Elevated Blood Pressure Occurs without Endothelial Dysfunction in a Rat Model of Pulmonary Emphysema
Abstract
1. Introduction
2. Results
2.1. Cardiac Function and Maximal Exercise Oxygen Uptake (V′O2 Max)
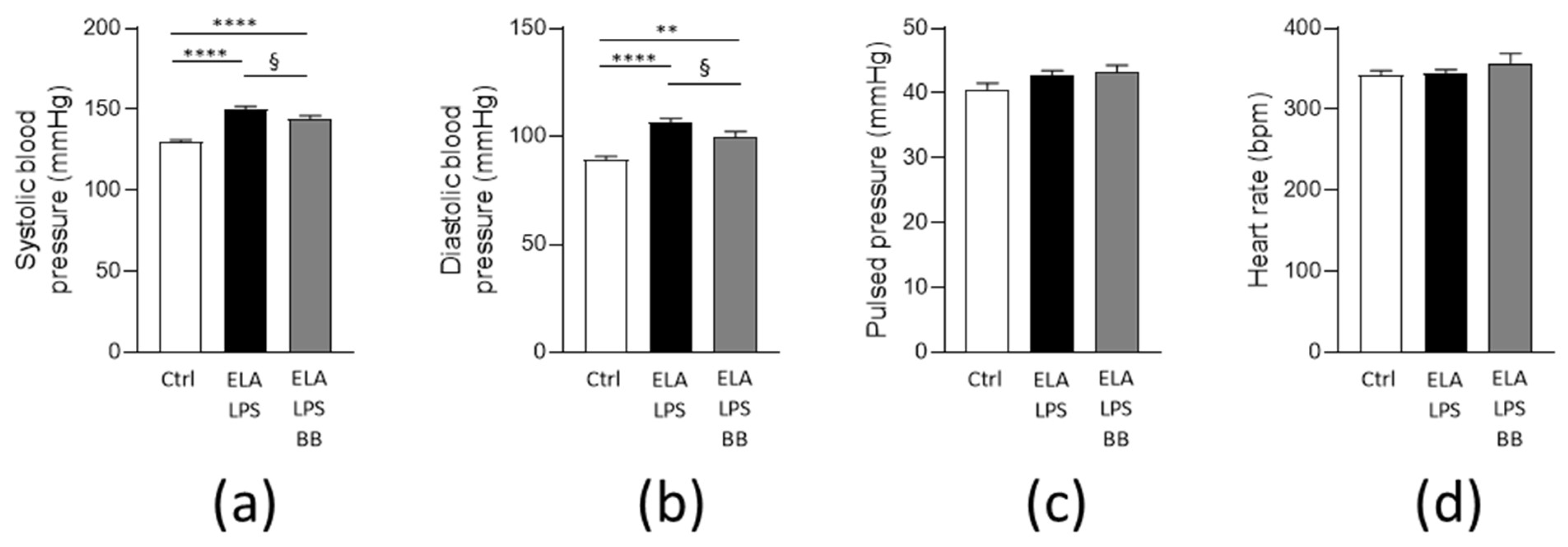
2.2. Blood Pressure
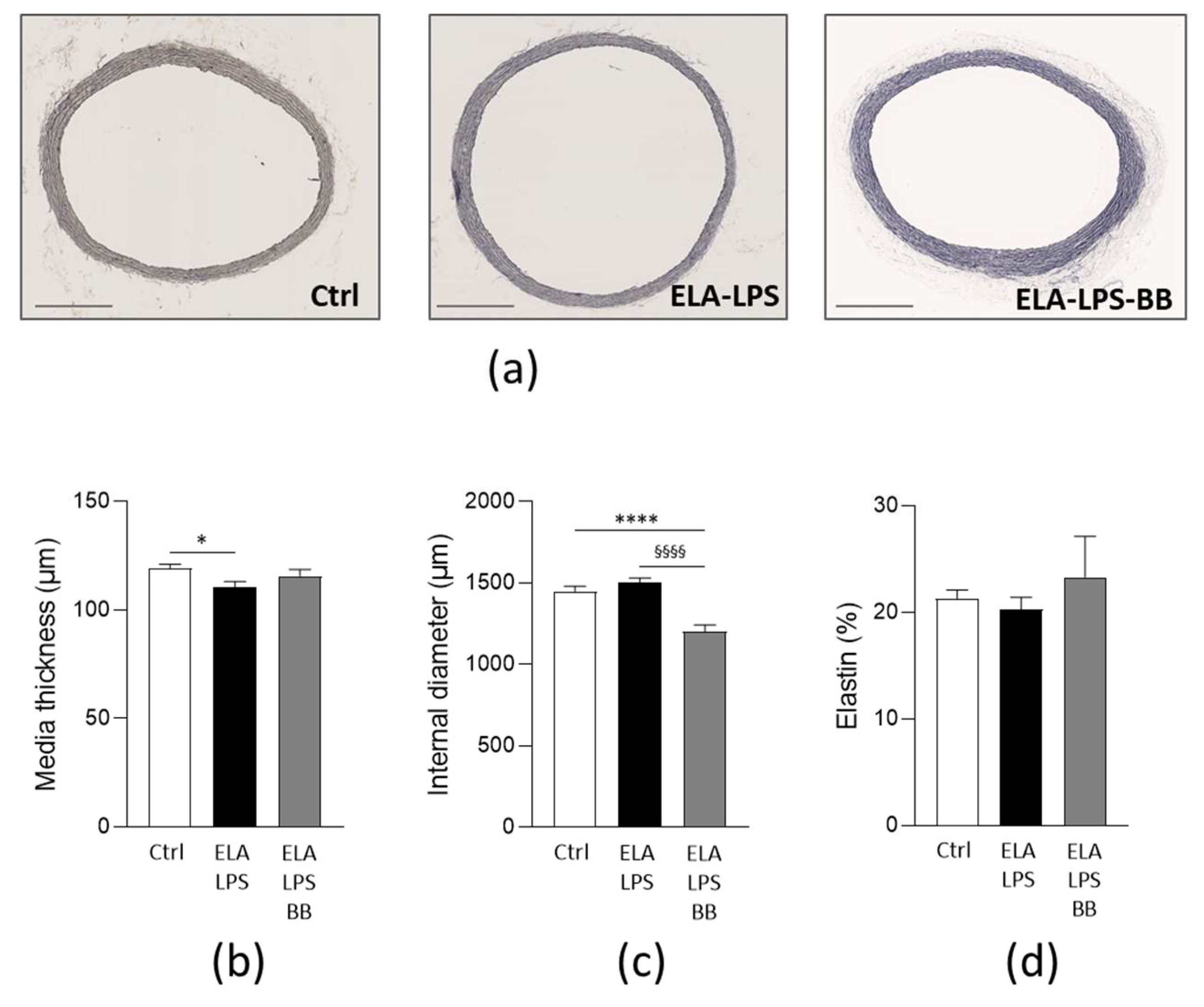
2.3. Morphological Remodeling of Rat Aorta
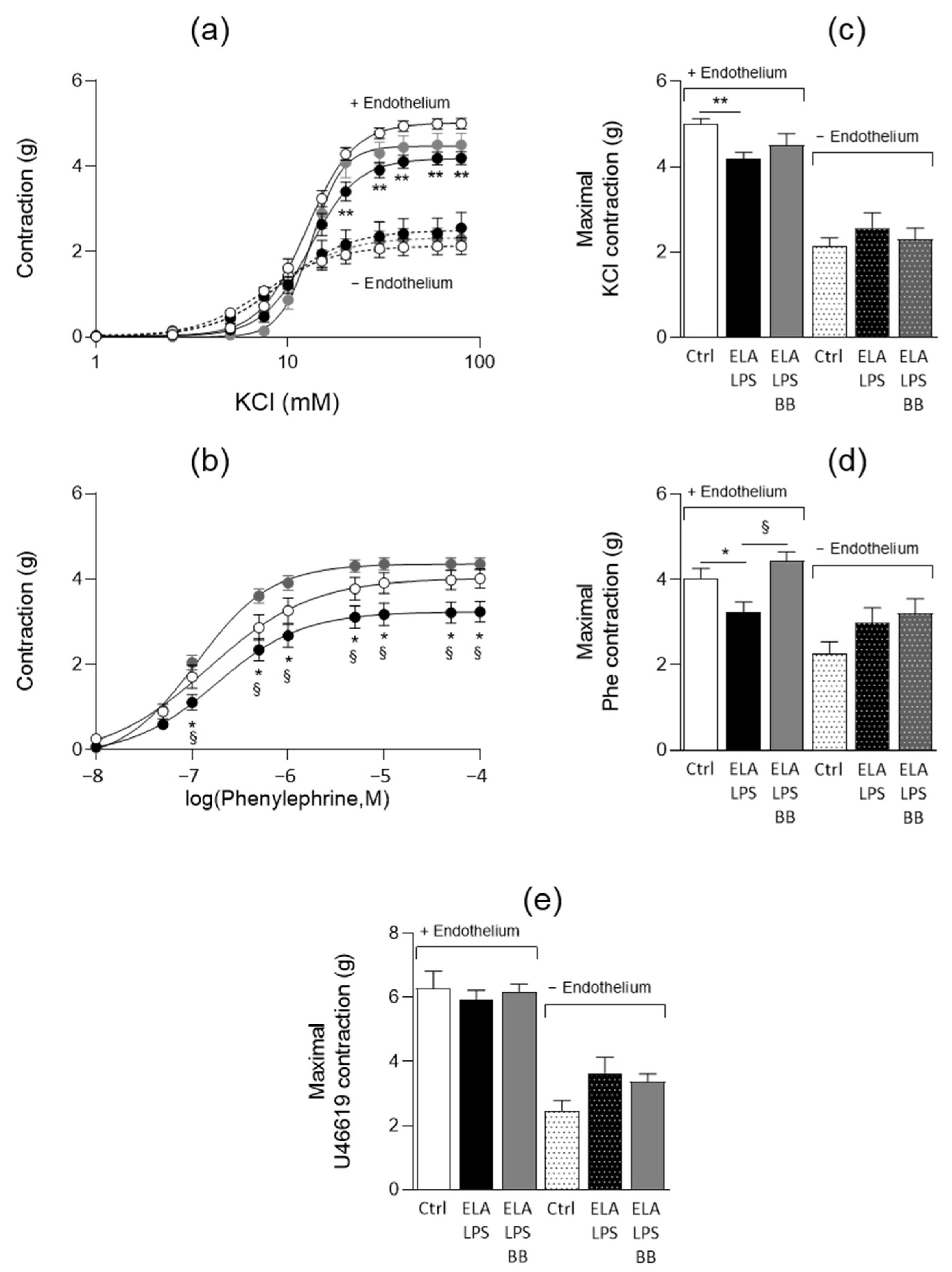
2.4. Evaluation of Vascular Contractile Properties of Aorta
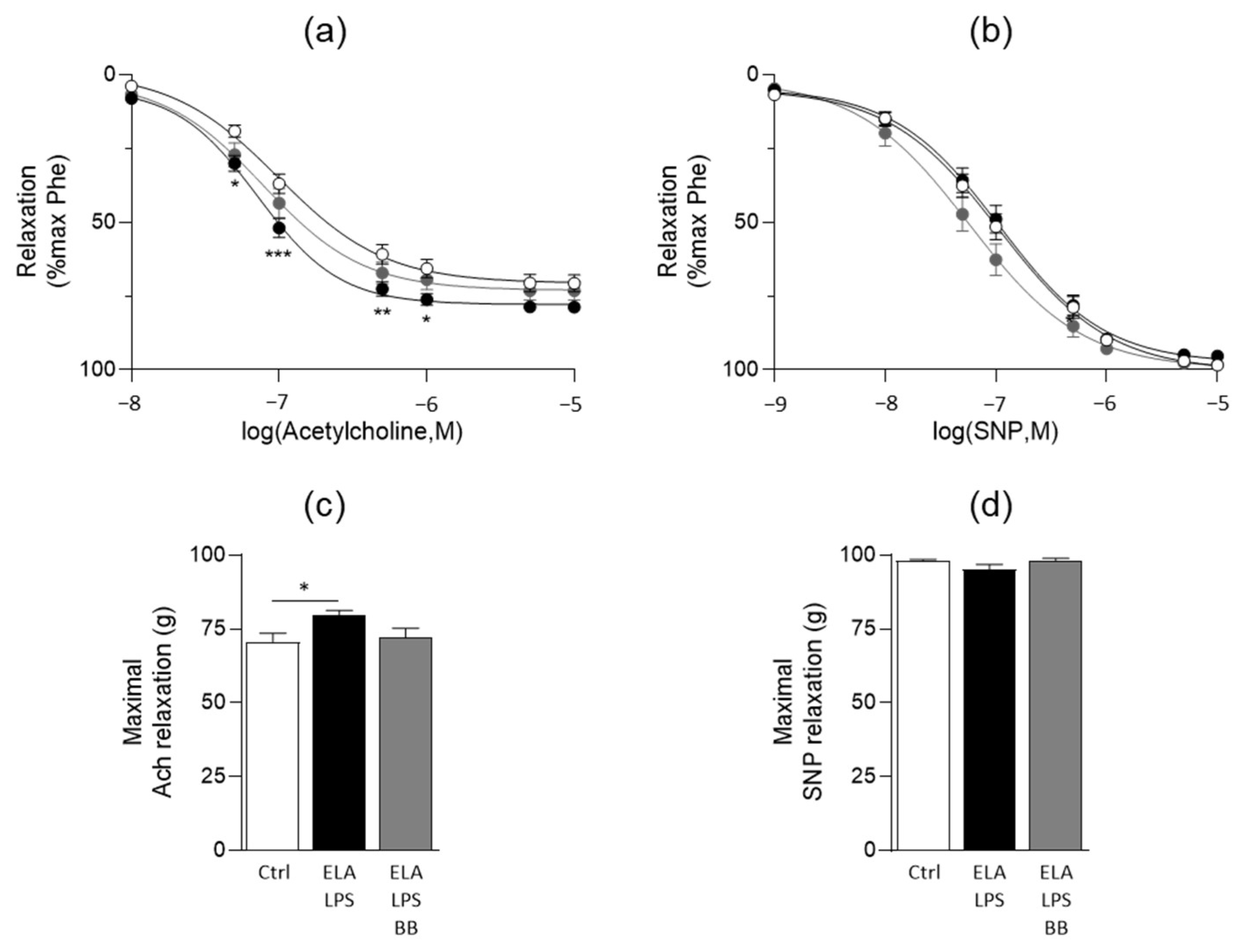
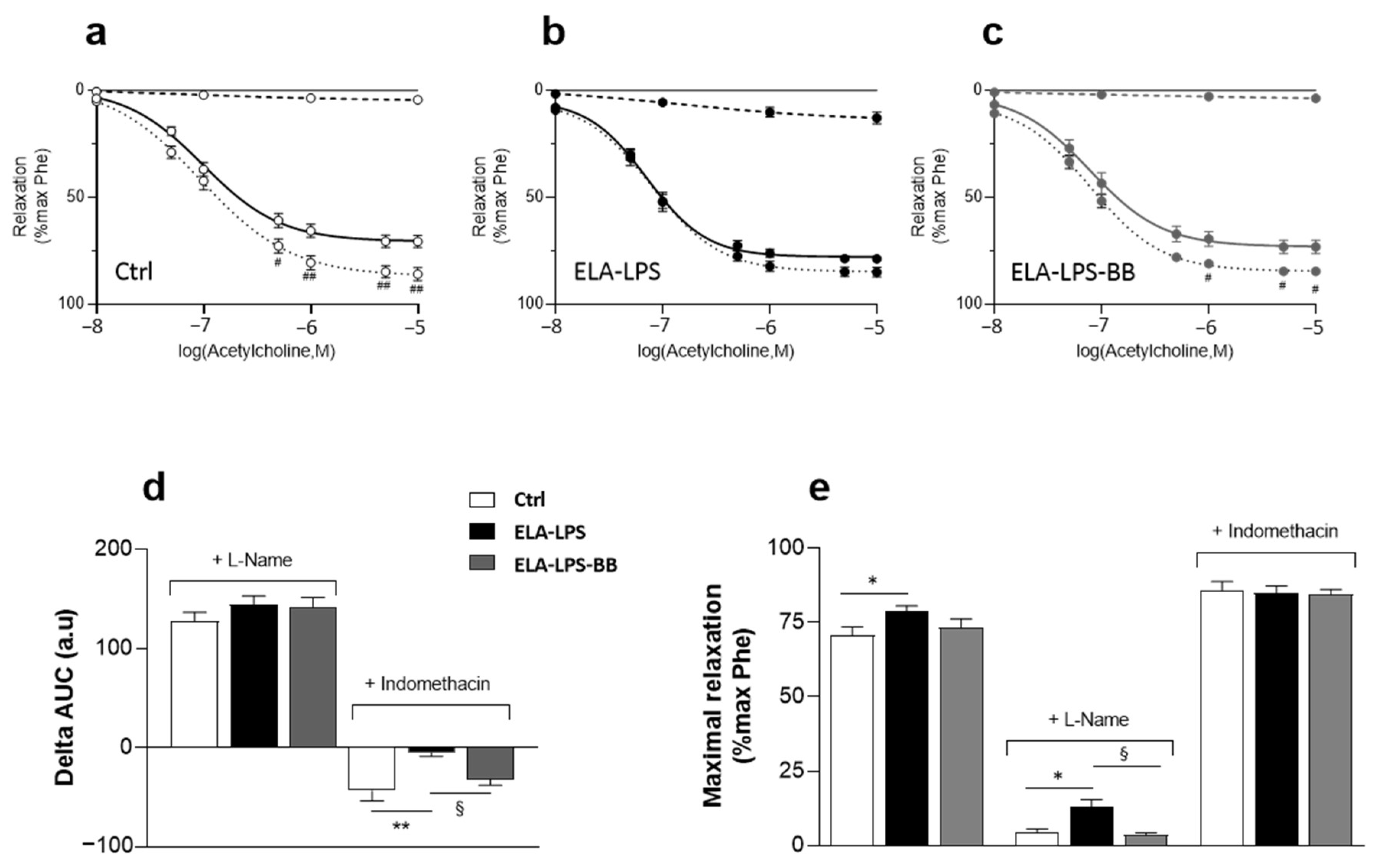
2.5. Evaluation of Vasorelaxant Capacity of Aorta
3. Discussion
4. Materials and Methods
4.1. Animal Ethics
4.2. Animal Model and Tissue Collection
4.3. Maximal Treadmill Exercise Test with Measurement of Maximal Oxygen Uptake (V′O2 Max)
4.4. Cardiac Function
4.5. Blood Pressure Determination
4.6. Aortic Diameter and Media Thickness
4.7. Vascular Reactivity
4.8. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, W.; Thomas, J.; Sadatsafavi, M.; FitzGerald, J.M. Risk of Cardiovascular Comorbidity in Patients with Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis. Lancet Respir. Med. 2015, 3, 631–639. [Google Scholar] [CrossRef]
- Morgan, A.D.; Rothnie, K.J.; Bhaskaran, K.; Smeeth, L.; Quint, J.K. Chronic Obstructive Pulmonary Disease and the Risk of 12 Cardiovascular Diseases: A Population-Based Study Using UK Primary Care Data. Thorax 2018, 73, 877–879. [Google Scholar] [CrossRef]
- Vanfleteren, L.E.G.W.; Spruit, M.A.; Groenen, M.; Gaffron, S.; van Empel, V.P.M.; Bruijnzeel, P.L.B.; Rutten, E.P.A.; Op’t Roodt, J.; Wouters, E.F.M.; Franssen, F.M.E. Clusters of Comorbidities Based on Validated Objective Measurements and Systemic Inflammation in Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2013, 187, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Di Daniele, N. Therapeutic Approaches of Uncomplicated Arterial Hypertension in Patients with COPD. Pulm. Pharmacol. Ther. 2015, 35, 1–7. [Google Scholar] [CrossRef]
- Vaes, A.W.; Spruit, M.A.; Theunis, J.; Goswami, N.; Vanfleteren, L.E.; Franssen, F.M.E.; Wouters, E.F.M.; De Boever, P. Endothelial Function in Patients with Chronic Obstructive Pulmonary Disease: A Systematic Review of Studies Using Flow Mediated Dilatation. Expert Rev. Respir. Med. 2017, 11, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Vaes, A.W.; De Boever, P.; Franssen, F.M.E.; Uszko-Lencer, N.H.M.K.; Vanfleteren, L.E.G.W.; Spruit, M.A. Endothelial Function in Patients with COPD: An Updated Systematic Review of Studies Using Flow-Mediated Dilatation. Expert Rev. Respir. Med. 2023, 17, 53–69. [Google Scholar] [CrossRef] [PubMed]
- Mills, N.L.; Miller, J.J.; Anand, A.; Robinson, S.D.; Frazer, G.A.; Anderson, D.; Breen, L.; Wilkinson, I.B.; McEniery, C.M.; Donaldson, K.; et al. Increased Arterial Stiffness in Patients with Chronic Obstructive Pulmonary Disease: A Mechanism for Increased Cardiovascular Risk. Thorax 2008, 63, 306–311. [Google Scholar] [CrossRef]
- Sabit, R.; Bolton, C.E.; Edwards, P.H.; Pettit, R.J.; Evans, W.D.; McEniery, C.M.; Wilkinson, I.B.; Cockcroft, J.R.; Shale, D.J. Arterial Stiffness and Osteoporosis in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2007, 175, 1259–1265. [Google Scholar] [CrossRef]
- Lahousse, L.; van den Bouwhuijsen, Q.J.A.; Loth, D.W.; Joos, G.F.; Hofman, A.; Witteman, J.C.M.; van der Lugt, A.; Brusselle, G.G.; Stricker, B.H. Chronic Obstructive Pulmonary Disease and Lipid Core Carotid Artery Plaques in the Elderly: The Rotterdam Study. Am. J. Respir. Crit. Care Med. 2013, 187, 58–64. [Google Scholar] [CrossRef]
- Fujikura, K.; Albini, A.; Barr, R.G.; Parikh, M.; Kern, J.; Hoffman, E.; Hiura, G.T.; Bluemke, D.A.; Carr, J.; Lima, J.A.C.; et al. Aortic Enlargement in Chronic Obstructive Pulmonary Disease (COPD) and Emphysema: The Multi-Ethnic Study of Atherosclerosis (MESA) COPD Study. Int. J. Cardiol. 2021, 331, 214–220. [Google Scholar] [CrossRef]
- Blervaque, L.; Passerieux, E.; Pomiès, P.; Catteau, M.; Héraud, N.; Blaquière, M.; Bughin, F.; Ayoub, B.; Molinari, N.; Cristol, J.-P.; et al. Impaired Training-Induced Angiogenesis Process with Loss of Pericyte-Endothelium Interactions Is Associated with an Abnormal Capillary Remodelling in the Skeletal Muscle of COPD Patients. Respir. Res. 2019, 20, 278. [Google Scholar] [CrossRef] [PubMed]
- Gouzi, F.; Maury, J.; Bughin, F.; Blaquière, M.; Ayoub, B.; Mercier, J.; Perez-Martin, A.; Pomiès, P.; Hayot, M. Impaired Training-Induced Adaptation of Blood Pressure in COPD Patients: Implication of the Muscle Capillary Bed. Int. J. Chron. Obstr. Pulm. Dis. 2016, 11, 2349–2357. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Harrison, D.G. Endothelial Dysfunction in Cardiovascular Diseases: The Role of Oxidant Stress. Available online: https://www.ahajournals.org/doi/epub/10.1161/01.RES.87.10.840 (accessed on 8 June 2023).
- Ambrosino, P.; Lupoli, R.; Iervolino, S.; De Felice, A.; Pappone, N.; Storino, A.; Di Minno, M.N.D. Clinical Assessment of Endothelial Function in Patients with Chronic Obstructive Pulmonary Disease: A Systematic Review with Meta-Analysis. Intern. Emerg. Med. 2017, 12, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Theodorakopoulou, M.P.; Alexandrou, M.E.; Bakaloudi, D.R.; Pitsiou, G.; Stanopoulos, I.; Kontakiotis, T.; Boutou, A.K. Endothelial Dysfunction in COPD: A Systematic Review and Meta-Analysis of Studies Using Different Functional Assessment Methods. ERJ Open Res. 2021, 7, 00983–02020. [Google Scholar] [CrossRef]
- Leitao Filho, F.S.; Sin, D.D. COPD and Cardiovascular Diseases: Now Is the Time for Action! Thorax 2018, 73, 799–800. [Google Scholar] [CrossRef]
- Jain, S.; Obeid, M.J.; Yenigalla, S.; Paravathaneni, M.; Gadela, N.V.; Singh, G.; Kulkarni, V.; Kondaveety, S.; Gade, K.C.; Lee, J.; et al. Impact of Chronic Obstructive Pulmonary Disease in Heart Failure with Preserved Ejection Fraction. Am. J. Cardiol. 2021, 149, 47–56. [Google Scholar] [CrossRef]
- Khedoe, P.P.S.J.; Rensen, P.C.N.; Berbée, J.F.P.; Hiemstra, P.S. Murine Models of Cardiovascular Comorbidity in Chronic Obstructive Pulmonary Disease. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 310, L1011–L1027. [Google Scholar] [CrossRef]
- Tanner, L.; Single, A.B. Animal Models Reflecting Chronic Obstructive Pulmonary Disease and Related Respiratory Disorders: Translating Pre-Clinical Data into Clinical Relevance. J. Innate Immun. 2020, 12, 203–225. [Google Scholar] [CrossRef]
- Ghorani, V.; Boskabady, M.H.; Khazdair, M.R.; Kianmeher, M. Experimental Animal Models for COPD: A Methodological Review. Tob. Induc. Dis. 2017, 15, 25. [Google Scholar] [CrossRef]
- Grillet, P.-E.; Desplanche, E.; Wynands, Q.; Gouzi, F.; Bideaux, P.; Fort, A.; Scheuermann, V.; Lacampagne, A.; Virsolvy, A.; Thireau, J.; et al. Diastolic Cardiomyopathy Secondary to Experimentally Induced Exacerbated Emphysema. Am. J. Respir. Cell Mol. Biol. 2023, 69, 230–241. [Google Scholar] [CrossRef]
- Maclay, J.D.; MacNee, W. Cardiovascular Disease in COPD: Mechanisms. Chest 2013, 143, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Canning, B.J.; Wright, J.L. Animal Models of Asthma and Chronic Obstructive Pulmonary Disease. Pulm. Pharmacol. Ther. 2008, 21, 695. [Google Scholar] [CrossRef] [PubMed]
- Kasner, M.; Westermann, D.; Steendijk, P.; Gaub, R.; Wilkenshoff, U.; Weitmann, K.; Hoffmann, W.; Poller, W.; Schultheiss, H.-P.; Pauschinger, M.; et al. Utility of Doppler Echocardiography and Tissue Doppler Imaging in the Estimation of Diastolic Function in Heart Failure with Normal Ejection Fraction: A Comparative Doppler-Conductance Catheterization Study. Circulation 2007, 116, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Paulus, W.J.; Tschöpe, C.; Sanderson, J.E.; Rusconi, C.; Flachskampf, F.A.; Rademakers, F.E.; Marino, P.; Smiseth, O.A.; De Keulenaer, G.; Leite-Moreira, A.F.; et al. How to Diagnose Diastolic Heart Failure: A Consensus Statement on the Diagnosis of Heart Failure with Normal Left Ventricular Ejection Fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur. Heart J. 2007, 28, 2539–2550. [Google Scholar] [CrossRef]
- Celli, B.R.; Cote, C.G.; Marin, J.M.; Casanova, C.; Montes de Oca, M.; Mendez, R.A.; Pinto Plata, V.; Cabral, H.J. The Body-Mass Index, Airflow Obstruction, Dyspnea, and Exercise Capacity Index in Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2004, 350, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Deb, P.P.; Ramamurthi, A. Spatiotemporal Mapping of Matrix Remodelling and Evidence of in Situ Elastogenesis in Experimental Abdominal Aortic Aneurysms. J. Tissue Eng. Regen. Med. 2017, 11, 231–245. [Google Scholar] [CrossRef][Green Version]
- Busch, A.; Holm, A.; Wagner, N.; Ergün, S.; Rosenfeld, M.; Otto, C.; Baur, J.; Kellersmann, R.; Lorenz, U. Extra- and Intraluminal Elastase Induce Morphologically Distinct Abdominal Aortic Aneurysms in Mice and Thus Represent Specific Subtypes of Human Disease. J. Vasc. Res. 2016, 53, 49–57. [Google Scholar] [CrossRef]
- Shu, J.; Lu, W.; Yang, K.; Zheng, Q.; Li, D.; Li, Y.; Kuang, M.; Liu, H.; Li, Z.; Chen, Y.; et al. Establishment and Evaluation of Chronic Obstructive Pulmonary Disease Model by Chronic Exposure to Motor Vehicle Exhaust Combined with Lipopolysaccharide Instillation. Exp. Physiol. 2018, 103, 1532–1542. [Google Scholar] [CrossRef]
- Briones, A.M.; Xavier, F.E.; Arribas, S.M.; González, M.C.; Rossoni, L.V.; Alonso, M.J.; Salaices, M. Alterations in Structure and Mechanics of Resistance Arteries from Ouabain-Induced Hypertensive Rats. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H193–H201. [Google Scholar] [CrossRef]
- Cipolla, M.J.; Sweet, J.G.; Chan, S.-L. Cerebral Vascular Adaptation to Pregnancy and Its Role in the Neurological Complications of Eclampsia. J. Appl. Physiol. 2011, 110, 329–339. [Google Scholar] [CrossRef]
- Ledingham, J.M.; Laverty, R. Basilar Artery Remodelling in the Genetically Hypertensive Rat: Effects of Nitric Oxide Synthase Inhibition and Treatment with Valsartan and Enalapril. Clin. Exp. Pharmacol. Physiol. 2000, 27, 642–646. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Hirooka, Y.; Matsuo, I.; Eshima, K.; Shigematsu, H.; Shimokawa, H.; Takeshita, A. Overexpression of ENOS in NTS Causes Hypotension and Bradycardia In Vivo. Hypertension 2000, 36, 1023–1028. [Google Scholar] [CrossRef][Green Version]
- Krum, H.; Viskoper, R.J.; Lacourciere, Y.; Budde, M.; Charlon, V. The Effect of an Endothelin-Receptor Antagonist, Bosentan, on Blood Pressure in Patients with Essential Hypertension. N. Engl. J. Med. 1998, 338, 784–791. [Google Scholar] [CrossRef]
- Khalil, R.A. Modulators of the Vascular Endothelin Receptor in Blood Pressure Regulation and Hypertension. Curr. Mol. Pharmacol. 2011, 4, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, J.; Wier, W.G.; Chen, L.; Blaustein, M.P. NO-Induced Vasodilation Correlates Directly with BP in Smooth Muscle-Na/Ca Exchanger-1-Engineered Mice: Elevated BP Does Not Attenuate Endothelial Function. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H221–H237. [Google Scholar] [CrossRef]
- Stratton, D.B.; Morrow, R.J.; Sanders, B.J. Vascular Responsiveness in the Unstressed Borderline Hypertensive Rat. Clin. Exp. Hypertens. 1994, 16, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Roso, M.; Montero, M.; Carrón, R.; Sevilla, M. Cardiovascular Changes in Spontaneously Hypertensive Rats Are Improved by Chronic Treatment with Zofenopril. Br. J. Pharmacol. 2009, 158, 1911–1921. [Google Scholar] [CrossRef][Green Version]
- Battault, S.; Singh, F.; Gayrard, S.; Zoll, J.; Reboul, C.; Meyer, G. Endothelial Function Does Not Improve with High-Intensity Continuous Exercise Training in SHR: Implications of ENOS Uncoupling. Hypertens. Res. 2016, 39, 70–78. [Google Scholar] [CrossRef]
- Oe, B. Bisoprolol (EMD 33512), a Highly Selective Beta 1-Adrenoceptor Antagonist: In Vitro and in Vivo Studies. J. Cardiovasc. Pharmacol. 1986, 8 (Suppl. 11), S29–S35. [Google Scholar] [CrossRef]
- AlHabeeb, W.; Mrabeti, S.; Abdelsalam, A.A.I. Therapeutic Properties of Highly Selective β-Blockers with or without Additional Vasodilator Properties: Focus on Bisoprolol and Nebivolol in Patients with Cardiovascular Disease. Cardiovasc. Drugs Ther. 2022, 36, 959–971. [Google Scholar] [CrossRef]
- Asmar, R.G.; Kerihuel, J.C.; Girerd, X.J.; Safar, M.E. Effect of Bisoprolol on Blood Pressure and Arterial Hemodynamics in Systemic Hypertension. Am. J. Cardiol. 1991, 68, 61–64. [Google Scholar] [CrossRef]
- Suojanen, L.; Haring, A.; Tikkakoski, A.; Koskela, J.K.; Tahvanainen, A.M.; Huhtala, H.; Kähönen, M.; Sipilä, K.; Eräranta, A.; Mustonen, J.T.; et al. Haemodynamic Influences of Bisoprolol in Hypertensive Middle-Aged Men: A Double-Blind, Randomized, Placebo-Controlled Cross-Over Study. Basic. Clin. Pharmacol. Toxicol. 2017, 121, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Suojanen, L.; Haring, A.; Tikkakoski, A.; Huhtala, H.; Kähönen, M.; Eräranta, A.; Mustonen, J.T.; Pörsti, I.H. Adverse Influence of Bisoprolol on Central Blood Pressure in the Upright Position: A Double-Blind Placebo-Controlled Cross-over Study. J. Hum. Hypertens. 2020, 34, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Cockburn, J.A.; Brett, S.E.; Guilcher, A.; Ferro, A.; Ritter, J.M.; Chowienczyk, P.J. Differential Effects of Beta-Adrenoreceptor Antagonists on Central and Peripheral Blood Pressure at Rest and during Exercise. Br. J. Clin. Pharmacol. 2010, 69, 329–335. [Google Scholar] [CrossRef]
- Esler, M.; Rumantir, M.; Kaye, D.; Lambert, G. The Sympathetic Neurobiology of Essential Hypertension: Disparate Influences of Obesity, Stress, and Noradrenaline Transporter Dysfunction? Am. J. Hypertens. 2001, 14, 139S–146S. [Google Scholar] [CrossRef] [PubMed]
- Oster, J.R.; Epstein, M. Use of Centrally Acting Sympatholytic Agents in the Management of Hypertension. Arch. Intern. Med. 1991, 151, 1638–1644. [Google Scholar] [CrossRef]
- Esler, M. The Sympathetic System and Hypertension. Am. J. Hypertens. 2000, 13, 99S–105S. [Google Scholar] [CrossRef]
- Sun, N.; Hong, T.; Zhang, R.; Yang, X. The Effects of Verapamil SR and Bisoprolol on Reducing the Sympathetic Nervous System’s Activity. Hypertens. Res. 2000, 23, 537–540. [Google Scholar] [CrossRef][Green Version]
- Kaye, D.M.; Nanayakkara, S.; Wang, B.; Shihata, W.; Marques, F.Z.; Esler, M.; Lambert, G.; Mariani, J. Characterization of Cardiac Sympathetic Nervous System and Inflammatory Activation in HFpEF Patients. JACC Basic. Transl. Sci. 2022, 7, 116–127. [Google Scholar] [CrossRef]
- Tracey, K.J. The Inflammatory Reflex. Nature 2002, 420, 853–859. [Google Scholar] [CrossRef]
- Maclay, J.D.; McAllister, D.A.; Mills, N.L.; Paterson, F.P.; Ludlam, C.A.; Drost, E.M.; Newby, D.E.; Macnee, W. Vascular Dysfunction in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2009, 180, 513–520. [Google Scholar] [CrossRef]
- de Matthaeis, A.; Greco, A.; Dagostino, M.P.; Paroni, G.; Fontana, A.; Vinciguerra, M.; Mazzoccoli, G.; Seripa, D.; Vendemiale, G. Effects of Hypercapnia on Peripheral Vascular Reactivity in Elderly Patients with Acute Exacerbation of Chronic Obstructive Pulmonary Disease. Clin. Interv. Aging 2014, 9, 871–878. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vaes, A.W.; Spruit, M.A.; Theunis, J.; Wouters, E.F.M.; De Boever, P. Peripheral Endothelial Function Is Positively Associated with Maximal Aerobic Capacity in Patients with Chronic Obstructive Pulmonary Disease. Respir. Med. 2018, 142, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Scherr, A.; Schumann, D.M.; Karakioulaki, M.; Franchetti, L.; Strobel, W.; Zellweger, M.; Tamm, M.; Stolz, D. Endothelial Dysfunction Is Not a Predictor of Outcome in Chronic Obstructive Pulmonary Disease. Respir. Res. 2020, 21, 90. [Google Scholar] [CrossRef] [PubMed]
- Minet, C.; Vivodtzev, I.; Tamisier, R.; Arbib, F.; Wuyam, B.; Timsit, J.-F.; Monneret, D.; Borel, J.-C.; Baguet, J.-P.; Lévy, P.; et al. Reduced Six-Minute Walking Distance, High Fat-Free-Mass Index and Hypercapnia Are Associated with Endothelial Dysfunction in COPD. Respir. Physiol. Neurobiol. 2012, 183, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Clarenbach, C.F.; Senn, O.; Sievi, N.A.; Camen, G.; van Gestel, A.J.R.; Rossi, V.A.; Puhan, M.A.; Thurnheer, R.; Russi, E.W.; Kohler, M. Determinants of Endothelial Function in Patients with COPD. Eur. Respir. J. 2013, 42, 1194–1204. [Google Scholar] [CrossRef]
- Eickhoff, P.; Valipour, A.; Kiss, D.; Schreder, M.; Cekici, L.; Geyer, K.; Kohansal, R.; Burghuber, O.C. Determinants of Systemic Vascular Function in Patients with Stable Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2008, 178, 1211–1218. [Google Scholar] [CrossRef]
- Polverino, F.; Celli, B.R.; Owen, C.A. COPD as an Endothelial Disorder: Endothelial Injury Linking Lesions in the Lungs and Other Organs? (2017 Grover Conference Series). Pulm. Circ. 2018, 8, 2045894018758528. [Google Scholar] [CrossRef]
- Morgan, A.D.; Zakeri, R.; Quint, J.K. Defining the Relationship between COPD and CVD: What Are the Implications for Clinical Practice? Ther. Adv. Respir. Dis. 2018, 12, 1753465817750524. [Google Scholar] [CrossRef]
- Teixeira-Coelho, F.; Fonseca, C.G.; Barbosa, N.H.S.; Vaz, F.F.; Cordeiro, L.M.d.S.; Coimbra, C.C.; Pires, W.; Soares, D.D.; Wanner, S.P. Effects of Manipulating the Duration and Intensity of Aerobic Training Sessions on the Physical Performance of Rats. PLoS ONE 2017, 12, e0183763. [Google Scholar] [CrossRef]
- Chakouri, N.; Farah, C.; Matecki, S.; Amedro, P.; Vincenti, M.; Saumet, L.; Vergely, L.; Sirvent, N.; Lacampagne, A.; Cazorla, O. Screening for In-Vivo Regional Contractile Defaults to Predict the Delayed Doxorubicin Cardiotoxicity in Juvenile Rat. Theranostics 2020, 10, 8130–8142. [Google Scholar] [CrossRef] [PubMed]
- Arthur-Ataam, J.; Bideaux, P.; Charrabi, A.; Sicard, P.; Fromy, B.; Liu, K.; Eddahibi, S.; Pasqualin, C.; Jouy, N.; Richard, S.; et al. Dietary Supplementation with Silicon-Enriched Spirulina Improves Arterial Remodeling and Function in Hypertensive Rats. Nutrients 2019, 11, 2574. [Google Scholar] [CrossRef] [PubMed]
| Ctrl (n = 20) | ELA-LPS (n = 20) | ELA-LPS-BB (n = 12) | |
|---|---|---|---|
| Weight (g) | 475 ± 7 | 466 ± 10 | 470 ± 13 |
| V′O2 max (mL/kg/min) | 66 ± 1 | 60 ± 1 *** | 64 ± 2 |
| Heart rate (bpm) | 357 ± 4 | 354 ± 5 | 357 ± 15 |
| LVEF (%) | 63.4 ± 1.5 | 65.6 ± 2.1 | 66.1 ± 2.4 |
| Cardiac output (mL/min) | 125 ± 32 | 114 ± 26 | 112 ± 16 |
| E/e′ | 31.5 ± 2.3 | 42.2 ± 3.9 *§ | 27.8 ± 4.8 |
| Ctrl (n = 20) | ELA-LPS (n = 20) | ELA-LPS-BB (n = 12) | |
|---|---|---|---|
| KCl (mM) | 12.7 ± 0.6 | 13.6 ± 1.1 | 13.56 ± 0.7 |
| Phe (nM) | 147 ± 60 | 180 ± 44 | 111 ± 11 § |
| SNP (nM) | 22.2 ± 4.4 | 18.7 ± 3.7 | 8.6 ± 2.2 |
| Ach (nM) | 122 ± 12 | 74 ± 5 * | 94 ± 18 |
| Ach + L-Name (nM) | 85 | 153 * | 82 § |
| Ach + indo | 117 ± 18 | 89 ± 12 | 84 ± 11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desplanche, E.; Grillet, P.-E.; Wynands, Q.; Bideaux, P.; Alburquerque, L.; Charrabi, A.; Bourdin, A.; Cazorla, O.; Gouzi, F.; Virsolvy, A. Elevated Blood Pressure Occurs without Endothelial Dysfunction in a Rat Model of Pulmonary Emphysema. Int. J. Mol. Sci. 2023, 24, 12609. https://doi.org/10.3390/ijms241612609
Desplanche E, Grillet P-E, Wynands Q, Bideaux P, Alburquerque L, Charrabi A, Bourdin A, Cazorla O, Gouzi F, Virsolvy A. Elevated Blood Pressure Occurs without Endothelial Dysfunction in a Rat Model of Pulmonary Emphysema. International Journal of Molecular Sciences. 2023; 24(16):12609. https://doi.org/10.3390/ijms241612609
Chicago/Turabian StyleDesplanche, Elodie, Pierre-Edouard Grillet, Quentin Wynands, Patrice Bideaux, Laurie Alburquerque, Azzouz Charrabi, Arnaud Bourdin, Olivier Cazorla, Fares Gouzi, and Anne Virsolvy. 2023. "Elevated Blood Pressure Occurs without Endothelial Dysfunction in a Rat Model of Pulmonary Emphysema" International Journal of Molecular Sciences 24, no. 16: 12609. https://doi.org/10.3390/ijms241612609
APA StyleDesplanche, E., Grillet, P.-E., Wynands, Q., Bideaux, P., Alburquerque, L., Charrabi, A., Bourdin, A., Cazorla, O., Gouzi, F., & Virsolvy, A. (2023). Elevated Blood Pressure Occurs without Endothelial Dysfunction in a Rat Model of Pulmonary Emphysema. International Journal of Molecular Sciences, 24(16), 12609. https://doi.org/10.3390/ijms241612609







