Cannabidiol and Cannabigerol Modify the Composition and Physicochemical Properties of Keratinocyte Membranes Exposed to UVA
Abstract
1. Introduction
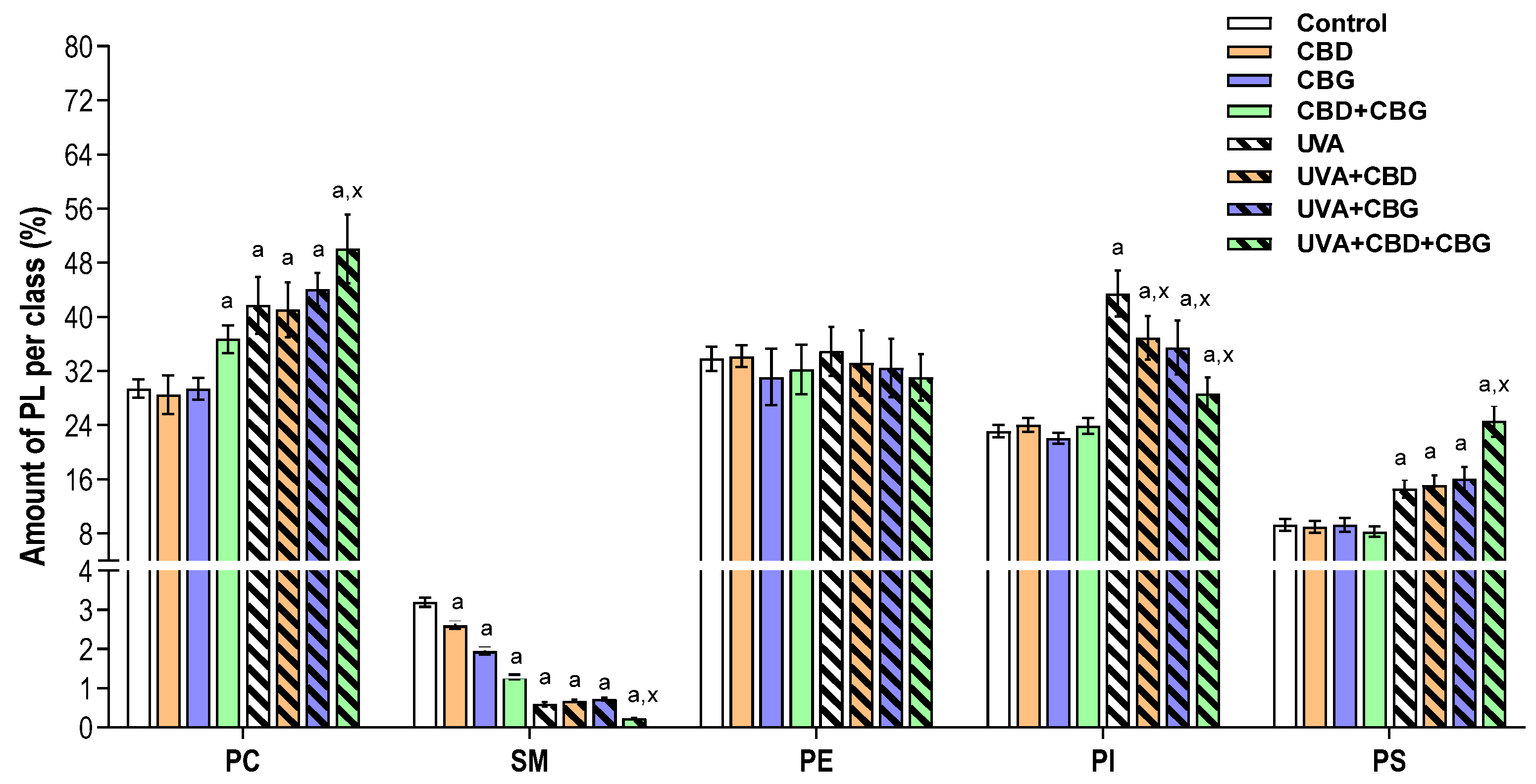
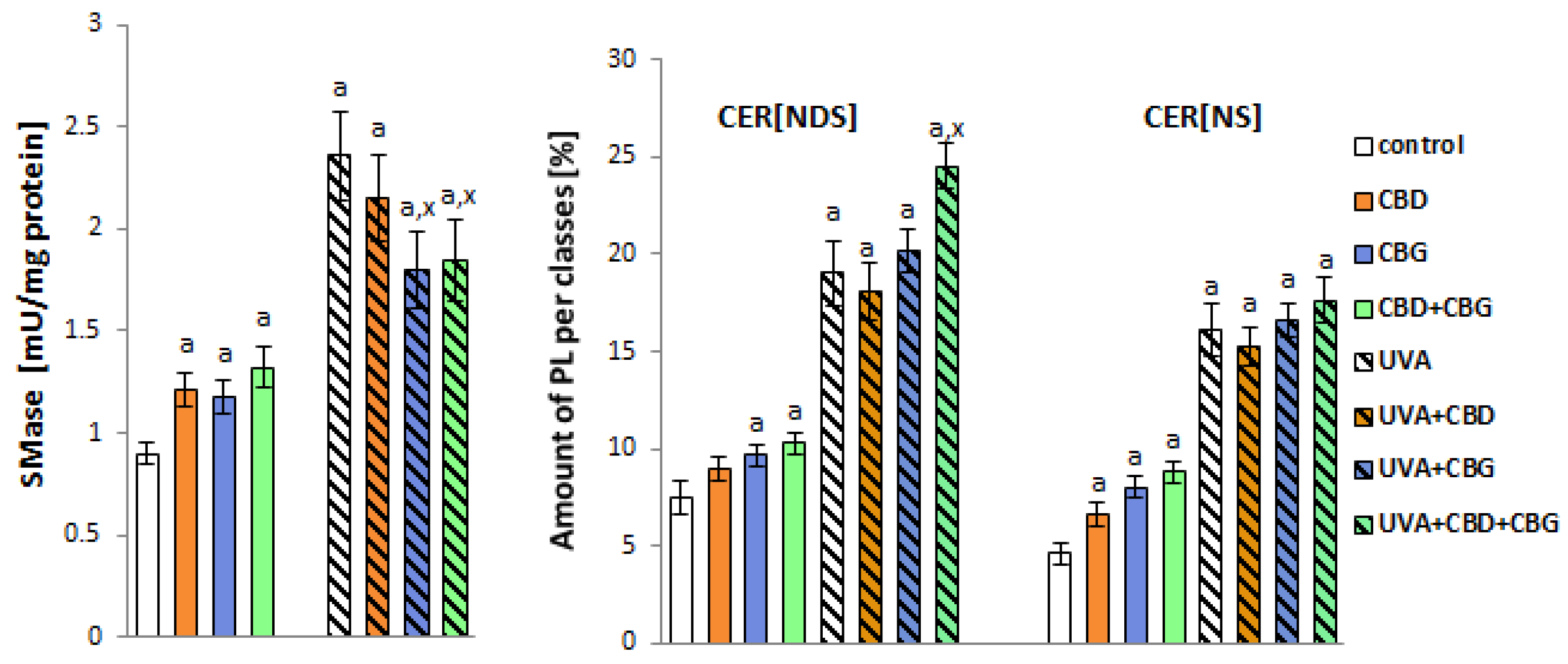
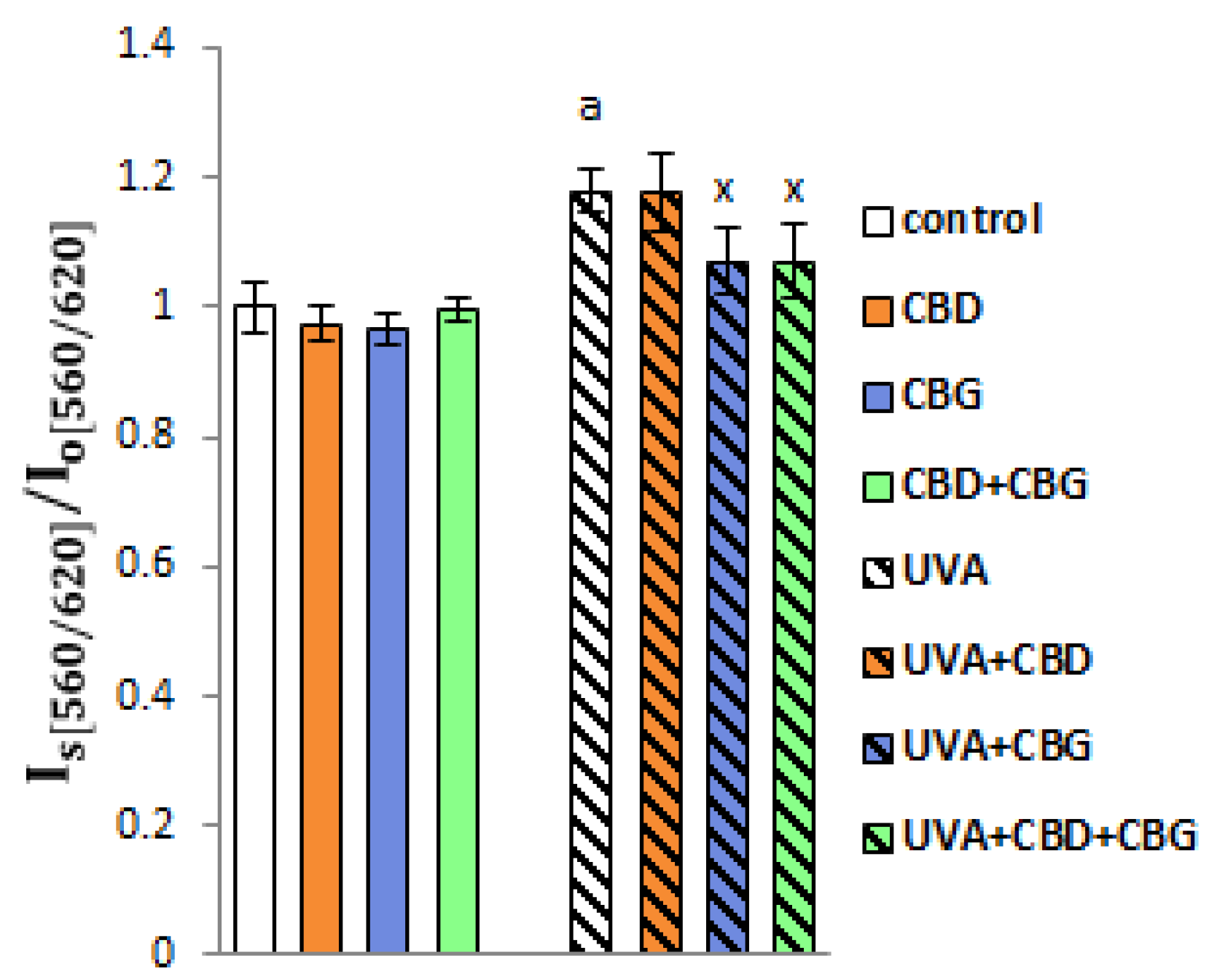
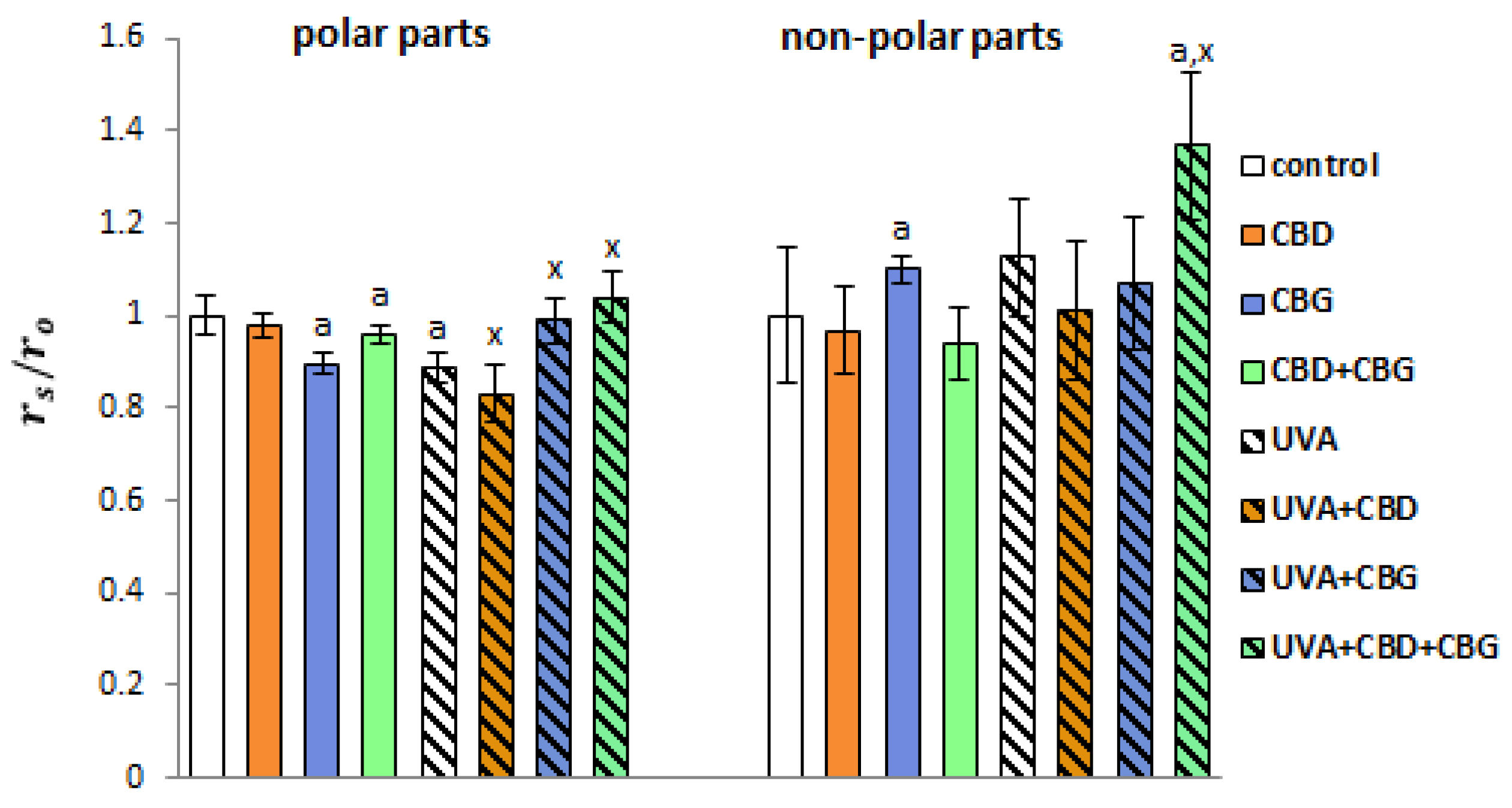
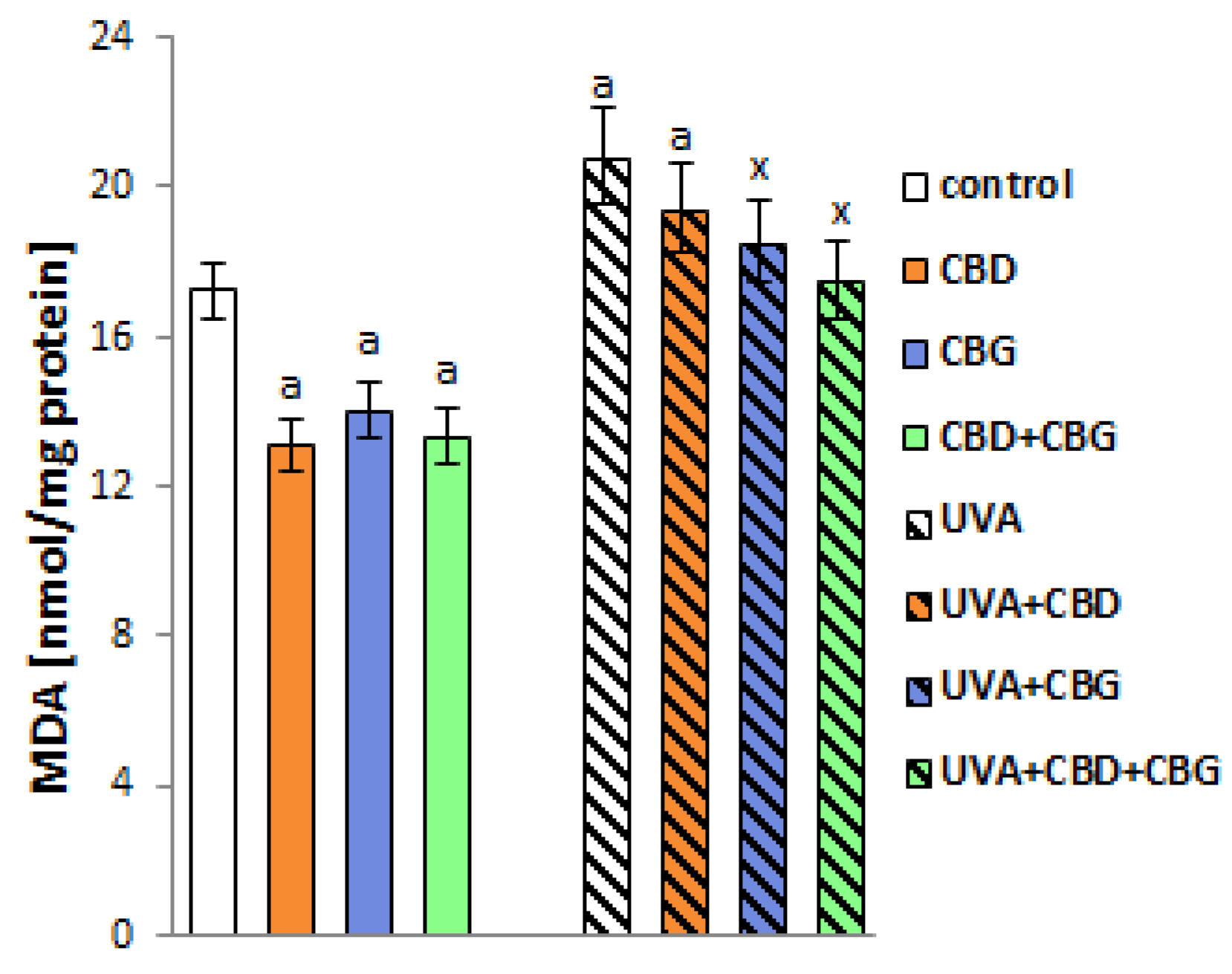
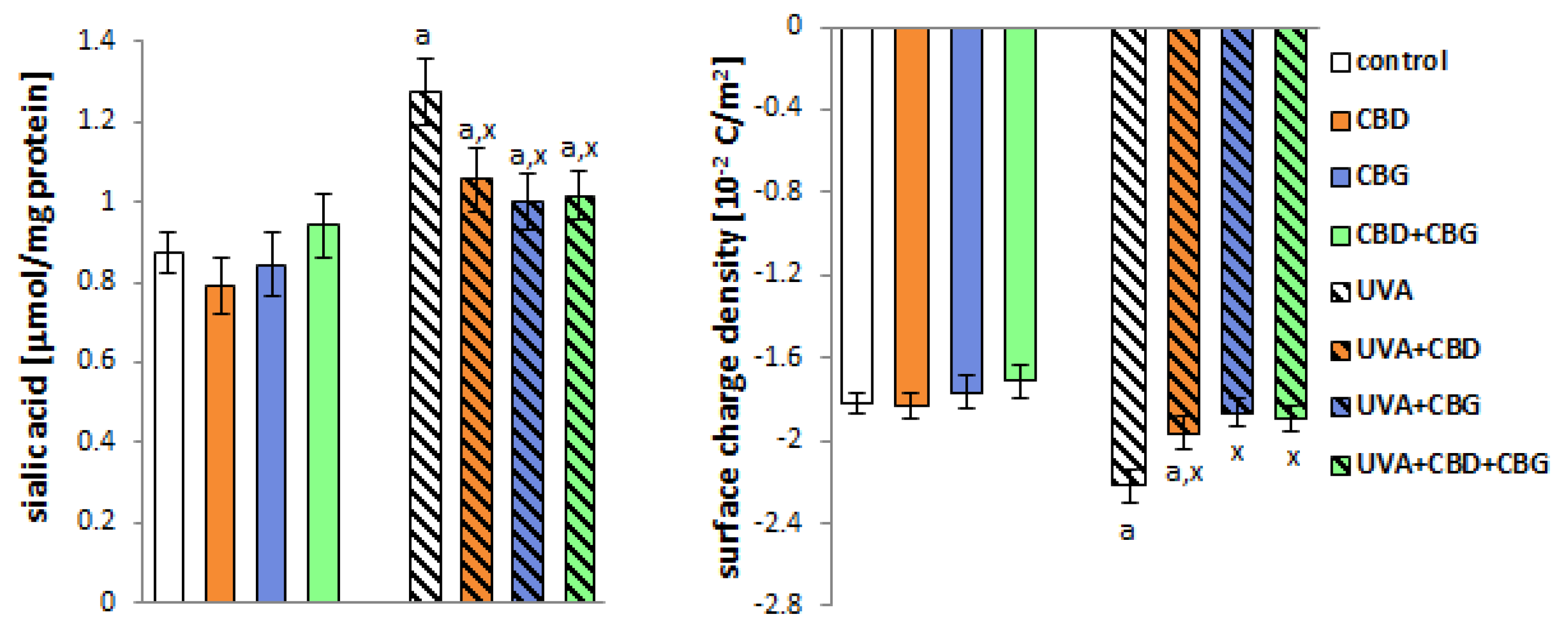
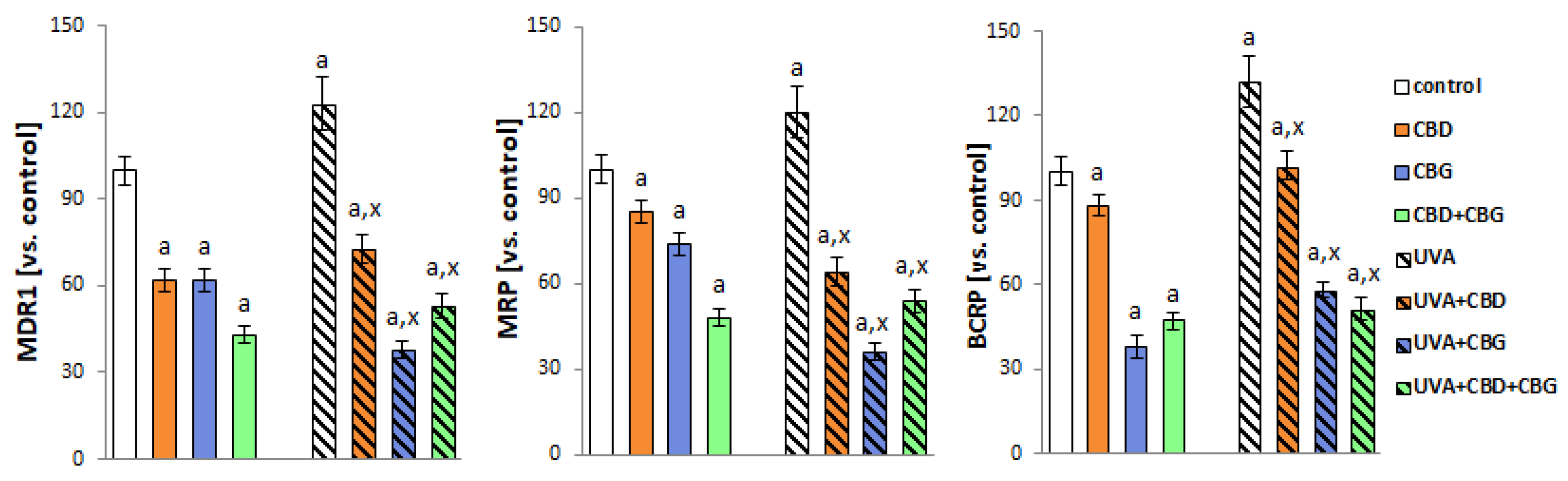
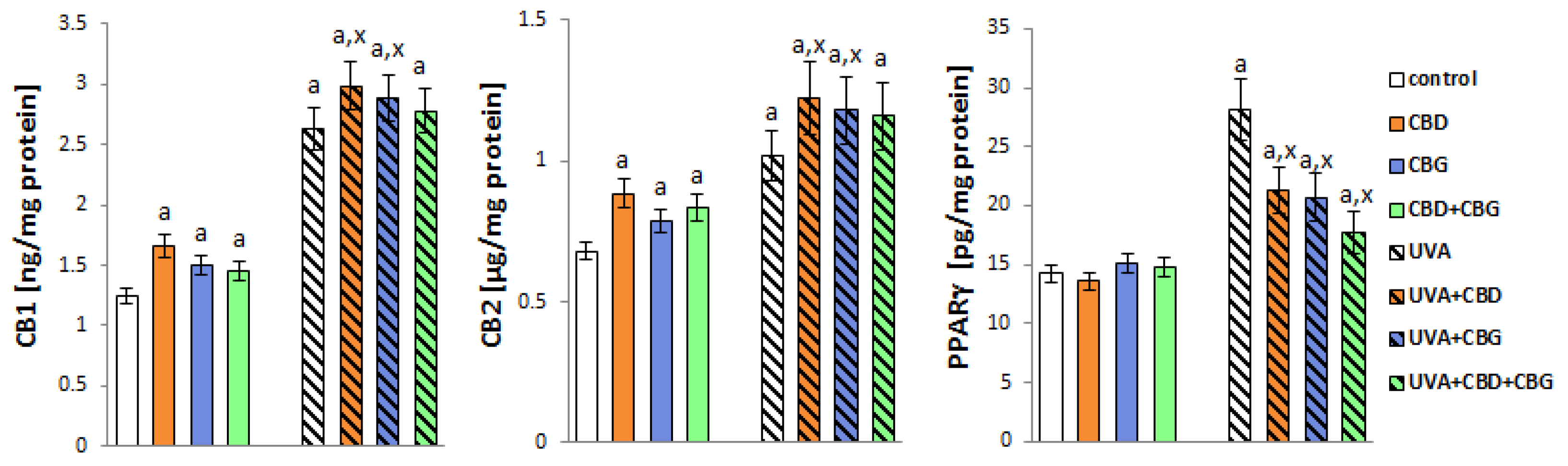
2. Results
3. Discussion
4. Materials and Methods
4.1. Cell Culturing and Treatment
4.2. Analysis of Keratinocyte Membrane Component Phospholipids
4.2.1. Lipid Extraction and Quantification of Total Phospholipid Content
4.2.2. Phospholipid Profiling by Hydrophilic Interaction Liquid Chromatography Coupled with High-Resolution Tandem Mass Spectrometry
4.2.3. Ceramide Profiling by Reversed-Phase Chromatography Coupled with High-Resolution Tandem Mass Spectrometry RPLC-MS/MS Analysis of Ceramides
4.2.4. Determination of Sialic Acid Level
4.3. Evaluation of Lipid Peroxidation
4.4. Determination of Surface Charge Density
4.5. Measurements of Membrane Fluidity
4.6. Analysis of Lipid Raft Formation Using Fluorescence Technique
4.7. Membrane Permeability
4.8. Transmembrane Transporter Activity
4.9. Membrane Receptor Levels
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2-AG | 2-Arachidonoylglycerol |
| AEA | Anandamide |
| ANOVA | Analysis of variance |
| BCRP | Breast cancer resistance protein |
| CBD | Cannabidiol |
| CBG | Cannabigerol |
| CER[NDS] | Ceramides containing non-hydroxy fatty acids and dihydrosphingosine |
| CER[NS] | Ceramides containing non-hydroxy fatty acids and sphingosine |
| COX-2 | Cyclooxygenase-2 |
| CRAC | Calcium-release-activated calcium channel |
| DPH | 1,6-Diphenyl-1,3,5-hexatriene |
| HDF | Human skin fibroblasts |
| IL | Interleukin |
| iNOS | Inducible nitric oxide synthase |
| Kv1.3 | Potassium channel |
| LDH | Lactate dehydrogenase |
| MAPK | Mitogen-activated protein kinase |
| MDA | Malondialdehyde |
| MDR1 | Multidrug resistance protein 1 |
| MRP | Multidrug-resistance-associated proteins |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NHEK | Normal human epidermal keratinocytes |
| factors Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| PC | Phosphatidylcholine |
| PI | Phosphatidylinositol |
| (PI3K)/AKT | Phosphatidylinositol 3-phosphatidyl kinase |
| PS | Phosphatidylserine |
| ROS | Reactive oxygen species |
| SM | Sphingomyelin |
| TMA-DPH | N,N,N-Trimethyl-4-(6-phenyl-1,3,5-hexatrien-1-yl)phenylammonium p-Toluenesulfonate |
| TNF-α | Tumor necrosis factor alpha |
| TNFR | Tumor necrosis factor receptor |
| UVA | Ultraviolet A |
References
- Gilaberte, Y.; Prieto-Torres, L.; Pastushenko, I.; Juarranz, Á. Anatomy and Function of the Skin. In Nanoscience in Dermatology; Hamblin, M.R., Avci, P., Prow, T.W., Eds.; Academic Press: Cambridge, MA, USA, 2016; Chapter 1; pp. 1–14. [Google Scholar]
- Nicolson, G.L.; Ferreira de Mattos, G. A brief introduction to some aspects of the fluid–mosaic model of cell membrane structure and its importance in membrane lipid replacement. Membranes 2021, 11, 947. [Google Scholar] [CrossRef] [PubMed]
- Sezgin, E.; Levental, I.; Mayor, S.; Eggeling, C. The mystery of membrane organization: Composition, regulation and roles of lipid rafts. Nat. Rev. Mol. Cell Biol. 2017, 18, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Szachowicz-Petelska, B.; Łuczaj, W.; Wroński, A.; Jastrząb, A.; Dobrzyńska, I. The differentia effect of cannabidiol on the composition and physicochemical properties of keratinocyte and fibroblast memnranes from psoriatic patients and healthy people. Membranes 2021, 11, 111. [Google Scholar] [CrossRef] [PubMed]
- Besaratinia, A.; Kim, S.I.; Pfeifer, G.P. Rapid repair of UVA-induced oxidized purines and persistence of UVB-induced dipyrimidine lesions determine the mutagenicity of sunlight in mouse cells. FASEB J. 2008, 22, 2379–2392. [Google Scholar] [CrossRef]
- Pavel, S. Light therapy (with UVA-1) for SLE patients: Is it a good or bad idea? Rheumatology 2006, 45, 653–655. [Google Scholar] [CrossRef]
- Juzeniene, A.; Moan, J. Beneficial effects of UV radiation other than via vitamin D production. Dermatoendocrinol 2012, 4, 109–117. [Google Scholar] [CrossRef]
- Gęgotek, A.; Biernacki, M.; Ambrożewicz, E.; Surażyński, A.; Wroński, A.; Skrzydlewska, E. The cross-talk between electrophiles, antioxidant defence and the endocannabinoid system in fibroblasts and keratinocytes after UVA and UVB irradiation. J. Dermatol. Sci. 2016, 81, 107–117. [Google Scholar] [CrossRef]
- Bickers, D.R.; Athar, M. Oxidative stress in the pathogenesis of skin disease. J. Investig. Dermatol. 2006, 126, 2565–2575. [Google Scholar] [CrossRef]
- Gęgotek, A.; Łuczaj, W.; Skrzydlewska, E. Effects of natural antioxidants on phospholipid and ceramide profiles of 3D-cultured skin fibroblasts exposed to UVA or UVB radiation. Antioxidants 2021, 10, 578. [Google Scholar] [CrossRef]
- Gęgotek, A.; Atalay, S.; Domingues, P.; Skrzydlewska, E. The differences in the proteome profile of cannabidiol-treated skin fibroblasts following UVA or UVB irradiation in 2D and 3D cell cultures. Cells 2019, 8, 995. [Google Scholar] [CrossRef]
- Łuczaj, W.; Jastrząb, A.; do Rosário Domingues, M.; Domingues, P.; Skrzydlewska, E. Changes in phospholipid/ceramide profiles and eicosanoid levels in the plasma of rats irradiated with UV rays and treated topically with cannabidiol. Int. J. Mol. Sci. 2021, 22, 8700. [Google Scholar] [CrossRef]
- Jarocka-Karpowicz, I.; Biernacki, M.; Wroński, A.; Gęgotek, A.; Skrzydlewska, E. Cannabidiol effects on phospholipid metabolism in keratinocytes from patients with psoriasis vulgaris. Biomolecules 2020, 10, 367. [Google Scholar] [CrossRef]
- Dobrzyńska, I.; Gęgotek, A.; Gajko, E.; Skrzydlewska, E.; Figaszewski, Z.A. Effects of rutin on the physicochemical properties of skin fibroblasts membrane disruption following UV radiation. Chem. Biol. Interact. 2018, 282, 29–35. [Google Scholar] [CrossRef]
- Coskun, U.; Simons, K. Cell membranes: The lipid perspective. Structure 2011, 19, 1543–1548. [Google Scholar] [CrossRef]
- Lundbaek, J.A. Regulation of membrane protein function by lipid bilayer elasticity—A single molecule technology to measure the bilayer properties experienced by an embedded protein. J. Phys. Condens. Matter. 2006, 18, S1305–S1344. [Google Scholar] [CrossRef]
- Atalay Ekiner, S.; Gęgotek, A.; Skrzydlewska, E. The molecular activity of cannabidiol in the regulation of Nrf2 system interacting with NF-κB pathway under oxidative stress. Redox Biol. 2022, 57, 102489. [Google Scholar] [CrossRef]
- Baswan, S.M.; E Klosner, A.; Glynn, K.; Rajgopal, A.; Malik, K.; Yim, S.; Stern, N. Therapeutic potential of cannabidiol (CBD) for skin health and disorders. Clin. Cosmet. Investig. Dermatol. 2020, 13, 927–942. [Google Scholar] [CrossRef]
- Perez, E.; Fernandez, J.R.; Fitzgerald, C.; Rouzard, K.; Tamura, M.; Savile, C. In vitro and clinical evaluation of cannabigerol (CBG) produced via yeast biosynthesis: A cannabinoid with a broad range of anti-inflammatory and skin health-boosting properties. Molecules 2022, 27, 491. [Google Scholar] [CrossRef]
- Pereira, S.R.; Hackett, B.; O’Driscoll, D.N.; Sun, M.C.; Downer, E.J. Cannabidiol modulation of oxidative stress and signalling. Neuronal Signal. 2021, 5, NS20200080. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cao, Z.; Guo, J.; Li, Q.; Zhu, W.; Kuang, Y.; Chen, X. Assessment of efficacy and safety of UV-based therapy for psoriasis: A network meta-analysis of randomized controlled trials. Ann. Med. 2022, 54, 159–169. [Google Scholar] [CrossRef]
- Casares, D.; Escribá, P.V.; Rosselló, C.A. Membrane lipid composition: Effect on membrane and organelle structure, function and compartmentalization and therapeutic avenues. Int. J. Mol. Sci. 2019, 20, 2167. [Google Scholar] [CrossRef] [PubMed]
- Jastrząb, A.; Jarocka-Karpowicz, I.; Markowska, A.; Wroński, A.; Gęgotek, A.; Skrzydlewska, E. Antioxidant and anti-inflammatory effect of cannabidiol contributes to the decreased lipid peroxidation of keratinocytes of rat skin exposed to UV radiation. Oxid. Med. Cell. Longev. 2021, 2021, 6647222. [Google Scholar] [CrossRef]
- Ansary, T.M.; Hossain, M.R.; Kamiya, K.; Komine, M.; Ohtsuki, M. Inflammatory molecules associated with ultraviolet radiation-mediated skin aging. Int. J. Mol. Sci. 2021, 22, 3974. [Google Scholar] [CrossRef] [PubMed]
- Łuczaj, W.; Dobrzyńska, I.; Wroński, A.; Domingues, M.R.; Domingues, P.; Skrzydlewska, E. Cannabidiol-mediated changes to the phospholipid profile of UVB-irradiated keratinocytes from psoriatic patients. Int. J. Mol. Sci. 2020, 21, 6592. [Google Scholar] [CrossRef] [PubMed]
- Dalmau, N.; Andrieu-Abadie, N.; Tauler, R.; Bedia, C. Phenotypic and lipidomic characterization of primary human epidermal keratinocytes exposed to simulated solar UV radiation. J. Dermatol. Sci. 2018, 92, 97–105. [Google Scholar] [CrossRef]
- Rozenova, K.A.; Deevska, G.M.; Karakashian, A.A.; Nikolova-Karakashian, M.N. Studies on the role of acid sphingomyelinase and ceramide in the regulation of tumor necrosis factor α (TNFα)-converting enzyme activity and TNFα secretion in macrophages. J. Biol. Chem. 2010, 285, 21103–21113. [Google Scholar] [CrossRef]
- Uchida, Y.; Hara, M.; Nishio, H.; Sidransky, E.; Inoue, S.; Otsuka, F.; Suzuki, A.; Elias, P.M.; Holleran, W.M.; Hamanaka, S. Epidermal sphingomyelins are precursors for selected stratum corneum ceramides. J. Lipid Res. 2000, 41, 2071–2082. [Google Scholar] [CrossRef]
- Gulshan, K.; Brubaker, G.; Wang, S.; Hazen, S.L.; Smith, J.D. Sphingomyelin depletion impairs anionic phospholipid inward translocation and induces cholesterol efflux. J. Biol. Chem. 2013, 288, 37166–37179. [Google Scholar] [CrossRef]
- Huang, H.; Kasumov, T.; Gatmaitan, P.; Heneghan, H.M.; Kashyap, S.R.; Schauer, P.R.; Brethauer, S.A.; Kirwan, J.P. Gastric bypass surgery reduces plasma ceramide subspecies and improves insulin sensitivity in severely obese patients. Obesity 2011, 19, 2235–2240. [Google Scholar] [CrossRef]
- Predescu, S.; Knezevic, I.; Bardita, C.; Neamu, R.F.; Brovcovych, V.; Predescu, D. Platelet activating factor-induced ceramide micro-domains drive endothelial NOS activation and contribute to barrier dysfunction. PLoS ONE 2013, 8, e75846. [Google Scholar] [CrossRef]
- Kim, S.; Kim, Y.; Lee, Y.; Chung, J.H. Ceramide accelerates ultraviolet-induced MMP-1 expression through JAK1/STAT-1 pathway in cultured human dermal fibroblasts. J. Lipid Res. 2008, 49, 2571–2581. [Google Scholar] [CrossRef]
- Stancevic, B.; Kolesnick, R. Ceramide-rich platforms in transmembrane signaling. FEBS Lett. 2010, 584, 1728–1740. [Google Scholar] [CrossRef]
- Moskot, M.; Bocheńska, K.; Jakóbkiewicz-Banecka, J.; Banecki, B.; Gabig-Cimińska, M. Abnormal sphingolipid world in inflammation specific for lysosomal storage diseases and skin disorders. Int. J. Mol. Sci. 2018, 19, 247. [Google Scholar] [CrossRef]
- Ren, Y.; Liu, X.; Geng, R.; Lu, Q.; Rao, R.; Tan, X.; Yang, X.; Liu, W. Increased level of α2,6-sialylated glycans on HaCaT cells induced by titanium dioxide nanoparticles under UV radiation. Nanomaterials 2018, 8, 253. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, G.-Y.; Han, Q.-L.; Wang, J.; Suriguga; Li, Y.; Yu, C.-H.; Li, Y.-R.; Yi, Z.-C. Phenolic metabolites of benzene induced caspase-dependent cytotoxicities to K562 cells accompanied with decrease in cell surface sialic acids. Environ. Toxicol. 2014, 29, 1437–1451. [Google Scholar] [CrossRef]
- Suzuki, O.; Abe, M. Galectin-1-mediated cell adhesion, invasion and cell death in human anaplastic large cell lymphoma: Regulatory roles of cell surface glycans. Int. J. Oncol. 2014, 44, 1433–1442. [Google Scholar] [CrossRef][Green Version]
- Meesmann, H.M.; Fehr, E.-M.; Kierschke, S.; Herrmann, M.; Bilyy, R.; Heyder, P.; Blank, N.; Krienke, S.; Lorenz, H.-M.; Schiller, M. Decrease of sialic acid residues as an eat-me signal on the surface of apoptotic lymphocytes. J. Cell Sci. 2010, 123, 3347–3356. [Google Scholar] [CrossRef]
- Jellusova, J.; Nitschke, L. Regulation of B cell functions by the sialic acid-binding receptors Siglec-G and CD22. Front. Immunol. 2012, 2, 96. [Google Scholar] [CrossRef]
- Dobrzyńska, I.; Szachowicz-Petelska, B.; Wroński, A.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Changes in the physicochemical properties of blood and skin cell membranes as a result of psoriasis vulgaris and psoriatic arthritis development. Int. J. Mol. Sci. 2020, 21, 9129. [Google Scholar] [CrossRef]
- Shlomovitz, I.; Speir, M.; Gerlic, M. Flipping the dogma–phosphatidylserine in non-apoptotic cell death. Cell Commun. Signal. 2019, 17, 139. [Google Scholar] [CrossRef]
- de la Haba, C.; Palacio, J.R.; Martínez, P.; Morros, A. Effect of oxidative stress on plasma membrane fluidity of THP-1 induced macrophages. Biochim. Biophys. Acta 2013, 1828, 357–364. [Google Scholar] [CrossRef] [PubMed]
- George, K.S.; Elyassaki, W.; Wu, Q.; Wu, S. The role of cholesterol in UV light B-induced apoptosis. Photochem. Photobiol. 2012, 88, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wu, S. Lipid rafts association and anti-apoptotic function of prohibitin in ultraviolet B light-irradiated HaCaT keratinocytes. Exp. Dermatol. 2012, 21, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Miller, Y.I.; Navia-Pelaez, J.M.; Corr, M.; Yaksh, T.L. Lipid rafts in glial cells: Role in neuroinflammation and pain processing. J. Lipid Res. 2020, 61, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Navia-Pelaez, J.M.; Agatisa-Boyle, C.; Choi, S.-H.; Kim, Y.S.; Li, S.; Alekseeva, E.; Weldy, K.; Miller, Y.I. differential expression of inflammarafts in macrophage foam cells and in nonfoamy macrophages in atherosclerotic lesions-brief report. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 323–329. [Google Scholar] [CrossRef]
- Lu, S.P.; Lin Feng, M.H.; Huang, H.L.; Huang, Y.C.; Tsou, W.I.; Lai, M.Z. Reactive oxygen species promote raft formation in T lymphocytes. Free Radic Biol Med. 2007, 42, 936–944. [Google Scholar] [CrossRef]
- Gęgotek, A.; Jastrząb, A.; Dobrzyńska, M.; Biernacki, M.; Skrzydlewska, E. Exogenous antioxidants impact on uv-induced changes in membrane phospholipids and the effectiveness of the endocannabinoid system in human skin cells. Antioxidants 2021, 10, 1260. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Gęgotek, A.; Skrzydlewska, E. Biological effect of protein modifications by lipid peroxidation products. Chem. Phys. Lipids 2019, 221, 46–52. [Google Scholar] [CrossRef]
- Gentile, F.; Arcaro, A.; Pizzimenti, S.; Daga, M.; Cetrangolo, G.P.; Dianzani, C.; Lepore, A.; Graf, M.; Ames, P.R.J.; Barrera, G. DNA damage by lipid peroxidation products: Implications in cancer, inflammation and autoimmunity. AIMS Genet. 2017, 4, 103–137. [Google Scholar] [CrossRef]
- Baron, J.M.; Höller, D.; Schiffer, R.; Frankenberg, S.; Neis, M.; Merk, H.F.; Jugert, F.K. Expression of multiple cytochrome p450 enzymes and multidrug resistance-associated transport proteins in human skin keratinocytes. J. Investig. Dermatol. 2001, 116, 541–548. [Google Scholar] [CrossRef]
- Ven, R.; Lindenberg, J.J.; Reurs, A.W.; Scheper, R.J.; Scheffer, G.L.; Gruijl, T.D. Preferential Langerhans cell differentiation from CD34+ precursors upon introduction of ABCG2 (BCRP). Immunol. Cell Biol. 2012, 90, 206–215. [Google Scholar] [CrossRef]
- Atalay, S.; Dobrzyńska, I.; Gęgotek, A.; Skrzydlewska, E. Cannabidiol protects keratinocyte cell membranes following exposure to UVB and hydrogen peroxide. Redox Biol. 2020, 36, 101613. [Google Scholar] [CrossRef]
- Buelna-Chontal, M.; Zazueta, C. Redox activation of Nrf2 & NF-κB: A double end sword? Cell Signal. 2013, 25, 2548–2557. [Google Scholar]
- Jastrząb, A.; Gęgotek, A.; Skrzydlewska, E. Cannabidiol regulates the expression of keratinocyte proteins involved in the inflammation process through transcriptional regulation. Cells 2019, 8, 827. [Google Scholar] [CrossRef]
- Wang, P.; Sachar, M.; Lu, J.; Shehu, A.I.; Zhu, J.; Chen, J.; Liu, K.; Anderson, K.E.; Xie, W.; Gonzalez, F.J.; et al. The essential role of the transporter ABCG2 in the pathophysiology of erythropoietic protoporphyria. Sci Adv. 2019, 5, eaaw6127. [Google Scholar] [CrossRef]
- Atalay, S.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Antioxidative and anti-inflammatory properties of cannabidiol. Antioxidants 2019, 9, 21. [Google Scholar] [CrossRef]
- Nachnani, R.; Raup-Konsavage, W.M.; Vrana, K.E. The pharmacological case for cannabigerol. J. Pharmacol. Exp. Ther. 2021, 376, 204–212. [Google Scholar] [CrossRef]
- Hamelink, C.; Hampson, A.; Wink, D.A.; Eiden, L.E.; Eskay, R.L. Comparison of cannabidiol, antioxidants, and diuretics in reversing binge ethanol-induced neurotoxicity. J. Pharmacol. Exp. Therapeut. 2005, 314, 780–788. [Google Scholar] [CrossRef]
- Borges, R.S.; da Silva, A. Cannabidiol as an antioxidant. In Handbook of Cannabis and Related Pathologies: Biology, Pharmacology, Diagnosis, and Treatment; Preedy, V.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 123–130. [Google Scholar]
- Zhao, X.R.; Gonzales, N.; Aronowski, J. Pleiotropic role of PPARγ in intracerebral hemorrhage: An intricate system involving Nrf2, RXR, and NF-κB. CNS Neurosci. Ther. 2015, 21, 357–366. [Google Scholar] [CrossRef]
- Wroński, A.; Jarocka-Karpowicz, I.; Stasiewicz, A.; Skrzydlewska, E. Phytocannabinoids in the pharmacotherapy of psoriasis. Molecules 2023, 28, 1192. [Google Scholar] [CrossRef] [PubMed]
- Han, K.H.; Lim, S.; Ryu, J.; Lee, C.-W.; Kim, Y.; Kang, J.-H.; Kang, S.-S.; Ahn, Y.K.; Park, C.-S.; Kim, J.J. CB1 and CB2 cannabinoid receptors differentially regulate the production of reactive oxygen species by macrophages. Cardiovasc. Res. 2009, 84, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Navarro, G.; Varani, K.; Lillo, A.; Vincenzi, F.; Rivas-Santisteban, R.; Raïch, I.; Reyes-Resina, I.; Ferreiro-Vera, C.; Borea, P.A.; de Medina, V.S.; et al. Pharmacological data of cannabidiol- and cannabigerol-type phytocannabinoids acting on cannabinoid CB1, CB2 and CB1/CB2 heteromer receptors. Pharmacol. Res. 2020, 159, 104940. [Google Scholar] [CrossRef] [PubMed]
- Jastrząb, A.; Jarocka-Karpowicz, I.; Skrzydlewska, E. The origin and biomedical relevance of cannabigerol. Int. J. Mol. Sci. 2022, 23, 7929. [Google Scholar] [CrossRef] [PubMed]
- Calapai, F.; Cardia, L.; Esposito, E.; Ammendolia, I.; Mondello, C.; Giudice, R.L.; Gangemi, S.; Calapai, G.; Mannucci, C. Pharmacological aspects and biological effects of cannabigerol and its synthetic derivatives. Evid. Based Complement. Alternat. Med. 2022, 2022, 3336516. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, S.E. An update on PPAR activation by cannabinoids. Br. J. Pharmacol. 2016, 173, 1899–1910. [Google Scholar] [CrossRef]
- O’Sullivan, S.E.; Kendall, D.A. Cannabinoid activation of peroxisome proliferator-activated receptors: Potential for modulation of inflammatory disease. Immunobiology 2010, 215, 611–616. [Google Scholar] [CrossRef]
- Callén, L.; Moreno, E.; Barroso-Chinea, P.; Moreno-Delgado, D.; Cortés, A.; Mallol, J.; Casadó, V.; Lanciego, J.L.; Franco, R.; Lluis, C.; et al. Cannabinoid receptors CB1 and CB2 form functional heteromers in brain. J. Biol. Chem. 2012, 287, 20851–20865. [Google Scholar] [CrossRef]
- Giacoppo, S.; Galuppo, M.; Pollastro, F.; Grassi, G.; Bramanti, P.; Mazzon, E. A new formulation of cannabidiol in cream shows therapeutic effects in a mouse model of experimental autoimmune encephalomyelitis. Daru 2015, 23, 48. [Google Scholar] [CrossRef]
- Scheau, C.; Badarau, I.A.; Mihai, L.-G.; Scheau, A.-E.; Costache, D.O.; Constantin, C.; Calina, D.; Caruntu, C.; Costache, R.S.; Caruntu, A. Cannabinoids in the pathophysiology of skin inflammation. Molecules 2020, 25, 652. [Google Scholar] [CrossRef]
- Guard, S.E.; Chapnick, D.A.; Poss, Z.C.; Ebmeier, C.C.; Jacobsen, J.; Nemkov, T.; Ball, K.A.; Webb, K.J.; Simpson, H.L.; Coleman, S.; et al. Old multiomic analysis reveals disruption of cholesterol homeostasis by cannabidiol in human cell lines. Mol. Cell Proteom. 2022, 21, 100262. [Google Scholar] [CrossRef]
- Luz-Veiga, M.; Amorim, M.; Pinto-Ribeiro, I.; Oliveira, A.L.S.; Silva, S.; Pimentel, L.L.; Rodríguez-Alcalá, L.M.; Madureira, R.; Pintado, M.; Azevedo-Silva, J.; et al. Cannabidiol and Cannabigerol Exert Antimicrobial Activity without Compromising Skin Microbiota. Int. J. Mol. Sci. 2023, 24, 2389. [Google Scholar] [CrossRef]
- Mammana, S.; Cavalli, E.; Gugliandolo, A.; Silvestro, S.; Pollastro, F.; Bramanti, P.; Mazzon, E. Could the combination of two non-psychotropic cannabinoids counteract neuroinflammation? Effectiveness of cannabidiol associated with cannabigerol. Medicina 2019, 55, 747. [Google Scholar] [CrossRef]
- Lah, T.T.; Novak, M.; Pena Almidon, M.A.; Marinelli, O.; Žvar Baškovič, B.; Majc, B.; Mlinar, M.; Bošnjak, R.; Breznik, B.; Zomer, R.; et al. Cannabigerol Is a Potential Therapeutic Agent in a Novel Combined Therapy for Glioblastoma. Cells 2021, 10, 340. [Google Scholar] [CrossRef]
- Fotakis, G.; Timbrell, J.A. In vitro cytotoxicity assays: Comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol. Lett. 2006, 160, 171–177. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Bartlett, E.M.; Lewis, D.H. Spectrophotometric determination of phosphate esters in the presence and absence of orthophosphate. Anal. Biochem. 1970, 36, 159–167. [Google Scholar] [CrossRef]
- Łuczaj, W.; Wroński, A.; Domingues, P.; Domingues, M.R.; Skrzydlewska, E. Lipidomic analysis reveals specific differences between fibroblast and keratinocyte ceramide profile of patients with psoriasis vulgaris. Molecules 2020, 25, 630. [Google Scholar] [CrossRef]
- Jourdian, G.W.; Dean, L.; Roseman, S. The sialic acids. XI. A periodate-resorcinol method for the quantitative estimation of free sialic acids and their glycosides. J. Biol. Chem. 1971, 246, 430–435. [Google Scholar] [CrossRef]
- Luo, X.P.; Yazdanpanah, M.; Bhooi, N.; Lehotay, D.C. Determination of aldehydes and other lipid peroxidation products in biological samples by gas chromatography-mass spectrometry. Anal. Biochem. 1995, 228, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Gęgotek, A.; Bielawska, K.; Biernacki, M.; Zaręba, I.; Surażyński, A.; Skrzydlewska, E. Comparison of protective effect of ascorbic acid on redox and endocannabinoid systems interactions in in vitro cultured human skin fibroblasts exposed to UV radiation and hydrogen peroxide. Arch. Dermatol. Res. 2017, 309, 285–303. [Google Scholar] [CrossRef] [PubMed]
- Dobrzyńska, I.; Szachowicz-Petelska, B.; Weresa, J.; Figaszewski, Z.A.; Skrzydlewska, E. Changes in physicochemical properties of kidney cells membrane as a consequence of hypertension and treatment of hypertensive rats with FAAH inhibitor. Chem. Biol. Interact. 2019, 299, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Olchowik-Grabarek, E.; Sekowski, S.; Kwiatek, A.; Płaczkiewicz, J.; Abdulladjanova, N.; Shlyonsky, V.; Swiecicka, I.; Zamaraeva, M. The Structural Changes in the Membranes of Staphylococcus aureus Caused by Hydrolysable Tannins Witness Their Antibacterial Activity. Membranes 2022, 12, 1124. [Google Scholar] [CrossRef]
- Czajkowska-Szczykowska, D.; Olchowik-Grabarek, E.; Sękowski, S.; Żarkowski, J.; Morzycki, J.W. Concise synthesis of E/F ring spiroethers from tigogenin. Carbaanalogs of steroidal sapogenins and their biological activity. J. Steroid Biochem. Mol. Biol. 2022, 224, 106174. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wroński, A.; Dobrzyńska, I.; Sękowski, S.; Łuczaj, W.; Olchowik-Grabarek, E.; Skrzydlewska, E. Cannabidiol and Cannabigerol Modify the Composition and Physicochemical Properties of Keratinocyte Membranes Exposed to UVA. Int. J. Mol. Sci. 2023, 24, 12424. https://doi.org/10.3390/ijms241512424
Wroński A, Dobrzyńska I, Sękowski S, Łuczaj W, Olchowik-Grabarek E, Skrzydlewska E. Cannabidiol and Cannabigerol Modify the Composition and Physicochemical Properties of Keratinocyte Membranes Exposed to UVA. International Journal of Molecular Sciences. 2023; 24(15):12424. https://doi.org/10.3390/ijms241512424
Chicago/Turabian StyleWroński, Adam, Izabela Dobrzyńska, Szymon Sękowski, Wojciech Łuczaj, Ewa Olchowik-Grabarek, and Elżbieta Skrzydlewska. 2023. "Cannabidiol and Cannabigerol Modify the Composition and Physicochemical Properties of Keratinocyte Membranes Exposed to UVA" International Journal of Molecular Sciences 24, no. 15: 12424. https://doi.org/10.3390/ijms241512424
APA StyleWroński, A., Dobrzyńska, I., Sękowski, S., Łuczaj, W., Olchowik-Grabarek, E., & Skrzydlewska, E. (2023). Cannabidiol and Cannabigerol Modify the Composition and Physicochemical Properties of Keratinocyte Membranes Exposed to UVA. International Journal of Molecular Sciences, 24(15), 12424. https://doi.org/10.3390/ijms241512424









