CC5 and CC8, Two Disintegrin Isoforms from Cerastes cerastes Snake Venom Decreased Inflammation Response In Vitro and In Vivo
Abstract
1. Introduction
2. Results
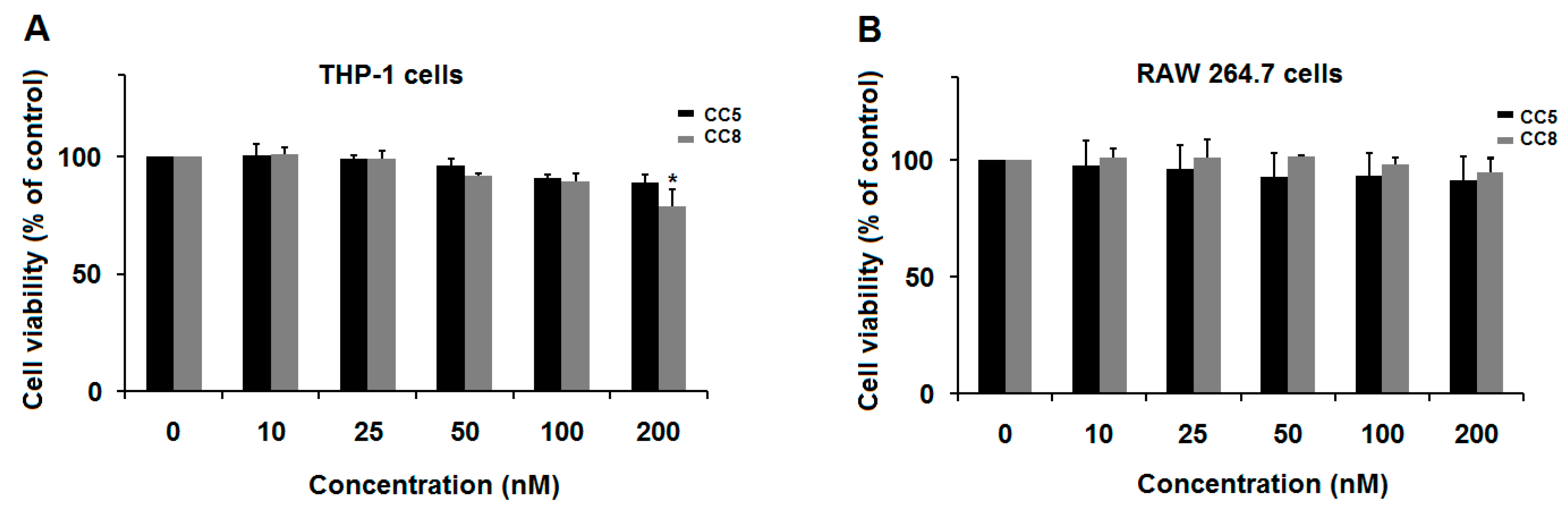
2.1. CC5 and CC8 Effect on THP-1-Derived Macrophages and RAW264.7 Cells Development
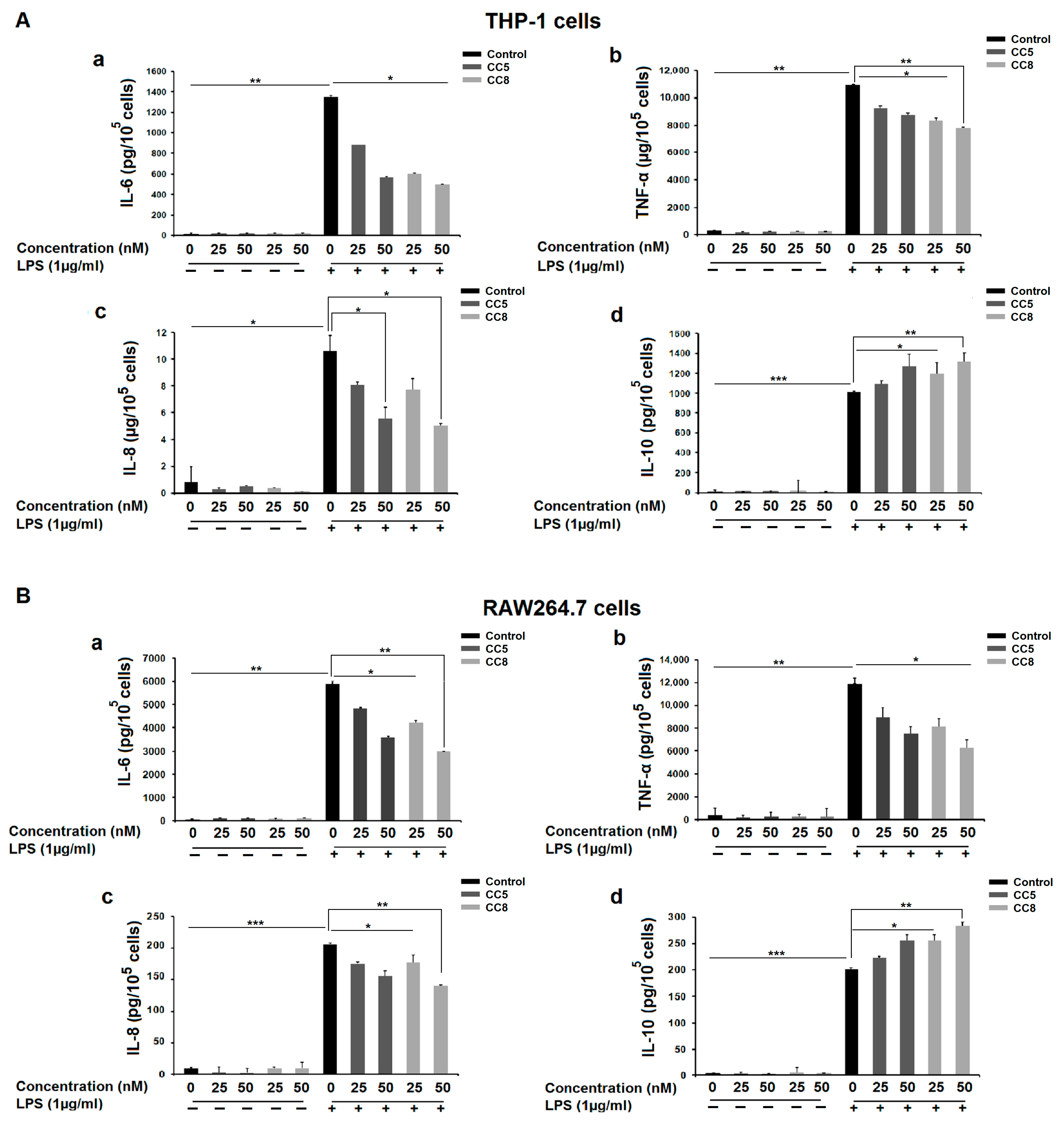
2.2. CC5 and CC8 Inhibited LPS-Induced Cytokine Production in THP-1 and RAW264.7 Cells
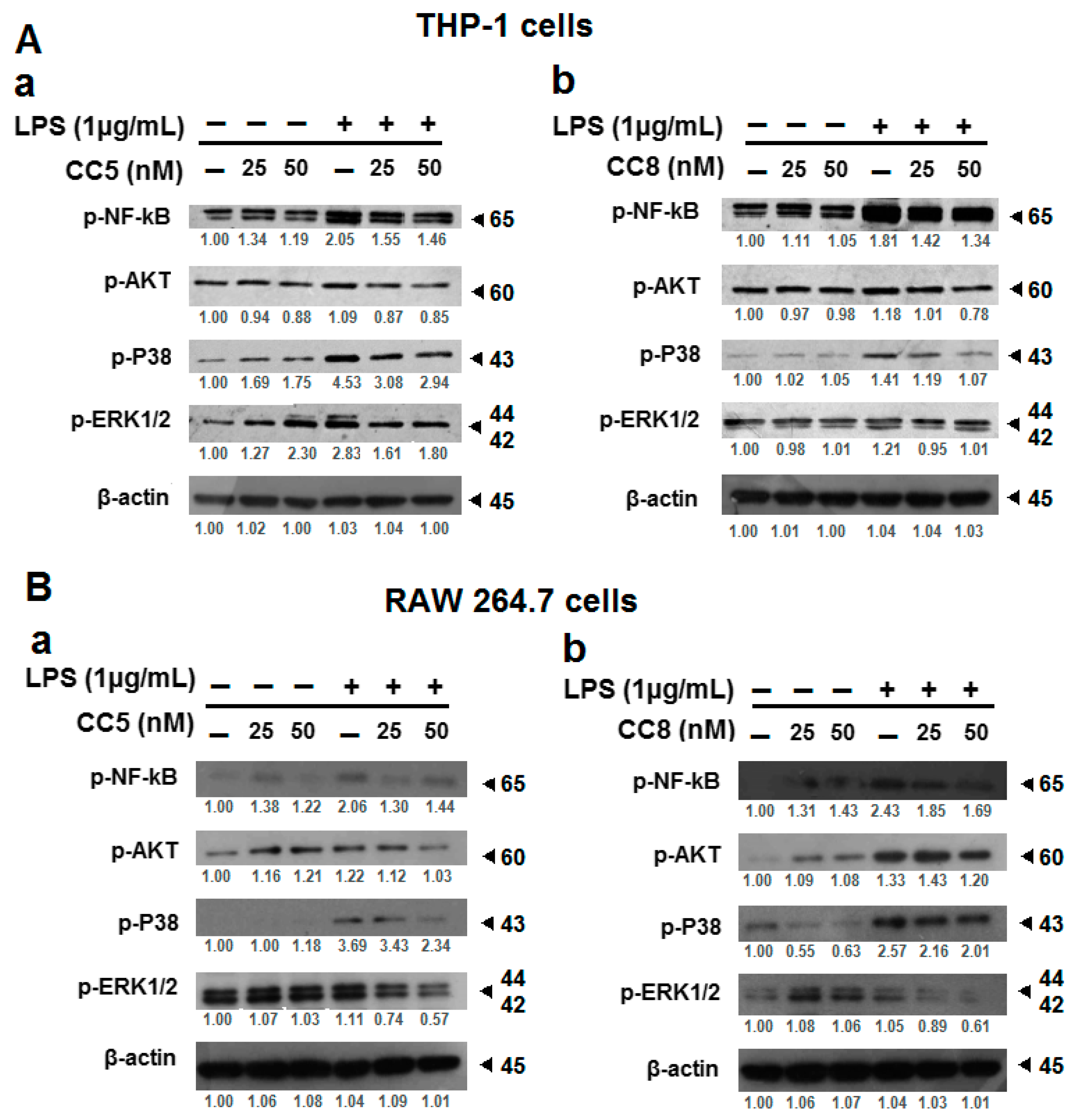
2.3. Modulation of LPS-Induced Inflammatory Cytokine Production by RAW264.7 and THP-1 Cells Is Mediated by NF-κB, ERK1/2, P38 MAPK, and Akt Inhibition
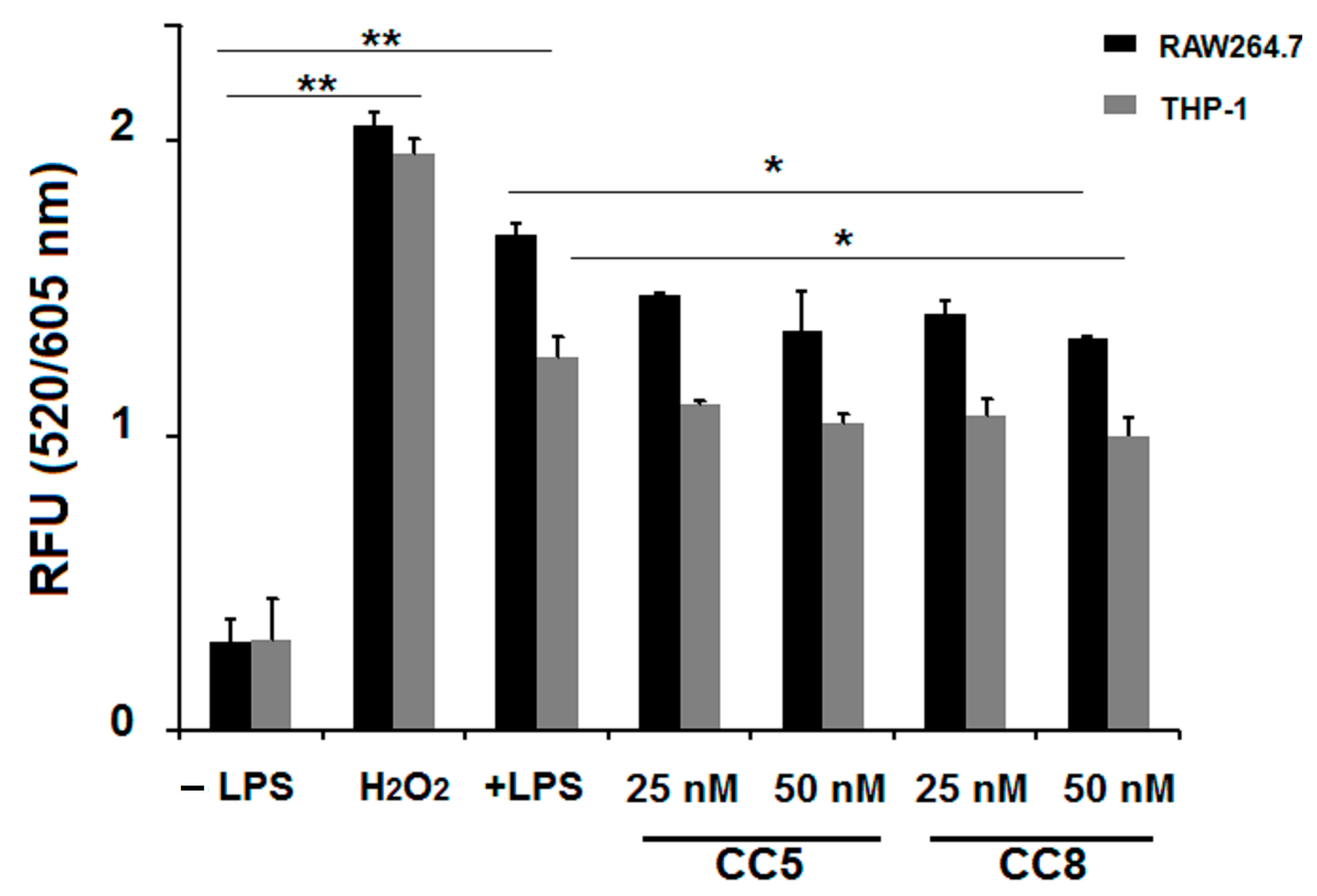
2.4. CC5 and CC8 Reduced ROS Production in LPS-Stimulated RAW264.7 and THP-1 Cells
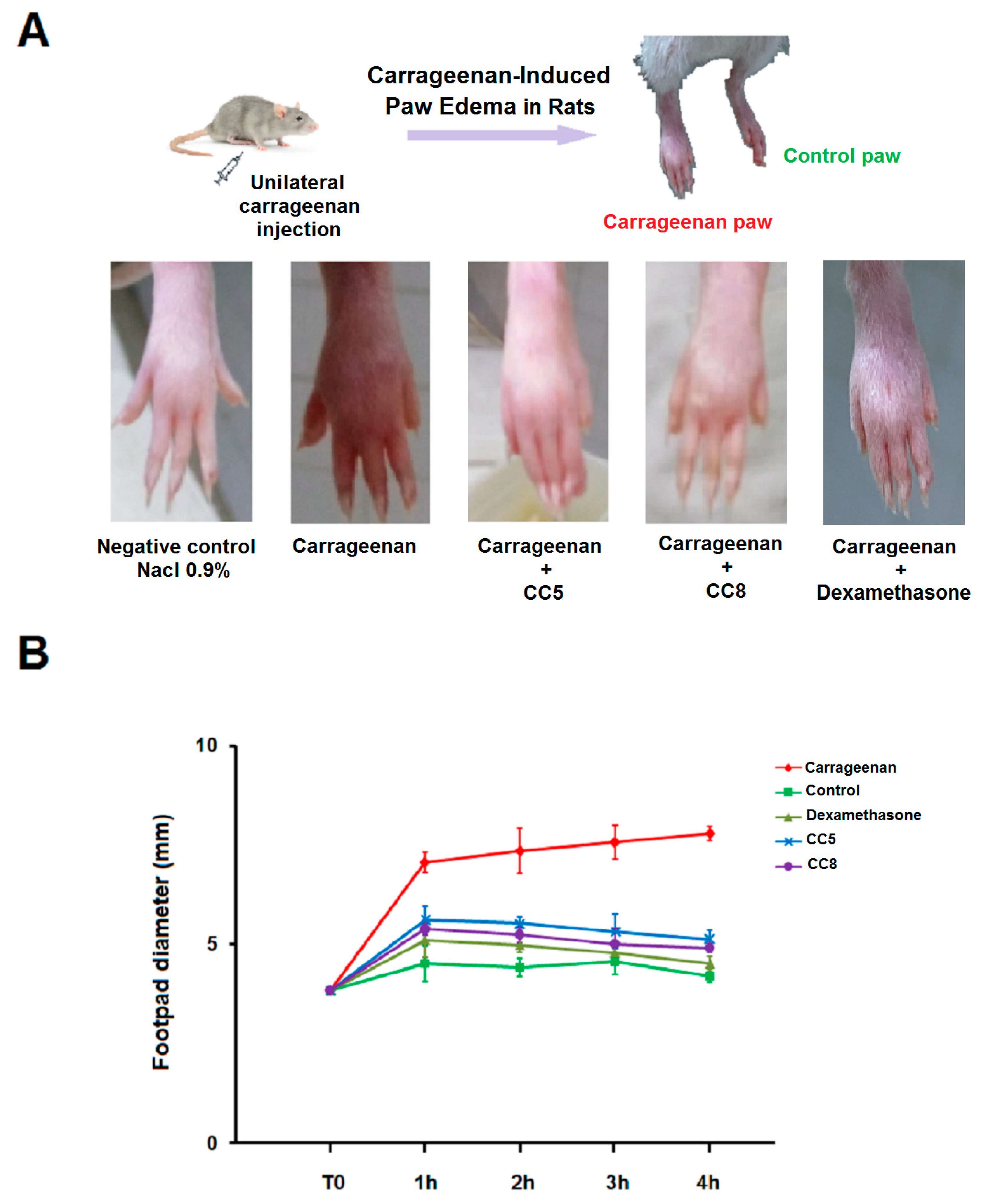
2.5. Effects of CC5 and CC8 on Carrageenan-Induced Paw Edema in Rats
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. CC5 and CC8 Disintegrin Purification
4.3. Cell Viability Assays
4.3.1. MTT Assay
4.3.2. Trypan Blue Exclusion Assay
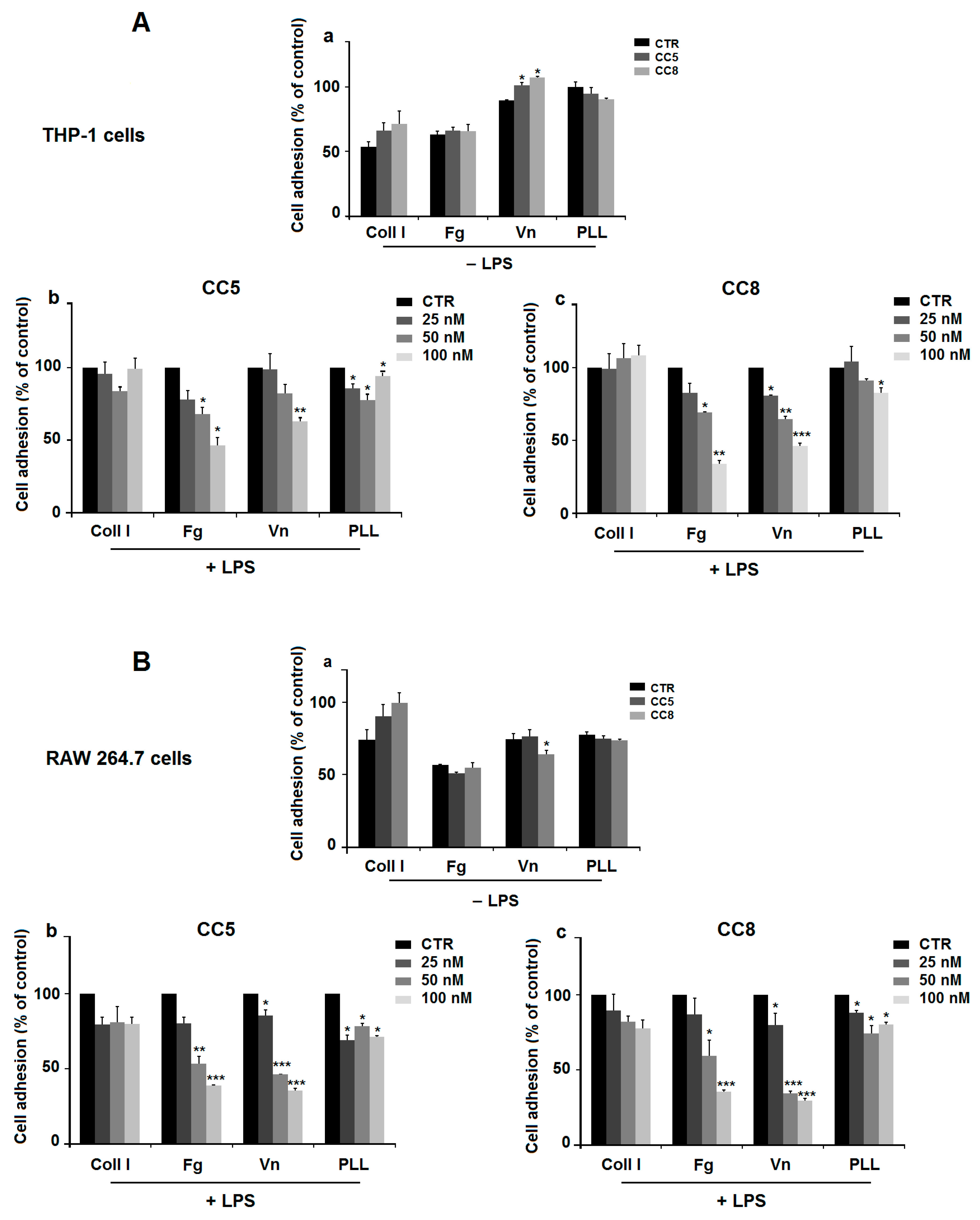
4.4. Adhesion Assay
4.5. Measurement of Cytokine Production in Culture Supernatants
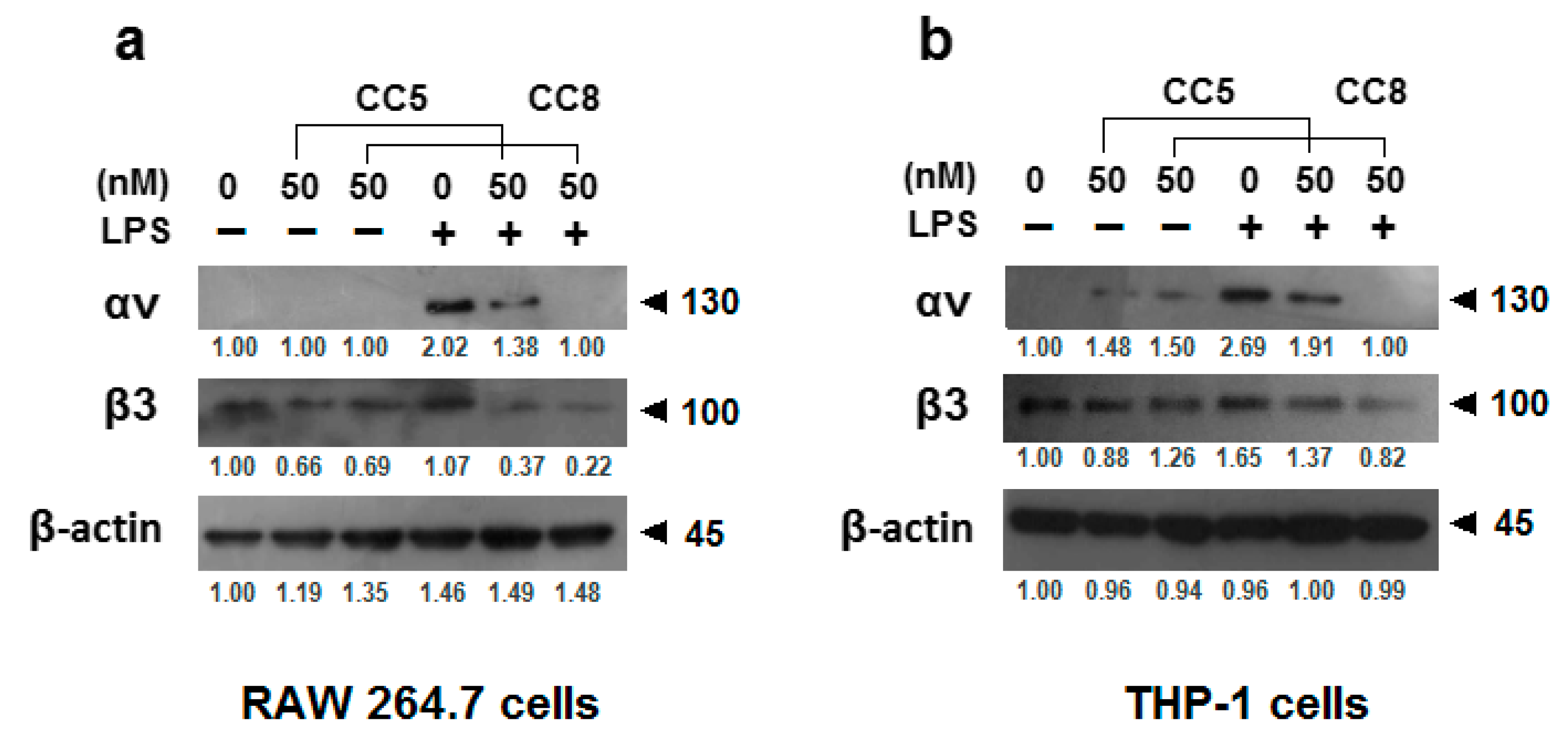
4.6. Western Blot Analysis
4.7. Measurement of Intracellular ROS Generation
4.8. Carrageenan-Induced Paw Edema
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Germolec, D.R.; Shipkowski, K.A.; Frawley, R.P.; Evans, E. Markers of Inflammation. Methods Mol. Biol. 2018, 1803, 57–79. [Google Scholar] [CrossRef]
- Medzhitov, R. Inflammation 2010: New adventures of an old flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef]
- Abdulkhaleq, L.A.; Assi, M.A.; Abdullah, R.; Zamri-Saad, M.; Taufiq-Yap, Y.H.; Hezmee, M.N.M. The crucial roles of inflammatory mediators in inflammation: A review. Vet. World 2018, 11, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2019, 1, a001651. [Google Scholar] [CrossRef]
- Kawabata, K.; Tung, N.H.; Shoyama, Y.; Sugie, S.; Mori, T.; Tanaka, T. Dietary Crocin Inhibits Colitis and Colitis-Associated Colorectal Carcinogenesis in Male ICR Mice. Evid. Based Complement. Altern. Med. 2012, 2012, 820415. [Google Scholar] [CrossRef]
- Aggarwal, B.B. Inflammation, a silent killer in cancer is not so silent! Curr. Opin. Pharmacol. 2009, 9, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, S.; Chen, J.; Xie, Z. The Interplay between Integrins and Immune Cells as a Regulator in Cancer Immunology. Int. J. Mol. Sci. 2023, 24, 6170. [Google Scholar] [CrossRef]
- Bae, H.B.; Zmijewski, J.W.; Deshane, J.S.; Zhi, D.; Thompson, L.C.; Peterson, C.B.; Chaplin, D.D.; Abraham, E. Vitronectin inhibits neutrophil apoptosis through activation of integrin-associated signaling pathways. Am. J. Respir. Cell Mol. Biol. 2012, 46, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Antonov, A.S.; Antonova, G.N.; Munn, D.H.; Mivechi, N.; Lucas, R.; Catravas, J.D.; Verin, A.D. αVβ3 integrin regulates macrophage inflammatory responses via PI3 kinase/Akt-dependent NF-κB activation. J. Cell. Physiol. 2011, 226, 469–476. [Google Scholar] [CrossRef]
- Dotan, I.; Allez, M.; Danese, S.; Keir, M.; Tole, S.; McBride, J. The role of integrins in the pathogenesis of inflammatory bowel disease: Approved and investigational anti-integrin therapies. Med. Res. Rev. 2020, 40, 245–262. [Google Scholar] [CrossRef]
- Gilroy, L.; Allen, P.B. Is there a role for vedolizumab in the treatment of ulcerative colitis and Crohn’s disease? Clin. Exp. Gastroenterol. 2014, 7, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Sarray, S.; Delamarre, E.; Marvaldi, J.; El Ayeb, M.; Marrakchi, N.; Luis, J. Lebectin and lebecetin, two C-type lectins from snake venom, inhibit alpha5beta1 and alphaV-containing integrins. Matrix Biol. 2007, 26, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Morjen, M.; Kallech-Ziri, O.; Bazaa, A.; Othman, H.; Mabrouk, K.; Zouari-Kessentini, R.; Sanz, L.; Calvete, J.J.; Srairi-Abid, N.; El Ayeb, M.; et al. PIVL, a new serine protease inhibitor from Macroviperalebetinatransmediterranea venom, impairs motility of human glioblastoma cells. Matrix Biol. 2013, 32, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Morjen, M.; Honoré, S.; Bazaa, A.; Abdelkafi-Koubaa, Z.; Ellafi, A.; Mabrouk, K.; Kovacic, H.; El Ayeb, M.; Marrakchi, N.; Luis, J. PIVL, a snake venom Kunitz-type serine protease inhibitor, inhibits in vitro and in vivo angiogenesis. Microvasc. Res. 2014, 95, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Calvete, J.J.; Fox, J.W.; Agelan, A.; Niewiarowski, S.; Marcinkiewicz, C. The presence of the WGD motif in CC8 heterodimeric disintegrin increases its inhibitory effect on alphaII(b)beta3, alpha(v)beta3, and alpha5beta1 integrins. Biochemistry 2002, 41, 2014–2021. [Google Scholar] [CrossRef] [PubMed]
- Calvete, J.J. Snake venomics: From the inventory of toxins to biology. Toxicon 2013, 75, 44–62. [Google Scholar] [CrossRef]
- Huang, T.F.; Holt, J.C.; Lukasiewicz, H.; Niewiarowski, S. Trigramin. A low molecular weight peptide inhibiting fibrinogen interaction with platelet receptors expressed on glycoprotein IIb-IIIa complex. J. Biol. Chem. 1987, 262, 16157–16163. [Google Scholar] [CrossRef]
- Gould, R.J.; Polokoff, M.A.; Friedman, P.A.; Huang, T.F.; Holt, J.C.; Cook, J.J.; Niewiarowski, S. Disintegrins: A family of integrin inhibitory proteins from viper venoms. Proc. Soc. Exp. Biol. Med. 1990, 195, 168–171. [Google Scholar] [CrossRef]
- McLane, M.A.; Sanchez, E.E.; Wong, A.; Paquette-Straub, C.; Perez, J.C. Disintegrins. Curr. Drug Targets Cardiovasc. Haematol. Disord. 2014, 4, 327–355. [Google Scholar] [CrossRef]
- Vasconcelos, A.A.; Estrada, J.C.; David, V.; Wermelinger, L.S.; Almeida, F.C.L.; Zingali, R.B. Structure-Function Relationship of the Disintegrin Family: Sequence Signature and Integrin Interaction. Front. Mol. Biosci. 2021, 8, 783301. [Google Scholar] [CrossRef]
- Ben-Mabrouk, H.; Zouari-Kessentini, R.; Montassar, F.; Koubaa, Z.A.; Messaadi, E.; Guillonneau, X.; ElAyeb, M.; Srairi-Abid, N.; Luis, J.; Micheau, O.; et al. CC5 and CC8, two homologous disintegrins from Cerastes cerastesvenom, inhibit in vitro and ex vivo angiogenesis. Int. J. Biol. Macromol. 2016, 86, 670–680. [Google Scholar] [CrossRef]
- Hung, Y.C.; Hsu, C.C.; Chung, C.H.; Huang, T.F. The disintegrin, trimucrin, suppresses LPS-induced activation of phagocytes primarily through blockade of NF-κB and MAPK activation. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2016, 389, 723–737. [Google Scholar] [CrossRef]
- Hsu, C.C.; Chuang, W.J.; Chang, C.H.; Tseng, Y.L.; Peng, H.C.; Huang, T.F. Improvements in endotoxemic syndromes using a disintegrin, rhodostomin, through integrin αvβ3-dependent pathway. J. Thromb. Haemost. 2011, 9, 593–602. [Google Scholar] [CrossRef]
- Min, C.K.; Lee, W.Y.; Min, D.J.; Lee, D.G.; Kim, Y.J.; Park, Y.H.; Kim, H.J.; Lee, S.; Kim, D.W.; Lee, J.W.; et al. The kinetics of circulating cytokines including IL-6, TNF-alpha, IL-8 and IL-10 following allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2001, 28, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Van Amersfoort, E.S.; Van Berkel, T.J.; Kuiper, J. Receptors, mediators, and mechanisms involved in bacterial sepsis and septic shock. Clin. Microbiol. Rev. 2003, 16, 379–414. [Google Scholar] [CrossRef]
- López-Bojórquez, L.N.; Dehesa, A.Z.; Reyes-Terán, G. Molecular mechanisms involved in the pathogenesis of septic shock. Arch. Med. Res. 2004, 35, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Schittenhelm, L.; Hilkens, C.M.; Morrison, V.L. β2 Integrins as Regulators of Dendritic Cell, Monocyte, and Macrophage Function. Front. Immunol. 2017, 8, 1866. [Google Scholar] [CrossRef] [PubMed]
- Mezu-Ndubuisi, O.J.; Maheshwari, A. The role of integrins in inflammation and angiogenesis. Pediatr. Res. 2021, 89, 1619–1626. [Google Scholar] [CrossRef]
- Su, G.; Atakilit, A.; Li, J.T.; Wu, N.; Bhattacharya, M.; Zhu, J.; Shieh, J.E.; Li, E.; Chen, R.; Sun, S.; et al. Absence of integrin αvβ3 enhances vascular leak in mice by inhibiting endothelial cortical actin formation. Am. J. Respir. Crit. Care Med. 2012, 185, 58–66. [Google Scholar] [CrossRef]
- Kumar, C.C. Integrin alpha v beta 3 as a therapeutic target for blocking tumor-induced angiogenesis. Curr. Drug Targets 2003, 4, 123–131. [Google Scholar] [CrossRef]
- Gu, Y.; Dong, B.; He, X.; Qiu, Z.; Zhang, J.; Zhang, M.; Liu, H.; Pang, X.; Cui, Y. The challenges and opportunities of αvβ3-based therapeutics in cancer: From bench to clinical trials. Pharmacol. Res. 2023, 189, 106694. [Google Scholar] [CrossRef] [PubMed]
- Marrocco, A.; Ortiz, L.A. Role of metabolic reprogramming in pro-inflammatory cytokine secretion from LPS or silica-activated macrophages. Front. Immunol. 2022, 13, 936167. [Google Scholar] [CrossRef]
- Nagata, K.; Nishiyama, C. IL-10 in Mast Cell-Mediated Immune Responses: Anti-Inflammatory and Proinflammatory Roles. Int. J. Mol. Sci. 2021, 22, 4972. [Google Scholar] [CrossRef]
- Berker, E.; Kantarci, A.; Hasturk, H.; Van Dyke, T.E. Blocking proinflammatory cytokine release modulates peripheral blood mononuclear cell response to Porphyromonasgingivalis. J. Periodontol. 2013, 84, 1337–1345. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.S.; Cheng, G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit. Rev. Immunol. 2012, 32, 23–63. [Google Scholar] [CrossRef]
- Jebali, J.; Zakraoui, O.; Aissaoui, D.; Abdelkafi-Koubaa, Z.; Srairi-Abid, N.; Marrakchi, N.; Essafi-Benkhadir, K. Lebecetin, a snake venom C-type lectin protein, modulates LPS-induced inflammatory cytokine production in human THP-1-derived macrophages. Toxicon 2020, 187, 144–150. [Google Scholar] [CrossRef]
- McGarry, T.; Biniecka, M.; Veale, D.J.; Fearon, U. Hypoxia, oxidative stress and inflammation. Free. Radic. Biol. Med. 2018, 125, 15–24. [Google Scholar] [CrossRef]
- Koutsaliaris, I.K.; Moschonas, I.C.; Pechlivani, L.M.; Tsouka, A.N.; Tselepis, A.D. Inflammation, Oxidative Stress, Vascular Aging and Atherosclerotic Ischemic Stroke. Curr. Med. Chem. 2022, 29, 5496–5509. [Google Scholar] [CrossRef]
- Wright, J.G.; Christman, J.W. The role of nuclear factor kappa B in the pathogenesis of pulmonary diseases: Implications for therapy. Am. J. Respir. Med. 2003, 2, 211–219. [Google Scholar] [CrossRef]
- He, Y.; Li, Z.; Xu, T.; Luo, D.; Chi, Q.; Zhang, Y.; Li, S. Polystyrene nanoplastics deteriorate LPS-modulated duodenal permeability and inflammation in mice via ROS drived-NF-κB/NLRP3 pathway. Chemosphere 2022, 307 Pt 1, 135662. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1988, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Shahabuddin, M.S.; Nambiar, M.; Choudhary, B.; Advirao, G.M.; Raghavan, S.C. A novel DNA intercalator, butylamino-pyrimido [4′,5′:4,5]selenolo(2,3-b)quinoline, induces cell cycle arrest and apoptosis in leukemic cells. Investig. New Drugs 2010, 28, 35–48. [Google Scholar] [CrossRef]
- Kadi, A.; Pichard, V.; Lehmann, M.; Briand, C.; Braguer, D.; Marvaldi, J.; Rognoni, J.B.; Luis, J. Effect of microtubule disruption on cell adhesion and spreading. Biochem. Biophys. Res. Commun. 1998, 246, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Winter, C.A.; Risley, E.A.; Nuss, G.W. Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proc. Soc. Exp. Biol. Med. 1962, 111, 544–547. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morjen, M.; Zakraoui, O.; Abdelkafi-Koubaa, Z.; Srairi-Abid, N.; Marrakchi, N.; Essafi-Benkhadir, K.; Jebali, J. CC5 and CC8, Two Disintegrin Isoforms from Cerastes cerastes Snake Venom Decreased Inflammation Response In Vitro and In Vivo. Int. J. Mol. Sci. 2023, 24, 12427. https://doi.org/10.3390/ijms241512427
Morjen M, Zakraoui O, Abdelkafi-Koubaa Z, Srairi-Abid N, Marrakchi N, Essafi-Benkhadir K, Jebali J. CC5 and CC8, Two Disintegrin Isoforms from Cerastes cerastes Snake Venom Decreased Inflammation Response In Vitro and In Vivo. International Journal of Molecular Sciences. 2023; 24(15):12427. https://doi.org/10.3390/ijms241512427
Chicago/Turabian StyleMorjen, Maram, Ons Zakraoui, Zaineb Abdelkafi-Koubaa, Najet Srairi-Abid, Naziha Marrakchi, Khadija Essafi-Benkhadir, and Jed Jebali. 2023. "CC5 and CC8, Two Disintegrin Isoforms from Cerastes cerastes Snake Venom Decreased Inflammation Response In Vitro and In Vivo" International Journal of Molecular Sciences 24, no. 15: 12427. https://doi.org/10.3390/ijms241512427
APA StyleMorjen, M., Zakraoui, O., Abdelkafi-Koubaa, Z., Srairi-Abid, N., Marrakchi, N., Essafi-Benkhadir, K., & Jebali, J. (2023). CC5 and CC8, Two Disintegrin Isoforms from Cerastes cerastes Snake Venom Decreased Inflammation Response In Vitro and In Vivo. International Journal of Molecular Sciences, 24(15), 12427. https://doi.org/10.3390/ijms241512427





