Matrix Metalloproteinase-9 Expression Is Associated with the Absence of Response to Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Cohort Study Design
2.2. Tissue Microarray Construction and IHC & ISH Techniques
2.3. Digital Image Analysis
2.4. Statistical Analysis
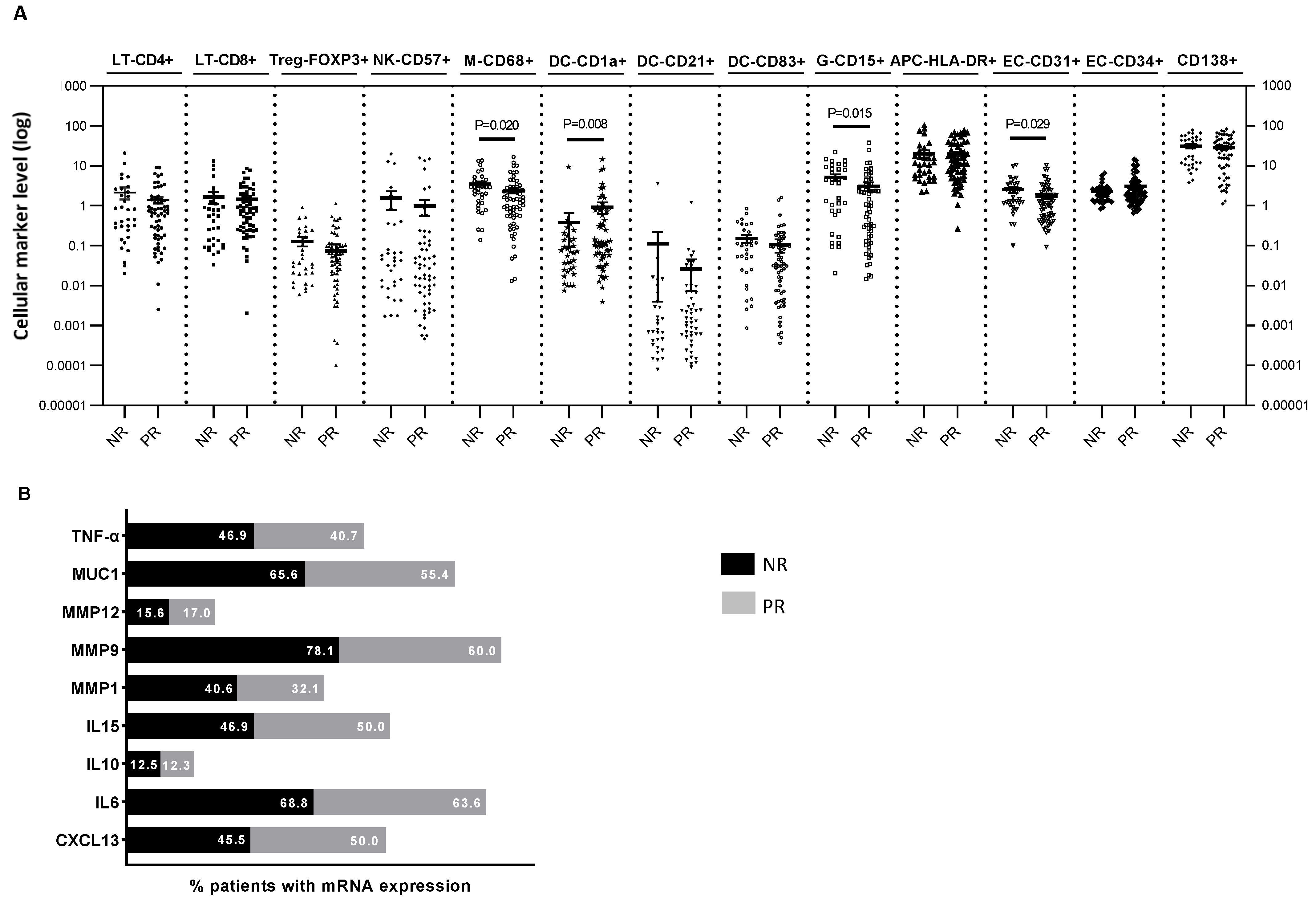
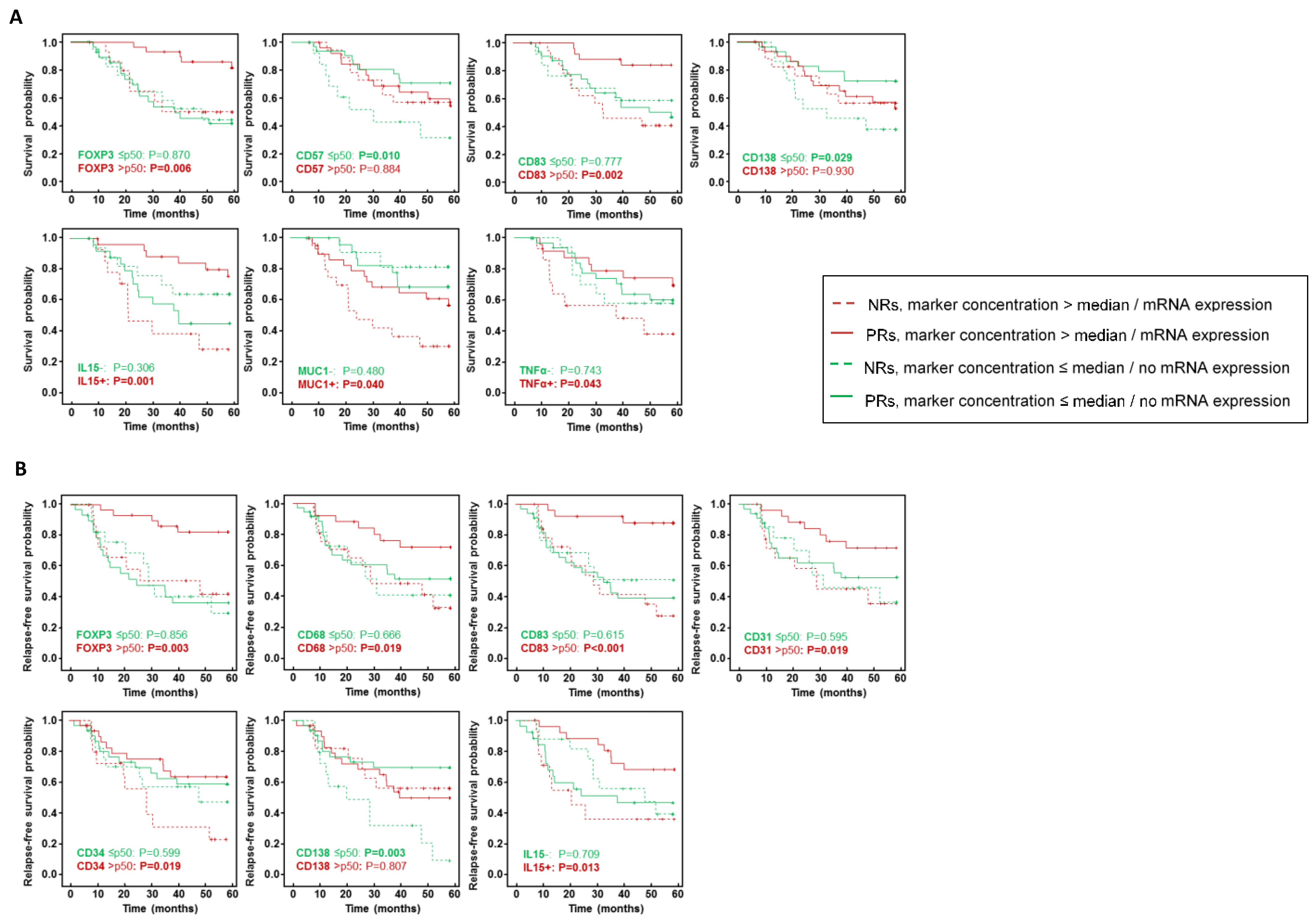
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yin, L.; Duan, J.J.; Bian, X.W.; Yu, S.C. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. BCR 2020, 22, 61. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.U.; Vanderplas, A.; Hughes, M.E.; Theriault, R.L.; Edge, S.B.; Wong, Y.N.; Blayney, D.W.; Niland, J.C.; Winer, E.P.; Weeks, J.C. Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer 2012, 118, 5463–5472. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.L.; Cardoso Nunes, N.C.; Izetti, P.; de Mesquita, G.G.; de Melo, A.C. Triple negative breast cancer: A thorough review of biomarkers. Crit. Rev. Oncol./Hematol. 2020, 145, 102855. [Google Scholar] [CrossRef] [PubMed]
- Lebert, J.M.; Lester, R.; Powell, E.; Seal, M.; McCarthy, J. Advances in the systemic treatment of triple-negative breast cancer. Curr. Oncol. 2018, 25, S142–S150. [Google Scholar] [CrossRef]
- Harbeck, N.; Gluz, O. Neoadjuvant therapy for triple negative and HER2-positive early breast cancer. Breast 2017, 34 (Suppl. S1), S99–S103. [Google Scholar] [CrossRef]
- Huang, M.; O’Shaughnessy, J.; Zhao, J.; Haiderali, A.; Cortes, J.; Ramsey, S.D.; Briggs, A.; Hu, P.; Karantza, V.; Aktan, G.; et al. Association of Pathologic Complete Response with Long-Term Survival Outcomes in Triple-Negative Breast Cancer: A Meta-Analysis. Cancer Res. 2020, 80, 5427–5434. [Google Scholar] [CrossRef]
- Hamy, A.S.; Darrigues, L.; Laas, E.; De Croze, D.; Topciu, L.; Lam, G.T.; Evrevin, C.; Rozette, S.; Laot, L.; Lerebours, F.; et al. Prognostic value of the Residual Cancer Burden index according to breast cancer subtype: Validation on a cohort of BC patients treated by neoadjuvant chemotherapy. PLoS ONE 2020, 15, e0234191. [Google Scholar] [CrossRef]
- Hanahan, D.; Coussens, L.M. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef]
- Uruena, C.; Lasso, P.; Bernal-Estevez, D.; Rubio, D.; Salazar, A.J.; Olaya, M.; Barreto, A.; Tawil, M.; Torregrosa, L.; Fiorentino, S. The breast cancer immune microenvironment is modified by neoadjuvant chemotherapy. Sci. Rep. 2022, 12, 7981. [Google Scholar] [CrossRef]
- Mao, Y.; Qu, Q.; Zhang, Y.; Liu, J.; Chen, X.; Shen, K. The value of tumor infiltrating lymphocytes (TILs) for predicting response to neoadjuvant chemotherapy in breast cancer: A systematic review and meta-analysis. PLoS ONE 2014, 9, e115103. [Google Scholar] [CrossRef]
- Derouane, F.; van Marcke, C.; Berliere, M.; Gerday, A.; Fellah, L.; Leconte, I.; Van Bockstal, M.R.; Galant, C.; Corbet, C.; Duhoux, F.P. Predictive Biomarkers of Response to Neoadjuvant Chemotherapy in Breast Cancer: Current and Future Perspectives for Precision Medicine. Cancers 2022, 14, 3876. [Google Scholar] [CrossRef]
- Lejeune, M.; Reverté, L.; Sauras, E.; Gallardo, N.; Bosch, R.; Roso, A.; Petit, A.; Peg, V.; Riu, F.; García-Fontgivell, J.; et al. Prognostic Implications of the Residual Tumor Microenvironment after Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer Patients without Pathological Complete Response. Cancers 2023, 15, 597. [Google Scholar] [CrossRef]
- Ogston, K.N.; Miller, I.D.; Payne, S.; Hutcheon, A.W.; Sarkar, T.K.; Smith, I.; Schofield, A.; Heys, S.D. A new histological grading system to assess response of breast cancers to primary chemotherapy: Prognostic significance and survival. Breast 2003, 12, 320–327. [Google Scholar] [CrossRef]
- Peintinger, F.; Sinn, B.; Hatzis, C.; Albarracin, C.; Downs-Kelly, E.; Morkowski, J.; Gould, R.; Symmans, W.F. Reproducibility of residual cancer burden for prognostic assessment of breast cancer after neoadjuvant chemotherapy. Mod. Pathol. 2015, 28, 913–920. [Google Scholar] [CrossRef]
- Lopez, C.; Callau, C.; Bosch, R.; Korzynska, A.; Jaen, J.; Garcia-Rojo, M.; Bueno, G.; Salvado, M.T.; Alvaro, T.; Onos, M.; et al. Development of automated quantification methodologies of immunohistochemical markers to determine patterns of immune response in breast cancer: A retrospective cohort study. BMJ Open 2014, 4, e005643. [Google Scholar] [CrossRef]
- Lejeune, M.; Plancoulaine, B.; Elie, N.; Bosch, R.; Fontoura, L.; de Villasante, I.; Korzynska, A.; Navarro, A.G.; Colon, E.S.; Lopez, C. How the variability between computer-assisted analysis procedures evaluating immune markers can influence patients’ outcome prediction. Histochem. Cell Biol. 2021, 156, 461–478. [Google Scholar] [CrossRef]
- Callau, C.; Lejeune, M.; Korzynska, A.; Garcia, M.; Bueno, G.; Bosch, R.; Jaen, J.; Orero, G.; Salvado, T.; Lopez, C. Evaluation of cytokeratin-19 in breast cancer tissue samples: A comparison of automatic and manual evaluations of scanned tissue microarray cylinders. Biomed. Eng. Online 2015, 14 (Suppl. S2), S2. [Google Scholar] [CrossRef]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef]
- van den Ende, N.S.; Nguyen, A.H.; Jager, A.; Kok, M.; Debets, R.; van Deurzen, C.H.M. Triple-Negative Breast Cancer and Predictive Markers of Response to Neoadjuvant Chemotherapy: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 2969. [Google Scholar] [CrossRef]
- Mehner, C.; Hockla, A.; Miller, E.; Ran, S.; Radisky, D.C.; Radisky, E.S. Tumor cell-produced matrix metalloproteinase 9 (MMP-9) drives malignant progression and metastasis of basal-like triple negative breast cancer. Oncotarget 2014, 5, 2736–2749. [Google Scholar] [CrossRef]
- Wang, R.X.; Chen, S.; Huang, L.; Shao, Z.M. Predictive and prognostic value of Matrix metalloproteinase (MMP)—9 in neoadjuvant chemotherapy for triple-negative breast cancer patients. BMC Cancer 2018, 18, 909. [Google Scholar] [CrossRef] [PubMed]
- Joseph, C.; Alsaleem, M.; Orah, N.; Narasimha, P.L.; Miligy, I.M.; Kurozumi, S.; Ellis, I.O.; Mongan, N.P.; Green, A.R.; Rakha, E.A. Elevated MMP9 expression in breast cancer is a predictor of shorter patient survival. Breast Cancer Res. Treat. 2020, 182, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, H. Prognostic values of tumoral MMP2 and MMP9 overexpression in breast cancer: A systematic review and meta-analysis. BMC Cancer 2021, 21, 149. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Tang, R.; Zhang, X.; Madushi, W.M.; Luo, D.; Dang, Y.; Li, Z.; Wei, K.; Chen, G. Overexpression of MMP Family Members Functions as Prognostic Biomarker for Breast Cancer Patients: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0135544. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.Q.; Zhang, K.J.; Wang, S.M.; Guo, L. YAP1/MMP7/CXCL16 axis affects efficacy of neoadjuvant chemotherapy via tumor environment immunosuppression in triple-negative breast cancer. Gland Surg. 2021, 10, 2799–2814. [Google Scholar] [CrossRef]
- Kaewkangsadan, V.; Verma, C.; Eremin, J.M.; Cowley, G.; Ilyas, M.; Satthaporn, S.; Eremin, O. The Differential Contribution of the Innate Immune System to a Good Pathological Response in the Breast and Axillary Lymph Nodes Induced by Neoadjuvant Chemotherapy in Women with Large and Locally Advanced Breast Cancers. J. Immunol. Res. 2017, 2017, 1049023. [Google Scholar] [CrossRef]
- Abdelrahman, A.E.; Rashed, H.E.; MostafaToam; Omar, A.; Abdelhamid, M.I.; Matar, I. Clinicopathological significance of the immunologic signature (PDL1, FOXP3+ Tregs, TILs) in early stage triple-negative breast cancer treated with neoadjuvant chemotherapy. Ann. Diagn. Pathol. 2021, 51, 151676. [Google Scholar] [CrossRef]
- Qiao, W.; Liu, H.; Guo, W.; Li, P.; Deng, M. Prognostic and clinical significance of syndecan-1 expression in breast cancer: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2019, 45, 1132–1137. [Google Scholar] [CrossRef]
- Julia, E.P.; Mordoh, J.; Levy, E.M. Cetuximab and IL-15 Promote NK and Dendritic Cell Activation In Vitro in Triple Negative Breast Cancer. Cells 2020, 9, 1573. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, H.; Pan, C.; He, G.; Cui, X.; Yu, X.; Zhang, X.; Wu, D.; Yang, J.; Wu, X.; et al. Integrated tumor genomic and immune microenvironment analysis identifies predictive biomarkers associated with the efficacy of neoadjuvant therapy for triple-negative breast cancer. Cancer Med. 2023, 12, 5846–5858. [Google Scholar] [CrossRef]

| Univariate Analysis | Multivariate Analysis | Multivariate Analysis after Bootstrapping | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Tumor diameter | 1.032 (1.009–1.056) | 0.006 | 1.041 (1.011–1.072) | 0.007 | 1.041 (1.011–1.072) | 0.003 |
| Node status Positive Negative | 11.177 (3.487–35.825) 1.0 | <0.001 | 10.073 (2.650–38.280) 1.0 | 0.001 | 10.073 (2.650–38.280) 1.0 | <0.001 |
| MMMP9 Presence Absence | 2.381 (0.879–6.451) 1.0 | 0.088 | 7.391 (1.818–30.052) 1.0 | 0.005 | 7.391 (1.818–30.052) 1.0 | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lejeune, M.; Reverté, L.; Gallardo, N.; Sauras, E.; Bosch, R.; Mata, D.; Roso, A.; Petit, A.; Peg, V.; Riu, F.; et al. Matrix Metalloproteinase-9 Expression Is Associated with the Absence of Response to Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer Patients. Int. J. Mol. Sci. 2023, 24, 11297. https://doi.org/10.3390/ijms241411297
Lejeune M, Reverté L, Gallardo N, Sauras E, Bosch R, Mata D, Roso A, Petit A, Peg V, Riu F, et al. Matrix Metalloproteinase-9 Expression Is Associated with the Absence of Response to Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer Patients. International Journal of Molecular Sciences. 2023; 24(14):11297. https://doi.org/10.3390/ijms241411297
Chicago/Turabian StyleLejeune, Marylène, Laia Reverté, Noèlia Gallardo, Esther Sauras, Ramon Bosch, Daniel Mata, Albert Roso, Anna Petit, Vicente Peg, Francisco Riu, and et al. 2023. "Matrix Metalloproteinase-9 Expression Is Associated with the Absence of Response to Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer Patients" International Journal of Molecular Sciences 24, no. 14: 11297. https://doi.org/10.3390/ijms241411297
APA StyleLejeune, M., Reverté, L., Gallardo, N., Sauras, E., Bosch, R., Mata, D., Roso, A., Petit, A., Peg, V., Riu, F., García-Fontgivell, J., Relea, F., Vieites, B., de la Cruz-Merino, L., Arenas, M., Rodriguez, V., Galera, J., Korzynska, A., Plancoulaine, B., ... López, C. (2023). Matrix Metalloproteinase-9 Expression Is Associated with the Absence of Response to Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer Patients. International Journal of Molecular Sciences, 24(14), 11297. https://doi.org/10.3390/ijms241411297







