Advances in the Management of Early-Stage Triple-Negative Breast Cancer
Abstract
1. Introduction
2. Systemic Therapy—New Insights
2.1. Advances in Chemotherapy
2.2. Immunotherapy
2.2.1. Other Checkpoint Inhibitors in Breast Cancer
2.2.2. Challenges and Future Directions
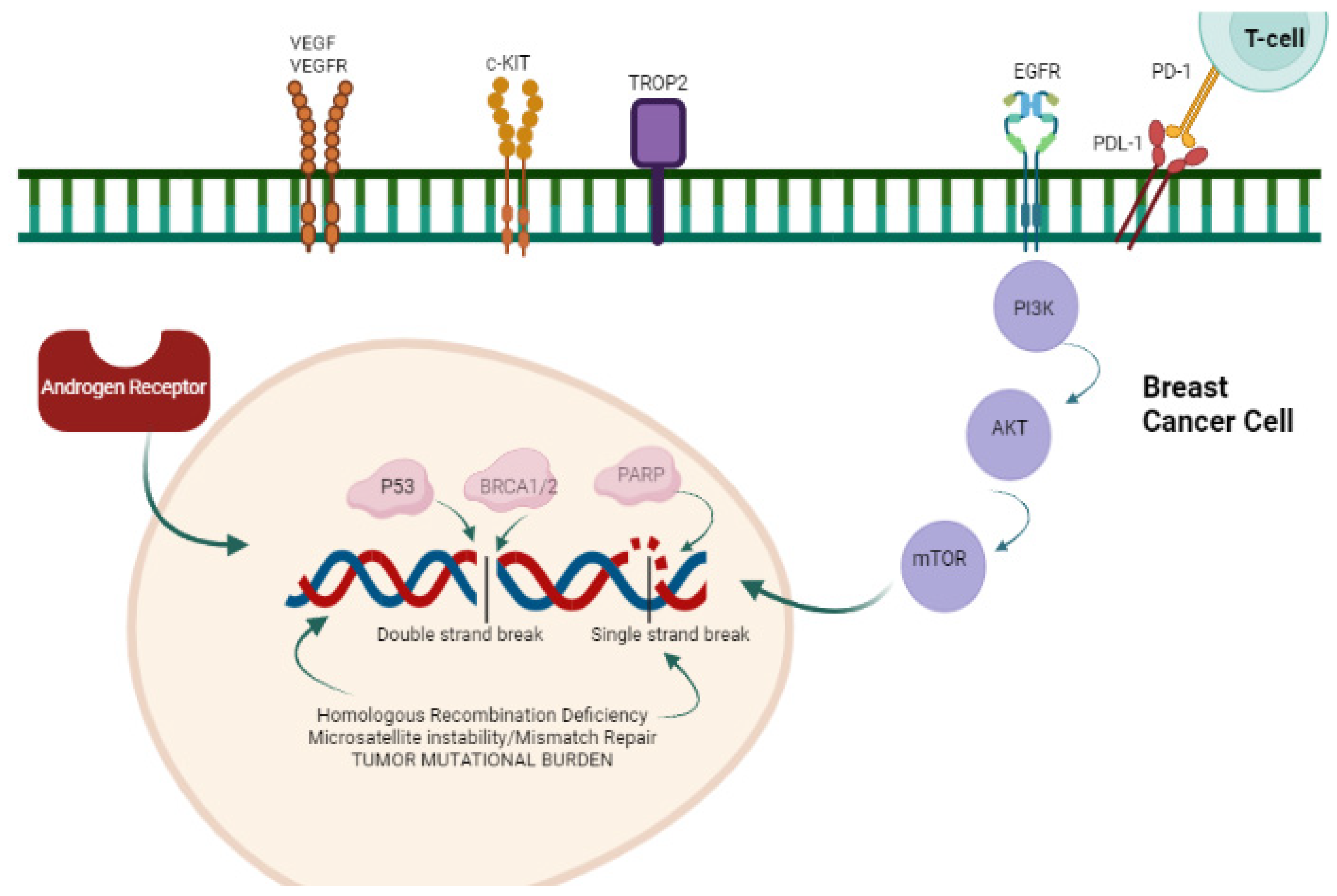
2.3. Targeted Agents
2.3.1. PARP Inhibitors (PARPi)
2.3.2. PIK3CA/AKT1/PTEN Pathway
2.3.3. Androgen Receptor (AR) Pathway
2.3.4. Receptor Tyrosine Kinase Family (HER2, VEGF)
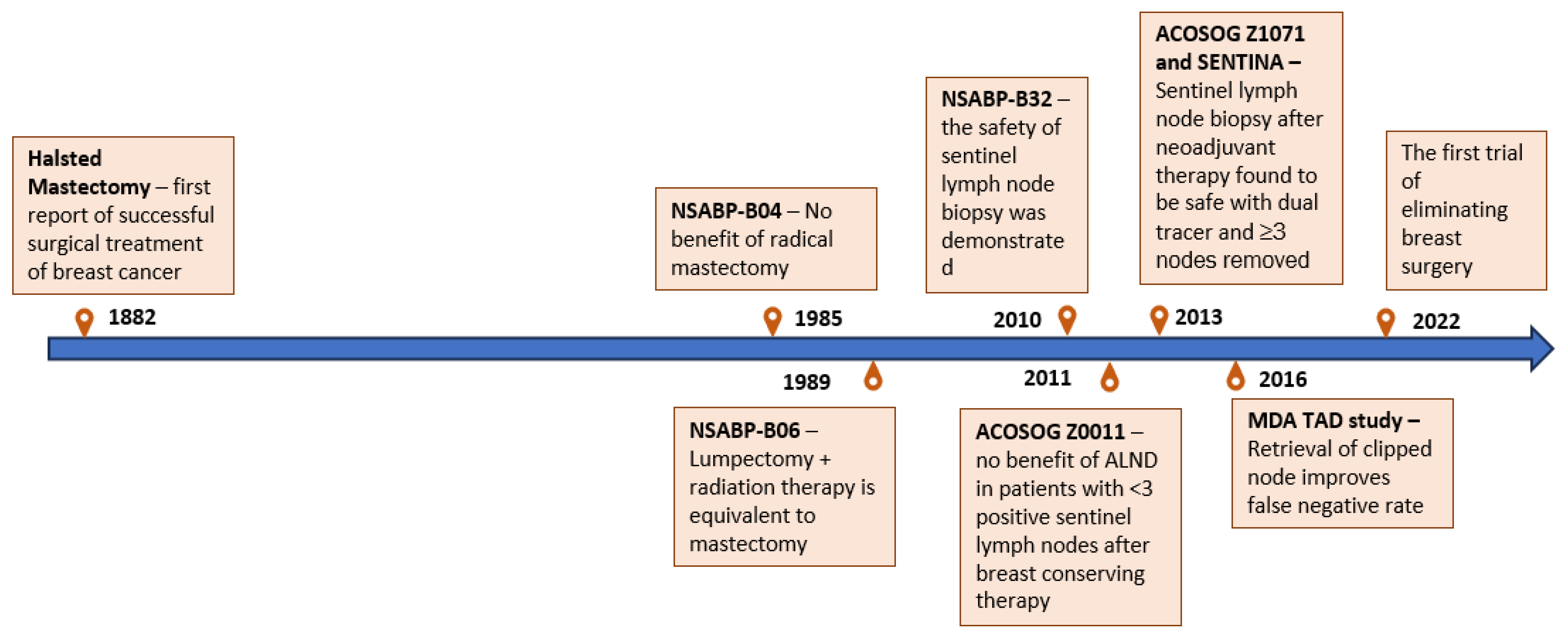
3. Advances in Surgery
4. Tumor Heterogeneity and the Future of Precision Medicine
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dent, R.; Hanna, W.M.; Trudeau, M.; Rawlinson, E.; Sun, P.; Narod, S.A. Pattern of Metastatic Spread in Triple-Negative Breast Cancer. Breast Cancer Res. Treat. 2009, 115, 423–428. [Google Scholar] [CrossRef]
- Gonzalez-Angulo, A.M.; Timms, K.M.; Liu, S.; Chen, H.; Litton, J.K.; Potter, J.; Lanchbury, J.S.; Stemke-Hale, K.; Hennessy, B.T.; Arun, B.K.; et al. Incidence and Outcome of BRCA Mutations in Unselected Patients with Triple Receptor-Negative Breast Cancer. Clin. Cancer Res. 2011, 17, 1082–1089. [Google Scholar] [CrossRef]
- Pereira, B.; Chin, S.-F.; Rueda, O.M.; Vollan, H.-K.M.; Provenzano, E.; Bardwell, H.A.; Pugh, M.; Jones, L.; Russell, R.; Sammut, S.-J.; et al. The Somatic Mutation Profiles of 2,433 Breast Cancers Refines Their Genomic and Transcriptomic Landscapes. Nat. Commun. 2016, 7, 11479. [Google Scholar] [CrossRef]
- Shah, S.P.; Roth, A.; Goya, R.; Oloumi, A.; Ha, G.; Zhao, Y.; Turashvili, G.; Ding, J.; Tse, K.; Haffari, G.; et al. The Clonal and Mutational Evolution Spectrum of Primary Triple Negative Breast Cancers. Nature 2012, 486, 395–399. [Google Scholar] [CrossRef]
- Bianchini, G.; Balko, J.M.; Mayer, I.A.; Sanders, M.E.; Gianni, L. Triple-Negative Breast Cancer: Challenges and Opportunities of a Heterogeneous Disease. Nat. Rev. Clin. Oncol. 2016, 13, 674–690. [Google Scholar] [CrossRef]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular Portraits of Human Breast Tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef]
- Sikov, W.M.; Barry, W.T.; Hoadley, K.A.; Pitcher, B.N.; Singh, B.; Tolaney, S.M.; Kuzma, C.S.; Pluard, T.J.; Somlo, G.; Port, E.R.; et al. Abstract S4-05: Impact of Intrinsic Subtype by PAM50 and Other Gene Signatures on Pathologic Complete Response (PCR) Rates in Triple-Negative Breast Cancer (TNBC) after Neoadjuvant Chemotherapy (NACT) +/− Carboplatin (Cb) or Bevacizumab (Bev): CALGB 40603/150709 (Allianc). Cancer Res. 2015, 75, S4-05. [Google Scholar] [CrossRef]
- Shepherd, J.H.; Ballman, K.; Polley, M.-Y.C.; Campbell, J.D.; Fan, C.; Selitsky, S.; Fernandez-Martinez, A.; Parker, J.S.; Hoadley, K.A.; Hu, Z.; et al. CALGB 40603 (Alliance): Long-Term Outcomes and Genomic Correlates of Response and Survival After Neoadjuvant Chemotherapy with or without Carboplatin and Bevacizumab in Triple-Negative Breast Cancer. J. Clin. Oncol. 2022, 40, 1323–1334. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Pietenpol, J.A. Identification and Use of Biomarkers in Treatment Strategies for Triple-Negative Breast Cancer Subtypes. J. Pathol. 2014, 232, 142–150. [Google Scholar] [CrossRef]
- Pinilla, K.; Drewett, L.M.; Lucey, R.; Abraham, J.E. Precision Breast Cancer Medicine: Early Stage Triple Negative Breast Cancer—A Review of Molecular Characterisation, Therapeutic Targets and Future Trends. Front. Oncol. 2022, 12, 866889. [Google Scholar] [CrossRef]
- Liu, Y.-R.; Jiang, Y.-Z.; Xu, X.-E.; Yu, K.-D.; Jin, X.; Hu, X.; Zuo, W.-J.; Hao, S.; Wu, J.; Liu, G.-Y.; et al. Comprehensive Transcriptome Analysis Identifies Novel Molecular Subtypes and Subtype-Specific RNAs of Triple-Negative Breast Cancer. Breast Cancer Res. 2016, 18, 33. [Google Scholar] [CrossRef]
- Rueda, O.M.; Sammut, S.-J.; Seoane, J.A.; Chin, S.-F.; Caswell-Jin, J.L.; Callari, M.; Batra, R.; Pereira, B.; Bruna, A.; Ali, H.R.; et al. Dynamics of Breast-Cancer Relapse Reveal Late-Recurring ER-Positive Genomic Subgroups. Nature 2019, 567, 399–404. [Google Scholar] [CrossRef]
- Curtis, C.; Shah, S.P.; Chin, S.-F.; Turashvili, G.; Rueda, O.M.; Dunning, M.J.; Speed, D.; Lynch, A.G.; Samarajiwa, S.; Yuan, Y.; et al. The Genomic and Transcriptomic Architecture of 2000 Breast Tumours Reveals Novel Subgroups. Nature 2012, 486, 346–352. [Google Scholar] [CrossRef]
- Prado-Vázquez, G.; Gámez-Pozo, A.; Trilla-Fuertes, L.; Arevalillo, J.M.; Zapater-Moros, A.; Ferrer-Gómez, M.; Díaz-Almirón, M.; López-Vacas, R.; Navarro, H.; Maín, P.; et al. A Novel Approach to Triple-Negative Breast Cancer Molecular Classification Reveals a Luminal Immune-Positive Subgroup with Good Prognoses. Sci. Rep. 2019, 9, 1538. [Google Scholar] [CrossRef]
- Burstein, M.D.; Tsimelzon, A.; Poage, G.M.; Covington, K.R.; Contreras, A.; Fuqua, S.A.W.; Savage, M.I.; Osborne, C.K.; Hilsenbeck, S.G.; Chang, J.C.; et al. Comprehensive Genomic Analysis Identifies Novel Subtypes and Targets of Triple-Negative Breast Cancer. Clin. Cancer Res. 2015, 21, 1688–1698. [Google Scholar] [CrossRef]
- Symmans, W.F.; Wei, C.; Gould, R.; Yu, X.; Zhang, Y.; Liu, M.; Walls, A.; Bousamra, A.; Ramineni, M.; Sinn, B.; et al. Long-Term Prognostic Risk after Neoadjuvant Chemotherapy Associated with Residual Cancer Burden and Breast Cancer Subtype. J. Clin. Oncol. 2017, 35, 1049–1060. [Google Scholar] [CrossRef]
- Thompson, A.M.; Moulder-Thompson, S.L. Neoadjuvant Treatment of Breast Cancer. Ann. Oncol. 2012, 23, x231–x236. [Google Scholar] [CrossRef]
- von Minckwitz, G.; Untch, M.; Blohmer, J.-U.; Costa, S.D.; Eidtmann, H.; Fasching, P.A.; Gerber, B.; Eiermann, W.; Hilfrich, J.; Huober, J.; et al. Definition and Impact of Pathologic Complete Response on Prognosis after Neoadjuvant Chemotherapy in Various Intrinsic Breast Cancer Subtypes. J. Clin. Oncol. 2012, 30, 1796–1804. [Google Scholar] [CrossRef]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Vala-gussa, P.; et al. Pathological Complete Response and Long-Term Clinical Benefit in Breast Cancer: The CTNeoBC Pooled Analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef]
- Spring, L.M.; Fell, G.; Arfe, A.; Sharma, C.; Greenup, R.; Reynolds, K.L.; Smith, B.L.; Alexander, B.; Moy, B.; Isakoff, S.J.; et al. Pathologic Complete Response after Neoadjuvant Chemotherapy and Impact on Breast Cancer Recurrence and Survival: A Com-prehensive Meta-Analysis. Clin. Cancer Res. 2020, 26, 2838–2848. [Google Scholar] [CrossRef]
- Huang, M.; O’Shaughnessy, J.; Zhao, J.; Haiderali, A.; Cortes, J.; Ramsey, S.; Briggs, A.; Karantza, V.; Aktan, G.; Qi, C.Z.; et al. Evaluation of Pathologic Complete Response as a Surrogate for Long-Term Survival Outcomes in Triple-Negative Breast Cancer. J. Natl. Compr. Cancer Netw. 2020, 18, 1096–1104. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Anthracycline-Containing and Taxane-Containing Chemotherapy for Early-Stage Operable Breast Cancer: A Patient-Level Meta-Analysis of 100,000 Women from 86 Randomised Trials. Lancet 2023, 401, 1277–1292. [Google Scholar] [CrossRef]
- Loibl, S.; O’Shaughnessy, J.; Untch, M.; Sikov, W.M.; Rugo, H.S.; McKee, M.D.; Huober, J.; Golshan, M.; von Minckwitz, G.; Maag, D.; et al. Addition of the PARP Inhibitor Veliparib plus Carboplatin or Carboplatin Alone to Standard Neoadjuvant Chem-otherapy in Triple-Negative Breast Cancer (BrighTNess): A Randomised, Phase 3 Trial. Lancet Oncol. 2018, 19, 497–509. [Google Scholar] [CrossRef]
- Sikov, W.M.; Berry, D.A.; Perou, C.M.; Singh, B.; Cirrincione, C.T.; Tolaney, S.M.; Kuzma, C.S.; Pluard, T.J.; Somlo, G.; Port, E.R.; et al. Impact of the Addition of Carboplatin and/or Bevacizumab to Neoadjuvant Once-per-Week Paclitaxel Followed by Dose-Dense Doxorubicin and Cyclophosphamide on Pathologic Complete Response Rates in Stage II to III Triple-Negative Breast Cancer: CALGB 40603 (Alliance). J. Clin. Oncol. 2015, 33, 13–21. [Google Scholar] [CrossRef]
- von Minckwitz, G.; Schneeweiss, A.; Loibl, S.; Salat, C.; Denkert, C.; Rezai, M.; Blohmer, J.U.; Jackisch, C.; Paepke, S.; Gerber, B.; et al. Neoadjuvant Carboplatin in Patients with Triple-Negative and HER2-Positive Early Breast Cancer (GeparSixto; GBG 66): A Randomised Phase 2 Trial. Lancet Oncol. 2014, 15, 747–756. [Google Scholar] [CrossRef]
- Geyer, C.E.; Sikov, W.M.; Huober, J.; Rugo, H.S.; Wolmark, N.; O’Shaughnessy, J.; Maag, D.; Untch, M.; Golshan, M.; Lorenzo, J.P.; et al. Long-Term Efficacy and Safety of Addition of Carboplatin with or without Veliparib to Standard Neoadjuvant Chemo-therapy in Triple-Negative Breast Cancer: 4-Year Follow-up Data from BrighTNess, a Randomized Phase III Trial. Ann. Oncol. 2022, 33, 384–394. [Google Scholar] [CrossRef]
- Poggio, F.; Bruzzone, M.; Ceppi, M.; Pondé, N.F.; La Valle, G.; Del Mastro, L.; de Azambuja, E.; Lambertini, M. Platinum-Based Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer: A Systematic Review and Meta-Analysis. Ann. Oncol. 2018, 29, 1497–1508. [Google Scholar] [CrossRef]
- Loibl, S.; Weber, K.E.; Timms, K.M.; Elkin, E.P.; Hahnen, E.; Fasching, P.A.; Lederer, B.; Denkert, C.; Schneeweiss, A.; Braun, S.; et al. Survival Analysis of Carboplatin Added to an Anthracycline/Taxane-Based Neoadjuvant Chemotherapy and HRD Score as Predictor of Response-Final Results from GeparSixto. Ann. Oncol. 2018, 29, 2341–2347. [Google Scholar] [CrossRef]
- Alba, E.; Chacon, J.I.; Lluch, A.; Anton, A.; Estevez, L.; Cirauqui, B.; Carrasco, E.; Calvo, L.; Segui, M.A.; Ribelles, N.; et al. A Randomized Phase II Trial of Platinum Salts in Basal-like Breast Cancer Patients in the Neoadjuvant Setting. Results from the GEICAM/2006-03, Multicenter Study. Breast Cancer Res. Treat. 2012, 136, 487–493. [Google Scholar] [CrossRef]
- Ando, M.; Yamauchi, H.; Aogi, K.; Shimizu, S.; Iwata, H.; Masuda, N.; Yamamoto, N.; Inoue, K.; Ohono, S.; Kuroi, K.; et al. Randomized Phase II Study of Weekly Paclitaxel with and without Carboplatin Followed by Cyclophospha-mide/Epirubicin/5-Fluorouracil as Neoadjuvant Chemotherapy for Stage II/IIIA Breast Cancer without HER2 Overexpression. Breast Cancer Res. Treat. 2014, 145, 401–409. [Google Scholar] [CrossRef]
- Möbus, V.; Jackisch, C.; Lück, H.J.; du Bois, A.; Thomssen, C.; Kuhn, W.; Nitz, U.; Schneeweiss, A.; Huober, J.; Harbeck, N.; et al. Ten-Year Results of Intense Dose-Dense Chemotherapy Show Superior Survival Compared with a Conventional Schedule in High-Risk Primary Breast Cancer: Final Results of AGO Phase III IddEPC Trial. Ann. Oncol. 2018, 29, 178–185. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Increasing the Dose Intensity of Chemotherapy by More Fre-quent Administration or Sequential Scheduling: A Patient-Level Meta-Analysis of 37,298 Women with Early Breast Cancer in 26 Randomised Trials. Lancet 2019, 393, 1440–1452. [Google Scholar] [CrossRef] [PubMed]
- Masuda, N.; Lee, S.-J.; Ohtani, S.; Im, Y.-H.; Lee, E.-S.; Yokota, I.; Kuroi, K.; Im, S.-A.; Park, B.-W.; Kim, S.-B.; et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N. Engl. J. Med. 2017, 376, 2147–2159. [Google Scholar] [CrossRef]
- Wang, X.; Wang, S.-S.; Huang, H.; Cai, L.; Zhao, L.; Peng, R.-J.; Lin, Y.; Tang, J.; Zeng, J.; Zhang, L.-H.; et al. Effect of Capecitabine Maintenance Therapy Using Lower Dosage and Higher Frequency vs. Observation on Disease-Free Survival Among Patients With Early-Stage Triple-Negative Breast Cancer Who Had Received Standard Treatment: The SYSUCC-001 Randomized Clinical Trial. JAMA 2021, 325, 50–58. [Google Scholar] [CrossRef]
- Lluch, A.; Barrios, C.H.; Torrecillas, L.; Ruiz-Borrego, M.; Bines, J.; Segalla, J.; Guerrero-Zotano, Á.; García-Sáenz, J.A.; Torres, R.; de la Haba, J.; et al. Phase III Trial of Adjuvant Capecitabine After Standard Neo-/Adjuvant Chemotherapy in Patients With Early Triple-Negative Breast Cancer (GEICAM/2003-11_CIBOMA/2004-01). J. Clin. Oncol. 2020, 38, 203–213. [Google Scholar] [CrossRef]
- García-Teijido, P.; Cabal, M.L.; Fernández, I.P.; Pérez, Y.F. Tumor-Infiltrating Lymphocytes in Triple Negative Breast Cancer: The Future of Immune Targeting. Clin. Med. Insights Oncol. 2016, 10, 31–39. [Google Scholar] [CrossRef]
- Stanton, S.E.; Adams, S.; Disis, M.L. Variation in the Incidence and Magnitude of Tumor-Infiltrating Lymphocytes in Breast Cancer Subtypes: A Systematic Review. JAMA Oncol. 2016, 2, 1354–1360. [Google Scholar] [CrossRef]
- Thomas, A.; Routh, E.D.; Pullikuth, A.; Jin, G.; Su, J.; Chou, J.W.; Hoadley, K.A.; Print, C.; Knowlton, N.; Black, M.A.; et al. Tumor Mutational Burden Is a Determinant of Immune-Mediated Survival in Breast Cancer. Oncoimmunology 2018, 7, e1490854. [Google Scholar] [CrossRef]
- Wimberly, H.; Brown, J.R.; Schalper, K.; Haack, H.; Silver, M.R.; Nixon, C.; Bossuyt, V.; Pusztai, L.; Lannin, D.R.; Rimm, D.L. PD-L1 Expression Correlates with Tumor-Infiltrating Lymphocytes and Response to Neoadjuvant Chemotherapy in Breast Cancer. Cancer Immunol. Res. 2015, 3, 326–332. [Google Scholar] [CrossRef]
- O’Meara, T.A.; Tolaney, S.M. Tumor Mutational Burden as a Predictor of Immunotherapy Response in Breast Cancer. Oncotarget 2021, 12, 394–400. [Google Scholar] [CrossRef]
- Cortes, J.; Cescon, D.W.; Rugo, H.S.; Nowecki, Z.; Im, S.-A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Holgado, E.; et al. Pembrolizumab plus Chemotherapy versus Placebo plus Chemotherapy for Previously Untreated Locally Recurrent Inoperable or Metastatic Triple-Negative Breast Cancer (KEYNOTE-355): A Randomised, Placebo-Controlled, Double-Blind, Phase 3 Clinical Trial. Lancet 2020, 396, 1817–1828. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Hegg, R.; Im, S.-A.; Shaw Wright, G.; et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, K.E.; Yost, S.E.; Chang, C.-W.; Johnson, R.M.; Carr, A.R.; McAdam, P.R.; Halligan, D.L.; Chang, C.-C.; Schmolze, D.; Liang, J.; et al. Comprehensive Profiling of Poor-Risk Paired Primary and Recurrent Triple-Negative Breast Cancers Reveals Immune Phenotype Shifts. Clin. Cancer Res. 2020, 26, 657–668. [Google Scholar] [CrossRef]
- Szekely, B.; Bossuyt, V.; Li, X.; Wali, V.B.; Patwardhan, G.A.; Frederick, C.; Silber, A.; Park, T.; Harigopal, M.; Pelekanou, V.; et al. Immunological Differences between Primary and Metastatic Breast Cancer. Ann. Oncol. 2018, 29, 2232–2239. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Dent, R.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; et al. Event-Free Survival with Pembrolizumab in Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2022, 386, 556–567. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Zhang, H.; Barrios, C.H.; Saji, S.; Jung, K.H.; Hegg, R.; Koehler, A.; Sohn, J.; Iwata, H.; Telli, M.L.; et al. Neoadjuvant Atezolizumab in Combination with Sequential Nab-Paclitaxel and Anthracycline-Based Chemotherapy versus Pla-cebo and Chemotherapy in Patients with Early-Stage Triple-Negative Breast Cancer (IMpassion031): A Randomised, Double-Blind, Phase 3 Trial. Lancet 2020, 396, 1090–1100. [Google Scholar] [CrossRef]
- Gianni, L.; Huang, C.S.; Egle, D.; Bermejo, B.; Zamagni, C.; Thill, M.; Anton, A.; Zambelli, S.; Bianchini, G.; Russo, S.; et al. Pathologic Complete Response (PCR) to Neoadjuvant Treatment with or without Atezolizumab in Triple-Negative, Early High-Risk and Locally Advanced Breast Cancer: NeoTRIP Michelangelo Randomized Study. Ann. Oncol. 2022, 33, 534–543. [Google Scholar] [CrossRef]
- Loibl, S.; Schneeweiss, A.; Huober, J.; Braun, M.; Rey, J.; Blohmer, J.-U.; Furlanetto, J.; Zahm, D.-M.; Hanusch, C.; Thomalla, J.; et al. Neoadjuvant Durvalumab Improves Survival in Early Triple-Negative Breast Cancer Independent of Pathological Complete Response. Ann. Oncol. 2022, 33, 1149–1158. [Google Scholar] [CrossRef]
- Nanda, R.; Liu, M.C.; Yau, C.; Shatsky, R.; Pusztai, L.; Wallace, A.; Chien, A.J.; Forero-Torres, A.; Ellis, E.; Han, H.; et al. Effect of Pembrolizumab Plus Neoadjuvant Chemotherapy on Pathologic Complete Response in Women with Early-Stage Breast Cancer: An Analysis of the Ongoing Phase 2 Adaptively Randomized I-SPY2 Trial. JAMA Oncol. 2020, 6, 676–684. [Google Scholar] [CrossRef]
- Loibl, S.; Jackisch, C.; Rastogi, P.; Seiler, S.; Lucas, P.C.; Denkert, C.; Costantino, J.; Nekljudova, V.; Wolmark, N.; Geyer, C. GeparDouze/NSABP B-59: A Randomized Double-Blind Phase III Clinical Trial of Neoadjuvant Chemotherapy with Atezolizumab or Placebo in Patients with Triple Negative Breast Cancer (TNBC) Followed by Adjuvant Atezolizumab or Placebo. Ann. Oncol. 2019, 30, iii38. [Google Scholar] [CrossRef]
- Saji, S.; McArthur, H.L.; Ignatiadis, M.; Bailey, A.; El-Abed, S.; Brandao, M.; Metzger, O.; Lai, C.; Guillaume, S.; Fumagalli, D.; et al. ALEXANDRA/IMpassion030: A Phase 3 Study of Standard Adjuvant Chemotherapy with or without Atezolizumab in Pa-tients with Early-Stage Triple-Negative Breast Cancer. J. Clin. Oncol. 2021, 39, TPS597. [Google Scholar] [CrossRef]
- Abstract OT1-02-04: SWOG S1418/NRG-BR006: A Randomized, Phase III Trial to Evaluate the Efficacy and Safety of MK-3475 as Adjuvant Therapy for Triple Receptor-Negative Breast Cancer with >1 cm Residual Invasive Cancer or Positive Lymph Nodes (>pN1mic) after Neoadjuvant Chemotherapy|Cancer Research|American Association for Cancer Research. Available online: https://aacrjournals.org/cancerres/article/78/4_Supplement/OT1-02-04/632005/Abstract-OT1-02-04-SWOG-S1418-NRG-BR006-A (accessed on 27 January 2023).
- Park, I.H.; Kim, G.M.; Kim, J.H.; Kim, H.; Park, K.H.; Park, Y.H.; Baek, S.K.; Sim, S.H.; Ahn, H.K.; Lee, G.-W.; et al. Ran-domized, Phase II Trial to Evaluate the Efficacy and Safety of Atezolizumab plus Capecitabine Adjuvant Therapy Compared to Capecitabine Monotherapy for Triple Receptor-Negative Breast Cancer (TNBC) with Residual Invasive Cancer after Neoadjuvant Chemotherapy (MIRINAE Trial, KCSG-BR18-21). J. Clin. Oncol. 2020, 38, TPS597. [Google Scholar] [CrossRef]
- Zhang, Q.; Luo, J.; Wu, S.; Si, H.; Gao, C.; Xu, W.; Abdullah, S.E.; Higgs, B.W.; Dennis, P.A.; van der Heijden, M.S.; et al. Prognostic and Predictive Impact of Circulating Tumor DNA in Patients with Advanced Cancers Treated with Immune Checkpoint Blockade. Cancer Discov. 2020, 10, 1842–1853. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, Y.-C.; Chen, Z.-H.; Zhou, Q.; Wu, Y.-L. Applications of Circulating Tumor DNA in Immune Checkpoint Inhibition: Emerging Roles and Future Perspectives. Front. Oncol. 2022, 12, 836891. [Google Scholar] [CrossRef] [PubMed]
- Tutt, A.N.J.; Garber, J.E.; Kaufman, B.; Viale, G.; Fumagalli, D.; Rastogi, P.; Gelber, R.D.; de Azambuja, E.; Fielding, A.; Balmaña, J.; et al. Adjuvant Olaparib for Patients with BRCA1- or BRCA2-Mutated Breast Cancer. N. Engl. J. Med. 2021, 384, 2394–2405. [Google Scholar] [CrossRef]
- Tarantino, P.; Corti, C.; Schmid, P.; Cortes, J.; Mittendorf, E.A.; Rugo, H.; Tolaney, S.M.; Bianchini, G.; Andrè, F.; Curigliano, G. Immunotherapy for Early Triple Negative Breast Cancer: Research Agenda for the next Decade. NPJ Breast Cancer 2022, 8, 23. [Google Scholar] [CrossRef]
- Hirsch, I.; Goldstein, D.A.; Tannock, I.F.; Butler, M.O.; Gilbert, D.C. Optimizing the Dose and Schedule of Immune Checkpoint Inhibitors in Cancer to Allow Global Access. Nat. Med. 2022, 28, 2236–2237. [Google Scholar] [CrossRef]
- Santa-Maria, C.A. Optimizing and Refining Immunotherapy in Breast Cancer. JCO Oncol. Pract. 2023, 19, 190–191. [Google Scholar] [CrossRef]
- Pusztai, L.; Denkert, C.; O’Shaughnessy, J.; Cortes, J.; Dent, R.A.; McArthur, H.L.; Kuemmel, S.; Bergh, J.C.S.; Park, Y.H.; Hui, R.; et al. Event-Free Survival by Residual Cancer Burden after Neoadjuvant Pembrolizumab + Chemotherapy versus Placebo + Chemotherapy for Early TNBC: Exploratory Analysis from KEYNOTE-522. J. Clin. Oncol. 2022, 40, 503. [Google Scholar] [CrossRef]
- German Breast Group. Phase III Postneoadjuvant Study Evaluating Sacituzumab Govitecan, an Antibody Drug Conjugate in Primary HER2-Negative Breast Cancer Patients with High Relapse Risk after Standard Neoadjuvant Treatment—SASCIA. 2023. Available online: https://www.clinicaltrials.gov/study/NCT04595565?cond=Sacituzumab%20Govitecan%20in%20Primary%20HER2-negative%20Breast%20Cancer%20(SASCIA)&rank=1 (accessed on 3 June 2023).
- Gilead Sciences. A Randomized, Open-Label, Phase 3 Study of Adjuvant Sacituzumab Govitecan and Pembrolizumab Versus Treatment of Physician’s Choice in Patients with Triple Negative Breast Cancer Who Have Residual Invasive Disease after Surgery and Neoadjuvant Therapy. 2023. Available online: https://www.clinicaltrials.gov/study/NCT05633654?cond=Who%20Have%20Residual%20Invasive%20Disease%20after%20Surgery%20and%20Neoadjuvant%20Therapy&rank=1 (accessed on 3 June 2023).
- Abdou, Y.; Goudarzi, A.; Yu, J.X.; Upadhaya, S.; Vincent, B.; Carey, L.A. Immunotherapy in Triple Negative Breast Cancer: Beyond Checkpoint Inhibitors. NPJ Breast Cancer 2022, 8, 121. [Google Scholar] [CrossRef]
- Soliman, H.; Hogue, D.; Han, H.; Mooney, B.; Costa, R.; Lee, M.C.; Niell, B.; Williams, A.; Chau, A.; Falcon, S.; et al. Oncolytic T-VEC Virotherapy plus Neoadjuvant Chemotherapy in Nonmetastatic Triple-Negative Breast Cancer: A Phase 2 Trial. Nat. Med. 2023, 29, 450–457. [Google Scholar] [CrossRef]
- Barchiesi, G.; Roberto, M.; Verrico, M.; Vici, P.; Tomao, S.; Tomao, F. Emerging Role of PARP Inhibitors in Metastatic Triple Negative Breast Cancer. Current Scenario and Future Perspectives. Front. Oncol. 2021, 11, 769280. [Google Scholar] [CrossRef] [PubMed]
- Geyer, C.E.; Garber, J.E.; Gelber, R.D.; Yothers, G.; Taboada, M.; Ross, L.; Rastogi, P.; Cui, K.; Arahmani, A.; Aktan, G.; et al. Overall Survival in the OlympiA Phase III Trial of Adjuvant Olaparib in Patients with Germline Pathogenic Variants in BRCA1/2 and High-Risk, Early Breast Cancer. Ann. Oncol. 2022, 33, 1250–1268. [Google Scholar] [CrossRef] [PubMed]
- Rugo, H.S.; Olopade, O.I.; DeMichele, A.; Yau, C.; van ’t Veer, L.J.; Buxton, M.B.; Hogarth, M.; Hylton, N.M.; Paoloni, M.; Perlmutter, J.; et al. Adaptive Randomization of Veliparib–Carboplatin Treatment in Breast Cancer. N. Engl. J. Med. 2016, 375, 23–34. [Google Scholar] [CrossRef]
- Litton, J.K.; Scoggins, M.E.; Hess, K.R.; Adrada, B.E.; Murthy, R.K.; Damodaran, S.; DeSnyder, S.M.; Brewster, A.M.; Bar-cenas, C.H.; Valero, V.; et al. Neoadjuvant Talazoparib for Patients with Operable Breast Cancer with a Germline BRCA Pathogenic Variant. J. Clin. Oncol. 2020, 38, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Fasching, P.A.; Link, T.; Hauke, J.; Seither, F.; Jackisch, C.; Klare, P.; Schmatloch, S.; Hanusch, C.; Huober, J.; Stefek, A.; et al. Neoadjuvant Paclitaxel/Olaparib in Comparison to Paclitaxel/Carboplatinum in Patients with HER2-Negative Breast Cancer and Homologous Recombination Deficiency (GeparOLA Study). Ann. Oncol. 2021, 32, 49–57. [Google Scholar] [CrossRef]
- Pusztai, L.; Yau, C.; Wolf, D.M.; Han, H.S.; Du, L.; Wallace, A.M.; String-Reasor, E.; Boughey, J.C.; Chien, A.J.; Elias, A.D.; et al. Durvalumab with Olaparib and Paclitaxel for High-Risk HER2-Negative Stage II/III Breast Cancer: Results from the Adaptively Randomized I-SPY2 Trial. Cancer Cell 2021, 39, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-Z.; Ma, D.; Suo, C.; Shi, J.; Xue, M.; Hu, X.; Xiao, Y.; Yu, K.-D.; Liu, Y.-R.; Yu, Y.; et al. Genomic and Transcriptomic Landscape of Triple-Negative Breast Cancers: Subtypes and Treatment Strategies. Cancer Cell 2019, 35, 428–440. [Google Scholar] [CrossRef]
- Koboldt, D.C.; Fulton, R.S.; McLellan, M.D.; Schmidt, H.; Kalicki-Veizer, J.; McMichael, J.F.; Fulton, L.L.; Dooling, D.J.; Ding, L.; Mardis, E.R.; et al. Comprehensive Molecular Portraits of Human Breast Tumours. Nature 2012, 490, 61–70. [Google Scholar] [CrossRef]
- Montero, J.C.; Esparís-Ogando, A.; Re-Louhau, M.F.; Seoane, S.; Abad, M.; Calero, R.; Ocaña, A.; Pandiella, A. Active Kinase Profiling, Genetic and Pharmacological Data Define MTOR as an Important Common Target in Triple-Negative Breast Cancer. Oncogene 2014, 33, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Saura, C.; Nuciforo, P.; Calvo, I.; Andersen, J.; Passos-Coelho, J.L.; Gil Gil, M.; Bermejo, B.; Patt, D.A.; Ciruelos, E.; et al. FAIRLANE, a Double-Blind Placebo-Controlled Randomized Phase II Trial of Neoadjuvant Ipatasertib plus Paclitaxel for Early Triple-Negative Breast Cancer. Ann. Oncol. 2019, 30, 1289–1297. [Google Scholar] [CrossRef]
- Jovanović, B.; Mayer, I.A.; Mayer, E.L.; Abramson, V.G.; Bardia, A.; Sanders, M.E.; Kuba, M.G.; Estrada, M.V.; Beeler, J.S.; Shaver, T.M.; et al. A Randomized Phase II Neoadjuvant Study of Cisplatin, Paclitaxel with or without Everolimus in Patients with Stage II/III Triple-Negative Breast Cancer (TNBC): Responses and Long-Term Outcome Correlated with Increased Frequency of DNA Damage Response Gene Mutations, TNBC Subtype, AR Status, and Ki67. Clin. Cancer Res. 2017, 23, 4035–4045. [Google Scholar] [CrossRef]
- Gonzalez-Angulo, A.M.; Akcakanat, A.; Liu, S.; Green, M.C.; Murray, J.L.; Chen, H.; Palla, S.L.; Koenig, K.B.; Brewster, A.M.; Valero, V.; et al. Open-Label Randomized Clinical Trial of Standard Neoadjuvant Chemotherapy with Paclitaxel Followed by FEC versus the Combination of Paclitaxel and Everolimus Followed by FEC in Women with Triple Receptor-Negative Breast Cancer†. Ann. Oncol. 2014, 25, 1122–1127. [Google Scholar] [CrossRef]
- Park, S.; Koo, J.; Park, H.S.; Kim, J.-H.; Choi, S.-Y.; Lee, J.H.; Park, B.-W.; Lee, K.S. Expression of Androgen Receptors in Primary Breast Cancer. Ann. Oncol. 2010, 21, 488–492. [Google Scholar] [CrossRef]
- Traina, T.A.; Miller, K.; Yardley, D.A.; Eakle, J.; Schwartzberg, L.S.; O’Shaughnessy, J.; Gradishar, W.; Schmid, P.; Winer, E.; Kelly, C.; et al. Enzalutamide for the Treatment of Androgen Receptor–Expressing Triple-Negative Breast Cancer. J. Clin. Oncol. 2018, 36, 884–890. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Abramson, V.G.; Sanders, M.E.; Mayer, E.L.; Haddad, T.C.; Nanda, R.; Van Poznak, C.; Storniolo, A.M.; Nangia, J.R.; Gonzalez-Ericsson, P.I.; et al. TBCRC 032 IB/II Multicenter Study: Molecular Insights to AR Antagonist and PI3K Inhibitor Efficacy in Patients with AR+ Metastatic Triple-Negative Breast Cancer. Clin. Cancer Res. 2020, 26, 2111–2123. [Google Scholar] [CrossRef]
- Bonnefoi, H.; Grellety, T.; Tredan, O.; Saghatchian, M.; Dalenc, F.; Mailliez, A.; L’Haridon, T.; Cottu, P.; Abadie-Lacourtoisie, S.; You, B.; et al. A Phase II Trial of Abiraterone Acetate plus Prednisone in Patients with Triple-Negative Androgen Receptor Positive Locally Advanced or Metastatic Breast Cancer (UCBG 12-1). Ann. Oncol. 2016, 27, 812–818. [Google Scholar] [CrossRef]
- Gucalp, A.; Tolaney, S.; Isakoff, S.J.; Ingle, J.N.; Liu, M.C.; Carey, L.A.; Blackwell, K.; Rugo, H.; Nabell, L.; Forero, A.; et al. Phase II Trial of Bicalutamide in Patients with Androgen Receptor-Positive, Estrogen Receptor-Negative Metastatic Breast Cancer. Clin. Cancer Res. 2013, 19, 5505–5512. [Google Scholar] [CrossRef]
- Wulfkuhle, J.D.; Yau, C.; Wolf, D.M.; Vis, D.J.; Gallagher, R.I.; Brown-Swigart, L.; Hirst, G.; Voest, E.E.; DeMichele, A.; Hylton, N.; et al. Evaluation of the HER/PI3K/AKT Family Signaling Network as a Predictive Biomarker of Pathologic Complete Response for Patients with Breast Cancer Treated with Neratinib in the I-SPY 2 TRIAL. JCO Precis. Oncol. 2018, 2, 1–20. [Google Scholar] [CrossRef]
- Wulfkuhle, J.D.; Yau, C.; Wolf, D.M.; Gallagher, R.I.; Deng, J.; Brown Swigart, L.; Hirst, G.; Liu, M.C.; Park, J.W.; Esserman, L.; et al. Protein Activation Mapping and Exploratory Predictive Markers for PCR in Triple-Negative Breast Cancer Patients Treated with Neratinib in the I-SPY 2 TRIAL. J. Clin. Oncol. 2015, 33, 1085. [Google Scholar] [CrossRef]
- Schettini, F.; Chic, N.; Brasó-Maristany, F.; Paré, L.; Pascual, T.; Conte, B.; Martínez-Sáez, O.; Adamo, B.; Vidal, M.; Barnadas, E.; et al. Clinical, Pathological, and PAM50 Gene Expression Features of HER2-Low Breast Cancer. NPJ Breast Cancer 2021, 7, 1. [Google Scholar] [CrossRef]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef]
- Earl, H.M.; Hiller, L.; Dunn, J.A.; Blenkinsop, C.; Grybowicz, L.; Vallier, A.-L.; Abraham, J.; Thomas, J.; Provenzano, E.; Hughes-Davies, L.; et al. Efficacy of Neoadjuvant Bevacizumab Added to Docetaxel Followed by Fluorouracil, Epirubicin, and Cyclophosphamide, for Women with HER2-Negative Early Breast Cancer (ARTemis): An Open-Label, Randomised, Phase 3 Trial. Lancet Oncol. 2015, 16, 656–666. [Google Scholar] [CrossRef]
- Earl, H.M.; Hiller, L.; Dunn, J.A.; Blenkinsop, C.; Grybowicz, L.; Vallier, A.-L.; Gounaris, I.; Abraham, J.E.; Hughes-Davies, L.; McAdam, K.; et al. Disease-Free and Overall Survival at 3.5 Years for Neoadjuvant Bevacizumab Added to Docetaxel Followed by Fluorouracil, Epirubicin and Cyclophosphamide, for Women with HER2 Negative Early Breast Cancer: ARTemis Trial. Ann. Oncol. 2017, 28, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Cameron, D.; Brown, J.; Dent, R.; Jackisch, C.; Mackey, J.; Pivot, X.; Steger, G.G.; Suter, T.M.; Toi, M.; Parmar, M.; et al. Adjuvant Bevacizumab-Containing Therapy in Triple-Negative Breast Cancer (BEATRICE): Primary Results of a Randomised, Phase 3 Trial. Lancet Oncol. 2013, 14, 933–942. [Google Scholar] [CrossRef]
- Colavito, S.A. AXL as a Target in Breast Cancer Therapy. J. Oncol. 2020, 2020, 5291952. [Google Scholar] [CrossRef]
- Ebrahimi, N.; Kharazmi, K.; Ghanaatian, M.; Miraghel, S.A.; Amiri, Y.; Seyedebrahimi, S.S.; Mobarak, H.; Yazdani, E.; Parkhideh, S.; Hamblin, M.R.; et al. Role of the Wnt and GTPase Pathways in Breast Cancer Tumorigenesis and Treatment. Cytokine Growth Factor Rev. 2022, 67, 11–24. [Google Scholar] [CrossRef]
- Campagna, R.; Pozzi, V.; Giorgini, S.; Morichetti, D.; Goteri, G.; Sartini, D.; Serritelli, E.N.; Emanuelli, M. Paraoxonase-2 Is Upregulated in Triple Negative Breast Cancer and Contributes to Tumor Progression and Chemoresistance. Hum. Cell 2023, 36, 1108–1119. [Google Scholar] [CrossRef]
- Spanheimer, P.M.; Carr, J.C.; Thomas, A.; Sugg, S.L.; Scott-Conner, C.E.; Liao, J.; Weigel, R.J. The Response to Neoadjuvant Chemotherapy Predicts Clinical Outcome and Increases Breast Conservation in Advanced Breast Cancer. Am. J. Surg. 2013, 206, 2–7. [Google Scholar] [CrossRef][Green Version]
- Golshan, M.; Loibl, S.; Wong, S.M.; Houber, J.B.; O’Shaughnessy, J.; Rugo, H.S.; Wolmark, N.; McKee, M.D.; Maag, D.; Sul-livan, D.M.; et al. Breast Conservation after Neoadjuvant Chemotherapy for Triple-Negative Breast Cancer. JAMA Surg. 2020, 155, e195410. [Google Scholar] [CrossRef]
- Donker, M.; van Tienhoven, G.; Straver, M.E.; Meijnen, P.; van de Velde, C.J.H.; Mansel, R.E.; Cataliotti, L.; Westenberg, A.H.; Klinkenbijl, J.H.G.; Orzalesi, L.; et al. Radiotherapy or Surgery of the Axilla after a Positive Sentinel Node in Breast Cancer (EORTC 10981-22023 AMAROS): A Randomised, Multicentre, Open-Label, Phase 3 Non-Inferiority Trial. Lancet Oncol. 2014, 15, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Fleissig, A.; Fallowfield, L.J.; Langridge, C.I.; Johnson, L.; Newcombe, R.G.; Dixon, J.M.; Kissin, M.; Mansel, R.E. Post-Operative Arm Morbidity and Quality of Life. Results of the ALMANAC Randomised Trial Comparing Sentinel Node Biopsy with Standard Axillary Treatment in the Management of Patients with Early Breast Cancer. Breast Cancer Res. Treat. 2006, 95, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Boughey, J.C.; Suman, V.J.; Mittendorf, E.A.; Ahrendt, G.M.; Wilke, L.G.; Taback, B.; Leitch, A.M.; Kuerer, H.M.; Bowling, M.; Flippo-Morton, T.S.; et al. Sentinel Lymph Node Surgery after Neoadjuvant Chemotherapy in Patients with Node-Positive Breast Cancer: The ACOSOG Z1071 (Alliance) Clinical Trial. JAMA 2013, 310, 1455–1461. [Google Scholar] [CrossRef]
- Kuehn, T.; Bauerfeind, I.; Fehm, T.; Fleige, B.; Hausschild, M.; Helms, G.; Lebeau, A.; Liedtke, C.; von Minckwitz, G.; Nekljudova, V.; et al. Sentinel-Lymph-Node Biopsy in Patients with Breast Cancer before and after Neoadjuvant Chemotherapy (SENTINA): A Prospective, Multicentre Cohort Study. Lancet Oncol. 2013, 14, 609–618. [Google Scholar] [CrossRef]
- Caudle, A.S.; Yang, W.T.; Krishnamurthy, S.; Mittendorf, E.A.; Black, D.M.; Gilcrease, M.Z.; Bedrosian, I.; Hobbs, B.P.; DeSnyder, S.M.; Hwang, R.F.; et al. Improved Axillary Evaluation Following Neoadjuvant Therapy for Patients With Node-Positive Breast Cancer Using Selective Evaluation of Clipped Nodes: Implementation of Targeted Axillary Dissection. J. Clin. Oncol. 2016, 34, 1072–1078. [Google Scholar] [CrossRef]
- Gallagher, K.K.; Iles, K.; Kuzmiak, C.; Louie, R.; McGuire, K.P.; Ollila, D.W. Prospective Evaluation of Radar-Localized Re-flector-Directed Targeted Axillary Dissection in Node-Positive Breast Cancer Patients after Neoadjuvant Systemic Therapy. J. Am. Coll. Surg. 2022, 234, 538–545. [Google Scholar] [CrossRef]
- Barrio, A.V.; Montagna, G.; Mamtani, A.; Sevilimedu, V.; Edelweiss, M.; Capko, D.; Cody, H.S.; El-Tamer, M.; Gemignani, M.L.; Heerdt, A.; et al. Nodal Recurrence in Patients with Node-Positive Breast Cancer Treated with Sentinel Node Biopsy Alone after Neoadjuvant Chemotherapy-A Rare Event. JAMA Oncol. 2021, 7, 1851–1855. [Google Scholar] [CrossRef]
- Giuliano, A.E.; Ballman, K.V.; McCall, L.; Beitsch, P.D.; Brennan, M.B.; Kelemen, P.R.; Ollila, D.W.; Hansen, N.M.; Whitworth, P.W.; Blumencranz, P.W.; et al. Effect of Axillary Dissection vs No Axillary Dissection on 10-Year Overall Survival Among Women with Invasive Breast Cancer and Sentinel Node Metastasis. JAMA 2017, 318, 918–926. [Google Scholar] [CrossRef]
- NSABP Foundation Inc. A Randomized Phase III Clinical Trial Evaluating Post-Mastectomy Chestwall and Regional Nodal XRT and Post-Lumpectomy Regional Nodal XRT in Patients With Positive Axillary Nodes Before Neoadjuvant Chemotherapy Who Convert to Pathologically Negative Axillary Nodes after Neoadjuvant Chemotherapy. 2022. Available online: https://www.clinicaltrials.gov/study/NCT01872975?cond=Phase%20III%20Clinical%20Trial%20Evaluating%20Post-Mastectomy%20Chestwall%20and%20Regional%20Nodal%20XRT%20&rank=1 (accessed on 3 June 2023).
- Kuerer, H.M.; Smith, B.D.; Krishnamurthy, S.; Yang, W.T.; Valero, V.; Shen, Y.; Lin, H.; Lucci, A.; Boughey, J.C.; White, R.L.; et al. Eliminating Breast Surgery for Invasive Breast Cancer in Exceptional Responders to Neoadjuvant Systemic Therapy: A Mul-ticentre, Single-Arm, Phase 2 Trial. Lancet Oncol. 2022, 23, 1517–1524. [Google Scholar] [CrossRef]
- Yin, L.; Duan, J.-J.; Bian, X.-W.; Yu, S. Triple-Negative Breast Cancer Molecular Subtyping and Treatment Progress. Breast Cancer Res. 2020, 22, 61. [Google Scholar] [CrossRef]
- Dagogo-Jack, I.; Shaw, A.T. Tumour Heterogeneity and Resistance to Cancer Therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef]
- Yu, C.; Mannan, A.M.; Yvone, G.M.; Ross, K.N.; Zhang, Y.-L.; Marton, M.A.; Taylor, B.R.; Crenshaw, A.; Gould, J.Z.; Tamayo, P.; et al. High-Throughput Identification of Genotype-Specific Cancer Vulnerabilities in Mixtures of Barcoded Tumor Cell Lines. Nat. Biotechnol. 2016, 34, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Tsherniak, A.; Vazquez, F.; Montgomery, P.G.; Weir, B.A.; Kryukov, G.; Cowley, G.S.; Gill, S.; Harrington, W.F.; Pantel, S.; Krill-Burger, J.M.; et al. Defining a Cancer Dependency Map. Cell 2017, 170, 564–576. [Google Scholar] [CrossRef]
- Shu, S.; Wu, H.-J.; Ge, J.Y.; Zeid, R.; Harris, I.S.; Jovanović, B.; Murphy, K.; Wang, B.; Qiu, X.; Endress, J.E.; et al. Synthetic Lethal and Resistance Interactions with BET Bromodomain Inhibitors in Triple-Negative Breast Cancer. Mol. Cell 2020, 78, 1096–1113.e8. [Google Scholar] [CrossRef]
- Rashid, N.S.; Hairr, N.S.; Murray, G.; Olex, A.L.; Leftwich, T.J.; Grible, J.M.; Reed, J.; Dozmorov, M.G.; Harrell, J.C. Identi-fication of Nuclear Export Inhibitor-Based Combination Therapies in Preclinical Models of Triple-Negative Breast Cancer. Transl. Oncol. 2021, 14, 101235. [Google Scholar] [CrossRef]
- Gambardella, G.; Viscido, G.; Tumaini, B.; Isacchi, A.; Bosotti, R.; di Bernardo, D. A Single-Cell Analysis of Breast Cancer Cell Lines to Study Tumour Heterogeneity and Drug Response. Nat. Commun. 2022, 13, 1714. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Z.; Al-Eryani, G.; Roden, D.L.; Junankar, S.; Harvey, K.; Andersson, A.; Thennavan, A.; Wang, C.; Torpy, J.R.; Bar-tonicek, N.; et al. A Single-Cell and Spatially Resolved Atlas of Human Breast Cancers. Nat. Genet. 2021, 53, 1334–1347. [Google Scholar] [CrossRef] [PubMed]
- Paik, S.; Tang, G.; Shak, S.; Kim, C.; Baker, J.; Kim, W.; Cronin, M.; Baehner, F.L.; Watson, D.; Bryant, J.; et al. Gene Expression and Benefit of Chemotherapy in Women with Node-Negative, Estrogen Receptor-Positive Breast Cancer. J. Clin. Oncol. 2006, 24, 3726–3734. [Google Scholar] [CrossRef]
- Paik, S.; Shak, S.; Tang, G.; Kim, C.; Baker, J.; Cronin, M.; Baehner, F.L.; Walker, M.G.; Watson, D.; Park, T.; et al. A Multigene Assay to Predict Recurrence of Tamoxifen-Treated, Node-Negative Breast Cancer. N. Engl. J. Med. 2004, 351, 2817–2826. [Google Scholar] [CrossRef]
- Parker, J.S.; Mullins, M.; Cheang, M.C.U.; Leung, S.; Voduc, D.; Vickery, T.; Davies, S.; Fauron, C.; He, X.; Hu, Z.; et al. Su-pervised Risk Predictor of Breast Cancer Based on Intrinsic Subtypes. J. Clin. Oncol. 2009, 27, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, H.; Mo, H.; Hu, X.; Gao, R.; Zhao, Y.; Liu, B.; Niu, L.; Sun, X.; Yu, X.; et al. Single-Cell Analyses Reveal Key Immune Cell Subsets Associated with Response to PD-L1 Blockade in Triple-Negative Breast Cancer. Cancer Cell 2021, 39, 1578–1593.e8. [Google Scholar] [CrossRef] [PubMed]
| Lehmann et al. [9] Classification n = 2347 | |
|---|---|
| Type | Main Molecular Characteristics |
| Basal-like 1 (BL1) | Enriched in: Cell cycle and proliferation genes (AURKA, AURKB, CENPA, BUB1, TTK, CCNA2, PRC1, MYC, NRAS, PLK1, BIRC5) DNA damage response genes (CHEK1, FANCA, FANCG, RAD54BP, RAD51, NBN, EXO1, MSH2, MCM10, RAD21, MDC1) High Ki-67 mRNA expression |
| Basal-like 2 (BL2) | Enriched in: Growth factor signaling genes (EGF, NGF, MET, Wnt/β-catenin, IGF1R pathways) Growth factor receptor genes (EGFR, MET, EPHA2) Myoepithelial markers (TP63 and MME or CD10) |
| Immunomodulatory (IM) | Enriched for gene ontologies in immune cell processes, including: Immune cell signaling (TH1/TH2, NK cell, BCR signaling, DC, T-cell receptor signaling pathway) Cytokine signaling (cytokine, IL-12, IL-7 pathway) Immune signal transduction (NFKB, TNF, JAK/STAT pathway) |
| Mesenchymal (M) | Enriched in: Cell motility (regulation of actin by Rho) ECM receptor interaction Cell differentiation pathways (Wnt/β-catenin, ALK, TGF-β signaling) |
| Mesenchymal stem-like (MSL) | Similar to M type. Also enriched in: Angiogenesis genes (VEGFR2, TEK, TIE1, EPAS1) Growth factor signaling pathways (including adipocytokine signaling, EGFR, PDGF, G-protein coupled receptor, ERK1/2) |
| Luminal androgen receptor (LAR) | Enriched in: Signaling pathway of androgen receptor (including FASN, APOD, CLDN8, DHCR24, ALCAM, FKBP5, PIP, SPDEF) |
| Trial | Design | Treatment | pCR (Carboplatin vs. No Carboplatin) | Survival Outcomes |
|---|---|---|---|---|
| CALGB-40603 (Alliance) [8,24] | Randomized phase II trial n = 443 | Weekly paclitaxel plus carboplatin followed by ddAC w/wo bevacizumab vs. wo carboplatin | 60% vs. 44% (p = 0.0018) | DFS: HR 0.94; 95% CI 0.67 to 1.32, p = 0.72 OS: HR 1.12; 95% CI 0.77 to 1.61, p = 0.56 |
| GeparSixto [25,28] | Randomized phase II trial n = 315 | Paclitaxel plus non-pegylated liposomal doxorubicin plus bevacizumab w/wo carboplatin | 53% vs. 37% (p = 0.005) | DFS: HR 0.56; 95% CI 0.34 to 0.93; p = 0.022 OS: HR 0.55; 95% CI 0.27 to 1.14, p = 0.10 |
| BrighTNess [23,26] | Randomized phase III trial n = 634 | Paclitaxel vs. paclitaxel plus veliparib plus carboplatin vs. paclitaxel plus carboplatin | 31% vs. 53% (p < 0.0001) 53% vs. 58% (p = 0.36) | EFS: 79.3% vs. 68.5%; HR 0.57, 95% CI 0.36 to 0.91; p = 0.018 OS: HR 0.63; 95% CI 0.33 to 1.21, p = 0.17 |
| GEICAM/2006–03 [29] | Randomized phase II trial n = 94 | Epirubicin plus cyclophosphamide followed by docetaxel w/wo carboplatin | 30% vs. 35% (p = 0.61) | Not assessed |
| Ando et al. [30] | Randomized phase II trial n = 181 | Paclitaxel w/wo carboplatin followed by cyclophosphamide plus epirubicin and fluorouracil | 61.2% vs. 26.3% (p = 0.003) in TNBC | Not assessed |
| Neoadjuvant Trials | ||||
|---|---|---|---|---|
| Trial | Design | Disease Setting | Treatment | Relevant Endpoint |
| Completed trials | ||||
| Impassion031 [47] | Phase III n = 455 | Neoadjuvant treatment of stage II–III TNBC | Nab-paclitaxel followed by doxorubicin/cyclophosphamide w/wo atezolizumab | pCR: 58% vs. 41% (rate difference 17%, 95% CI 6 to 27; p = 0.0044) |
| NeoTrip [48] | Phase III n = 280 | Neoadjuvant treatment of stage II–III TNBC | Carboplatin plus nab-paclitaxel with or without atezolizumab followed by adjuvant anthracycline | pCR: 48.6% vs. 44.4% (OR 1.18, 95% CI 0.74 to 1.89; p = 0.48) EFS pending |
| GeparNuevo [49] | Phase II n = 174 | Neoadjuvant treatment of cT1b-cT4a-d TNBC | Durvalumab or placebo plus epirubicin/cyclophosphamide | pCR: 53.4% vs. 44.2% (OR 1.45; 95% CI 0.80 to 2.63, p = 0.22) 3y DFS: 85.6% vs. 77.2%, p = 0.036 OS: 95.2% vs. 83.5%, p = 0.006 |
| I-SPY2 [50] | Adaptive Phase II n = 250 (29 with TNBC) | Neoadjuvant treatment of high-risk stage II–III breast cancer, including TNBC | Taxane and anthracycline w/wo pembrolizumab | pCR: 60% vs. 22% in the TNBC cohort |
| Ongoing trials | ||||
| GeparDouze [51] | Phase III n = 1520 | Neoadjuvant plus adjuvant treatment of high-risk (cT2-3N0 or cT1c-3N+) TNBC | NAT with atezolizumab vs. placebo plus paclitaxel/carboplatin followed by AC, six months of postoperative atezolizumab or placebo | EFS, pCR pending |
| Adjuvant trials | ||||
| IMpassion030 [52] | Phase III n = 2300 | Postoperative treatment of operable stage II–III TNBC | Atezolizumab vs. placebo plus anthracycline/taxane | iDFS pending |
| SWOG S1418 [53] | Phase III n = 1155 | Postoperative treatment of stage II–III TNBC with residual disease (>1 cm) or lymph node-positive disease (ypN+ including micrometastatic disease) after NAT | 12 months of pembrolizumab vs. observation postoperatively | iDFS pending |
| MIRINAE [54] | Phase II n = 284 | Postoperative treatment of TNBC with residual disease (>1 cm) or macroscopic positive lymph nodes (ypN+) after NAT | Capecitabine w/wo atezolizumab | 5y iDFS pending |
| Trial | Trial Characteristics | Setting | Treatment | Result |
|---|---|---|---|---|
| NEOTALA [69] | Phase II, non-randomized, single arm trial | Early-stage gBRCA1/2-mutated HER2- breast cancer | 24 weeks of neoadjuvant talazoparib monotherapy 1 mg daily followed by surgery | pCR was 49% |
| GeparOLA [70] | Phase II, randomized, open-label trial | Early-stage HER2- breast cancer with either gBRCA1/2 mutation or high HRD score | Neoadjuvant Paclitaxel plus olaparib versus paclitaxel plus carboplatin, both followed by epirubicin/cyclophosphamide | pCR was 55.1% vs. 48.6% In TNBC subgroup, pCR was 56% vs. 59.3% |
| I-SPY2 [71] | Phase II, adaptive trial | Stage II/III HER2- breast cancer | Neoadjuvant Durvalumab, olaparib, and weekly paclitaxel vs. chemotherapy alone | pCR in the TNBC group higher (27–47%) |
| I-SPY2 [68] | Phase II, adaptive trial | >2.5 cm stage II/III HER2- breast cancer | Neoadjuvant veliparib with carboplatin plus taxol vs. taxol | pCR rate was higher in the TNBC group at 51% vs. 26%. |
| BrighTNess [23] | Phase III, randomized, double-blind | Stage II/III TNBC | One of three: Taxol plus carboplatin (AUC6) plus veliparib vs. taxol plus carboplatin (AUC6) plus veliparib placebo vs. taxol plus carboplatin placebo plus veliparib placebo | pCR was 53% vs. 58% vs. 31% |
| Trial | Phase | Setting | Treatment | Primary Endpoint | |
|---|---|---|---|---|---|
| EGFR | NCT05177796 | II | Neoadjuvant inflammatory BC | Panitumumab plus pembrolizumab plus chemotherapy | pCR |
| FGFR/VEGFR/PDGFR | NCT04427293 | I | Neoadjuvant | Lenvatinib plus pembrolizumab | Infiltration of CD8+ TILs |
| NeoATCT [NCT04914390] | II | Neoadjuvant | Tislelizumab plus Anlotinib plus anthracycline/nab-paclitaxel | pCR | |
| JAK2/STAT3 | NCT02876302 | II | Neoadjuvant | Ruxolitinib plus paclitaxel followed by AC | Effect on pStat3+ Expression |
| NCT02041429 | II | Neoadjuvant inflammatory BC | Ruxolitinib plus paclitaxel followed by AC | Maximum Tolerated Dose | |
| TROP2 | NeoSTAR [NCT04230109] | II | Neoadjuvant | Sacituzumab govitecan | pCR rate |
| SASCIA [NCT04595565] | III | Adjuvant, HER2- BC with residual disease | Sacituzumab govitecan | iDFS | |
| ASPRIA [NCT04434040] | II | Adjuvant TNBC with residual disease | Atezolizumab and Sacituzumab govitecan | Undetectable circulating cfDNA | |
| ASCENT-05 [NCT05633654] | III | Adjuvant TNBC with residual disease | Sacituzumab govitecan plus pembrolizumab vs. pembrolizumab or pembrolizumab/capecitabine | iDFS | |
| TROPION-Breast03 [NCT05629585] | III | Adjuvant TNBC with residual disease | Datopotamab plus durvalumab vs. Capecitabine w/wo pembrolizumab | iDFS | |
| CDK4/6 | CAREGIVER [NCT05067530] | II | Neoadjuvant | Palbociclib vs. paclitaxel vs. palbociclib plus paclitaxel vs. carboplatin vs. carboplatin plus paclitaxel | Early metabolic response |
| NCT03979508 | II | Neoadjuvant | NAT, then abemaciclib, then surgery | Change from CD8/FOXP3 ratio <1.6 to >1.6 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhardwaj, P.V.; Wang, Y.; Brunk, E.; Spanheimer, P.M.; Abdou, Y.G. Advances in the Management of Early-Stage Triple-Negative Breast Cancer. Int. J. Mol. Sci. 2023, 24, 12478. https://doi.org/10.3390/ijms241512478
Bhardwaj PV, Wang Y, Brunk E, Spanheimer PM, Abdou YG. Advances in the Management of Early-Stage Triple-Negative Breast Cancer. International Journal of Molecular Sciences. 2023; 24(15):12478. https://doi.org/10.3390/ijms241512478
Chicago/Turabian StyleBhardwaj, Prarthna V., Yue Wang, Elizabeth Brunk, Philip M. Spanheimer, and Yara G. Abdou. 2023. "Advances in the Management of Early-Stage Triple-Negative Breast Cancer" International Journal of Molecular Sciences 24, no. 15: 12478. https://doi.org/10.3390/ijms241512478
APA StyleBhardwaj, P. V., Wang, Y., Brunk, E., Spanheimer, P. M., & Abdou, Y. G. (2023). Advances in the Management of Early-Stage Triple-Negative Breast Cancer. International Journal of Molecular Sciences, 24(15), 12478. https://doi.org/10.3390/ijms241512478






