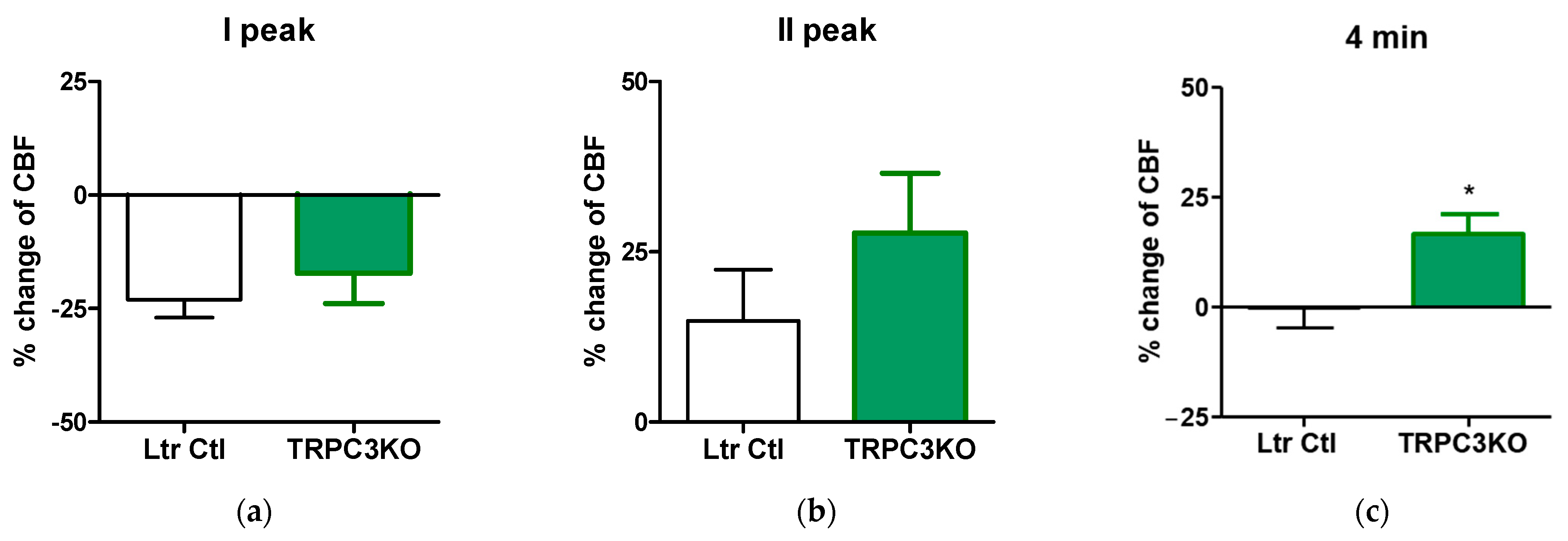
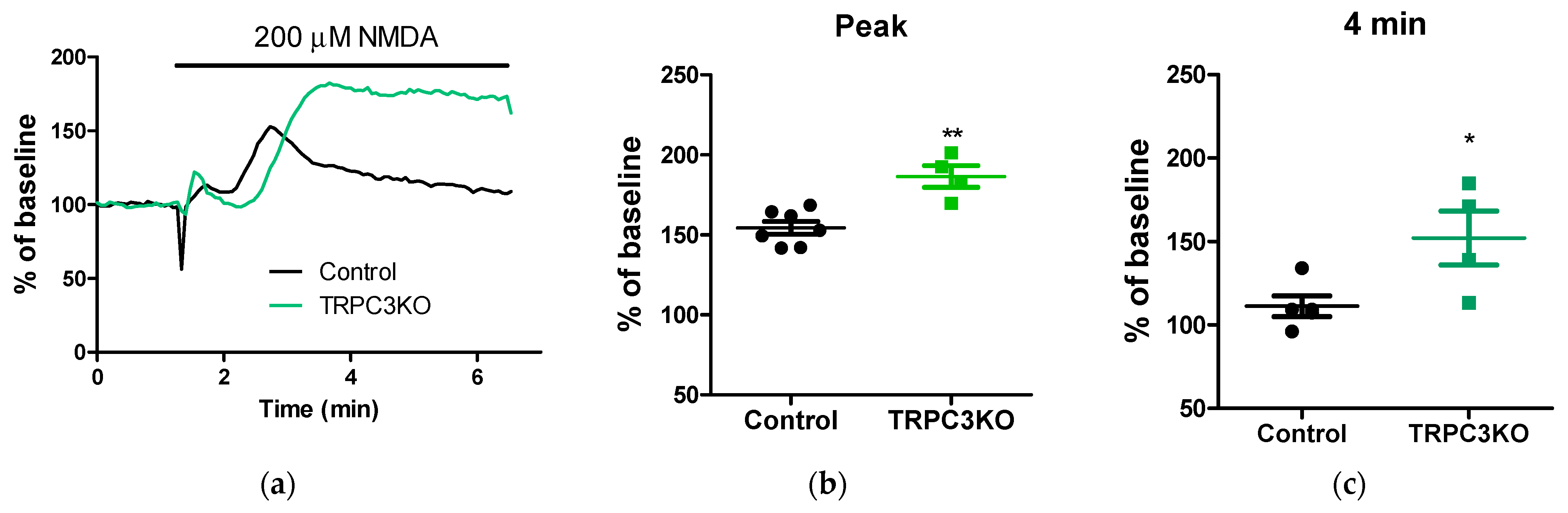
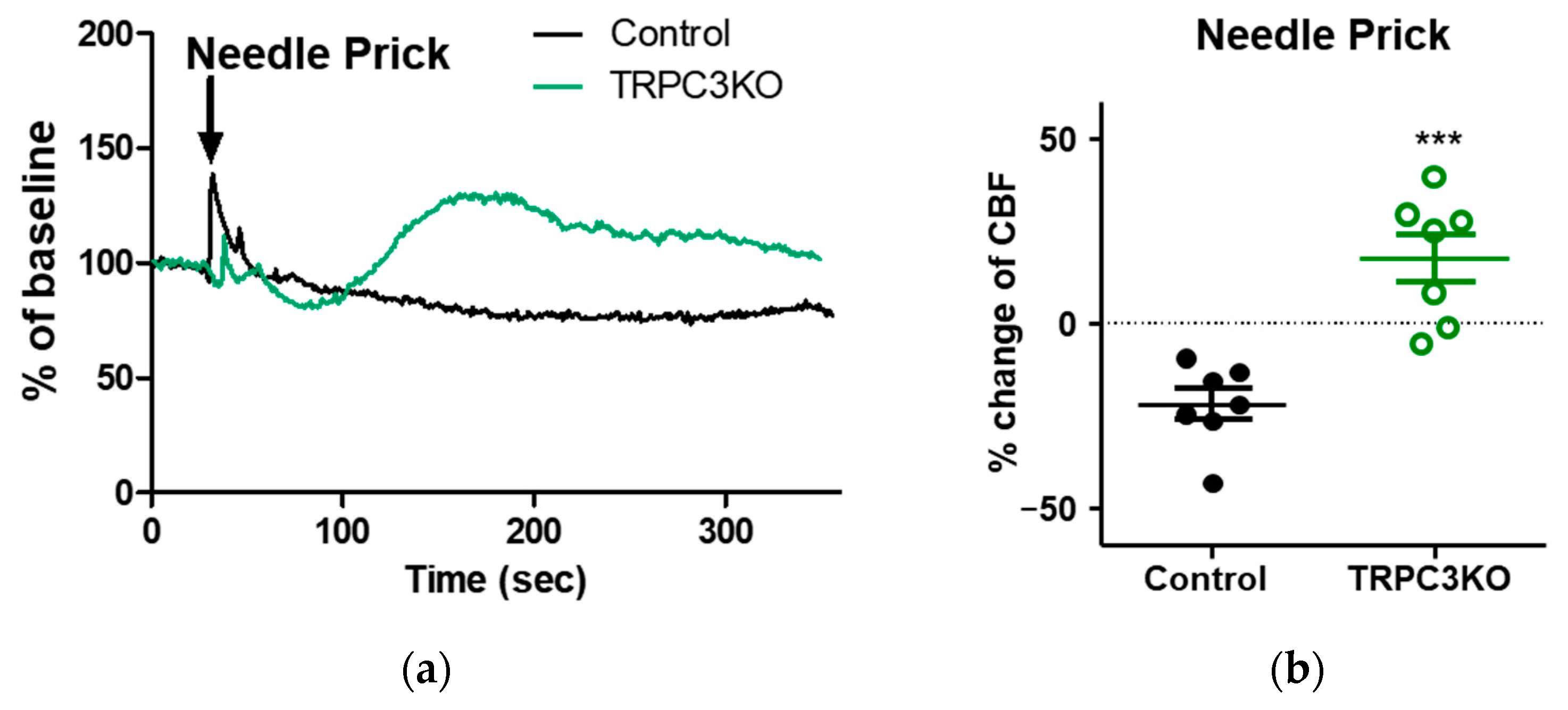
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumagai, T.; Walberer, M.; Nakamura, H.; Endepols, H.; Sué, M.; Vollmar, S.; Adib, S.; Mies, G.; Yoshimine, T.; Schroeter, M.; et al. Distinct Spatiotemporal Patterns of Spreading Depolarizations during Early Infarct Evolution: Evidence from Real-Time Imaging. J. Cereb. Blood Flow Metab. 2011, 31, 580–592. [Google Scholar] [CrossRef]
- Dreier, J.P. The Role of Spreading Depression, Spreading Depolarization and Spreading Ischemia in Neurological Disease. Nat. Med. 2011, 17, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, M.; Dreier, J.P.; Fabricius, M.; Hartings, J.A.; Graf, R.; Strong, A.J. Clinical Relevance of Cortical Spreading Depression in Neurological Disorders: Migraine, Malignant Stroke, Subarachnoid and Intracranial Hemorrhage, and Traumatic Brain Injury. J. Cereb. Blood Flow Metab. 2011, 31, 17–35. [Google Scholar] [CrossRef] [PubMed]
- Toth, P.; Szarka, N.; Farkas, E.; Ezer, E.; Czeiter, E.; Amrein, K.; Ungvari, Z.; Hartings, J.A.; Buki, A.; Koller, A. Traumatic Brain Injury-Induced Autoregulatory Dysfunction and Spreading Depression-Related Neurovascular Uncoupling: Pathomechanisms, Perspectives, and Therapeutic Implications. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H1118–H1131. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, M. Pathophysiology of the Migraine Aura. Brain 1994, 117, 199–210. [Google Scholar] [CrossRef]
- Fabricius, M.; Fuhr, S.; Willumsen, L.; Dreier, J.P.; Bhatia, R.; Boutelle, M.G.; Hartings, J.A.; Bullock, R.; Strong, A.J.; Lauritzen, M. Association of Seizures with Cortical Spreading Depression and Peri-Infarct Depolarisations in the Acutely Injured Human Brain. Clin. Neurophysiol. 2008, 119, 1973–1984. [Google Scholar] [CrossRef]
- Dohmen, C.; Sakowitz, O.W.; Fabricius, M.; Bosche, B.; Reithmeier, T.; Ernestus, R.I.; Brinker, G.; Dreier, J.P.; Woitzik, J.; Strong, A.J.; et al. Spreading Depolarizations Occur in Human Ischemic Stroke with High Incidence. Ann. Neurol. 2008, 63, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Unekawa, M.; Tomita, Y.; Toriumi, H.; Osada, T.; Masamoto, K.; Kawaguchi, H.; Itoh, Y.; Kanno, I.; Suzuki, N. Hyperperfusion Counteracted by Transient Rapid Vasoconstriction Followed by Long-Lasting Oligemia Induced by Cortical Spreading Depression in Anesthetized Mice. J. Cereb. Blood Flow Metab. 2015, 35, 689–698. [Google Scholar] [CrossRef]
- Ayata, C. Spreading Depression and Neurovascular Coupling. Stroke 2013, 44, S87–S89. [Google Scholar] [CrossRef][Green Version]
- Ayata, C.; Lauritzen, M. Spreading Depression, Spreading Depolarizations, and the Cerebral Vasculature. Physiol. Rev. 2015, 95, 953–993. [Google Scholar] [CrossRef]
- Vazquez, G.; Wedel, B.J.; Aziz, O.; Trebak, M.; Putney, J.W. The Mammalian TRPC Cation Channels. Biochim. Biophys. Acta—Mol. Cell Res. 2004, 1742, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Minke, B. The History of the Drosophila TRP Channel: The Birth of a New Channel Superfamily. J. Neurogenet. 2010, 24, 216–233. [Google Scholar] [CrossRef] [PubMed]
- Birnbaumer, L. From GTP and G Proteins to TRPC Channels: A Personal Account. J. Mol. Med. 2015, 93, 941–953. [Google Scholar] [CrossRef] [PubMed]
- Birnbaumer, L.; Yidirim, E.; Abramowitz, J. A Comparison of the Genes Coding for Canonical TRP Channels and Their M, V and P Relatives. Cell Calcium 2003, 33, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Owsianik, G.; Voets, T.; Peters, J.A. Transient Receptor Potential Cation Channels in Disease. Physiol. Rev. 2007, 87, 165–217. [Google Scholar] [CrossRef]
- Kollewe, A.; Schwarz, Y.; Oleinikov, K.; Raza, A.; Haupt, A.; Wartenberg, P.; Wyatt, A.; Boehm, U.; Ectors, F.; Bildl, W.; et al. Subunit Composition, Molecular Environment, and Activation of Native TRPC Channels Encoded by Their Interactomes. Neuron 2022, 110, 4162–4175.e7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Reboreda, A.; Alonso, A.; Barker, P.A.; Séguéla, P. TRPC Channels Underlie Cholinergic Plateau Potentials and Persistent Activity in Entorhinal Cortex. Hippocampus 2011, 21, 386–397. [Google Scholar] [CrossRef]
- Chung, Y.H.; Sun Ahn, H.; Kim, D.; Hoon Shin, D.; Su Kim, S.; Yong Kim, K.; Bok Lee, W.; Ik Cha, C. Immunohistochemical Study on the Distribution of TRPC Channels in the Rat Hippocampus. Brain Res. 2006, 1085, 132–137. [Google Scholar] [CrossRef]
- Kochukov, M.Y.; Balasubramanian, A.; Abramowitz, J.; Birnbaumer, L.; Marrelli, S.P. Activation of Endothelial Transient Receptor Potential C3 Channel Is Required for Small Conductance Calcium-Activated Potassium Channel Activation and Sustained Endothelial Hyperpolarization and Vasodilation of Cerebral Artery. J. Am. Heart Assoc. 2014, 3, e000913. [Google Scholar] [CrossRef]
- Phelan, K.D.; Shwe, U.T.; Cozart, M.A.; Wu, H.; Mock, M.M.; Abramowitz, J.; Birnbaumer, L.; Zheng, F. TRPC3 Channels Play a Critical Role in the Theta Component of Pilocarpine-Induced Status Epilepticus in Mice. Epilepsia 2017, 58, 247–254. [Google Scholar] [CrossRef]
- Vanlandewijck, M.; He, L.; Mäe, M.A.; Andrae, J.; Ando, K.; Del Gaudio, F.; Nahar, K.; Lebouvier, T.; Laviña, B.; Gouveia, L.; et al. A Molecular Atlas of Cell Types and Zonation in the Brain Vasculature. Nature 2018, 554, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Cozart, M.A.; Phelan, K.D.; Wu, H.; Mu, S.; Birnbaumer, L.; Rusch, N.J.; Zheng, F. Vascular Smooth Muscle TRPC3 Channels Facilitate the Inverse Hemodynamic Response during Status Epilepticus. Sci. Rep. 2020, 10, 812. [Google Scholar] [CrossRef] [PubMed]
- Piilgaard, H.; Lauritzen, M. Persistent Increase in Oxygen Consumption and Impaired Neurovascular Coupling after Spreading Depression in Rat Neocortex. J. Cereb. Blood Flow Metab. 2009, 29, 1517–1527. [Google Scholar] [CrossRef] [PubMed]
- Earley, S.; Brayden, J.E. Transient Receptor Potential Channels in the Vasculature. Physiol. Rev. 2015, 95, 645–690. [Google Scholar] [CrossRef]
- You, J.; Edvinsson, L.; Bryan, R.M. Neuropeptide Y-Mediated Constriction and Dilation in Rat Middle Cerebral Arteries. J. Cereb. Blood Flow Metab. 2001, 21, 77–84. [Google Scholar] [CrossRef]
- Cauli, B.; Tong, X.-K.; Rancillac, A.; Serluca, N.; Lambolez, B.; Rossier, J.; Hamel, E. Cortical GABA Interneurons in Neurovascular Coupling: Relays for Subcortical Vasoactive Pathways. J. Neurosci. 2004, 24, 8940–8949. [Google Scholar] [CrossRef]
- Ramamoorthy, P.; Whim, M.D. Trafficking and Fusion of Neuropeptide Y-Containing Dense-Core Granules in Astrocytes. J. Neurosci. 2008, 28, 13815–13827. [Google Scholar] [CrossRef]
- Bao, L.; Kopp, J.; Zhang, X.; Xu, Z.Q.; Zhang, L.F.; Wong, H.; Walsh, J.; Hökfelt, T. Localization of Neuropeptide Y Y1 Receptors in Cerebral Blood Vessels. Proc. Natl. Acad. Sci. USA 1997, 94, 12661–12666. [Google Scholar] [CrossRef]
- Misra, S.; Murthy, K.S.; Zhou, H.; Grider, J.R. Coexpression of Y1, Y2, and Y4 Receptors in Smooth Muscle Coupled to Distinct Signaling Pathways. J. Pharmacol. Exp. Ther. 2004, 311, 1154–1162. [Google Scholar] [CrossRef]
- Rubaiy, H.N. Treasure Troves of Pharmacological Tools to Study Transient Receptor Potential Canonical 1/4/5 Channels. Br. J. Pharmacol. 2019, 176, 832–846. [Google Scholar] [CrossRef]
- Minard, A.; Bauer, C.C.; Wright, D.J.; Rubaiy, H.N.; Muraki, K.; Beech, D.J.; Bon, R.S. Remarkable Progress with Small-Molecule Modulation of TRPC1/4/5 Channels: Implications for Understanding the Channels in Health and Disease. Cells 2018, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Bon, R.S.; Beech, D.J. In Pursuit of Small Molecule Chemistry for Calcium-Permeable Non-Selective TRPC Channels—Mirage or Pot of Gold? Br. J. Pharmacol. 2013, 170, 459–474. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, F. Canonical Transient Receptor Potential Channel 3 Contributes to Cerebral Blood Flow Changes Associated with Cortical Spreading Depression in Mice. Int. J. Mol. Sci. 2023, 24, 12611. https://doi.org/10.3390/ijms241612611
Zheng F. Canonical Transient Receptor Potential Channel 3 Contributes to Cerebral Blood Flow Changes Associated with Cortical Spreading Depression in Mice. International Journal of Molecular Sciences. 2023; 24(16):12611. https://doi.org/10.3390/ijms241612611
Chicago/Turabian StyleZheng, Fang. 2023. "Canonical Transient Receptor Potential Channel 3 Contributes to Cerebral Blood Flow Changes Associated with Cortical Spreading Depression in Mice" International Journal of Molecular Sciences 24, no. 16: 12611. https://doi.org/10.3390/ijms241612611
APA StyleZheng, F. (2023). Canonical Transient Receptor Potential Channel 3 Contributes to Cerebral Blood Flow Changes Associated with Cortical Spreading Depression in Mice. International Journal of Molecular Sciences, 24(16), 12611. https://doi.org/10.3390/ijms241612611





