Polarization of Melatonin-Modulated Colostrum Macrophages in the Presence of Breast Tumor Cell Lines
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Colostrum Sampling and Separation of Colostral Cells
4.2. Melatonin Determination
4.3. Melatonin Receptor 1A (MTNR1A) and Melatonin Receptor 1B (MTNR1B) Determination
4.4. Tumor Cell Lines and Cell Culture
4.5. Melatonin Hormone Modulation
4.6. Immunophenotyping and Macrophage Identification and Polarization
4.7. Cytokine Determination
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Noy, R.; Pollard, J.W. Tumor-associated macrophages: From mechanisms to therapy. Immunity 2014, 17, 49–61. [Google Scholar] [CrossRef]
- Cassetta, L.; Pollard, J.W. Repolarizing macrophages improves breast cancer therapy. Cell Res. 2017, 27, 963–964. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T.; Natoli, G. Transcriptional regulation of macrophage polarization: Enabling diversity with identity. Nat. Rev. Immunol. 2011, 11, 750–761. [Google Scholar] [CrossRef] [PubMed]
- Rippaus, N.; Taggart, D.; Williams, J.; Andreou, T.; Wurdak, H.; Wronski, K.; Lorger, M. Metastatic site-specific polarization of macrophages in intracranial breast cancer metastases. Oncotarget 2016, 7, 41473–41487. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.-H.; Chen, F.-M.; Lin, Y.-C.; Tsai, M.-L.; Wang, S.-L.; Chen, Y.-C.; Chen, Y.-T.; Hou, M.-F. Altered monocyte differentiation and macrophage polarization patterns in patients with breast cancer. BMC Cancer 2018, 18, 366–375. [Google Scholar] [CrossRef]
- Vallerand, D.; Massonnet, G.; Kébir, F.; Gentien, D.; Maciorowski, Z.; De la Grange, P.; Sigal-Zafrani, B.; Richardson, M.; Humbert, S.; Thuleau, A.; et al. Characterization of Breast Cancer Preclinical Models Reveals a Specific Pattern of Macrophage Polarization. PLoS ONE 2016, 11, e0157670. [Google Scholar] [CrossRef]
- Ma, Y.; Ren, Y.; Dai, Z.-J.; Wu, C.-J.; Ji, Y.-H.; Xu, J. IL-6, IL-8 and TNF-α levels correlate with disease stage in breast cancer patients. Adv. Clin. Exp. Med. 2017, 3, 421–426. [Google Scholar] [CrossRef]
- Morais, T.C.; Honorio-França, A.C.; Silva, R.R.; Fujimori, M.; Fagundes, D.L.G.; França, E.L. Temporal fluctuations of cytokine concentrations in human milk. Biol. Rhythm. Res. 2015, 46, 811–821. [Google Scholar] [CrossRef]
- Fujimori, M.; França, E.L.; Morais, T.C.; Fiorin, V.; de Abreu, L.C.; Honório-França, A.C. Cytokine and adipokine are biofactors can act in blood and colostrum of obese mothers. Biofactors 2017, 23, 45–51. [Google Scholar] [CrossRef]
- Fagundes, D.L.G.; França, E.L.; Gonzatti, M.A.B.; Rudge, M.V.C.; Calderon, I.M.P.; Honorio-França, A.C. The modulatory role of cytokines IL-4 and IL-17 in the functional activity of phagocytes in diabetic pregnant women. Apmis 2018, 126, 56–64. [Google Scholar] [CrossRef]
- Fagundes, D.L.G.; França, E.L.; Hara, C.C.P.; Honorio-França, A.C. Immunomodulatory effects of poly (Etilene Glicol) Mi-crospheres adsorbed with cortisol on the activity of colostrum phagocytes. Int. J. Pharmacol. 2012, 1, 510–518. [Google Scholar] [CrossRef]
- Honorio-França, A.C.; Hara, C.C.P.; Ormonde, J.V.S.; Nunes, G.T.; França, E.L. Human colostrum melatonin exhibits a day-night variation and modulates the activity of colostral phagocytes. J. Appl. Biomed. 2013, 11, 153–162. [Google Scholar] [CrossRef]
- Pereira, Q.L.C.; Hara, C.C.P.; Fernandes, R.T.S.; Fagundes, D.L.G.; França-Botelho, A.C.; Gomes, M.A.; França, E.L.; Honorio-França, A.C. Human colostrum action against Giardia lamblia infection influenced by hormones and advanced maternal age. Parasitol. Res. 2018, 117, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Ekmekcioglu, C. Melatonin receptors in humans: Biological role and clinical relevance. Biomed. Pharmacother. 2006, 60, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R.; Cardinali, D.P.; Srinivasan, V.; Spence, D.W.; Brown, G.M.; Pandi-Perumal, S.R. Melatonin—A pleiotropic, orchestrating regulator molecule. Prog. Neurobiol. 2011, 93, 350–384. [Google Scholar] [CrossRef]
- Li, D.Y.; Smith, D.G.; Hardeland, R.; Yang, M.Y.; Xu, H.L.; Zhang, L.; Yin, H.D.; Zhu, Q. Melatonin Receptor Genes in Vertebrates. Int. J. Mol. Sci. 2013, 14, 11208–11223. [Google Scholar] [CrossRef]
- França, E.L.; Honorio-França, A.C.; Fernandes, R.T.S.; Marins, C.M.F.; Pereira, C.C.S.; Varotti, F.P. The Effect of Melatonin Adsorbed to Polyethylene Glycol Microspheres on the Survival of MCF-7 Cells. Neuroimmunomodulation 2016, 23, 27–32. [Google Scholar] [CrossRef]
- Nicholals, C.; Pinto, A.R.; Li, H.; Li, L.; Wang, L.; Simpson, R.; Liu, J.-P. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) induces cancer cell senescence by interacting with telomerase RNA component. Proc. Natl. Acad. Sci. USA 2012, 109, 13308–13313. [Google Scholar] [CrossRef]
- Jiang, X.; Shapiro, D.J. The immune system and inflammation in breast cancer. Mol. Cell. Endocrinol. 2014, 382, 673–682. [Google Scholar] [CrossRef]
- Ribeiro, A.A.L.; Silva, F.H.; Cotrim, A.C.M.; Deluque, A.L.; Marchi, P.G.F.; Fagundes, D.L.G.; Sousa, P.C.; Franca, E.L.; Honorio-Franca, A.C. Herbal Mixture Adsorbed to Polyethylene Glycol Microspheres Induces Apoptotic Effects on Breast Cancer Cells. Curr. Drug Deliv. 2018, 14, 227–234. [Google Scholar] [CrossRef]
- Silva, F.H.; Ribeiro, A.A.L.; Deluque, A.L.; Cotrim, A.C.M.; de Marchi, P.G.F.; França, E.L.; Honorio-França, A.C. Effects of barium chloride adsorbed to polyethylene glycol (PEG) microspheres on co-culture of human blood mononuclear cell and breast cancer cell lines (MCF-7). Immunopharmacol. Immunotoxicol. 2018, 40, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Honorio-frança, A.C.; Fernandes, R.T.S.; Tozetti, I.A.; Fuhjimori, M.; Pinho, C.L.C.; Fagundes-Triches, D.L.G.; França, E.L. Mechanism Anti-Tumor of IgA-based Delivery System on the Human Colostral Mononuclear Cells via Fcα Receptor. Biointerface Res. Appl. Chem. 2021, 11, 14906–14917. [Google Scholar]
- Honorio-França, A.C.; Carvalho, M.P.; Isaac, L.; Trabulsi, L.R.; Carneiro-Sampaio, M.M.S. Colostral Mononuclear Phagocytes are Able to Kill Enteropathogenic Escherichia coli Opsonized with Colostral IgA. Scand. J. Immunol. 1997, 46, 59–66. [Google Scholar] [CrossRef]
- Honorio-França, A.C.; Launay, P.; Carneiro-Sampaio, M.M.; Monteiro, R.C. Colostral neutrophils express IgA Fc receptors (CD89) lacking y chain association that mediate non-inflammatory properties of secretory IgA. J. Leuk. Biol. 2001, 69, 289–296. [Google Scholar] [CrossRef]
- Russo, J.; Balogh, G.A.; Russo, I.H. Full-term Pregnancy Induces a Specific Genomic Signature in the Human Breast. Cancer Epidemiol. Biomarkers Prev. 2008, 17, 17–51. [Google Scholar] [CrossRef] [PubMed]
- Russo, J.; Moral, R.; Balogh, G.A. The protective role of pregnancy in breast cancer. Breast Cancer Res. 2005, 7, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Jacobsen, K.H. A Systematic Review of the Association between Breastfeeding and Breast Cancer. J. Women’s Health 2008, 17, 1635–1645. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.T.S.; França, E.L.; Triches, D.L.G.F.; Fujimori, M.; Machi, P.G.F.; Massmman, P.F.; Tozetti, I.A.; Honor-io-França, A.C. Nanodoses of melatonin induces apoptosis on human breast cancer cells co-cultured with colostrum cells. Biointerface Res. 2019, 9, 4416–4423. [Google Scholar] [CrossRef]
- Witt-Enderby, P.A.; Radio, N.M.; Doctor, J.S.; Davis, V.L. Therapeutic treatments potentially mediated by melatonin receptors: Potential clinical uses in the prevention of osteoporosis, cancer and as an adjuvant therapy. J. Pineal Res. 2006, 41, 297–305. [Google Scholar] [CrossRef]
- Liu, J.; Clough, S.J.; Hutchinson, A.J.; Adamah-Biassi, E.B.; Popovska-Gorevski, M.; Dubocovich, M.L. MT1 and MT2 Melatonin Receptors: A Therapeutic Perspective. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 361–383. [Google Scholar] [CrossRef]
- Morceli, G.; Honorio-França, A.C.; Fagundes, D.L.G.; Calderon, I.M.P.; França, E.L. Antioxidant Effect of Melatonin on the Functional Activity of Colostral Phagocytes in Diabetic Women. PLoS ONE 2013, 8, e56915. [Google Scholar] [CrossRef] [PubMed]
- Gerdin, M.J.; Masana, M.I.; Rivera-Bermúdez, M.A.; Hudson, R.L.; Earnest, D.J.; Gillette, M.U.; Dubocovich, M.L. Melatonin desensitizes endogenous MT2 melatonin receptors in the rat suprachiasmatic nucleus: Relevance for defining the periods of sensitivity of the mammalian circadian clock to melatonin. FASEB J. 2004, 18, 1646–1656. [Google Scholar] [CrossRef] [PubMed]
- Skytthe, M.K.; Graversen, J.H.; Moestrup, S.K. Targeting of CD163+ Macrophages in Inflammatory and Malignant Diseases. Int. J. Mol. Sci. 2020, 21, 5497. [Google Scholar] [CrossRef]
- Yao, Y.; Xu, X.-H.; Jin, L. Macrophage Polarization in Physiological and Pathological Pregnancy. Front. Immunol. 2019, 10, 792. [Google Scholar] [CrossRef]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef]
- Murray, P.J. Macrophage Polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef]
- Appari, M.; Channon, K.M.; McNeill, E. Metabolic Regulation of Adipose Tissue Macrophage Function in Obesity and Diabetes. Antioxid. Redox Signal. 2018, 29, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Jayasingam, S.D.; Citartan, M.; Thang, T.H.; Mat Zin, A.A.; Ang, K.C.; Ch’ng, E.S. Evaluating the Polarization of Tumor-Associated Macrophages Into M1 and M2 Phenotypes in Human Cancer Tissue: Technicalities and Challenges in Routine Clinical Practice. Front. Oncol. 2020, 9, 1512. [Google Scholar] [CrossRef]
- Garg, S.K.; Delaney, C.; Shi, H.; Yung, R. Changes in Adipose Tissue Macrophages and T Cells During Aging. Crit. Rev. Immunol. 2014, 34, 1–14. [Google Scholar] [CrossRef]
- Evans, B.J.; Haskard, D.O.; Sempowksi, G.; Landis, R.C. Evolution of the Macrophage CD163 Phenotype and Cytokine Profiles in a Human Model of Resolving Inflammation. Int. J. Inflamm. 2013, 2013, 780502. [Google Scholar] [CrossRef]
- Hara, C.C.P.; Honorio-França, A.C.; Fagundes, D.L.G.; Guimarães, P.C.L.; França, E.L. Melatonin Nanoparticles Adsorbed to Polyethylene Glycol Microspheres as Activators of Human Colostrum Macrophages. J. Nanomater. 2013, 2013, 973179. [Google Scholar] [CrossRef]
- França, E.L.; Bitencourt, R.V.; Fujimori, M.; Morais, T.C.; Calderon, M.P.; Honorio-França, A.C. Human colostral phagocytes eliminate enterotoxigenic Escherichia coli opsonized by colostrum supernatant. J. Microbiol. Immunol. Infect. 2011, 44, 1–7. [Google Scholar] [CrossRef]
- Panahipour, L.; Kochergina, E.; Kreissl, A.; Haiden, N.; Gruber, R. Milk modulates macrophage polarization in vitro. Cytokine: X 2019, 1, 100009. [Google Scholar] [CrossRef]
- Anhe, G.F.; Caperuto, L.C.; Souza, L.C. Melatonin induces tyrosine phosphorylation of IRS-1 and JAK-2 in the rat pré-optic hypothalamic area in vivo. Diabetes 2002, 51, 453–454. [Google Scholar]
- Xia, Y.; Chen, S.; Zeng, S.; Zhao, Y.; Zhu, C.; Deng, B.; Zhu, G.; Yin, Y.; Wang, W.; Hardeland, R.; et al. Melatonin in macrophage biology: Current understanding and future perspectives. J. Pineal Res. 2019, 66, e12547. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Medeiros, T.X.; Sové, R.J.; Annex, B.H.; Popel, A.S. A data-driven computational model enables integrative and mechanistic characterization of dynamic macrophage polarization. iScience 2021, 24, 102112. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Liu, M.; Xu, Z.; Li, Y.; Guo, H.; Ge, Y.; Liu, Y.; Zheng, D.; Shi, J. A double feedback loop mediated by microRNA-23a/27a/24-2 regulation M1 versus M2 macrophage polarization and thus regulates cancer progression. Oncotarget 2016, 7, 13502–13519. [Google Scholar] [CrossRef]
- Kartikasari, A.E.R.; Huertas, C.S.; Mitchell, A.; Plebanski, M. Tumor-Induced Inflammatory Cytokines and the Emerging Diagnostic Devices for Cancer Detection and Prognosis. Front. Oncol. 2021, 12, 692142. [Google Scholar] [CrossRef]
- Liu, Z.-W.; Zhang, Y.-M.; Zhang, L.-Y.; Zhou, T.; Li, Y.-Y.; Zhou, G.-C.; Miao, Z.-M.; Shang, M.; He, J.-P.; Ding, N.; et al. Duality of Interactions Between TGF-β and TNF-α During Tumor Formation. Front. Immunol. 2022, 12, 810286. [Google Scholar] [CrossRef]
- Zheng, X.; Turkowski, K.; Mora, J.; Brüne, B.; Seeger, W.; Weigert, A.; Savai, R. Redirecting tumor-associated macrophages to become tumoricidal effectors as a novel strategy for cancer therapy. Oncotarget 2017, 8, 48436–48452. [Google Scholar] [CrossRef]
- Shen, Z.; Zhou, R.; Liu, C.; Wang, Y.; Zhan, W.; Shaos, Z.; Liu, J.; Zhang, F.; Xu, L.; Zhou, X.; et al. MicroRNA-105 is involved in TNF-α-related tumor microenvironment enhanced colorectal cancer progression. Cell Death Dis. 2017, 8, 3213. [Google Scholar] [CrossRef]
- Si, H.; Lu, H.; Yang, X.; Mattox, A.; Jang, M.; Bian, Y.; Sano, E.; Viadiu, H.; Yan, B.; Yau, C.L.; et al. TNF-α modulates genome-wide redistribution of Np63α/TAp73 and NF-κB c-REL interactive binding on TP53 and AP-1 motifs to promote an oncogenic gene program in squamous cancer. Oncogene 2016, 35, 5781–5794. [Google Scholar] [CrossRef] [PubMed]
- Laha, D.; Grant, R.; Mishra, P.; Nilubol, N. The Role of Tumor Necrosis Factor in Manipulating the Immunological Response of Tumor Microenvironment. Front. Immunol. 2021, 12, 656908. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wei, Y.; Yang, W.; Huang, Q.; Chen, Y.; Zeng, K.; Chen, J. IL-6: The Link Between Inflammation, Immunity and Breast Cancer. Front. Oncol. 2022, 12, 903800. [Google Scholar] [CrossRef]
- Lee, O.K.; Park, Y.; Seo, M.W.; Lee, D.S. IL-1β induces IL-6 production and increases invasiveness and estrogen-independent growth in a TG2-dependent manner in human breast cancer cells. BMC Cancer 2016, 16, 724. [Google Scholar] [CrossRef]
- Carrillo-Vico, A.; Lardone, P.J.; Alvarez-Sánchez, N.; Rodríguez-Rodríguez, A.; Guerrero, J.M. Melatonina: Protegendo o sistema imunológico. Int. J. Mol. Sci. 2013, 14, 8638–8683. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Cui, P.-L.; Yao, S.-W.; Xu, Y.-Q.; Yang, Z.-X. Melatonin inhibits the expression of vascular endothelial growth factor in pancreatic cancer cells. Chin. J. Cancer Res. 2012, 24, 310–316. [Google Scholar] [CrossRef]
- Esposito, E.; Cuzzocrea, S. Anti-inflammatory Activity of Melatonin in Central Nervous System. Curr. Neuropharmacol. 2010, 8, 228–242. [Google Scholar] [CrossRef]
- Qiu, X.; Liang, T.; Wu, Z.; Zhu, Y.; Gao, W.; Gao, B.; Qiu, J.; Wang, X.; Chen, T.; Deng, Z.; et al. Melatonin reverses tumor necrosis factor-alpha-induced metabolic disturbance of human nucleus pulposus cells via MTNR1B/Gαi2/YAP signaling. Int. J. Biol. Sci. 2022, 18, 2202–2219. [Google Scholar] [CrossRef]
- Russo, R.C.; Garcia, C.C.; Teixeira, M.M.; Amaral, F.A. The CXCL8/IL-8 chemokine family and its receptors in inflammatory diseases. Expert Rev. Clin. Immunol. 2014, 10, 593–619. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, C.; Varì, R.; Scazzocchio, B.; Di Benedetto, R.; Files, C.; Masella, R. Polyphenols, intracellular signalling and in-flammation. Ann. Ist. Super Sanita 2007, 43, 394–405. [Google Scholar] [PubMed]
- Deng, F.; Weng, Y.; Li, X.; Wang, T.; Fan, M.; Shi, Q. Overexpression of IL-8 promotes cell migration via PI3K-Akt signaling pathway and EMT in triple-negative breast cancer. Pathol. Res. Pr. 2020, 223, 152824. [Google Scholar] [CrossRef] [PubMed]
- Hanker, L.C.; Rody, A.; Holtrich, U.; Pusztai, L.; Ruckhaeberle, E.; Liedtke, C.; Ahr, A.; Heinrich, T.M.; Sänger, N.; Becker, S.; et al. Prognostic evaluation of the B cell/IL-8 metagene in different intrinsic breast cancer subtypes. Breast Cancer Res. Treat. 2013, 137, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Todorović-Raković, N.; Milovanović, J. Interleukin-8 in Breast Cancer Progression. J. Interf. Cytokine Res. 2013, 33, 563–570. [Google Scholar] [CrossRef]
- Polat, A.; Tunc, T.; Erdem, G.; Yerebasmaz, N.; Tas, A.; Beken, S.; Basbozkurt, G.; Saldir, M.; Zenciroglu, A.; Yaman, H. Interleukin-8 and Its Receptors in Human Milk from Mothers of Full-Term and Premature Infants. Breastfeed. Med. 2016, 11, 247–251. [Google Scholar] [CrossRef]
- Ustundag, B.; Yilmaz, E.; Dogan, Y.; Akarsu, S.; Canatan, H.; Halifeoglu, I.; Cikim, G.; Denizmen Aygun, A. Levels of cytokines (IL-1β, IL-2, IL-6, IL-8, TNF-α) and trace elements (Zn, Cu) in breast milk from mothers of preterm and term infants. Mediat. Inflamm. 2005, 2005, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Hirahara, K.; Vahedi, G.; Ghoreschi, K.; Yang, X.-P.; Nakayamada, S.; Kanno, Y.; O’shea, J.J.; Laurence, A. Helper T-cell differentiation and plasticity: Insights from epigenetics. Immunology 2011, 134, 235–245. [Google Scholar] [CrossRef]
- Xie, K. Interleukin-8 and human cancer biology. Cytokine Growth Factor Rev. 2001, 12, 375–391. [Google Scholar] [CrossRef]
- Kim, G.-D.; Lee, S.E.; Kim, T.-H.; Jin, Y.-H.; Park, Y.S.; Park, C.-S. Melatonin suppresses acrolein-induced IL-8 production in human pulmonary fibroblasts. J. Pineal Res. 2011, 52, 356–364. [Google Scholar] [CrossRef]
- Benoit, M.; Desnues, B.; Mege, J.-L. Macrophage Polarization in Bacterial Infections. J. Immunol. 2008, 181, 3733–3739. [Google Scholar] [CrossRef]
- Kwiecień, I.; Polubiec-Kownacka, M.; Dziedzic, D.; Wołosz, D.; Rzepecki, P.; Domagała-Kulawik, J. CD163 and CCR7 as markers for macrophage polarization in lung cancer microenvironment. Central Eur. J. Immunol. 2019, 44, 395–402. [Google Scholar] [CrossRef] [PubMed]
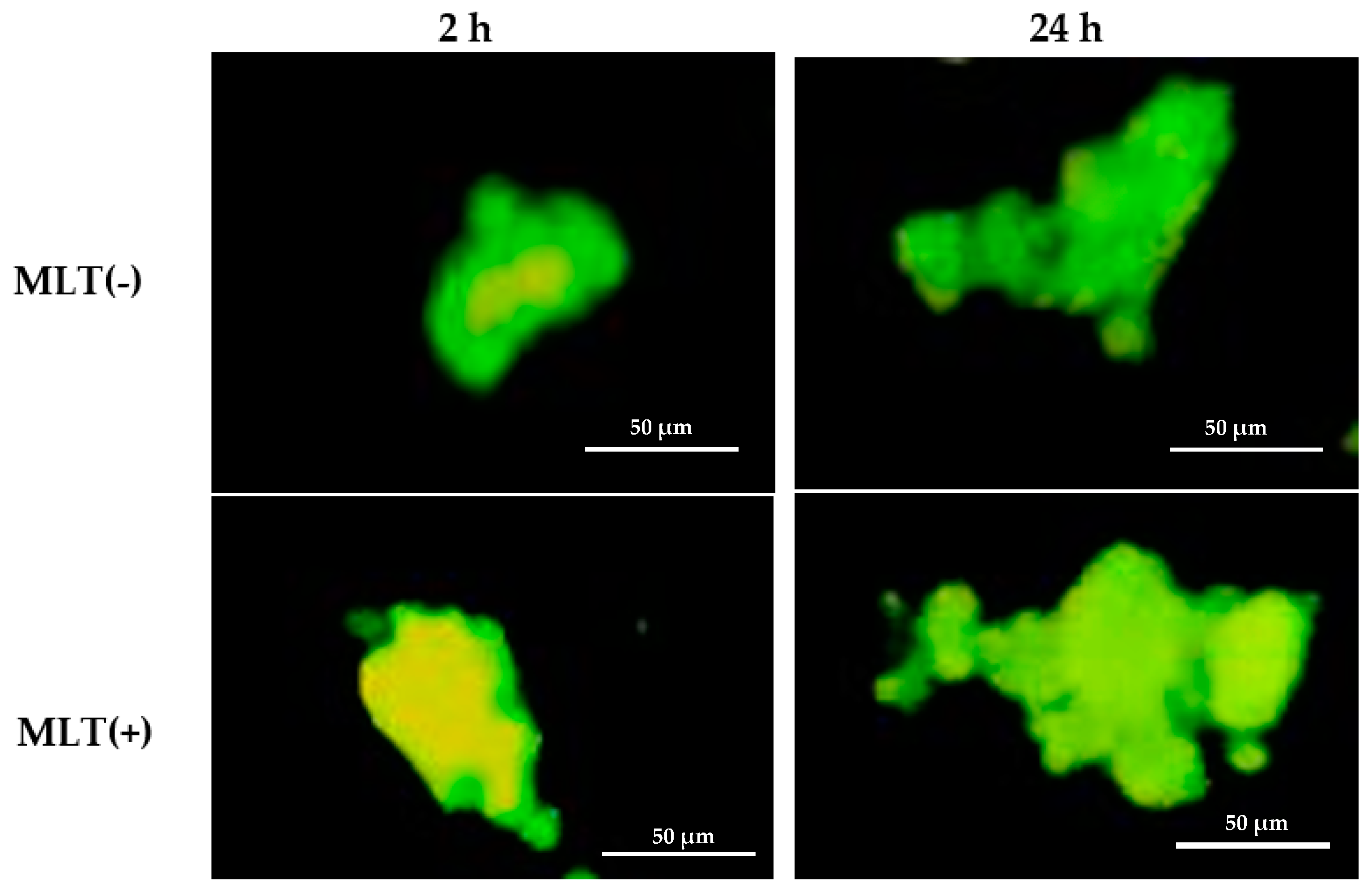

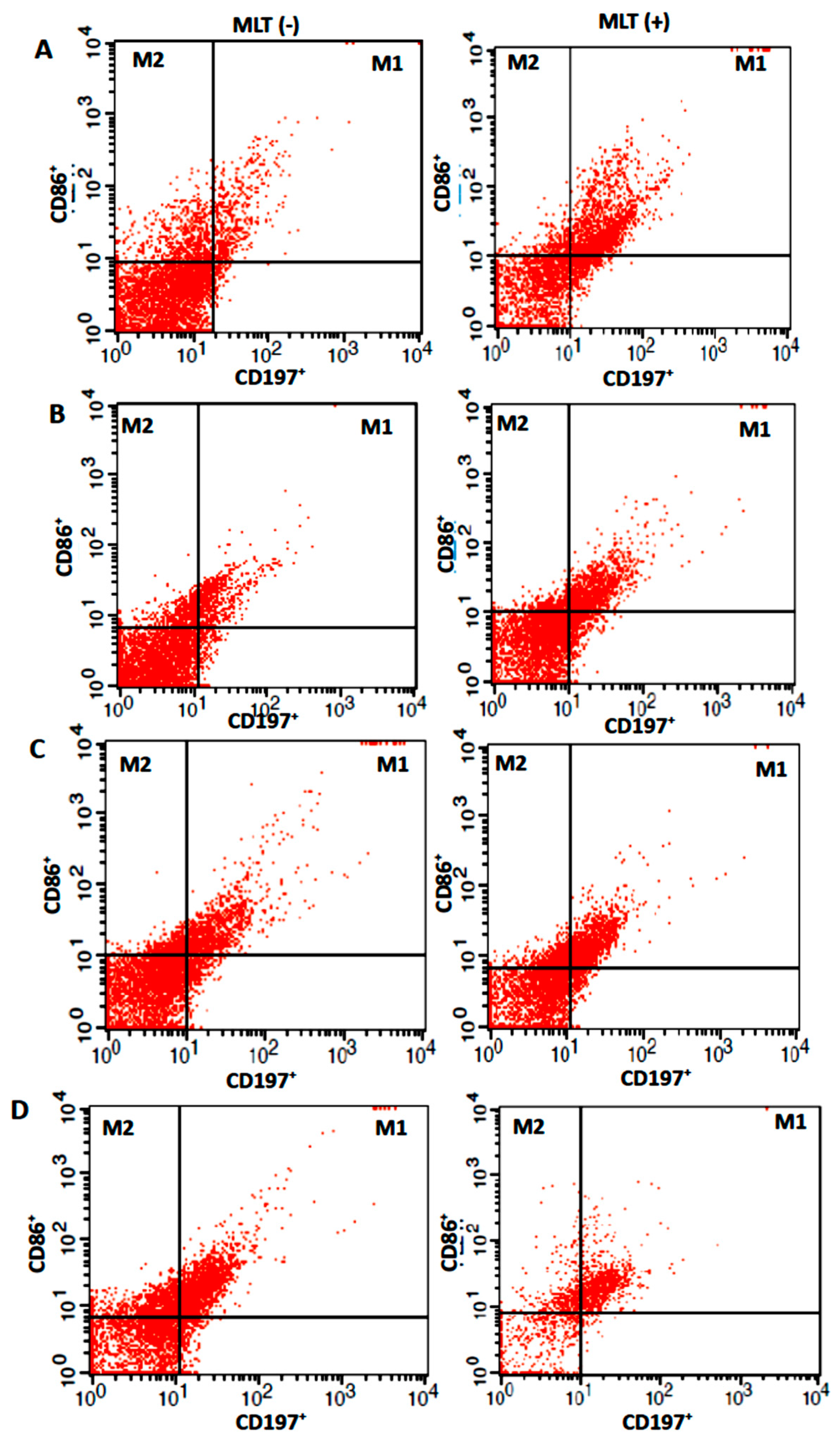


| Parameters | pg/mL |
|---|---|
| MLT | 183 ± 33 |
| MT1 in cells lysed 199 medium | 277 ± 135 |
| MT1 cells lysed + MLT | 850 ± 138 * |
| MT2 in cells lysed medium 199 | 670 ± 58 |
| MT2 in cells lysed + MLT | 952 ± 110 * |
| MLT/MT1 cells lysed ratio | 0.7 ± 0.2 |
| MLT/MT2 cells lysed ratio | 0.3 ± 0.1 # |
| Colostrum | Melatonin | 2 h | 24 h | |
|---|---|---|---|---|
| CD14+ | No | 10.8 ± 4.7 | 26.9 ± 7.0 + | |
| Cells | Yes | 6.5 ± 1.9 * | 16.4 ± 7.1 *+ | |
| CD14+CD163+ | No | 15.0 ± 2.5 | 26.3 ± 6.1 + | |
| Yes | 16.3 ± 7.0 | 16.9 ± 7.1 * | ||
| CD14+ | No | 10.7 ± 5.7 | 22.5 ± 5.2 + | |
| Coculture | Yes | 6.4 ± 2.0 * | 23.7 ± 5.9 + | |
| CD14+CD163+ | No | 15.0 ± 2.5 | 16.1 ± 6.6 | |
| Yes | 16.2 ± 7.7 | 15.6 ± 6.4 |
| Colostrum | Melatonin | 2 h | 24 h | |
|---|---|---|---|---|
| M1 | No | 11.3 ± 5.5 | 31.0 ± 2.2 + | |
| Cells | Yes | 21.4 ± 7.1 * | 27 ± 4.0 | |
| M2 | No | 28.5 ± 4.2 # | 4.8 ± 1.4 #+ | |
| Yes | 22.3 ± 7.2 | 5.5 ± 1.8 #+ | ||
| M1 | No | 24.4 ± 5.2 | 24.1 ± 9.4 | |
| Coculture | Yes | 18.3 ± 5.0 | 3.5 ± 0.5 *+ | |
| M2 | No | 24.8 ± 10.0 | 24.8 ± 10.0 | |
| Yes | 17.1 ± 4.3 | 5.2 ± 2.0 *+ |
| Macrophages | M1 MLT (−) | M1 MLT (+) | M2 MLT (−) | M2 MLT (+) |
|---|---|---|---|---|
| M1 MLT (−) | __ | r = 0.4709 p = 0.3458 | r = −0.2864 p = 0.9821 | __ |
| M1 MLT (+) | r = 0.4709 p = 0.3458 | __ | __ | r = 0.7231 p = 0.1043 |
| M2 MLT (−) | r = 0.2864 p = 0.9821 | __ | __ | r = 0.7098 p = 0.0498 |
| M2 MLT (+) | __ | r = 0.7231 p = 0.1043 | r = −0.7098 p = 0.0498 | __ |
| Cells | MLT | Time | IL-1 β | IL-6 | IL-8 | IL-10 | TNF-α | IL-12P70 |
|---|---|---|---|---|---|---|---|---|
| MN | No | 24 h | 55.5 ± 17.2 | 10.7 ± 10.4 | 85.1 ± 68.5 | 28.4 ± 6.7 | 13.5 ± 7.5 | 23.3 ± 15. |
| Yes | 24 h | 43.3 ± 19.8 | 14.1 ± 9.3 | 102.5 ± 78.0 * | 31.3 ± 7.3 | 22.2 ± 7.4 # | 39.2 ± 8.6 | |
| MN+MCF-7 | No | 24 h | 48.2 ± 17.5 | 16.9 ± 8.2 | 64.7 ± 12.1 # | 33.8 ± 9.6 | 15.7 ± 5.9 | 28.7 ± 15.5 |
| Yes | 24 h | 47.4 ± 12.6 | 17.5 ± 8.9 | 41.5 ± 5.7 #* | 27.7 ± 2.6 | 10.8 ± 6.4 # | 22.1 ± 16.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, K.M.R.; França, D.C.H.; de Queiroz, A.A.; Fagundes-Triches, D.L.G.; de Marchi, P.G.F.; Morais, T.C.; Honorio-França, A.C.; França, E.L. Polarization of Melatonin-Modulated Colostrum Macrophages in the Presence of Breast Tumor Cell Lines. Int. J. Mol. Sci. 2023, 24, 12400. https://doi.org/10.3390/ijms241512400
Silva KMR, França DCH, de Queiroz AA, Fagundes-Triches DLG, de Marchi PGF, Morais TC, Honorio-França AC, França EL. Polarization of Melatonin-Modulated Colostrum Macrophages in the Presence of Breast Tumor Cell Lines. International Journal of Molecular Sciences. 2023; 24(15):12400. https://doi.org/10.3390/ijms241512400
Chicago/Turabian StyleSilva, Kenia Maria Rezende, Danielle Cristina Honório França, Adriele Ataídes de Queiroz, Danny Laura Gomes Fagundes-Triches, Patrícia Gelli Feres de Marchi, Tassiane Cristina Morais, Adenilda Cristina Honorio-França, and Eduardo Luzía França. 2023. "Polarization of Melatonin-Modulated Colostrum Macrophages in the Presence of Breast Tumor Cell Lines" International Journal of Molecular Sciences 24, no. 15: 12400. https://doi.org/10.3390/ijms241512400
APA StyleSilva, K. M. R., França, D. C. H., de Queiroz, A. A., Fagundes-Triches, D. L. G., de Marchi, P. G. F., Morais, T. C., Honorio-França, A. C., & França, E. L. (2023). Polarization of Melatonin-Modulated Colostrum Macrophages in the Presence of Breast Tumor Cell Lines. International Journal of Molecular Sciences, 24(15), 12400. https://doi.org/10.3390/ijms241512400






