A Brief Review on the Potential of Psychedelics for Treating Alzheimer’s Disease and Related Depression
Abstract
1. Introduction
2. Depression and Apathy in AD
3. Serotonin in AD
4. Dopamine in AD
5. Psychedelics for Treatment of AD and AD-Related Depression
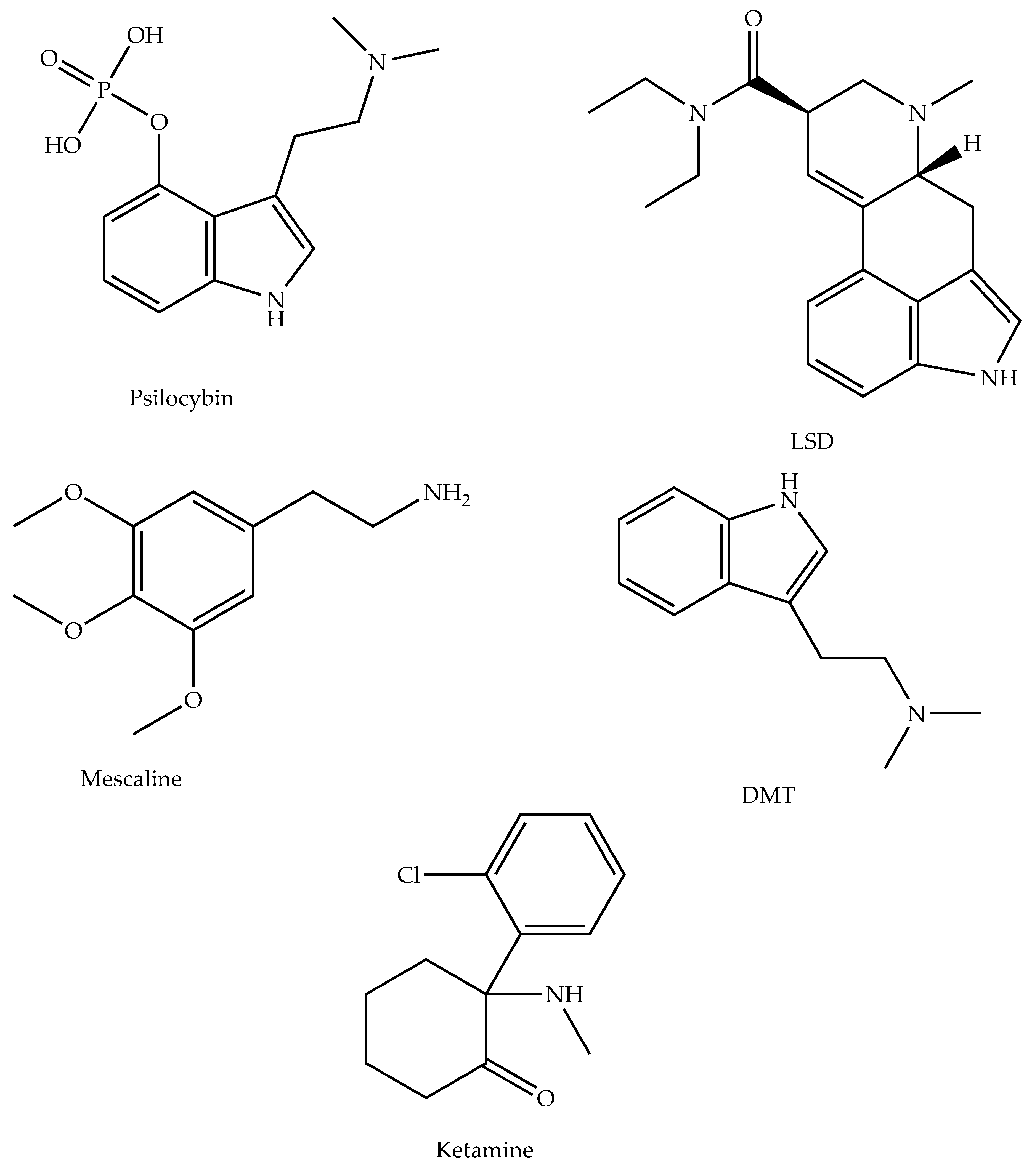
5.1. Psilocybin
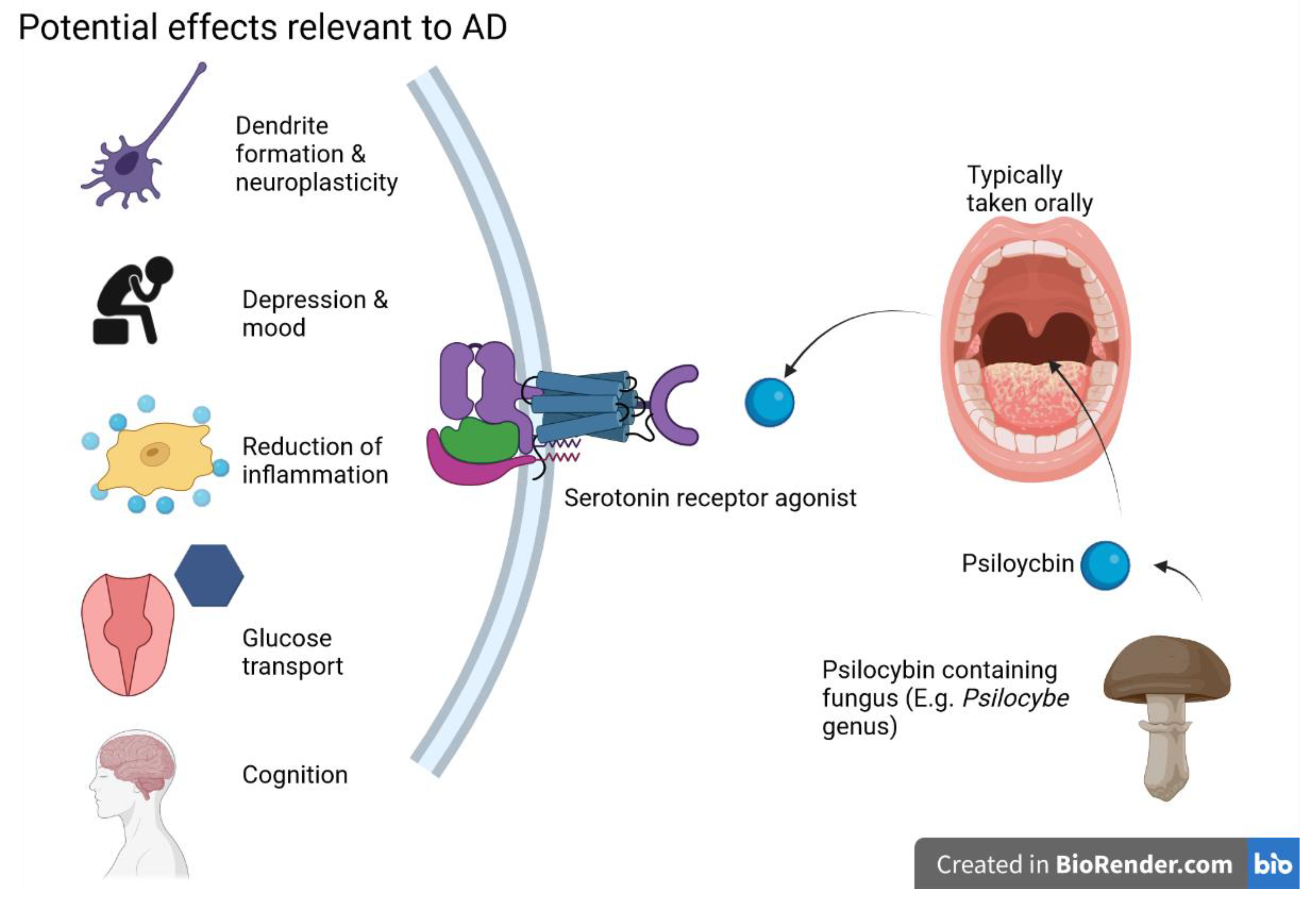
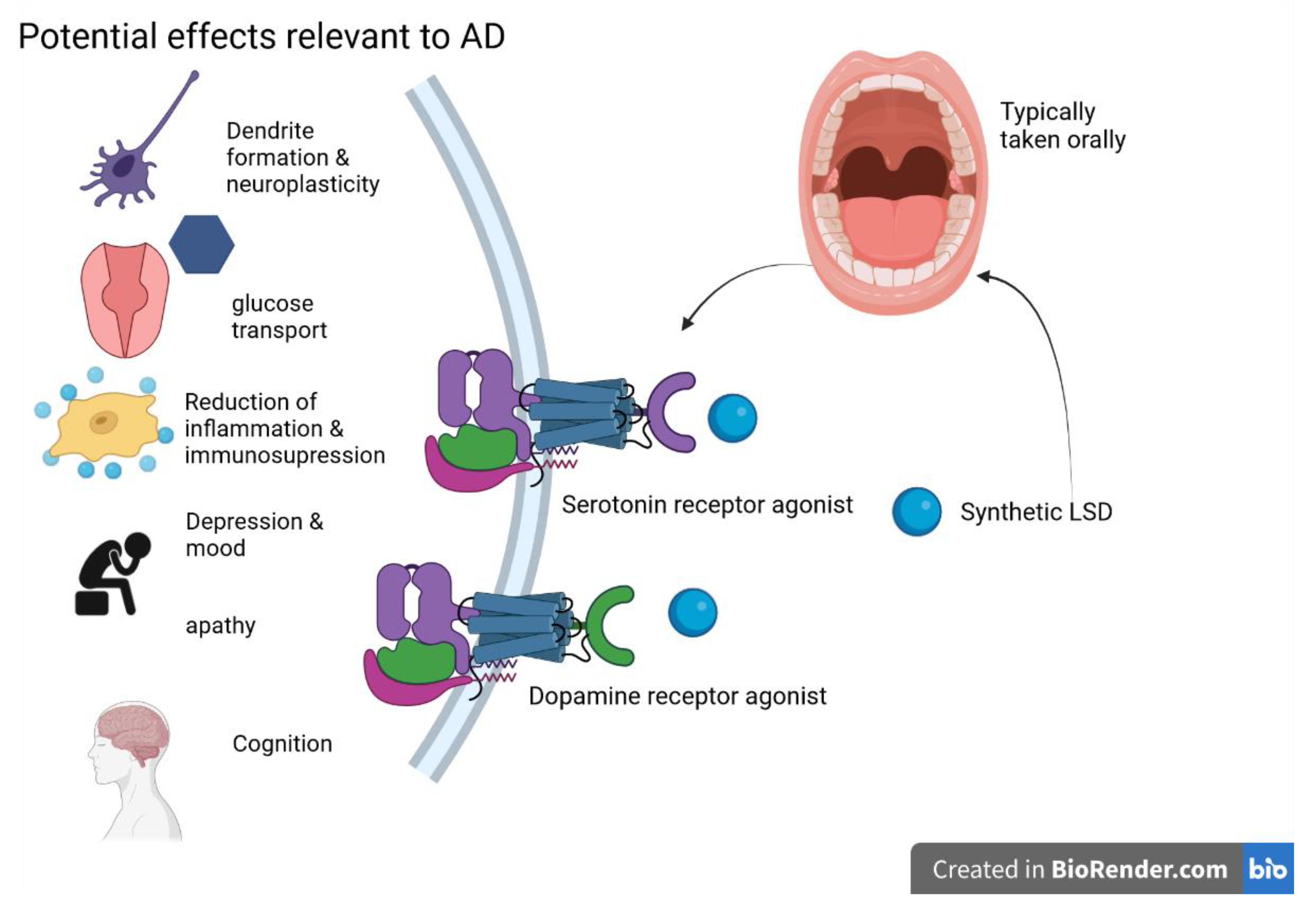
5.2. D-Lysergic Acid Diethylamide (LSD)
5.3. Mescaline
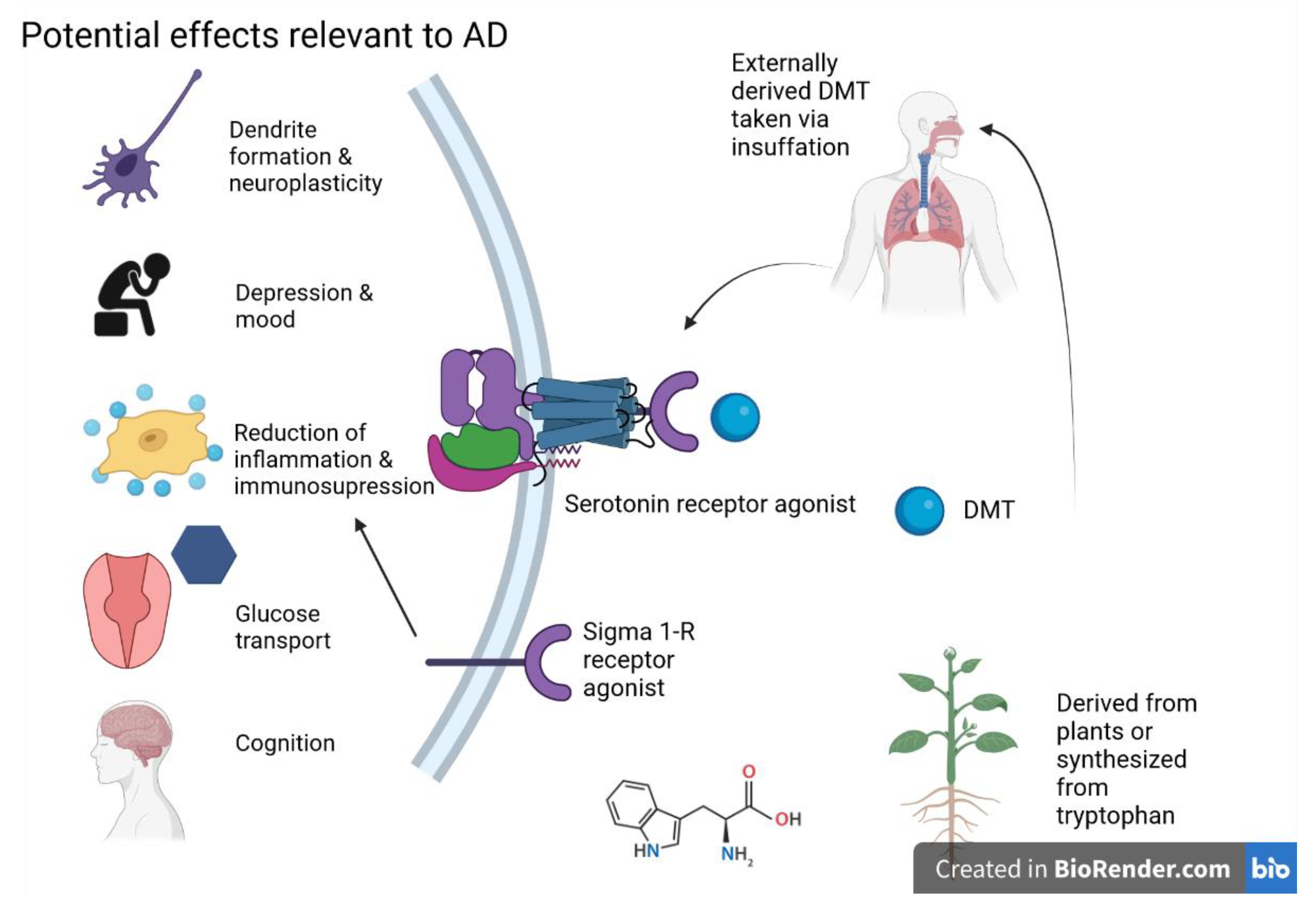
5.4. Dimethyltryptamine (DMT)
5.5. Ketamine
6. Potential of Microdosing
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s Disease |
| aMCI | Amnestic Mild Cognitive Impairment |
| Aβ | Amyloid Beta |
| BBB | Blood–Brain Barrier |
| 5D-ASC | Five Dimensional Altered States of Consciousness |
| DMT | Dimethyltryptamine |
| DMX | Dextromethorphan |
| DSM | Diagnostic and Statistical Manual of Mental Disorders |
| 5-HT | 5-Hydroxytryptamine |
| LSD | D-Lysergic Acid Diethylamide |
| MMSE | Mini-Mental State Examination |
| MOA | Mechanism of Action |
| NCD | Neurocognitive Disorder |
| NFTs | Neurofibrillary Tangles |
| NMDA | N-Methyl-D-Aspartate |
| OMM | Outer Mitochondria Membrane |
| PD | Parkinson’s Disease |
| PET | Positron Emission Tomography |
| PBR | Peripheral Benzodiazepine Receptor |
| S1R | Sigma-1 Receptor |
| SI | Suicidal Ideation |
| SSRIs | Selective Serotonin Reuptake Inhibitors |
| TMPA | 3,4,5-Tri Methoxyphenyl Acetic Acid |
| TSPO | Translocator Protein |
References
- Haaland, K.Y.; Price, L.; Larue, A. What does the WMS-III tell us about memory changes with normal aging? J. Int. Neuropsychol. Soc. 2003, 9, 89–96. [Google Scholar] [CrossRef]
- Darowski, E.S.; Helder, E.; Zacks, R.T.; Hasher, L.; Hambrick, D.Z. Age-related differences in cognition: The role of distraction control. Neuropsychology 2008, 22, 638–644. [Google Scholar] [CrossRef]
- Salthouse, T.A.; Mitchell, D.R.; Skovronek, E.; Babcock, R.L. Effects of adult age and working memory on reasoning and spatial abilities. J. Exp. Psychol. Learn. Mem. Cogn. 1989, 15, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Larrieu, S.; Letenneur, L.; Orgogozo, J.M.; Fabrigoule, C.; Amieva, H.; Le Carret, N.; Barberger-Gateau, P.; Dartigues, J.F. Incidence and outcome of mild cognitive impairment in a population-based prospective cohort. Neurology 2002, 59, 1594–1599. [Google Scholar] [CrossRef] [PubMed]
- Aerts, L.; Heffernan, M.; Kochan, N.A.; Crawford, J.D.; Draper, B.; Trollor, J.N.; Sachdev, P.S.; Brodaty, H. Effects of MCI subtype and reversion on progression to dementia in a community sample. Neurology 2017, 88, 2225–2232. [Google Scholar] [CrossRef] [PubMed]
- 2022 Alzheimer’s disease facts and figures. Alzheimers Dement. 2022, 18, 700–789. [CrossRef] [PubMed]
- Schneider, L. A resurrection of aducanumab for Alzheimer’s disease. Lancet Neurol. 2020, 19, 111–112. [Google Scholar] [CrossRef] [PubMed]
- Casey, D.A.; Antimisiaris, D.; O’Brien, J. Drugs for Alzheimer’s disease: Are they effective? Pharm. Ther. 2010, 35, 208. [Google Scholar]
- Carhart-Harris, R.L.; Goodwin, G.M. The therapeutic potential of psychedelic drugs: Past, present, and future. Neuropsychopharmacology 2017, 42, 2105–2113. [Google Scholar] [CrossRef] [PubMed]
- Kuypers, K.P.; Ng, L.; Erritzoe, D.; Knudsen, G.M.; Nichols, C.D.; Nichols, D.E.; Pani, L.; Soula, A.; Nutt, D. Microdosing psychedelics: More questions than answers? An overview and suggestions for future research. J. Psychopharmacol. 2019, 33, 1039–1057. [Google Scholar] [CrossRef]
- Lyketsos, C.G.; Olin, J. Depression in Alzheimer’s disease: Overview and treatment. Biol. Psychiatry 2002, 52, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.L.; Charney, D.S.; Lewis, L.; Golden, R.N.; Gorman, J.M.; Krishnan, K.R.R.; Nemeroff, C.B.; Bremner, J.D.; Carney, R.M.; Coyne, J.C. Mood disorders in the medically ill: Scientific review and recommendations. Biol. Psychiatry 2005, 58, 175–189. [Google Scholar]
- Enache, D.; Winblad, B.; Aarsland, D. Depression in dementia: Epidemiology, mechanisms, and treatment. Curr. Opin. Psychiatry 2011, 24, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Ballard, C.; Bannister, C.; Solis, M.; Oyebode, F.; Wilcock, G. The prevalence, associations and symptoms of depression amongst dementia sufferers. J. Affect. Disord. 1996, 36, 135–144. [Google Scholar] [CrossRef]
- Schmutte, T.; Olfson, M.; Maust, D.T.; Xie, M.; Marcus, S.C. Suicide risk in first year after dementia diagnosis in older adults. Alzheimer’s Dement. 2021, 18, 262–271. [Google Scholar] [CrossRef]
- Baptista, M.A.T.; Santos, R.L.; Kimura, N.; Lacerda, I.B.; Dourado, M.C.N. Disease awareness may increase risk of suicide in young onset dementia: A case report. Dement. Neuropsychol. 2017, 11, 308–311. [Google Scholar]
- Conejero, I.; Navucet, S.; Keller, J.; Olié, E.; Courtet, P.; Gabelle, A. A complex relationship between suicide, dementia, and amyloid: A narrative review. Front. Neurosci. 2018, 12, 371. [Google Scholar] [PubMed]
- Caselli, R.J.; Langbaum, J.B.; Marchant, G.E.; Lindor, R.A.; Hunt, K.S.; Henslin, B.R.; Dueck, A.C.; Robert, J.S. P2-356: Predictive Testing for alzheimer’s disease: Suicidal ideation among healthy participants. Alzheimer’s Dement. 2014, 10, P610. [Google Scholar]
- Starkstein, S.E.; Ingram, L.; Garau, M.; Mizrahi, R. On the overlap between apathy and depression in dementia. J. Neurol. Neurosurg. Psychiatry 2005, 76, 1070–1074. [Google Scholar] [CrossRef]
- Starkstein, S.E.; Mizrahi, R.; Garau, L. Specificity of symptoms of depression in Alzheimer disease: A longitudinal analysis. Am. J. Geriatr. Psychiatry 2005, 13, 802–807. [Google Scholar] [CrossRef]
- Lyketsos, C.G.; Steele, C.; Baker, L.; Galik, E.; Kopunek, S.; Steinberg, M.; Warren, A. Major and minor depression in Alzheimer’s disease: Prevalence and impact. J. Neuropsychiatry Clin. Neurosci. 1997, 9, 556–561. [Google Scholar] [PubMed]
- El Haj, M.; Altintas, E.; Chapelet, G.; Kapogiannis, D.; Gallouj, K. High depression and anxiety in people with Alzheimer’s disease living in retirement homes during the covid-19 crisis. Psychiatry Res. 2020, 291, 113294. [Google Scholar] [CrossRef] [PubMed]
- Caraci, F.; Copani, A.; Nicoletti, F.; Drago, F. Depression and Alzheimer’s disease: Neurobiological links and common pharmacological targets. Eur. J. Pharmacol. 2010, 626, 64–71. [Google Scholar] [PubMed]
- Rovner, B.W.; Broadhead, J.; Spencer, M.; Carson, K.; Folstein, M.F. Depression and Alzheimer’s disease. Am. J. Psychiatry 1989, 146, 350–353. [Google Scholar]
- Orgeta, V.; Tabet, N.; Nilforooshan, R.; Howard, R. Efficacy of antidepressants for depression in Alzheimer’s disease: Systematic review and meta-analysis. J. Alzheimer’s Dis. 2017, 58, 725–733. [Google Scholar] [CrossRef]
- Vollenweider, F.X.; Vollenweider-Scherpenhuyzen, M.F.; Bäbler, A.; Vogel, H.; Hell, D. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport 1998, 9, 3897–3902. [Google Scholar]
- Chen, C.P.; Eastwood, S.L.; Hope, T.; McDonald, B.; Francis, P.T.; Esiri, M.M. Immunocytochemical study of the dorsal and median raphe nuclei in patients with Alzheimer’s disease prospectively assessed for behavioural changes. Neuropathol. Appl. Neurobiol. 2000, 26, 347–355. [Google Scholar] [CrossRef]
- Hendricksen, M.; Thomas, A.J.; Ferrier, I.N.; Ince, P.; O’Brien, J.T. Neuropathological study of the dorsal raphe nuclei in late-life depression and Alzheimer’s disease with and without depression. Am. J. Psychiatry 2004, 161, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Metaxas, A.; Anzalone, M.; Vaitheeswaran, R.; Petersen, S.; Landau, A.M.; Finsen, B. Neuroinflammation and amyloid-beta 40 are associated with reduced serotonin transporter (SERT) activity in a transgenic model of familial Alzheimer’s disease. Alzheimer’s Res. Ther. 2019, 11, 38. [Google Scholar] [CrossRef]
- Cirrito, J.R.; Disabato, B.M.; Restivo, J.L.; Verges, D.K.; Goebel, W.D.; Sathyan, A.; Hayreh, D.; D’Angelo, G.; Benzinger, T.; Yoon, H. Serotonin signaling is associated with lower amyloid-β levels and plaques in transgenic mice and humans. Proc. Natl. Acad. Sci. USA 2011, 108, 14968–14973. [Google Scholar] [CrossRef]
- Claeysen, S.; Bockaert, J.; Giannoni, P. Serotonin: A new hope in Alzheimer’s disease? ACS Chem. Neurosci. 2015, 6, 940–943. [Google Scholar] [PubMed]
- Kepe, V.; Barrio, J.R.; Huang, S.-C.; Ercoli, L.; Siddarth, P.; Shoghi-Jadid, K.; Cole, G.M.; Satyamurthy, N.; Cummings, J.L.; Small, G.W. Serotonin 1A receptors in the living brain of Alzheimer’s disease patients. Proc. Natl. Acad. Sci. USA 2006, 103, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.; Tsang, S.; Alder, J.; Keene, J.; Hope, T.; Esiri, M.; Francis, P.; Chen, C. Loss of serotonin 5-HT 2A receptors in the postmortem temporal cortex correlates with rate of cognitive decline in Alzheimer’s disease. Psychopharmacology 2005, 179, 673–677. [Google Scholar] [CrossRef]
- Rosier, A.; Dupont, P.; Peuskens, J.; Bormans, G.; Vandenberghe, R.; Maes, M.; de Groot, T.; Schiepers, C.; Verbruggen, A.; Mortelmans, L. Visualisation of loss of 5-HT2A receptors with age in healthy volunteers using [18F] altanserin and positron emission tomographic imaging. Psychiatry Res. Neuroimaging 1996, 68, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Fox, N.; Warrington, E.; Freeborough, P.; Hartikainen, P.; Kennedy, A.; Stevens, J.; Rossor, M.N. Presymptomatic hippocampal atrophy in Alzheimer’s disease: A longitudinal MRI study. Brain 1996, 119, 2001–2007. [Google Scholar] [CrossRef]
- Bremner, J.D.; Narayan, M.; Anderson, E.R.; Staib, L.H.; Miller, H.L.; Charney, D.S. Hippocampal volume reduction in major depression. Am. J. Psychiatry 2000, 157, 115–118. [Google Scholar]
- Videbech, P.; Ravnkilde, B. Hippocampal volume and depression: A meta-analysis of MRI studies. Am. J. Psychiatry 2004, 161, 1957–1966. [Google Scholar] [PubMed]
- Artin, H.; Zisook, S.; Ramanathan, D. How do serotonergic psychedelics treat depression: The potential role of neuroplasticity. World J. Psychiatry 2021, 11, 201–214. [Google Scholar] [CrossRef]
- Vythilingam, M.; Vermetten, E.; Anderson, G.M.; Luckenbaugh, D.; Anderson, E.R.; Snow, J.; Staib, L.H.; Charney, D.S.; Bremner, J.D. Hippocampal volume, memory, and cortisol status in major depressive disorder: Effects of treatment. Biol. Psychiatry 2004, 56, 101–112. [Google Scholar] [CrossRef]
- Colle, R.; Cury, C.; Chupin, M.; Deflesselle, E.; Hardy, P.; Nasser, G.; Falissard, B.; Ducreux, D.; Colliot, O.; Corruble, E. Hippocampal volume predicts antidepressant efficacy in depressed patients without incomplete hippocampal inversion. NeuroImage Clin. 2016, 12, 949–955. [Google Scholar] [CrossRef]
- Béchade, C.; d’Andrea, I.; Etienne, F.; Verdonk, F.; Moutkine, I.; Banas, S.M.; Kolodziejczak, M.; Diaz, S.L.; Parkhurst, C.N.; Gan, W.B. The serotonin 2B receptor is required in neonatal microglia to limit neuroinflammation and sickness behavior in adulthood. Glia 2020, 69, 638–654. [Google Scholar] [CrossRef] [PubMed]
- Whitford, M. Alzheimer’s disease and serotonin: A review. Neuropsychobiology 1986, 15, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Holmes, C.; Arranz, M.; Collier, D.; Powell, J.; Lovestone, S. Depression in Alzheimer’s disease: The effect of serotonin receptor gene variation. Am. J. Med. Genet. Part. B Neuropsychiatr. Genet. 2003, 119, 40–43. [Google Scholar]
- Krebs, T.S.; Johansen, P.-Ø. Psychedelics and mental health: A population study. PLoS ONE 2013, 8, e63972. [Google Scholar]
- Piert, M.; Koeppe, R.A.; Giordani, B.; Berent, S.; Kuhl, D.E. Diminished glucose transport and phosphorylation in Alzheimer’s disease determined by dynamic FDG-PET. J. Nucl. Med. 1996, 37, 201–208. [Google Scholar]
- Jagust, W.J.; Seab, J.P.; Huesman, R.H.; Valk, P.E.; Mathis, C.A.; Reed, B.R.; Coxson, P.G.; Budinger, T.F. Diminished glucose transport in Alzheimer’s disease: Dynamic PET studies. J. Cereb. Blood Flow. Metab. 1991, 11, 323–330. [Google Scholar] [CrossRef]
- Wirth, M.; Madison, C.M.; Rabinovici, G.D.; Oh, H.; Landau, S.M.; Jagust, W.J. Alzheimer’s Disease Neurodegenerative Biomarkers Are Associated with Decreased Cognitive Function but Not-Amyloid in Cognitively Normal Older Individuals. J. Neurosci. 2013, 33, 5553–5563. [Google Scholar] [CrossRef]
- Dwyer, D.S.; Ardizzone, T.D.; Bradley, R.J. Psychoactive drugs affect glucose transport and the regulation of glucose metabolism. Int. Rev. Neurobiol. 2002, 51, 503–530. [Google Scholar]
- Hajduch, E.; Rencurel, F.; Balendran, A.; Batty, I.H.; Downes, C.P.; Hundal, H.S. Serotonin (5-hydroxytryptamine), a novel regulator of glucose transport in rat skeletal muscle. J. Biol. Chem. 1999, 274, 13563–13568. [Google Scholar] [CrossRef]
- Lotharius, J.; Brundin, P. Pathogenesis of Parkinson’s disease: Dopamine, vesicles and α-synuclein. Nat. Rev. Neurosci. 2002, 3, 932–942. [Google Scholar] [CrossRef]
- Pan, X.; Kaminga, A.C.; Wen, S.W.; Wu, X.; Acheampong, K.; Liu, A. Dopamine and dopamine receptors in Alzheimer’s disease: A systematic review and network meta-analysis. Front. Aging Neurosci. 2019, 11, 175. [Google Scholar] [PubMed]
- Cross, A.J.; Crow, T.J.; Perry, E.K.; Perry, R.H.; Blessed, G.; Tomlinson, B.E. Reduced dopamine-beta-hydroxylase activity in Alzheimer’s disease. Br. Med. J. 1981, 282, 93–94. [Google Scholar] [CrossRef] [PubMed]
- Martorana, A.; Koch, G. Is dopamine involved in Alzheimer’s disease? Front. Aging Neurosci. 2014, 6, 252. [Google Scholar]
- Chen, J.-Y.; Stern, Y.; Sano, M.; Mayeux, R. Cumulative risks of developing extrapyramidal signs, psychosis, or myoclonus in the course of Alzheimer’s disease. Arch. Neurol. 1991, 48, 1141–1143. [Google Scholar] [CrossRef] [PubMed]
- Stern, Y.; Albert, M.; Brandt, J.; Jacobs, D.; Tang, M.; Marder, K.; Bell, K.; Sano, M.; Devanand, D.P.; Bylsma, F. Utility of extrapyramidal signs and psychosis as predictors of cognitive and functional decline, nursing home admission, and death in Alzheimer’s disease: Prospective analyses from the Predictors Study. Neurology 1994, 44, 2300. [Google Scholar] [PubMed]
- Chui, H.C.; Lyness, S.A.; Sobel, E.; Schneider, L.S. Extrapyramidal signs and psychiatric symptoms predict faster cognitive decline in Alzheimer’s disease. Arch. Neurol. 1994, 51, 676–681. [Google Scholar] [CrossRef]
- Mitchell, R.A.; Herrmann, N.; Lanctôt, K.L. The role of dopamine in symptoms and treatment of apathy in Alzheimer’s disease. CNS Neurosci. Ther. 2011, 17, 411–427. [Google Scholar] [CrossRef]
- Udo, N.; Hashimoto, N.; Toyonaga, T.; Isoyama, T.; Oyanagi, Y.; Narita, H.; Shiga, T.; Nakagawa, S.; Kusumi, I. Apathy in Alzheimer’s disease correlates with the dopamine transporter level in the caudate nuclei. Dement. Geriatr. Cogn. Disord. Extra 2020, 10, 86–93. [Google Scholar] [CrossRef]
- Nieoullon, A. Dopamine and the regulation of cognition and attention. Progress. Neurobiol. 2002, 67, 53–83. [Google Scholar] [CrossRef]
- Goldman-Rakic, P.S. The cortical dopamine system: Role in memory and cognition. Adv. Pharmacol. 1997, 42, 707–711. [Google Scholar]
- Wise, R.A. Dopamine, learning and motivation. Nat. Rev. Neurosci. 2004, 5, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Eisenegger, C.; Naef, M.; Linssen, A.; Clark, L.; Gandamaneni, P.K.; Müller, U.; Robbins, T.W. Role of dopamine D2 receptors in human reinforcement learning. Neuropsychopharmacology 2014, 39, 2366–2375. [Google Scholar] [CrossRef] [PubMed]
- Shiner, T.; Seymour, B.; Wunderlich, K.; Hill, C.; Bhatia, K.P.; Dayan, P.; Dolan, R.J. Dopamine and performance in a reinforcement learning task: Evidence from Parkinson’s disease. Brain 2012, 135, 1871–1883. [Google Scholar] [CrossRef]
- Xia, Q.-P.; Cheng, Z.-Y.; He, L. The modulatory role of dopamine receptors in brain neuroinflammation. Int. Immunopharmacol. 2019, 76, 105908. [Google Scholar] [CrossRef]
- Shao, L.-X.; Liao, C.; Gregg, I.; Davoudian, P.A.; Savalia, N.K.; Delagarza, K.; Kwan, A.C. Psilocybin induces rapid and persistent growth of dendritic spines in frontal cortex in vivo. Neuron 2021, 109, 2535–2544.e4. [Google Scholar] [CrossRef]
- Gao, H.-M.; Kotzbauer, P.T.; Uryu, K.; Leight, S.; Trojanowski, J.Q.; Lee, V.M.-Y. Neuroinflammation and oxidation/nitration of α-synuclein linked to dopaminergic neurodegeneration. J. Neurosci. 2008, 28, 7687–7698. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhou, F.-M.; Dani, J.A. Cholinergic drugs for Alzheimer’s disease enhance in vitro dopamine release. Mol. Pharmacol. 2004, 66, 538–544. [Google Scholar] [CrossRef]
- Li, J.; Zhu, M.; Manning-Bog, A.B.; Di Monte, D.A.; Fink, A.L. Dopamine and L-dopa disaggregate amyloid fibrils: Implications for Parkinson’s and Alzheimer’s disease. FASEB J. 2004, 18, 962–964. [Google Scholar] [CrossRef]
- Lawn, T.; Dipasquale, O.; Vamvakas, A.; Tsougos, I.; Mehta, M.A.; Howard, M.A. Differential contributions of serotonergic and dopaminergic functional connectivity to the phenomenology of LSD. Psychopharmacology 2022, 239, 1797–1808. [Google Scholar] [CrossRef]
- Passie, T.; Seifert, J.; Schneider, U.; Emrich, H.M. The pharmacology of psilocybin. Addict. Biol. 2002, 7, 357–364. [Google Scholar] [CrossRef]
- Baloyannis, S. Dendritic pathology in Alzheimer’s disease. J. Neurol. Sci. 2009, 283, 153–157. [Google Scholar]
- Barrett, F.S.; Carbonaro, T.M.; Hurwitz, E.; Johnson, M.W.; Griffiths, R.R. Double-blind comparison of the two hallucinogens psilocybin and dextromethorphan: Effects on cognition. Psychopharmacology 2018, 235, 2915–2927. [Google Scholar]
- Carter, O.L.; Burr, D.C.; Pettigrew, J.D.; Wallis, G.M.; Hasler, F.; Vollenweider, F.X. Using psilocybin to investigate the relationship between attention, working memory, and the serotonin 1A and 2A receptors. J. Cogn. Neurosci. 2005, 17, 1497–1508. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, S.B.; Pace, B.T.; Nicholas, C.R.; Raison, C.L.; Hutson, P.R. The experimental effects of psilocybin on symptoms of anxiety and depression: A meta-analysis. Psychiatry Res. 2020, 284, 112749. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Bolstridge, M.; Rucker, J.; Day, C.M.; Erritzoe, D.; Kaelen, M.; Bloomfield, M.; Rickard, J.A.; Forbes, B.; Feilding, A. Psilocybin with psychological support for treatment-resistant depression: An open-label feasibility study. Lancet Psychiatry 2016, 3, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Carhart-Harris, R.; Giribaldi, B.; Watts, R.; Baker-Jones, M.; Murphy-Beiner, A.; Murphy, R.; Martell, J.; Blemings, A.; Erritzoe, D.; Nutt, D.J. Trial of psilocybin versus escitalopram for depression. N. Engl. J. Med. 2021, 384, 1402–1411. [Google Scholar] [CrossRef]
- Roseman, L.; Nutt, D.J.; Carhart-Harris, R.L. Quality of acute psychedelic experience predicts therapeutic efficacy of psilocybin for treatment-resistant depression. Front. Pharmacol. 2018, 8, 974. [Google Scholar]
- Griffiths, R.R.; Johnson, M.W.; Carducci, M.A.; Umbricht, A.; Richards, W.A.; Richards, B.D.; Cosimano, M.P.; Klinedinst, M.A. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. J. Psychopharmacol. 2016, 30, 1181–1197. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.; Bossis, A.; Guss, J.; Agin-Liebes, G.; Malone, T.; Cohen, B.; Mennenga, S.E.; Belser, A.; Kalliontzi, K.; Babb, J. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: A randomized controlled trial. J. Psychopharmacol. 2016, 30, 1165–1180. [Google Scholar] [CrossRef]
- Family, N.; Maillet, E.L.; Williams, L.T.J.; Krediet, E.; Carhart-Harris, R.L.; Williams, T.M.; Nichols, C.D.; Goble, D.J.; Raz, S. Safety, tolerability, pharmacokinetics, and pharmacodynamics of low dose lysergic acid diethylamide (LSD) in healthy older volunteers. Psychopharmacology 2020, 237, 841–853. [Google Scholar] [CrossRef]
- Bershad, A.K.; Schepers, S.T.; Bremmer, M.P.; Lee, R.; de Wit, H. Acute subjective and behavioral effects of microdoses of lysergic acid diethylamide in healthy human volunteers. Biol. Psychiatry 2019, 86, 792–800. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Kaelen, M.; Bolstridge, M.; Williams, T.; Williams, L.; Underwood, R.; Feilding, A.; Nutt, D.J. The paradoxical psychological effects of lysergic acid diethylamide (LSD). Psychol. Med. 2016, 46, 1379–1390. [Google Scholar] [CrossRef]
- Nichols, C.D.; Sanders-Bush, E. A single dose of lysergic acid diethylamide influences gene expression patterns within the mammalian brain. Neuropsychopharmacology 2002, 26, 634–642. [Google Scholar] [CrossRef]
- González-Maeso, J.; Yuen, T.; Ebersole, B.J.; Wurmbach, E.; Lira, A.; Zhou, M.; Weisstaub, N.; Hen, R.; Gingrich, J.A.; Sealfon, S.C. Transcriptome fingerprints distinguish hallucinogenic and nonhallucinogenic 5-hydroxytryptamine 2A receptor agonist effects in mouse somatosensory cortex. J. Neurosci. 2003, 23, 8836–8843. [Google Scholar] [CrossRef]
- Cini, F.A.; Ornelas, I.; Marcos, E.; Goto-Silva, L.; Nascimento, J.; Ruschi, S.; Salerno, J.; Karmirian, K.; Costa, M.; Sequerra, E. d-Lysergic acid diethylamide has major potential as a cognitive enhancer. bioRxiv 2019, 866814. [Google Scholar]
- Speth, J.; Speth, C.; Kaelen, M.; Schloerscheidt, A.M.; Feilding, A.; Nutt, D.J.; Carhart-Harris, R.L. Decreased mental time travel to the past correlates with default-mode network disintegration under lysergic acid diethylamide. J. Psychopharmacol. 2016, 30, 344–353. [Google Scholar] [CrossRef]
- Grof, S.; Goodman, L.E.; Richards, W.A.; Kurland, A. LSD-assisted psychotherapy in patients with terminal cancer. Int. Pharmacopsychiatry 1973, 8, 129–144. [Google Scholar] [CrossRef]
- Gasser, P.; Kirchner, K.; Passie, T. LSD-assisted psychotherapy for anxiety associated with a life-threatening disease: A qualitative study of acute and sustained subjective effects. J. Psychopharmacol. 2015, 29, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Gasser, P.; Holstein, D.; Michel, Y.; Doblin, R.; Yazar-Klosinski, B.; Passie, T.; Brenneisen, R. Safety and efficacy of lysergic acid diethylamide-assisted psychotherapy for anxiety associated with life-threatening diseases. J. Nerv. Ment. Dis. 2014, 202, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Abbott, K.L.; Flannery, P.C.; Gill, K.S.; Boothe, D.M.; Dhanasekaran, M.; Mani, S.; Pondugula, S.R. Adverse pharmacokinetic interactions between illicit substances and clinical drugs. Drug Metab. Rev. 2020, 52, 44–65. [Google Scholar] [CrossRef] [PubMed]
- Halpern, J.H.; Sherwood, A.R.; Hudson, J.I.; Yurgelun-Todd, D.; Pope Jr, H.G. Psychological and cognitive effects of long-term peyote use among Native Americans. Biol. Psychiatry 2005, 58, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Dinis-Oliveira, R.J.; Pereira, C.L.; da Silva, D.D. Pharmacokinetic and pharmacodynamic aspects of peyote and mescaline: Clinical and forensic repercussions. Curr. Mol. Pharmacol. 2019, 12, 184. [Google Scholar] [PubMed]
- Carstairs, S.D.; Cantrell, F.L. Peyote and mescaline exposures: A 12-year review of a statewide poison center database. Clin. Toxicol. 2010, 48, 350–353. [Google Scholar] [CrossRef]
- Callaway, J.C.; McKenna, D.J.; Grob, C.S.; Brito, G.S.; Raymon, L.; Poland, R.E.; Andrade, E.; Andrade, E.; Mash, D.C. Pharmacokinetics of Hoasca alkaloids in healthy humans. J. Ethnopharmacol. 1999, 65, 243–256. [Google Scholar] [CrossRef]
- Barker, S.A. N, N-Dimethyltryptamine (DMT), an endogenous hallucinogen: Past, present, and future research to determine its role and function. Front. Neurosci. 2018, 12, 536. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.-W.; Jiang, X.-L.; Yu, A.-M. Nonlinear pharmacokinetics of 5-methoxy-N, N-dimethyltryptamine in mice. Drug Metab. Dispos. 2011, 39, 1227–1234. [Google Scholar] [CrossRef]
- Keiser, M.J.; Setola, V.; Irwin, J.J.; Laggner, C.; Abbas, A.I.; Hufeisen, S.J.; Jensen, N.H.; Kuijer, M.B.; Matos, R.C.; Tran, T.B.; et al. Predicting new molecular targets for known drugs. Nature 2009, 462, 175–181. [Google Scholar] [CrossRef]
- Fontanilla, D.; Johannessen, M.; Hajipour, A.R.; Cozzi, N.V.; Jackson, M.B.; Ruoho, A.E. The hallucinogen N, N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator. Science 2009, 323, 934–937. [Google Scholar] [CrossRef]
- Bourrie, B.; Bouaboula, M.; Benoit, J.M.; Derocq, J.M.; Esclangon, M.; Le Fur, G.; Casellas, P. Enhancement of endotoxin-induced interleukin-10 production by SR 31747A, a sigma ligand. Eur. J. Immunol. 1995, 25, 2882–2887. [Google Scholar] [CrossRef]
- Carayon, P.; Bouaboula, M.; Loubet, J.F.; Bourrie, B.; Petitpretre, G.; Le Fur, G.; Casellas, P. The sigma ligand SR 31747 prevents the development of acute graft-versus-host disease in mice by blocking IFN-gamma and GM-CSF mRNA expression. Int. J. Immunopharmacol. 1995, 17, 753–761. [Google Scholar] [CrossRef]
- Zhu, F.; Panwar, B.; Dodge, H.H.; Li, H.; Hampstead, B.M.; Albin, R.L.; Paulson, H.L.; Guan, Y. COMPASS: A computational model to predict changes in MMSE scores 24-months after initial assessment of Alzheimer’s disease. Sci. Rep. 2016, 6, srep34567. [Google Scholar] [CrossRef]
- Szabo, A.; Rajnavolgyi, E. The brain-immune-gut triangle: Innate immunity in psychiatric and neurological disorders. Curr. Immunol. Rev. 2013, 9, 241–248. [Google Scholar]
- Cameron, L.P.; Benson, C.J.; DeFelice, B.C.; Fiehn, O.; Olson, D.E. Chronic, intermittent microdoses of the psychedelic N, N-dimethyltryptamine (DMT) produce positive effects on mood and anxiety in rodents. ACS Chem. Neurosci. 2019, 10, 3261–3270. [Google Scholar] [CrossRef] [PubMed]
- Morgan, C.J.; Curran, H.V.; Drugs, I.S.C.o. Ketamine use: A review. Addiction 2012, 107, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Berman, R.M.; Cappiello, A.; Anand, A.; Oren, D.A.; Heninger, G.R.; Charney, D.S.; Krystal, J.H. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry 2000, 47, 351–354. [Google Scholar] [CrossRef]
- McIntyre, R.S.; Carvalho, I.P.; Lui, L.M.; Majeed, A.; Masand, P.S.; Gill, H.; Rodrigues, N.B.; Lipsitz, O.; Coles, A.C.; Lee, Y. The effect of intravenous, intranasal, and oral ketamine/esketamine in mood disorders: A meta-analysis. J. Affect. Disord. 2020, 276, 576–584. [Google Scholar] [CrossRef]
- Short, B.; Fong, J.; Galvez, V.; Shelker, W.; Loo, C.K. Side-effects associated with ketamine use in depression: A systematic review. Lancet Psychiatry 2018, 5, 65–78. [Google Scholar]
- Malhotra, A.K.; Pinals, D.A.; Weingartner, H.; Sirocco, K.; Missar, C.D.; Pickar, D.; Breier, A. NMDA receptor function and human cognition: The effects of ketamine in healthy volunteers. Neuropsychopharmacology 1996, 14, 301–307. [Google Scholar] [CrossRef]
- Pfenninger, E.G.; Durieux, M.E.; Himmelseher, S. Cognitive impairment after small-dose ketamine isomers in comparison to equianalgesic racemic ketamine in human volunteers. J. Am. Soc. Anesthesiol. 2002, 96, 357–366. [Google Scholar] [CrossRef]
- Morgan, C.J.; Dodds, C.M.; Furby, H.; Pepper, F.; Fam, J.; Freeman, T.P.; Hughes, E.; Doeller, C.; King, J.; Howes, O. Long-term heavy ketamine use is associated with spatial memory impairment and altered hippocampal activation. Front. Psychiatry 2014, 5, 149. [Google Scholar] [CrossRef]
- Kessels, R.; Feijen, J.; Postma, A. Implicit and explicit memory for spatial information in Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2005, 20, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Smalheiser, N.R. Ketamine: A Neglected Therapy for Alzheimer Disease. Front. Aging Neurosci. 2019, 11, 186. [Google Scholar] [CrossRef] [PubMed]
- Mohammad Shehata, I.; Masood, W.; Nemr, N.; Anderson, A.; Bhusal, K.; Edinoff, A.N.; Cornett, E.M.; Kaye, A.M.; Kaye, A.D. The Possible Application of Ketamine in the Treatment of Depression in Alzheimer’s Disease. Neurol. Int. 2022, 14, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Carbonaro, T.M.; Bradstreet, M.P.; Barrett, F.S.; MacLean, K.A.; Jesse, R.; Johnson, M.W.; Griffiths, R.R. Survey study of challenging experiences after ingesting psilocybin mushrooms: Acute and enduring positive and negative consequences. J. Psychopharmacol. 2016, 30, 1268–1278. [Google Scholar]
- Cameron, L.P.; Nazarian, A.; Olson, D.E. Psychedelic Microdosing: Prevalence and Subjective Effects. J. Psychoact. Drugs 2020, 52, 113–122. [Google Scholar] [CrossRef]
- Anderson, T.; Petranker, R.; Christopher, A.; Rosenbaum, D.; Weissman, C.; Dinh-Williams, L.-A.; Hui, K.; Hapke, E. Psychedelic microdosing benefits and challenges: An empirical codebook. Harm Reduct. J. 2019, 16, 1–10. [Google Scholar] [CrossRef]
- Bornemann, J. The viability of microdosing psychedelics as a strategy to enhance cognition and well-being-an early review. J. Psychoact. Drugs 2020, 52, 300–308. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pilozzi, A.; Foster, S.; Mischoulon, D.; Fava, M.; Huang, X. A Brief Review on the Potential of Psychedelics for Treating Alzheimer’s Disease and Related Depression. Int. J. Mol. Sci. 2023, 24, 12513. https://doi.org/10.3390/ijms241512513
Pilozzi A, Foster S, Mischoulon D, Fava M, Huang X. A Brief Review on the Potential of Psychedelics for Treating Alzheimer’s Disease and Related Depression. International Journal of Molecular Sciences. 2023; 24(15):12513. https://doi.org/10.3390/ijms241512513
Chicago/Turabian StylePilozzi, Alexander, Simmie Foster, David Mischoulon, Maurizio Fava, and Xudong Huang. 2023. "A Brief Review on the Potential of Psychedelics for Treating Alzheimer’s Disease and Related Depression" International Journal of Molecular Sciences 24, no. 15: 12513. https://doi.org/10.3390/ijms241512513
APA StylePilozzi, A., Foster, S., Mischoulon, D., Fava, M., & Huang, X. (2023). A Brief Review on the Potential of Psychedelics for Treating Alzheimer’s Disease and Related Depression. International Journal of Molecular Sciences, 24(15), 12513. https://doi.org/10.3390/ijms241512513






