Analysis of Under-Diagnosed Malignancy during Fine Needle Aspiration Cytology of Lymphadenopathies
Abstract
1. Introduction
2. Results
2.1. Comparison of FNAC and Tissue Biopsy
2.2. RNA-Seq Analysis of Under-Diagnosed Patients’ Samples
2.3. Functional Classification of DEGs Associated with Under-Diagnoses
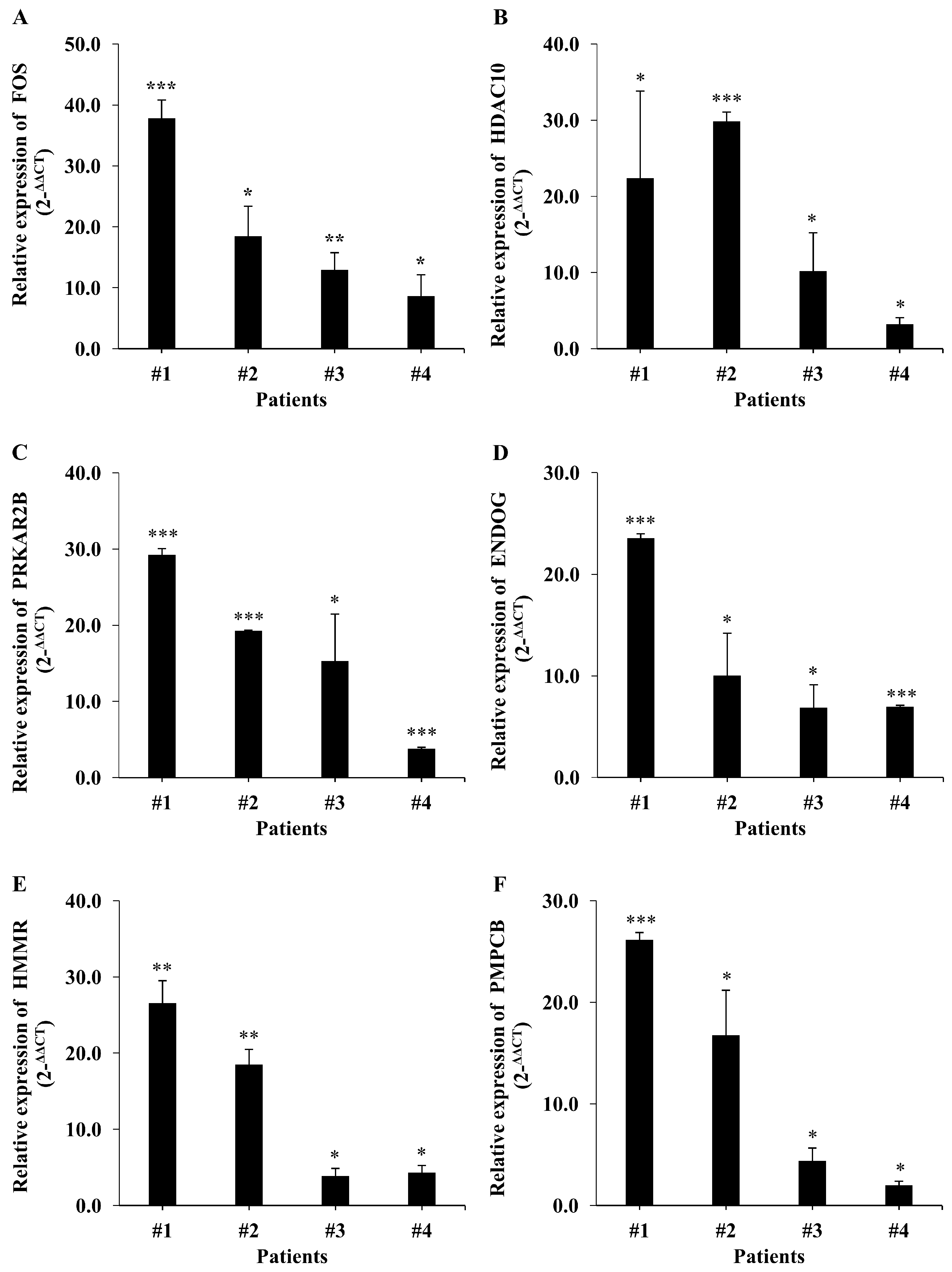
2.4. Validation of Candidates
3. Discussion
4. Materials and Methods
4.1. Obtaining Specimens and Ancillary Tests
4.2. RNA Isolation and RNA Sequencing
4.3. Identification of Differentially Expressed Genes and Data Analysis
4.4. Gene Set Enrichment Analysis (GSEA)
4.5. Heatmap with MeV
4.6. Gene Ontology with DAVID
4.7. Significant Module Analysis with Cytoscape
4.8. ClueGO
4.9. Validation of Genetic Alterations in Candidate Genes
4.10. Reverse Transcription-Polymerase Chain Reaction (RT-PCR) and Quantitative RT-PCR (qRT-PCR)
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hafez, N.H.; Tahoun, N.S. Reliability of fine needle aspiration cytology (FNAC) as a diagnostic tool in cases of cervical lymphadenopathy. J. Egypt. Natl. Cancer Inst. 2011, 23, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Bazemore, A.W.; Smucker, D.R. Lymphadenopathy and malignancy. Am. Fam. Physician 2002, 66, 2103–2110. [Google Scholar] [PubMed]
- Gaddey, H.L.; Riegel, A.M. Unexplained Lymphadenopathy: Evaluation and Differential Diagnosis. Am. Fam. Physician 2016, 94, 896–903. [Google Scholar]
- Wilkinson, A.R.; Mahore, S.D.; Maimoon, S.A. FNAC in the diagnosis of lymph node malignancies: A simple and sensitive tool. Indian J. Med. Paediatr. Oncol. 2012, 33, 21–24. [Google Scholar] [CrossRef]
- Katz, R.L. Cytologic Diagnosis of Leukemia and Lymphoma: Values and Limitations. Clin. Lab. Med. 1991, 11, 469–499. [Google Scholar] [CrossRef]
- Faro, R.O.; Mohammed, A.Z.; Atanda, A.T. Diagnostic Utility of Fine Needle Aspiration Cytology in the Evaluation of Peripheral Lymphadenopathy. West Afr. J. Med. 2018, 35, 162–167. [Google Scholar] [PubMed]
- Haque, M.A.; Talukder, S.I. Evaluation of fine needle aspiration cytology (FNAC) of lymph node in Mymensingh. Mymensingh Med. J. 2003, 12, 33–35. [Google Scholar]
- Winter, J.N.; Peterson, L.C. Lymphocytosis, lymphadenopathy: Benign or malignant? Hemato. Am. Soc. Hematol. Educ. Program 2015, 2015, 106–110. [Google Scholar] [CrossRef]
- Jeffers, M.D.; Milton, J.; Herriot, R.; McKean, M. Fine needle aspiration cytology in the investigation on non-Hodgkin’s lymphoma. J. Clin. Pathol. 1998, 51, 189–196. [Google Scholar] [CrossRef]
- Ha, H.J.; Lee, J.; Kim, D.Y.; Kim, J.-S.; Shin, M.-S.; Noh, I.; Koh, J.S.; Kim, E.J.; Lee, S.-S. Utility and Limitations of Fine-Needle Aspiration Cytology in the Diagnosis of Lymphadenopathy. Diagnostics 2023, 13, 728. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Eisen, M.B.; Spellman, P.T.; Brown, P.O.; Botstein, D. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. USA 1998, 95, 14863–14868. [Google Scholar] [CrossRef] [PubMed]
- The Gene Ontology Consortium. Gene Ontology Consortium: Going forward. Nucleic Acids Res. 2015, 43, D1049–D1056. [Google Scholar] [CrossRef] [PubMed]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.-H.; Pagès, F.; Trajanoski, Z.; Galon, J. ClueGO: A Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef]
- Osterman, T.J.; Terry, M.; Miller, R.S. Improving Cancer Data Interoperability: The Promise of the Minimal Common Oncology Data Elements (mCODE) Initiative. JCO Clin. Cancer Inform. 2020, 4, 993–1001. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef]
- Chantziantoniou, N.; Donnelly, A.D.; Mukherjee, M.; Boon, M.E.; Austin, R.M. Inception and Development of the Papanicolaou Stain Method. Acta Cytol. 2017, 61, 266–280. [Google Scholar] [CrossRef] [PubMed]
- Bonora, M.; Missiroli, S.; Perrone, M.; Fiorica, F.; Pinton, P.; Giorgi, C. Mitochondrial Control of Genomic Instability in Cancer. Cancers 2021, 13, 1914. [Google Scholar] [CrossRef] [PubMed]
- Criscuolo, D.; Avolio, R.; Matassa, D.S.; Esposito, F. Targeting Mitochondrial Protein Expression as a Future Approach for Cancer Therapy. Front. Oncol. 2021, 11, 797265. [Google Scholar] [CrossRef]
- Grasso, D.; Zampieri, L.X.; Capeloa, T.; Van de Velde, J.A.; Sonveaux, P. Mitochondria in cancer. Cell Stress 2020, 4, 114–146. [Google Scholar] [CrossRef]
- Lu, T.; Zheng, Y.; Gong, X.; Lv, Q.; Chen, J.; Tu, Z.; Lin, S.; Pan, J.; Guo, Q.; Li, J. High Expression of Hyaluronan-Mediated Motility Receptor Predicts Adverse Outcomes: A Potential Therapeutic Target for Head and Neck Squamous Cell Carcinoma. Front. Oncol. 2021, 11, 608842. [Google Scholar] [CrossRef]
- Schmitt, F.; Lozano, M.D. Molecular/biomarker testing in lung cytology: A practical approach. Diagn. Cytopathol. 2023, 51, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Liu, Z.; Yu, S.; Wang, X.; Shi, J. CCND2 and miR-206 as potential biomarkers in the clinical diagnosis of thyroid carcinoma by fine-needle aspiration cytology. World J. Surg. Oncol. 2023, 21, 1–6. [Google Scholar] [CrossRef]
- Manzo, J.L.; Cuda, J.; Pantanowitz, L.; Xing, J.; Yu, J.; Beasley, H.S.; Dhir, R.; Monaco, S.E. Clinical trial cytology: Use of on-site evaluation of small biopsy and FNA samples for clinical trials and biomarker research studies. Cancer Cytopathol. 2018, 126, 481–489. [Google Scholar] [CrossRef]
- Rodriques, S.G.; Stickels, R.R.; Goeva, A.; Martin, C.A.; Murray, E.; Vanderburg, C.R.; Welch, J.; Chen, L.M.; Chen, F.; Macosko, E.Z. Slide-seq: A scalable technology for measuring genome-wide expression at high spatial resolution. Science 2019, 363, 1463–1467. [Google Scholar] [CrossRef]
- Sun, R.; Medeiros, L.J.; Young, K.H. Diagnostic and predictive biomarkers for lymphoma diagnosis and treatment in the era of precision medicine. Mod. Pathol. 2016, 29, 1118–1142. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, A.R.; Hall, I.M. BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics 2010, 26, 841–842. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. EdgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Lee, J.; Kwon, J.; Kim, D.; Park, M.; Kim, K.; Bae, I.; Kim, H.; Kong, J.; Kim, Y.; Shin, U.; et al. Gene Expression Profiles Associated with Radio-Responsiveness in Locally Advanced Rectal Cancer. Biology 2021, 10, 500. [Google Scholar] [CrossRef] [PubMed]
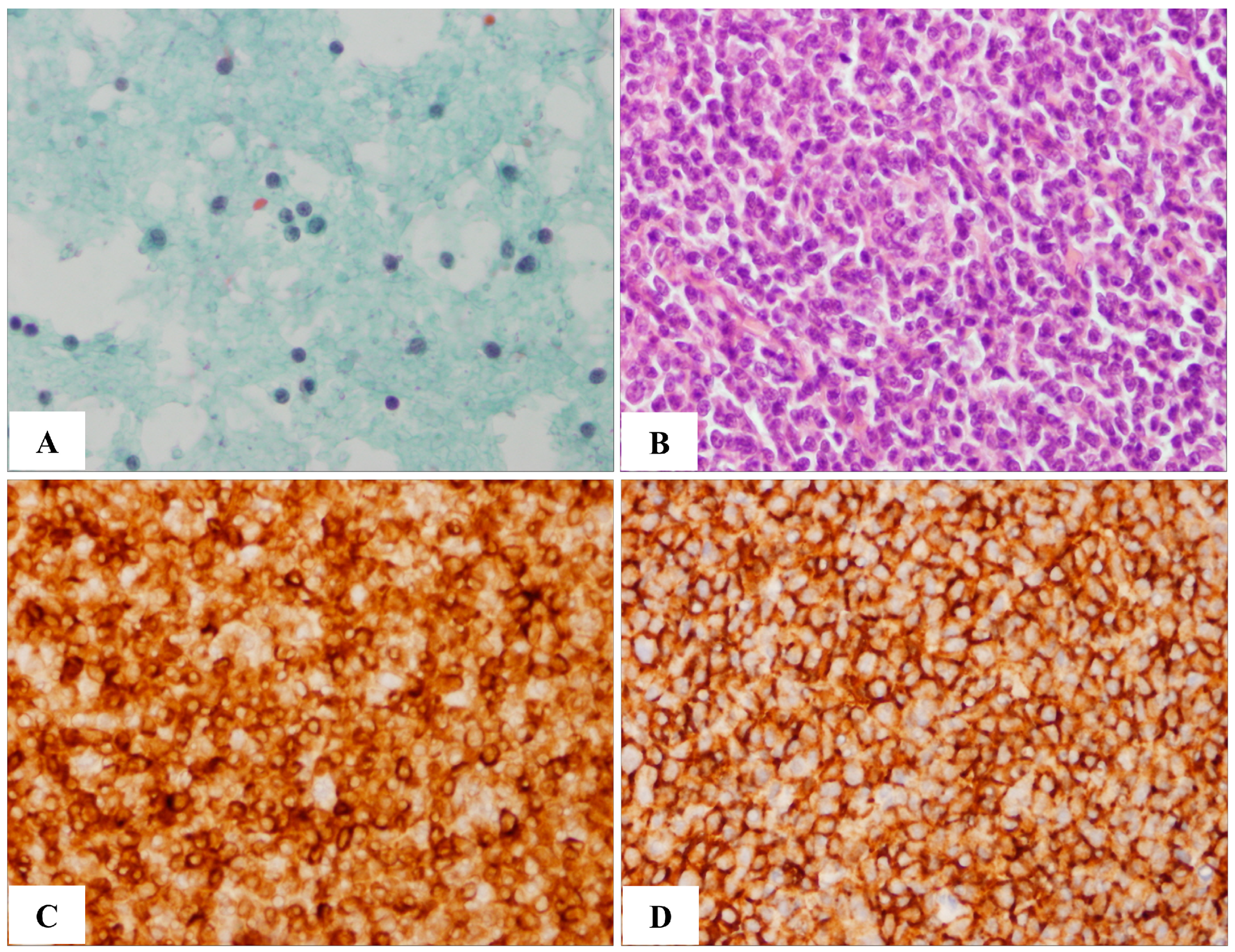
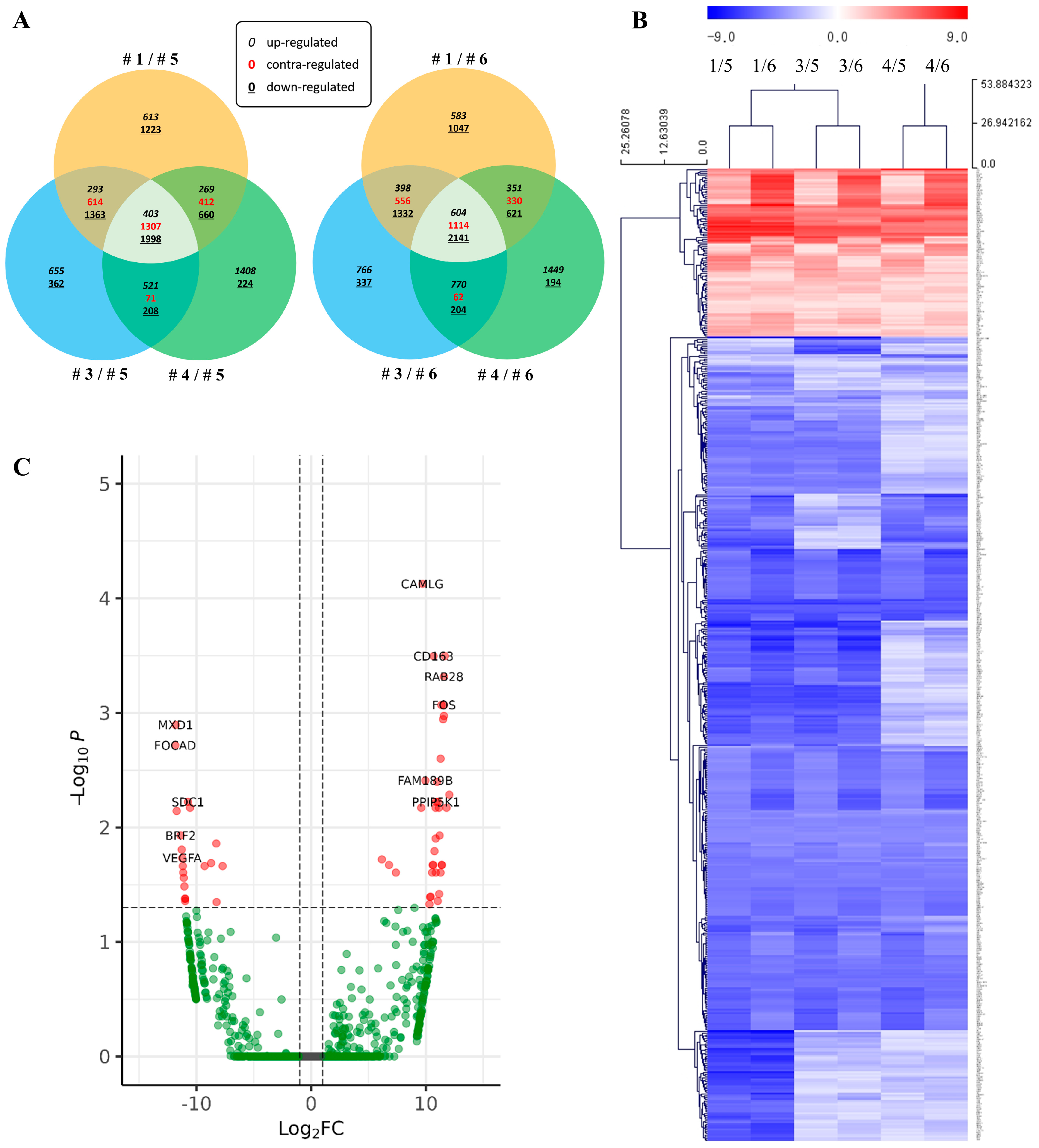
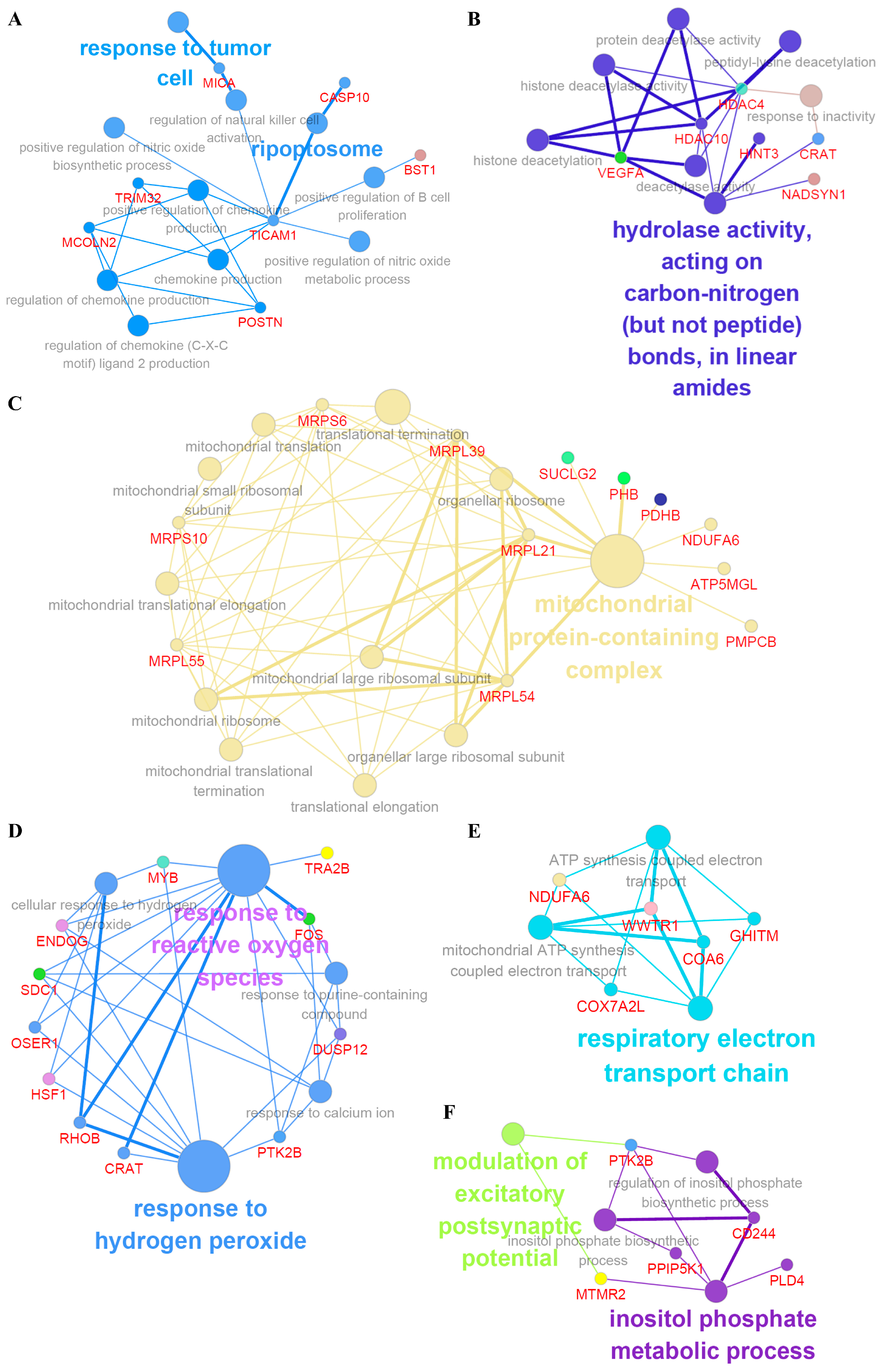
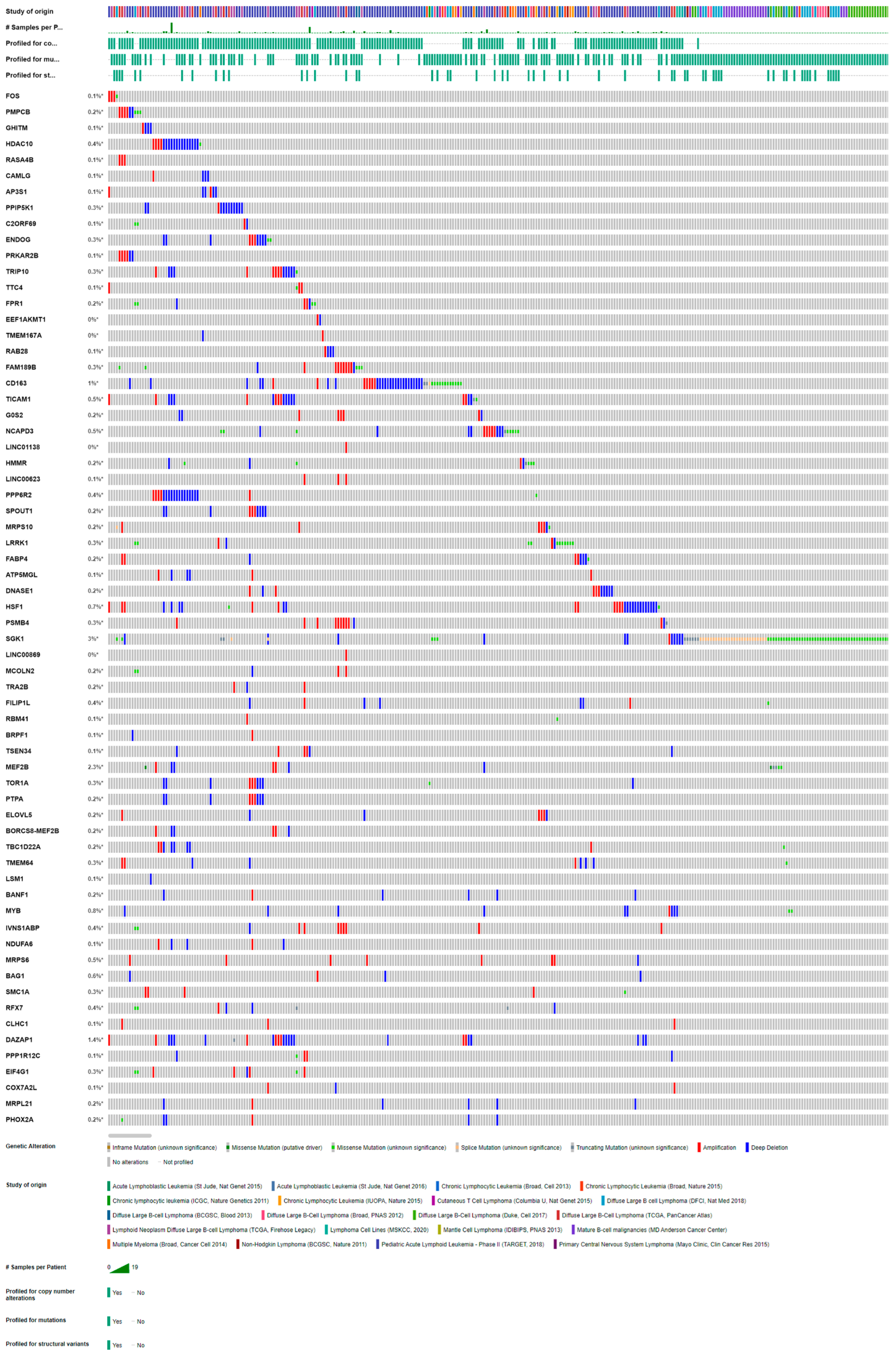
| Sample No. | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 |
|---|---|---|---|---|---|---|
| Sex | F | M | F | F | M | F |
| Age (years) | 67 | 59 | 78 | 76 | 59 | 64 |
| History | Palpable, painless | TB | HL, Colon Ca. | NSL | NSL | Rectal Ca. |
| Duration | ||||||
| <4 weeks | ||||||
| ≥4 weeks | o | o | o | o | o | o |
| Tumor length | ||||||
| <1 cm | o | o | o | o | ||
| >1 cm | o | o | ||||
| Number of enlarged LN | ||||||
| 1 | o | |||||
| 2 or more | o | o | o | o | o | |
| Location | Axillary | Cervical | Axillary | Cervical | Submandibular | Inguinal |
| Pathological Diagnosis | DLBCL | MCL | DLBCL | FL | RH | RH |
| Gene Sets Details | SIZE | ES | NES | NOM p-Value | FDR q-Value |
|---|---|---|---|---|---|
| KEGG_METABOLISM_OF_XENOBIOTICS_BY_CYTOCHROME_P450 | 70 | 0.696 | 2.05 | 0.000 | 0.000 |
| KEGG_DRUG_METABOLISM_CYTOCHROME_P450 | 72 | 0.669 | 2.01 | 0.000 | 0.001 |
| KEGG_PEROXISOME | 78 | 0.602 | 1.82 | 0.000 | 0.005 |
| KEGG_PROXIMAL_TUBULE_BICARBONATE_RECLAMATION | 23 | 0.751 | 1.81 | 0.000 | 0.005 |
| KEGG_PPAR_SIGNALING_PATHWAY | 69 | 0.613 | 1.81 | 0.000 | 0.004 |
| KEGG_GLUTATHIONE_METABOLISM | 50 | 0.635 | 1.81 | 0.000 | 0.004 |
| KEGG_PENTOSE_AND_GLUCURONATE_INTERCONVERSIONS | 28 | 0.704 | 1.77 | 0.000 | 0.006 |
| KEGG_ASCORBATE_AND_ALDARATE_METABOLISM | 25 | 0.722 | 1.75 | 0.002 | 0.009 |
| KEGG_ARACHIDONIC_ACID_METABOLISM | 58 | 0.595 | 1.74 | 0.000 | 0.010 |
| KEGG_RETINOL_METABOLISM | 64 | 0.595 | 1.73 | 0.002 | 0.011 |
| KEGG_DRUG_METABOLISM_OTHER_ENZYMES | 51 | 0.610 | 1.72 | 0.000 | 0.011 |
| KEGG_STEROID_HORMONE_BIOSYNTHESIS | 55 | 0.594 | 1.70 | 0.002 | 0.016 |
| KEGG_PORPHYRIN_AND_CHLOROPHYLL_METABOLISM | 41 | 0.626 | 1.69 | 0.004 | 0.015 |
| KEGG_GLYCOLYSIS_GLUCONEOGENESIS | 62 | 0.582 | 1.69 | 0.002 | 0.015 |
| KEGG_PYRUVATE_METABOLISM | 40 | 0.613 | 1.65 | 0.008 | 0.022 |
| KEGG_ARGININE_AND_PROLINE_METABOLISM | 54 | 0.584 | 1.65 | 0.004 | 0.021 |
| KEGG_SELENOAMINO_ACID_METABOLISM | 26 | 0.661 | 1.61 | 0.008 | 0.030 |
| KEGG_ALANINE_ASPARTATE_AND_GLUTAMATE_METABOLISM | 32 | 0.629 | 1.60 | 0.011 | 0.035 |
| KEGG_STARCH_AND_SUCROSE_METABOLISM | 52 | 0.552 | 1.57 | 0.009 | 0.044 |
| Category | Term | Description | Count | p-Value |
|---|---|---|---|---|
| BP | GO:0007229 | Integrin-mediated signaling pathway | 7 | 1.9 × 10−3 |
| GO:0032543 | Mitochondrial translation | 6 | 5.6 × 10−3 | |
| GO:0042493 | Response to drug | 10 | 8.2 × 10−3 | |
| GO:0043129 | Surfactant homeostasis | 3 | 1.5 × 10−2 | |
| GO:0006355 | Regulation of transcription, DNA-templated | 20 | 2.4 × 10−2 | |
| GO:1990830 | Cellular response to leukemia inhibitory factor | 5 | 3.3 × 10−2 | |
| GO:0007155 | Cell adhesion | 13 | 3.3 × 10−2 | |
| GO:0048026 | Positive regulation of mRNA splicing, via spliceosome | 3 | 3.7 × 10−2 | |
| GO:0007160 | Cell-matrix adhesion | 5 | 3.8 × 10−2 | |
| GO:0001570 | Vasculogenesis | 4 | 3.8 × 10−2 | |
| GO:0006886 | Intracellular protein transport | 9 | 4.1 × 10−2 | |
| GO:0021773 | Striatal medium spiny neuron differentiation | 2 | 4.6 × 10−2 | |
| GO:0060271 | Cilium assembly | 7 | 4.8 × 10−2 | |
| GO:0048010 | Vascular endothelial growth factor receptor signaling pathway | 3 | 4.8 × 10−2 | |
| CC | GO:0005739 | Mitochondrion | 34 | 1.5 × 10−4 |
| GO:0005829 | Cytosol | 86 | 1.6 × 10−3 | |
| GO:0009986 | Cell surface | 17 | 3.5 × 10−3 | |
| GO:0005856 | Cytoskeleton | 15 | 4.7 × 10−3 | |
| GO:0005737 | Cytoplasm | 83 | 9.1 × 10−3 | |
| GO:0005769 | Early endosome | 10 | 1.1 × 10−2 | |
| GO:0005743 | Mitochondrial inner membrane | 12 | 2.1 × 10−2 | |
| GO:0043025 | Neuronal cell body | 11 | 2.2 × 10−2 | |
| GO:0034686 | Integrin alphav-beta8 complex | 2 | 2.3 × 10−2 | |
| GO:0016021 | Integral component of membrane | 78 | 2.6 × 10−2 | |
| GO:0005762 | Mitochondrial large ribosomal subunit | 4 | 2.9 × 10−2 | |
| GO:0005790 | Smooth endoplasmic reticulum | 3 | 3.9 × 10−2 | |
| MF | GO:0004725 | Protein tyrosine phosphatase activity | 6 | 9.1 × 10−3 |
| GO:0008138 | Protein tyrosine/serine/threonine phosphatase activity | 4 | 1.0 × 10−2 | |
| GO:0005515 | Protein binding | 163 | 4.0 × 10−2 | |
| GO:0016787 | Hydrolase activity | 8 | 4.5 × 10−2 | |
| GO:0050839 | Cell adhesion molecule binding | 4 | 5.1 × 10−2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Ha, H.J.; Kim, D.Y.; Koh, J.S.; Kim, E.J. Analysis of Under-Diagnosed Malignancy during Fine Needle Aspiration Cytology of Lymphadenopathies. Int. J. Mol. Sci. 2023, 24, 12394. https://doi.org/10.3390/ijms241512394
Lee J, Ha HJ, Kim DY, Koh JS, Kim EJ. Analysis of Under-Diagnosed Malignancy during Fine Needle Aspiration Cytology of Lymphadenopathies. International Journal of Molecular Sciences. 2023; 24(15):12394. https://doi.org/10.3390/ijms241512394
Chicago/Turabian StyleLee, Jeeyong, Hwa Jeong Ha, Da Yeon Kim, Jae Soo Koh, and Eun Ju Kim. 2023. "Analysis of Under-Diagnosed Malignancy during Fine Needle Aspiration Cytology of Lymphadenopathies" International Journal of Molecular Sciences 24, no. 15: 12394. https://doi.org/10.3390/ijms241512394
APA StyleLee, J., Ha, H. J., Kim, D. Y., Koh, J. S., & Kim, E. J. (2023). Analysis of Under-Diagnosed Malignancy during Fine Needle Aspiration Cytology of Lymphadenopathies. International Journal of Molecular Sciences, 24(15), 12394. https://doi.org/10.3390/ijms241512394






