A Potential Association between Ribonuclease 1 Dynamics in the Blood and the Outcome in COVID-19 Patients
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
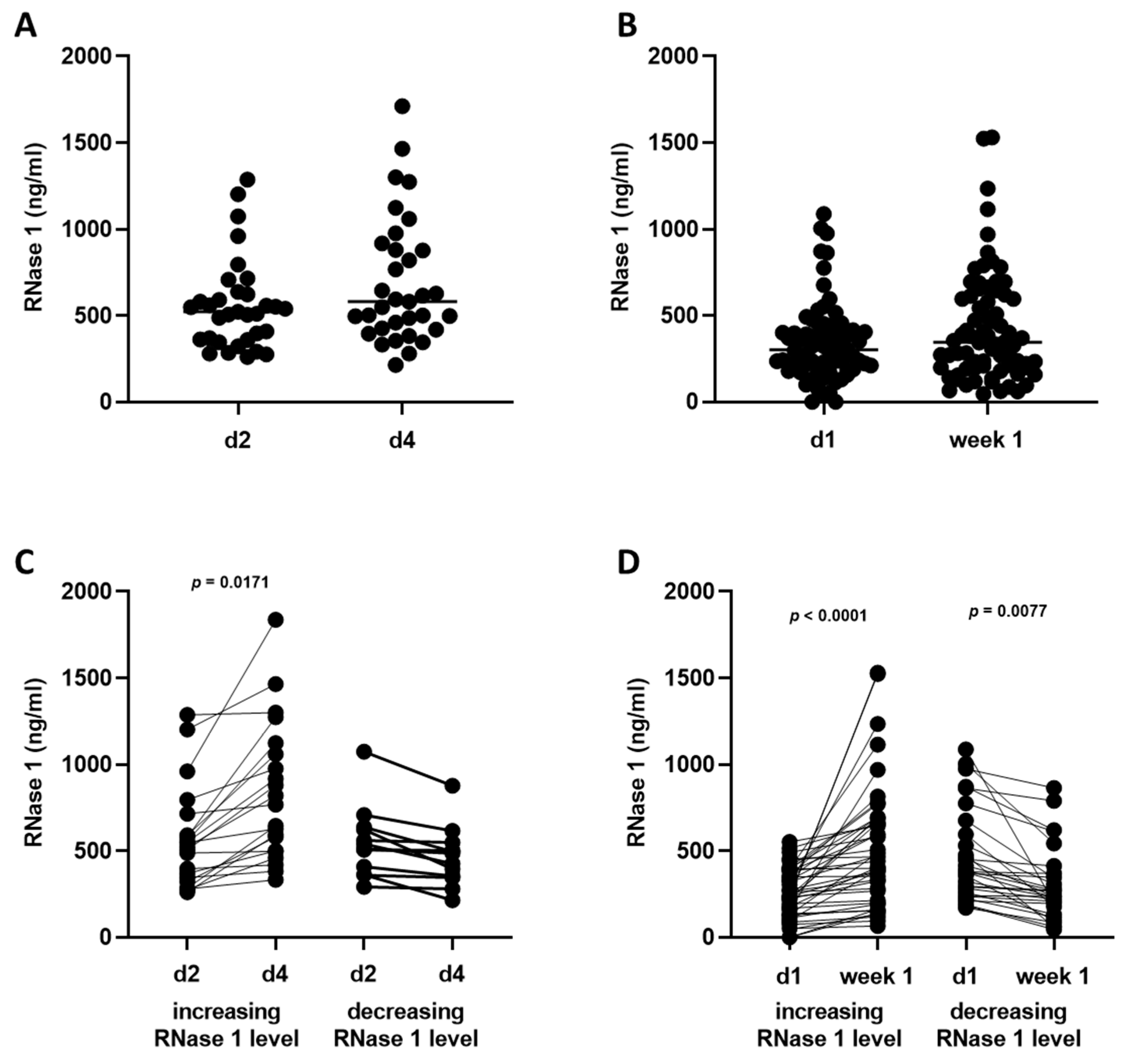
2.2. RNase 1 Serum Levels
2.3. Correlation of RNase 1 Serum Levels with 28-Day Mortality
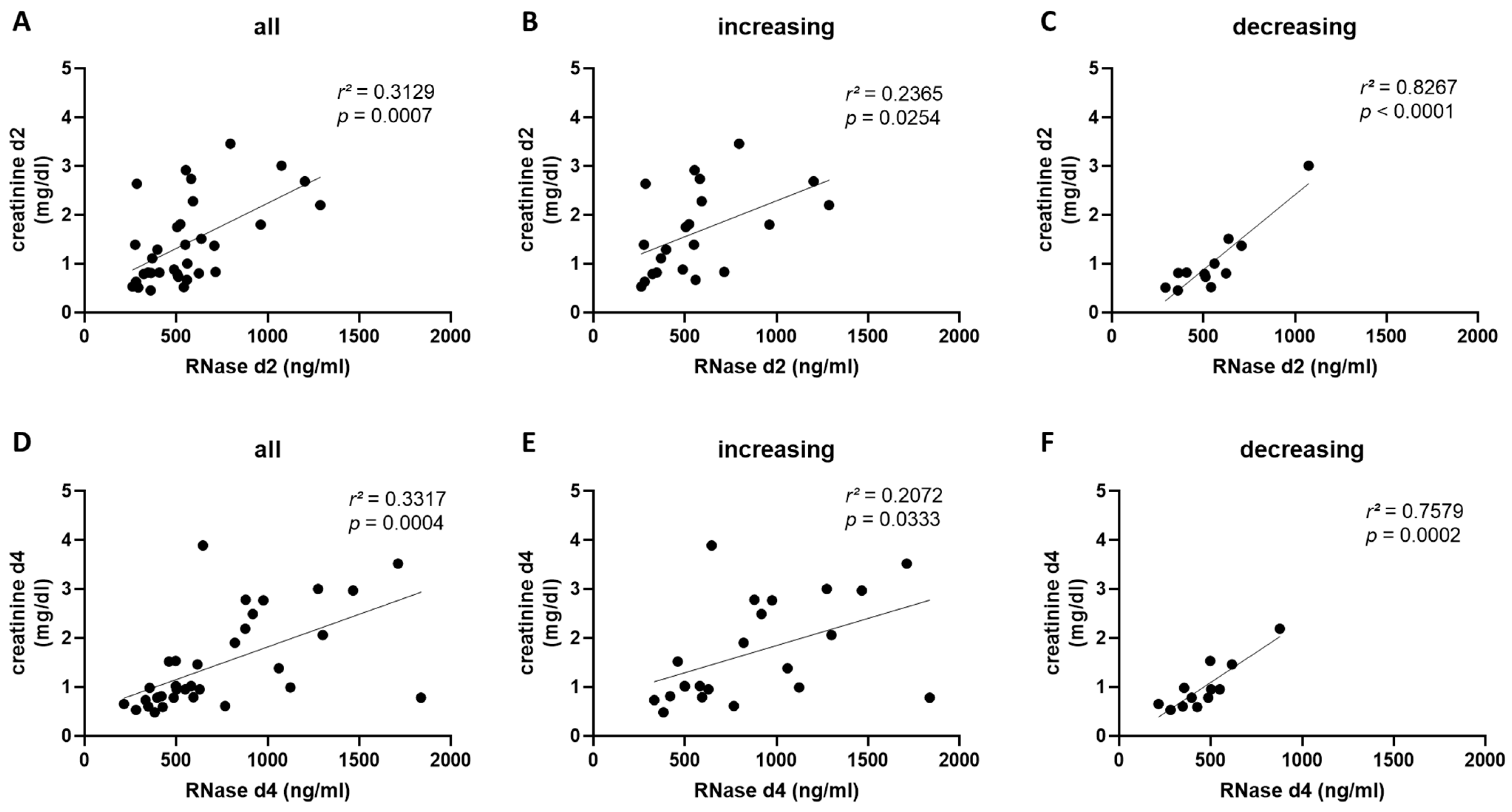
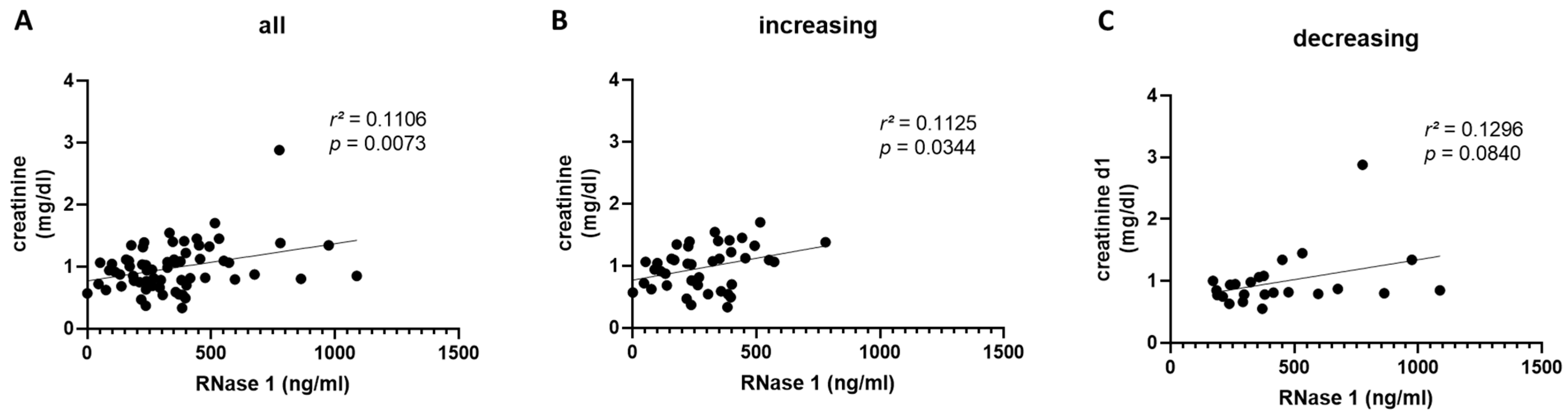
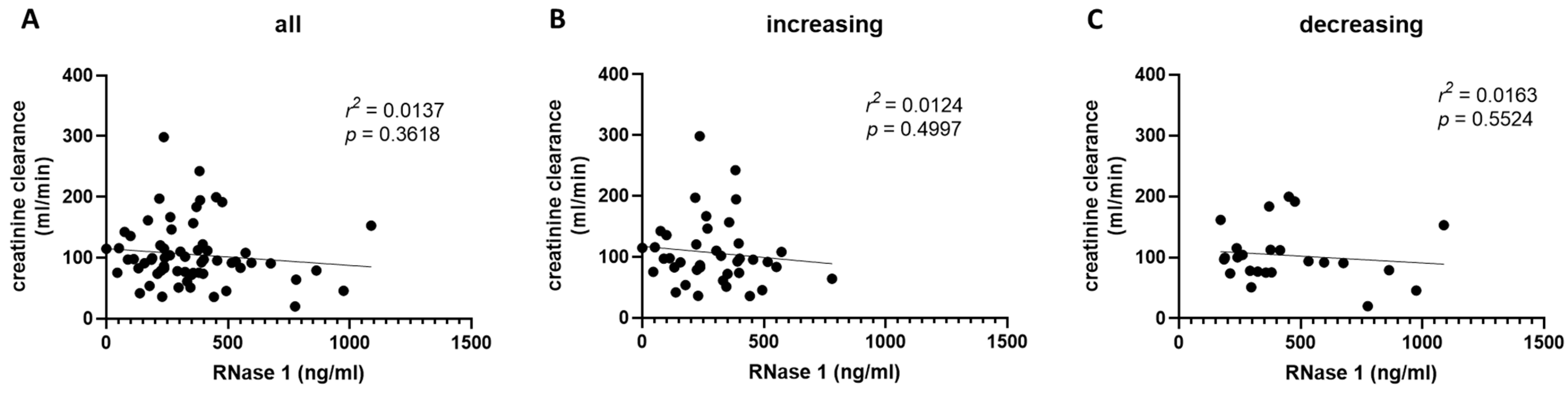
2.4. RNase 1 Serum Levels Correlate with Creatinine Serum Levels and Creatinine Clearance in COVID-19 Patients
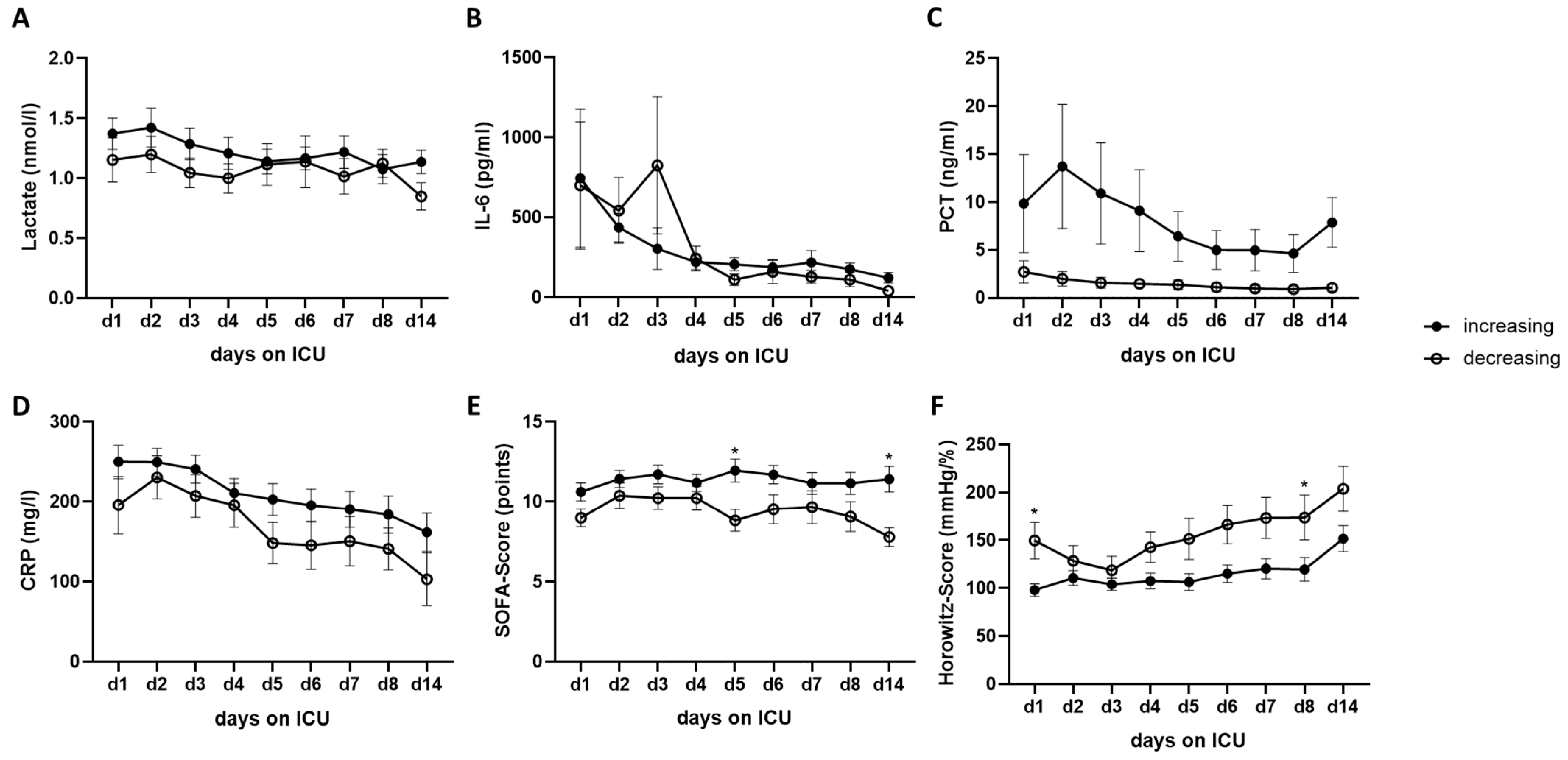
2.5. Time Course of Biomarkers and Scores over 14 Days and the Correlations of RNase 1 Levels with Clinical Parameters of ICU Patients with SARS-CoV-2 Infection
3. Discussion
4. Materials and Methods
4.1. Study Design/Population
4.2. Serum Sampling
4.3. Human Enzyme-Linked Immunosorbent Assay
4.4. Statistics
5. Limitation/Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organisation. WHO Coronavirus Disease (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 12 October 2021).
- Hasöksüz, M.; Kiliç, S.; Saraç, F. Coronaviruses and SARS-CoV-2. Turk. J. Med. Sci. 2020, 50, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Lee-Huang, S.; Huang, P.L.; Sun, Y.; Huang, P.L.; Kung, H.F.; Blithe, D.L.; Chen, H.C. Lysozyme and RNases as anti-HIV components in beta-core preparations of human chorionic gonadotropin. Proc. Natl. Acad. Sci. USA 1999, 96, 2678–2681. [Google Scholar] [CrossRef]
- Rugeles, M.T.; Trubey, C.M.; Bedoya, V.I.; Pinto, L.A.; Oppenheim, J.J.; Rybak, S.M.; Shearer, G.M. Ribonuclease is partly responsible for the HIV-1 inhibitory effect activated by HLA alloantigen recognition. AIDS 2003, 17, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Koczera, P.; Martin, L.; Marx, G.; Schuerholz, T. The Ribonuclease A Superfamily in Humans: Canonical RNases as the Buttress of Innate Immunity. Int. J. Mol. Sci. 2016, 17, 1278. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Feng, Y.; Zou, L.; Wang, L.; Chen, H.H.; Cai, J.Y.; Xu, J.M.; Sosnovik, D.E.; Chao, W. Role of extracellular RNA and TLR3-Trif signaling in myocardial ischemia-reperfusion injury. J. Am. Heart Assoc. 2014, 3, e000683. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Chen, H.; Cai, J.; Zou, L.; Yan, D.; Xu, G.; Li, D.; Chao, W. Cardiac RNA induces inflammatory responses in cardiomyocytes and immune cells via Toll-like receptor 7 signaling. J. Biol. Chem. 2015, 290, 26688–26698. [Google Scholar] [CrossRef] [PubMed]
- Zechendorf, E.; O’Riordan, C.E.; Stiehler, L.; Wischmeyer, N.; Chiazza, F.; Collotta, D.; Denecke, B.; Ernst, S.; Muller-Newen, G.; Coldewey, S.M.; et al. Ribonuclease 1 attenuates septic cardiomyopathy and cardiac apoptosis in a murine model of polymicrobial sepsis. JCI Insight 2020, 5, e131571. [Google Scholar] [CrossRef]
- Li, J.; Boix, E. Host Defence RNases as Antiviral Agents against Enveloped Single Stranded RNA Viruses. Virulence 2021, 12, 444–469. [Google Scholar] [CrossRef]
- Ireland, D.D.; Stohlman, S.A.; Hinton, D.R.; Kapil, P.; Silverman, R.H.; Atkinson, R.A.; Bergmann, C.C. RNase L mediated protection from virus induced demyelination. PLoS Pathog. 2009, 5, e1000602. [Google Scholar] [CrossRef]
- Legrand, M.; Bell, S.; Forni, L.; Joannidis, M.; Koyner, J.L.; Liu, K.; Cantaluppi, V. Pathophysiology of COVID-19-associated acute kidney injury. Nat. Rev. Nephrol. 2021, 17, 751–764. [Google Scholar] [CrossRef]
- Kaye, A.D.; Okeagu, C.N.; Tortorich, G.; Pham, A.D.; Ly, E.I.; Brondeel, K.C.; Eng, M.R.; Luedi, M.M.; Urman, R.D.; Cornett, E.M. COVID-19 impact on the renal system: Pathophysiology and clinical outcomes. Best Pract. Res. Clin. Anaesthesiol. 2021, 35, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Copur, S.; Berkkan, M.; Basile, C.; Tuttle, K.; Kanbay, M. Post-acute COVID-19 syndrome and kidney diseases: What do we know? J. Nephrol. 2022, 35, 795–805. [Google Scholar] [CrossRef] [PubMed]
- Zechendorf, E.; Gombert, A.; Bülow, T.; Frank, N.; Beckers, C.; Peine, A.; Kotelis, D.; Jacobs, M.J.; Marx, G.; Martin, L. The Role of Ribonuclease 1 and Ribonuclease Inhibitor 1 in Acute Kidney Injury after Open and Endovascular Thoracoabdominal Aortic Aneurysm Repair. J. Clin. Med. 2020, 9, 3292. [Google Scholar] [CrossRef] [PubMed]
- Ilinskaya, O.N.; Mahmud, R.S. Ribonucleases as antiviral agents. Mol. Biol. 2014, 48, 615–623. [Google Scholar] [CrossRef]
- Humphrey, R.L.; Karpetsky, T.P.; Neuwelt, E.A.; Levy, C.C. Levels of serum ribonuclease as an indicator of renal insufficiency in patients with leukemia. Cancer Res. 1977, 37, 2015–2022. [Google Scholar]
- Martin, L.; Koczera, P.; Simons, N.; Zechendorf, E.; Hoeger, J.; Marx, G.; Schuerholz, T. The Human Host Defense Ribonucleases 1, 3 and 7 Are Elevated in Patients with Sepsis after Major Surgery—A Pilot Study. Int. J. Mol. Sci. 2016, 17, 294. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef]
- Ards Definition Task Force; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef]
- Takahashi, T.; Ellingson, M.K.; Wong, P.; Israelow, B.; Lucas, C.; Klein, J.; Silva, J.; Mao, T.; Oh, J.E.; Tokuyama, M.; et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature 2020, 588, 315–320. [Google Scholar] [CrossRef]
- Meng, Y.; Wu, P.; Lu, W.; Liu, K.; Ma, K.; Huang, L.; Cai, J.; Zhang, H.; Qin, Y.; Sun, H.; et al. Sex-specific clinical characteristics and prognosis of coronavirus disease-19 infection in Wuhan, China: A retrospective study of 168 severe patients. PLoS Pathog. 2020, 16, e1008520. [Google Scholar] [CrossRef] [PubMed]
- Shoeb, F.; Mahdi, F.; Hussain, I. Gender Differences Associated with Hyper-Inflammatory Conditions in COVID-19 Patients. Aging Dis. 2023, 14, 299–308. [Google Scholar] [CrossRef]
- Bleilevens, C.; Soppert, J.; Hoffmann, A.; Breuer, T.; Bernhagen, J.; Martin, L.; Stiehler, L.; Marx, G.; Dreher, M.; Stoppe, C.; et al. Macrophage Migration Inhibitory Factor (MIF) Plasma Concentration in Critically Ill COVID-19 Patients: A Prospective Observational Study. Diagnostics 2021, 11, 332. [Google Scholar] [CrossRef]
- Zechendorf, E.; Schröder, K.; Stiehler, L.; Frank, N.; Beckers, C.; Kraemer, S.; Dreher, M.; Kersten, A.; Thiemermann, C.; Marx, G.; et al. The Potential Impact of Heparanase Activity and Endothelial Damage in COVID-19 Disease. J. Clin. Med. 2022, 11, 5261. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.P.; Stoppe, C.; Breuer, T.; Stiehler, L.; Dreher, M.; Kersten, A.; Kluge, S.; Karakas, M.; Zechendorf, E.; Marx, G.; et al. Prognostic Value of Bioactive Adrenomedullin in Critically Ill Patients with COVID-19 in Germany: An Observational Cohort Study. J. Clin. Med. 2021, 10, 1667. [Google Scholar] [CrossRef] [PubMed]


| All (n = 35) | Increasing RNase 1 Levels (n = 22) | Decreasing RNase 1 Levels (n = 13) | p-Value | |
|---|---|---|---|---|
| Age (year) (IQR) | 61.00 (56.00–67.00) | 60.50 (56.75–72.25) | 62.00 (54.00–66.00) | 0.4467 |
| Male sex (%) | 26 (74.29) | 18 (81.82) | 8 (61.54) | 0.1954 |
| BMI (kg/m2) (IQR) | 29.40 (26.30–32.30) | 30.15 (26.53–33.03) | 29.30 (24.25–30.80) | 0.7779 |
| Diabetes mellitus (%) | 8 (22.86) | 8 (36.36) | 0 (0) | * 0.0124 |
| Chronic renal failure (%) | 5 (14.29) | 2 (9.09) | 3 (23.08) | 0.2663 |
| Smoker (%) | 2 (5.71) | 2 (9.09) | 0 (0) | 0.2762 |
| Ex-smoker (%) | 3 (8.57) | 2 (9.09) | 1 (7.69) | 0.8905 |
| LOS (days) (IQR) | 38.00 (26.00–55.00) | 38.50 (27.50–52.75) | 30.00 (18.00–56.00) | 0.5713 |
| LOS ICU (days) (IQR) | 19.00 (16.00–35.00) | 23.50 (17.50–46.25) | 17.00 (12.50–23.00) | * 0.0443 |
| 28-day mortality (%) | 12 (34.29) | 9 (40.91) | 3 (23.08) | 0.2967 |
| All (n = 80) | Increasing RNase 1 Levels (n = 48) | Decreasing RNase 1 Levels (n = 32) | p-Value | |
|---|---|---|---|---|
| Age (year) (IQR) | 64.00 (52.00–75.00) | 63.00 (55.00–71.75) | 68.00 (51.00–78.25) | 0.5802 |
| Male sex (%) | 51 (63.75) | 30 (62.5) | 21 (65.63) | 0.7791 |
| BMI (kg/m2) (IQR) | 28.49 (26.10–31.95) | 27.73 (25.45–31.22) | 29.40 (26.64–32.89) | 0.5656 |
| LOS (days) (IQR) | 18.00 (12.25–34.00) | 18.50 (12.25–38.50) | 16.50 (10.75–27.00) | 0.5515 |
| LOS ICU (days) (IQR) | 19.00 (12.00–38.75) | 18.00 (12.00–35.00) | 21.00 (12.50–44.50) | 0.9218 |
| ICU patients (%) | 44 (55.00) | 31 (64.58) | 13 (40.63) | * 0.0351 |
| 28-day mortality (%) | 22 (27.50) | 18 (37.50) | 4 (12.5) | * 0.0138 |
| Increasing RNase 1 d2 | Decreasing RNase 1 d2 | |||
| Variable | Pearson r | p-Value | Pearson r | p-Value |
| CRP | 0.0360 | 0.8909 | −0.5112 | 0.1080 |
| PCT | 0.5570 | * 0.0132 | 0.1131 | 0.7263 |
| IL-6 | −0.2412 | 0.3197 | −0.2364 | 0.4839 |
| Leucocytes | 0.3793 | 0.0899 | −0.3282 | 0.2977 |
| days of dialysis | 0.5038 | * 0.0199 | 0.2462 | 0.4406 |
| Diuresis | −0.6085 | * 0.0044 | −0.2475 | 0.4379 |
| Increasing RNase 1 d4 | Decreasing RNase 1 d4 | |||
| Variable | Pearson r | p-value | Pearson r | p-value |
| CRP | −0.5576 | * 0.0200 | 0.1304 | 0.7023 |
| PCT | 0.3239 | 0.1414 | 0.1752 | 0.5859 |
| IL-6 | 0.1298 | 0.5855 | 0.3544 | 0.2849 |
| Leucocytes | 0.5044 | * 0.0167 | −0.0142 | 0.9650 |
| days of dialysis | 0.5812 | * 0.0046 | 0.1999 | 0.5334 |
| Diuresis | −0.6414 | * 0.0013 | −0.3839 | 0.2179 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zechendorf, E.; Beckers, C.; Frank, N.; Kraemer, S.; Neu, C.; Breuer, T.; Dreher, M.; Dahl, E.; Marx, G.; Martin, L.; et al. A Potential Association between Ribonuclease 1 Dynamics in the Blood and the Outcome in COVID-19 Patients. Int. J. Mol. Sci. 2023, 24, 12428. https://doi.org/10.3390/ijms241512428
Zechendorf E, Beckers C, Frank N, Kraemer S, Neu C, Breuer T, Dreher M, Dahl E, Marx G, Martin L, et al. A Potential Association between Ribonuclease 1 Dynamics in the Blood and the Outcome in COVID-19 Patients. International Journal of Molecular Sciences. 2023; 24(15):12428. https://doi.org/10.3390/ijms241512428
Chicago/Turabian StyleZechendorf, Elisabeth, Christian Beckers, Nadine Frank, Sandra Kraemer, Carolina Neu, Thomas Breuer, Michael Dreher, Edgar Dahl, Gernot Marx, Lukas Martin, and et al. 2023. "A Potential Association between Ribonuclease 1 Dynamics in the Blood and the Outcome in COVID-19 Patients" International Journal of Molecular Sciences 24, no. 15: 12428. https://doi.org/10.3390/ijms241512428
APA StyleZechendorf, E., Beckers, C., Frank, N., Kraemer, S., Neu, C., Breuer, T., Dreher, M., Dahl, E., Marx, G., Martin, L., & Simon, T.-P. (2023). A Potential Association between Ribonuclease 1 Dynamics in the Blood and the Outcome in COVID-19 Patients. International Journal of Molecular Sciences, 24(15), 12428. https://doi.org/10.3390/ijms241512428







