Synthesis, Properties, and Biomedical Application of Dicationic Gemini Surfactants with Dodecane Spacer and Carbamate Fragments
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Synthesis of GS with Carbamate Fragment
4.3. Tensiometry
4.4. Conductometry
4.5. Electrode Potential Measurement
4.6. Fluorescence Spectroscopic Measurement
4.7. Spectrophotometry
4.8. Antimicrobial Activity
4.9. Hemolysis
4.10. Turbidimetry
4.11. Dynamic and Electrophoretic Light Scattering
4.12. Liposome Preparation
4.13. Transmission Electron Microscopy
4.14. Encapsulation Efficiency and Release Rate of Substrate
4.15. In Vitro Cholinesterase Inhibitory Activity
4.16. Animals
4.17. Histology Analysis of Liposome Penetration into the Brain
4.18. Novel Object Recognition Test
4.19. Thioflavin S Staining Procedure
4.20. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sheikhpour, M.; Barani, L.; Kasaeian, A. Biomimetics in Drug Delivery Systems: A Critical Review. J. Control. Release 2017, 253, 97–109. [Google Scholar] [CrossRef]
- Harmatys, K.M.; Chen, J.; Charron, D.M.; MacLaughlin, C.M.; Zheng, G. Multipronged Biomimetic Approach to Create Optically Tunable Nanoparticles. Angew. Chem. Int. Ed. 2018, 57, 8125–8129. [Google Scholar] [CrossRef]
- Oroojalian, F.; Beygi, M.; Baradaran, B.; Mokhtarzadeh, A.; Shahbazi, M. Immune Cell Membrane-Coated Biomimetic Nanoparticles for Targeted Cancer Therapy. Small 2021, 17, 2006484. [Google Scholar] [CrossRef] [PubMed]
- Kashapov, R.; Gaynanova, G.; Gabdrakhmanov, D.; Kuznetsov, D.; Pavlov, R.; Petrov, K.; Zakharova, L.; Sinyashin, O. Self-Assembly of Amphiphilic Compounds as a Versatile Tool for Construction of Nanoscale Drug Carriers. Int. J. Mol. Sci. 2020, 21, 6961. [Google Scholar] [CrossRef] [PubMed]
- Zakharova, L.; Pashirova, T.; Doktorovova, S.; Fernandes, A.; Sanchez-Lopez, E.; Silva, A.; Souto, S.; Souto, E. Cationic Surfactants: Self-Assembly, Structure-Activity Correlation and Their Biological Applications. Int. J. Mol. Sci. 2019, 20, 5534. [Google Scholar] [CrossRef]
- Gonçalves, R.A.; Holmberg, K.; Lindman, B. Cationic Surfactants: A Review. J. Mol. Liq. 2023, 375, 121335. [Google Scholar] [CrossRef]
- Zakharova, L.Y.; Vasilieva, E.A.; Mirgorodskaya, A.B.; Zakharov, S.V.; Pavlov, R.V.; Kashapova, N.E.; Gaynanova, G.A. Hydrotropes: Solubilization of Nonpolar Compounds and Modification of Surfactant Solutions. J. Mol. Liq. 2023, 370, 120923. [Google Scholar] [CrossRef]
- Ghosh, S.; Ray, A.; Pramanik, N. Self-Assembly of Surfactants: An Overview on General Aspects of Amphiphiles. Biophys. Chem. 2020, 265, 106429. [Google Scholar] [CrossRef]
- Khaliq, N.U.; Park, D.Y.; Yun, B.M.; Yang, D.H.; Jung, Y.W.; Seo, J.H.; Hwang, C.S.; Yuk, S.H. Pluronics: Intelligent Building Units for Targeted Cancer Therapy and Molecular Imaging. Int. J. Pharm. 2019, 556, 30–44. [Google Scholar] [CrossRef]
- Solomonov, A.V.; Marfin, Y.S.; Rumyantsev, E.V.; Ragozin, E.; Zahavi, T.S.; Gellerman, G.; Tesler, A.B.; Muench, F.; Kumagai, A.; Miyawaki, A. Self-Assembled Micellar Clusters Based on Triton-X-Family Surfactants for Enhanced Solubilization, Encapsulation, Proteins Permeability Control, and Anticancer Drug Delivery. Mater. Sci. Eng. 2019, 99, 794–804. [Google Scholar] [CrossRef]
- Lundberg, D.; Stjerndahl, M.; Holmberg, K. Ester-based Surfactants: Are They Stable Enough? J. Surfactants Deterg. 2023, 26, 229–236. [Google Scholar] [CrossRef]
- Kushnazarova, R.A.; Mirgorodskaya, A.B.; Lukashenko, S.S.; Voloshina, A.D.; Sapunova, A.S.; Nizameev, I.R.; Kadirov, M.K.; Zakharova, L.Y. Novel Cationic Surfactants with Cleavable Carbamate Fragment: Tunable Morphological Behavior, Solubilization of Hydrophobic Drugs and Cellular Uptake Study. J. Mol. Liq. 2020, 318, 113894. [Google Scholar] [CrossRef]
- Bhadani, A.; Kafle, A.; Ogura, T.; Akamatsu, M.; Sakai, K.; Sakai, H.; Abe, M. Current Perspective of Sustainable Surfactants Based on Renewable Building Blocks. Curr. Opin. Colloid Interface Sci. 2020, 45, 124–135. [Google Scholar] [CrossRef]
- Gallezot, P. Conversion of Biomass to Selected Chemical Products. Chem. Soc. Rev. 2012, 41, 1538–1558. [Google Scholar] [CrossRef]
- Ahmady, A.R.; Hosseinzadeh, P.; Solouk, A.; Akbari, S.; Szulc, A.M.; Brycki, B.E. Cationic Gemini Surfactant Properties, Its Potential as a Promising Bioapplication Candidate, and Strategies for Improving Its Biocompatibility: A Review. Adv. Colloid Interface Sci. 2022, 299, 102581. [Google Scholar] [CrossRef] [PubMed]
- Menger, F.M.; Keiper, J.S. Gemini Surfactants. Angew. Chem. Int. Ed. 2000, 39, 1906–1920. [Google Scholar] [CrossRef]
- Wettig, S.; Verrall, R.; Foldvari, M. Gemini Surfactants: A New Family of Building Blocks for Non-Viral Gene Delivery Systems. Curr. Gene Ther. 2008, 8, 9–23. [Google Scholar] [CrossRef]
- Zakharova, L.Y.; Gabdrakhmanov, D.R.; Ibragimova, A.R.; Vasilieva, E.A.; Nizameev, I.R.; Kadirov, M.K.; Ermakova, E.A.; Gogoleva, N.E.; Faizullin, D.A.; Pokrovsky, A.G. Structural, Biocomplexation and Gene Delivery Properties of Hydroxyethylated Gemini Surfactants with Varied Spacer Length. Colloids Surf. B 2016, 140, 269–277. [Google Scholar] [CrossRef]
- Bombelli, C.; Giansanti, L.; Luciani, P.; Mancini, G. Gemini Surfactant Based Carriers in Gene and Drug Delivery. Curr. Med. Chem. 2009, 16, 171–183. [Google Scholar] [CrossRef]
- Pisárčik, M.; Polakovičová, M.; Markuliak, M.; Lukáč, M.; Devínsky, F. Self-Assembly Properties of Cationic Gemini Surfactants with Biodegradable Groups in the Spacer. Molecules 2019, 24, 1481. [Google Scholar] [CrossRef]
- Pavlov, R.V.; Gaynanova, G.A.; Kuznetsov, D.M.; Ivanov, Y.A.; Amerkhanova, S.K.; Lyubina, A.P.; Voloshina, A.D.; Zakharova, L.Y. A Study Involving PC-3 Cancer Cells and Novel Carbamate Gemini Surfactants: Is Zeta Potential the Key to Control Adhesion to Cells? Smart Mater. Med. 2023, 4, 123–133. [Google Scholar] [CrossRef]
- Tehrani-Bagha, A.R.; Singh, R.G.; Holmberg, K. Solubilization of Two Organic Dyes by Cationic Ester-Containing Gemini Surfactants. J. Colloid Interface Sci. 2012, 376, 112–118. [Google Scholar] [CrossRef]
- Sęk, A.; Perczyk, P.; Wydro, P.; Gruszecki, W.I.; Szcześ, A. Effect of Trace Amounts of Ionic Surfactants on the Zeta Potential of DPPC Liposomes. Chem. Phys. Lipids 2021, 235, 105059. [Google Scholar] [CrossRef] [PubMed]
- Saengkrit, N.; Saesoo, S.; Srinuanchai, W.; Phunpee, S.; Ruktanonchai, U.R. Influence of Curcumin-Loaded Cationic Liposome on Anticancer Activity for Cervical Cancer Therapy. Colloids Surf. B 2014, 114, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Battista, S.; Campitelli, P.; Galantini, L.; Köber, M.; Vargas-Nadal, G.; Ventosa, N.; Giansanti, L. Use of N-Oxide and Cationic Surfactants to Enhance Antioxidant Properties of (+)-Usnic Acid Loaded Liposomes. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124154. [Google Scholar] [CrossRef]
- Liu, P.; Chen, G.; Zhang, J. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef] [PubMed]
- Thapa Magar, K.; Boafo, G.F.; Li, X.; Chen, Z.; He, W. Liposome-Based Delivery of Biological Drugs. Chin. Chem. Lett. 2022, 33, 587–596. [Google Scholar] [CrossRef]
- Apolinário, A.C.; Hauschke, L.; Nunes, J.R.; Lopes, L.B. Lipid Nanovesicles for Biomedical Applications: ‘What Is in a Name’? Prog. Lipid Res. 2021, 82, 101096. [Google Scholar] [CrossRef]
- Eloy, J.O.; Claro De Souza, M.; Petrilli, R.; Barcellos, J.P.A.; Lee, R.J.; Marchetti, J.M. Liposomes as Carriers of Hydrophilic Small Molecule Drugs: Strategies to Enhance Encapsulation and Delivery. Colloids Surf. B 2014, 123, 345–363. [Google Scholar] [CrossRef]
- Gaynanova, G.; Vasileva, L.; Kashapov, R.; Kuznetsova, D.; Kushnazarova, R.; Tyryshkina, A.; Vasilieva, E.; Petrov, K.; Zakharova, L.; Sinyashin, O. Self-Assembling Drug Formulations with Tunable Permeability and Biodegradability. Molecules 2021, 26, 6786. [Google Scholar] [CrossRef]
- Kashapov, R.; Ibragimova, A.; Pavlov, R.; Gabdrakhmanov, D.; Kashapova, N.; Burilova, E.; Zakharova, L.; Sinyashin, O. Nanocarriers for Biomedicine: From Lipid Formulations to Inorganic and Hybrid Nanoparticles. Int. J. Mol. Sci. 2021, 22, 7055. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.Y.; Li, Z.; Loh, X.J. Small Molecule Therapeutic-Loaded Liposomes as Therapeutic Carriers: From Development to Clinical Applications. RSC Adv. 2016, 6, 70592–70615. [Google Scholar] [CrossRef]
- Caritá, A.C.; Resende De Azevedo, J.; Chevalier, Y.; Arquier, D.; Buri, M.V.; Riske, K.A.; Ricci Leonardi, G.; Bolzinger, M.-A. Elastic Cationic Liposomes for Vitamin C Delivery: Development, Characterization and Skin Absorption Study. Int. J. Pharm. 2023, 638, 122897. [Google Scholar] [CrossRef] [PubMed]
- Zoughaib, M.; Pavlov, R.V.; Gaynanova, G.A.; Garifullin, R.; Evtugyn, V.G.; Abdullin, T.I. Amphiphilic RGD and GHK Peptides Synergistically Enhance Liposomal Delivery into Cancer and Endothelial Cells. Mater. Adv. 2021, 2, 7715–7730. [Google Scholar] [CrossRef]
- Kuznetsova, D.A.; Vasilieva, E.A.; Kuznetsov, D.M.; Lenina, O.A.; Filippov, S.K.; Petrov, K.A.; Zakharova, L.Y.; Sinyashin, O.G. Enhancement of the Transdermal Delivery of Nonsteroidal Anti-Inflammatory Drugs Using Liposomes Containing Cationic Surfactants. ACS Omega 2022, 7, 25741–25750. [Google Scholar] [CrossRef]
- Duangjit, S.; Opanasopit, P.; Rojanarata, T.; Obata, Y.; Takayama, K.; Ngawhirunpat, T.; Pamornpathomkul, B. Role of the Charge, Carbon Chain Length, and Content of Surfactant on the Skin Penetration of Meloxicam-Loaded Liposomes. Int. J. Nanomed. 2014, 9, 2005–2017. [Google Scholar] [CrossRef]
- Kuznetsova, D.A.; Gaynanova, G.A.; Vasileva, L.A.; Sibgatullina, G.V.; Samigullin, D.V.; Sapunova, A.S.; Voloshina, A.D.; Galkina, I.V.; Petrov, K.A.; Zakharova, L.Y. Mitochondria-Targeted Cationic Liposomes Modified with Alkyltriphenylphosphonium Bromides Loaded with Hydrophilic Drugs: Preparation, Cytotoxicity and Colocalization Assay. J. Mater. Chem. B 2019, 7, 7351–7362. [Google Scholar] [CrossRef]
- Kamidate, T.; Niwa, S.; Nakata, N. Application of Cationic Liposomes Containing Surfactants to an Enhancer in Firefly Bioluminescent Assay of Adenosine 5′-Triphosphate. Anal. Chim. Acta 2000, 424, 169–175. [Google Scholar] [CrossRef]
- Sobral, C.N.C.; Soto, M.A.; Carmona-Ribeiro, A.M. Characterization of DODAB/DPPC Vesicles. Chem. Phys. Lipids 2008, 152, 38–45. [Google Scholar] [CrossRef]
- Aihua, Z.; Qiang, G.; Chunwei, Y.; Rong, G. Liposome Formation in Mixed Aqueous Solution of Tripple-Tailed Cationic Surfactant and Phosphatidylcholine. J. Dispers. Sci. Technol. 2005, 25, 789–794. [Google Scholar] [CrossRef]
- Chen, Y.; Qiao, F.; Fan, Y.; Han, Y.; Wang, Y. Interactions of Phospholipid Vesicles with Cationic and Anionic Oligomeric Surfactants. J. Phys. Chem. B 2017, 121, 7122–7132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Qi, R.; Li, H.; Guan, B.; Liu, Y.; Han, Y.; Wang, Y. Interaction of Phospholipid Vesicles with Gemini Surfactants of Different Lysine Spacer Lengths. Soft Matter 2019, 15, 9458–9467. [Google Scholar] [CrossRef]
- Kuznetsova, D.A.; Vasileva, L.A.; Gaynanova, G.A.; Pavlov, R.V.; Sapunova, A.S.; Voloshina, A.D.; Sibgatullina, G.V.; Samigullin, D.V.; Petrov, K.A.; Zakharova, L.Y.; et al. Comparative Study of Cationic Liposomes Modified with Triphenylphosphonium and Imidazolium Surfactants for Mitochondrial Delivery. J. Mol. Liq. 2021, 330, 115703. [Google Scholar] [CrossRef]
- Boddapati, S.V.; D’Souza, G.G.M.; Erdogan, S.; Torchilin, V.P.; Weissig, V. Organelle-Targeted Nanocarriers: Specific Delivery of Liposomal Ceramide to Mitochondria Enhances Its Cytotoxicity in Vitro and in Vivo. Nano Lett. 2008, 8, 2559–2563. [Google Scholar] [CrossRef] [PubMed]
- Vasileva, L.; Gaynanova, G.; Valeeva, F.; Belyaev, G.; Zueva, I.; Bushmeleva, K.; Sibgatullina, G.; Samigullin, D.; Vyshtakalyuk, A.; Petrov, K.; et al. Mitochondria-Targeted Delivery Strategy of Dual-Loaded Liposomes for Alzheimer’s Disease Therapy. Int. J. Mol. Sci. 2023, 24, 10494. [Google Scholar] [CrossRef] [PubMed]
- Muzzalupo, R.; Pérez, L.; Pinazo, A.; Tavano, L. Pharmaceutical Versatility of Cationic Niosomes Derived from Amino Acid-Based Surfactants: Skin Penetration Behavior and Controlled Drug Release. Int. J. Pharm. 2017, 529, 245–252. [Google Scholar] [CrossRef]
- Pashirova, T.N.; Zhukova, N.A.; Lukashenko, S.S.; Valeeva, F.G.; Burilova, E.A.; Sapunova, A.S.; Voloshina, A.D.; Mirgorodskaya, A.B.; Zakharova, L.Y.; Sinyashin, O.G.; et al. Multi-Targeted Approach by 2-Benzimidazolylquinoxalines-Loaded Cationic Arginine Liposomes against Сervical Cancer Cells in Vitro. Colloids Surf. B 2019, 178, 317–328. [Google Scholar] [CrossRef]
- Kuznetsova, D.A.; Gaynanova, G.A.; Vasilieva, E.A.; Pavlov, R.V.; Zueva, I.V.; Babaev, V.M.; Kuznetsov, D.M.; Voloshina, A.D.; Petrov, K.A.; Zakharova, L.Y.; et al. Oxime Therapy for Brain AChE Reactivation and Neuroprotection after Organophosphate Poisoning. Pharmaceutics 2022, 14, 1950. [Google Scholar] [CrossRef]
- Kuznetsova, D.A.; Gabdrakhmanov, D.R.; Gaynanova, G.A.; Vasileva, L.A.; Kuznetsov, D.M.; Lukashenko, S.S.; Voloshina, A.D.; Sapunova, A.S.; Nizameev, I.R.; Sibgatullina, G.V.; et al. Novel Biocompatible Liposomal Formulations for Encapsulation of Hydrophilic Drugs—Chloramphenicol and Cisplatin. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125673. [Google Scholar] [CrossRef]
- Kuznetsova, D.A.; Gabdrakhmanov, D.R.; Lukashenko, S.S.; Ahtamyanova, L.R.; Nizameev, I.R.; Kadirov, M.K.; Zakharova, L.Y. Novel Hybrid Liposomal Formulations Based on Imidazolium-Containing Amphiphiles for Drug Encapsulation. Colloids Surf. B 2019, 178, 352–357. [Google Scholar] [CrossRef]
- Kuznetsova, D.A.; Vasileva, L.A.; Gaynanova, G.A.; Vasilieva, E.A.; Lenina, O.A.; Nizameev, I.R.; Kadirov, M.K.; Petrov, K.A.; Zakharova, L.Y.; Sinyashin, O.G. Cationic Liposomes Mediated Transdermal Delivery of Meloxicam and Ketoprofen: Optimization of the Composition, in Vitro and in Vivo Assessment of Efficiency. Int. J. Pharm. 2021, 605, 120803. [Google Scholar] [CrossRef]
- Barenholz, Y.; Bombelli, C.; Bonicelli, M.G.; Profio, P.D.; Giansanti, L.; Mancini, G.; Pascale, F. Influence of Lipid Composition on the Thermotropic Behavior and Size Distribution of Mixed Cationic Liposomes. J. Colloid Interface Sci. 2011, 356, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Vasileva, L.; Gaynanova, G.; Zueva, I.; Lyubina, A.; Amerhanova, S.; Buzyurova, D.; Babaev, V.; Voloshina, A.; Petrov, K.; Zakharova, L. Transdermal Delivery of 2-PAM as a Tool to Increase the Effectiveness of Traditional Treatment of Organophosphate Poisoning. Int. J. Mol. Sci. 2022, 23, 14992. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.; Kaur, G.; Chaudhary, G.R.; Gawali, S.L.; Hassan, P.A. Fabrication of Metalosomes (Metal Containing Cationic Liposomes) Using Single Chain Surfactants as a Precursor via Formation of Inorganic Organic Hybrids. Phys. Chem. Chem. Phys. 2017, 19, 25764–25773. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Arca, V.; Sabín, J.; García-Río, L.; Bastos, M.; Taboada, P.; Barbosa, S.; Prieto, G. On the Structure and Stability of Novel Cationic DPPC Liposomes Doped with Gemini Surfactants. J. Mol. Liq. 2022, 366, 120230. [Google Scholar] [CrossRef]
- Pavlov, R.V.; Gaynanova, G.A.; Kuznetsova, D.A.; Vasileva, L.A.; Zueva, I.V.; Sapunova, A.S.; Buzyurova, D.N.; Babaev, V.M.; Voloshina, A.D.; Lukashenko, S.S.; et al. Biomedical Potentialities of Cationic Geminis as Modulating Agents of Liposome in Drug Delivery across Biological Barriers and Cellular Uptake. Int. J. Pharm. 2020, 587, 119640. [Google Scholar] [CrossRef]
- Bombelli, C.; Caracciolo, G.; Di Profio, P.; Diociaiuti, M.; Luciani, P.; Mancini, G.; Mazzuca, C.; Marra, M.; Molinari, A.; Monti, D.; et al. Inclusion of a Photosensitizer in Liposomes Formed by DMPC/Gemini Surfactant: Correlation between Physicochemical and Biological Features of the Complexes. J. Med. Chem. 2005, 48, 4882–4891. [Google Scholar] [CrossRef]
- Aleandri, S.; Bonicelli, M.G.; Bordi, F.; Casciardi, S.; Diociaiuti, M.; Giansanti, L.; Leonelli, F.; Mancini, G.; Perrone, G.; Sennato, S. How Stereochemistry Affects the Physicochemical Features of Gemini Surfactant Based Cationic Liposomes. Soft Matter 2012, 8, 5904. [Google Scholar] [CrossRef]
- Pashirova, T.N.; Zueva, I.V.; Petrov, K.A.; Lukashenko, S.S.; Nizameev, I.R.; Kulik, N.V.; Voloshina, A.D.; Almasy, L.; Kadirov, M.K.; Masson, P.; et al. Mixed Cationic Liposomes for Brain Delivery of Drugs by the Intranasal Route: The Acetylcholinesterase Reactivator 2-PAM as Encapsulated Drug Model. Colloids Surf. B 2018, 171, 358–367. [Google Scholar] [CrossRef]
- Migliore, M.M.; Vyas, T.K.; Campbell, R.B.; Amiji, M.M.; Waszczak, B.L. Brain Delivery of Proteins by the Intranasal Route of Administration: A Comparison of Cationic Liposomes versus Aqueous Solution Formulations. J. Pharm. Sci. 2010, 99, 1745–1761. [Google Scholar] [CrossRef]
- Le, M.Q.; Carpentier, R.; Lantier, I.; Ducournau, C.; Dimier-Poisson, I.; Betbeder, D. Residence Time and Uptake of Porous and Cationic Maltodextrin-Based Nanoparticles in the Nasal Mucosa: Comparison with Anionic and Cationic Nanoparticles. Int. J. Pharm. 2018, 550, 316–324. [Google Scholar] [CrossRef]
- Laffleur, F.; Bauer, B. Progress in Nasal Drug Delivery Systems. Int. J. Pharm. 2021, 607, 120994. [Google Scholar] [CrossRef]
- Zakharova, L.; Gaynanova, G.; Vasilieva, E.; Vasileva, L.; Pavlov, R.; Kashapov, R.; Petrov, K.; Sinyashin, O. Recent Nanoscale Carriers for Therapy of Alzheimer’s Disease:Current Strategies and Perspectives. Curr. Med. Chem. 2023, 30, 3743–3774. [Google Scholar] [CrossRef]
- Barage, S.H.; Sonawane, K.D. Amyloid Cascade Hypothesis: Pathogenesis and Therapeutic Strategies in Alzheimer’s Disease. Neuropeptides 2015, 52, 1–18. [Google Scholar] [CrossRef]
- Kametani, F.; Hasegawa, M. Reconsideration of Amyloid Hypothesis and Tau Hypothesis in Alzheimer’s Disease. Front. Neurosci. 2018, 12, 25. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, R.H.; Burns, J.M.; Khan, S.M. The Alzheimer’s Disease Mitochondrial Cascade Hypothesis: Progress and Perspectives. Biochim. Biophys. Acta Mol. Basis Dis. 2014, 1842, 1219–1231. [Google Scholar] [CrossRef] [PubMed]
- Ringheim, G.; Szczepanik, A. Brain Inflammation, Cholesterol, and Glutamate as Interconnected Participants in the Pathology of Alzheimers Disease. Curr. Pharm. Des. 2006, 12, 719–738. [Google Scholar] [CrossRef] [PubMed]
- Conway, M.E. Alzheimer’s Disease: Targeting the Glutamatergic System. Biogerontology 2020, 21, 257–274. [Google Scholar] [CrossRef]
- Farlow, M.P. Utilizing Combination Therapy in the Treatment of Alzheimer’s Disease. Expert Rev. Neurother. 2004, 4, 799–808. [Google Scholar] [CrossRef]
- Carreiras, M.; Mendes, E.; Perry, M.; Francisco, A.; Marco-Contelles, J. The Multifactorial Nature of Alzheimer’s Disease for Developing Potential Therapeutics. Curr. Top. Med. Chem. 2013, 13, 1745–1770. [Google Scholar] [CrossRef]
- Ghosh, S.; Khatua, D.; Dey, J. Interaction between Zwitterionic and Anionic Surfactants: Spontaneous Formation of Zwitanionic Vesicles. Langmuir 2011, 27, 5184–5192. [Google Scholar] [CrossRef] [PubMed]
- Schott, H. Solubilization of a Water-Insoluble Dye as a Method for Determining Micellar Molecular Weights. J. Phys. Chem. 1966, 70, 2966–2973. [Google Scholar] [CrossRef]
- Asadov, Z.H.; Ahmadova, G.A.; Rahimov, R.A.; Hashimzade, S.-Z.F.; Nasibova, S.M.; Ismailov, E.H.; Suleymanova, S.A.; Muradova, S.A.; Asadova, N.Z.; Zubkov, F.I. Surface Properties and Premicellar Aggregation Behavior of Cationic Gemini Surfactants with Mono- and Di-(2-Hydroxypropyl)Ammonium Head Groups. Colloids Surf. A Physicochem. Eng. Asp. 2019, 575, 212–221. [Google Scholar] [CrossRef]
- Sarıkaya, İ.; Bilgen, S.; Ünver, Y.; İnan Bektaş, K.; Akbaş, H. Synthesis, Characterization, Antibacterial Activity, and Interfacial and Micellar Features of Novel Cationic Gemini Surfactants with Different Spacers. J. Surfactants Deterg. 2021, 24, 909–921. [Google Scholar] [CrossRef]
- Zhang, S.; Ding, S.; Yu, J.; Chen, X.; Lei, Q.; Fang, W. Antibacterial Activity, in Vitro Cytotoxicity, and Cell Cycle Arrest of Gemini Quaternary Ammonium Surfactants. Langmuir 2015, 31, 12161–12169. [Google Scholar] [CrossRef]
- Dam, T.; Engberts, J.B.F.N.; Karthäuser, J.; Karaborni, S.; Van Os, N.M. Synthesis, Surface Properties and Oil Solubilisation Capacity of Cationic Gemini Surfactants. Colloids Surf. A Physicochem. Eng. Asp. 1996, 118, 41–49. [Google Scholar] [CrossRef]
- Borse, M.S.; Devi, S. Dependence of Aggregation Behavior and Physicochemical Properties of Bis-Cationic Surfactants on the Polarity of Surfactant Head Group. Colloids Surf. A Physicochem. Eng. Asp. 2004, 245, 1–8. [Google Scholar] [CrossRef]
- Lu, T.; Lan, Y.; Liu, C.; Huang, J.; Wang, Y. Surface Properties, Aggregation Behavior and Micellization Thermodynamics of a Class of Gemini Surfactants with Ethyl Ammonium Headgroups. J. Colloid Interface Sci. 2012, 377, 222–230. [Google Scholar] [CrossRef]
- Ao, M.; Xu, G.; Zhu, Y.; Bai, Y. Synthesis and Properties of Ionic Liquid-Type Gemini Imidazolium Surfactants. J. Colloid Interface Sci. 2008, 326, 490–495. [Google Scholar] [CrossRef]
- Asadov, Z.H.; Nasibova, S.M.; Ahmadova, G.A.; Zubkov, F.I.; Rahimov, R.A. Head-Group Effect of Surfactants of Cationic Type in Interaction with Propoxylated Sodium Salt of Polyacrylic Acid in Aqueous Solution. Colloids Surf. A Physicochem. Eng. Asp. 2017, 527, 95–100. [Google Scholar] [CrossRef]
- Quagliotto, P.; Barbero, N.; Barolo, C.; Costabello, K.; Marchese, L.; Coluccia, S.; Kalyanasundaram, K.; Viscardi, G. Characterization of Monomeric and Gemini Cationic Amphiphilic Molecules by Fluorescence Intensity and Anisotropy. Dyes Pigm. 2009, 82, 124–129. [Google Scholar] [CrossRef]
- Dong, D.C.; Winnik, M.A. The Py scale of solvent polarities. Solvent effects on the vibronic fine structure of pyrene fluorescence and empirical correlations with E T and Y values. Photochem. Photobiol. 1982, 35, 17–21. [Google Scholar] [CrossRef]
- Aguiar, J.; Carpena, P.; Molina-Bolívar, J.A.; Carnero Ruiz, C. On the Determination of the Critical Micelle Concentration by the Pyrene 1:3 Ratio Method. J. Colloid Interface Sci. 2003, 258, 116–122. [Google Scholar] [CrossRef]
- Zana, R.; In, M.; Lévy, H.; Duportail, G. Alkanediyl-α,ω-Bis(Dimethylalkylammonium Bromide). 7. Fluorescence Probing Studies of Micelle Micropolarity and Microviscosity. Langmuir 1997, 13, 5552–5557. [Google Scholar] [CrossRef]
- Gainanova, G.A.; Vagapova, G.I.; Syakaev, V.V.; Ibragimova, A.R.; Valeeva, F.G.; Tudriy, E.V.; Galkina, I.V.; Kataeva, O.N.; Zakharova, L.Y.; Latypov, S.K.; et al. Self-Assembling Systems Based on Amphiphilic Alkyltriphenylphosphonium Bromides: Elucidation of the Role of Head Group. J. Colloid Interface Sci. 2012, 367, 327–336. [Google Scholar] [CrossRef]
- Lianos, P.; Zana, R. Fluorescence Probe Studies of the Effect of Concentration on the State of Aggregation of Surfactants in Aqueous Solution. J. Colloid Interface Sci. 1981, 84, 100–107. [Google Scholar] [CrossRef]
- Liang, Y.; Liang, D.; Hu, Z.; Cao, D. Synthesis and Physicochemical Characterization of Chiral Pyrrolidinium-Based Surfactants. J. Dispers. Sci. Technol. 2015, 36, 831–837. [Google Scholar] [CrossRef]
- Zhong, X.; Guo, J.; Feng, L.; Xu, X.; Zhu, D. Cationic Gemini Surfactants Based on Adamantane: Synthesis, Surface Activity and Aggregation Properties. Colloids Surf. A Physicochem. Eng. Asp. 2014, 441, 572–580. [Google Scholar] [CrossRef]
- Tehrani-Bagha, A.R.; Kärnbratt, J.; Löfroth, J.-E.; Holmberg, K. Cationic Ester-Containing Gemini Surfactants: Determination of Aggregation Numbers by Time-Resolved Fluorescence Quenching. J. Colloid Interface Sci. 2012, 376, 126–132. [Google Scholar] [CrossRef]
- Fatma, N.; Panda, M.; Kabir-ud-Din; Beg, M. Ester-Bonded Cationic Gemini Surfactants: Assessment of Their Cytotoxicity and Antimicrobial Activity. J. Mol. Liq. 2016, 222, 390–394. [Google Scholar] [CrossRef]
- Kuznetsova, D.A.; Kuznetsov, D.M.; Amerhanova, S.K.; Buzmakova, E.V.; Lyubina, A.P.; Syakaev, V.V.; Nizameev, I.R.; Kadirov, M.K.; Voloshina, A.D.; Zakharova, L.Y. Cationic Imidazolium Amphiphiles Bearing a Methoxyphenyl Fragment: Synthesis, Self-Assembly Behavior, and Antimicrobial Activity. Langmuir 2022, 38, 4921–4934. [Google Scholar] [CrossRef] [PubMed]
- Amerkhanova, S.K.; Voloshina, A.D.; Mirgorodskaya, A.B.; Lyubina, A.P.; Kuznetsova, D.A.; Kushnazarova, R.A.; Mikhailov, V.A.; Zakharova, L.Y. Antimicrobial Properties and Cytotoxic Effect of Imidazolium Geminis with Tunable Hydrophobicity. Int. J. Mol. Sci. 2021, 22, 13148. [Google Scholar] [CrossRef] [PubMed]
- Valeeva, F.G.; Karimova, T.R.; Pavlov, R.V.; Bakhtiyarov, D.I.; Sapunova, A.S.; Ivshin, K.A.; Kataeva, O.N.; Gaynanova, G.A.; Syakaev, V.V.; Voloshina, A.D.; et al. Introduction of Isothiuronium Surfactant Series: Synthesis, Structure-Dependent Aggregation Overview and Biological Activity. J. Mol. Liq. 2021, 324, 114721. [Google Scholar] [CrossRef]
- Chen, W.; Duša, F.; Witos, J.; Ruokonen, S.-K.; Wiedmer, S.K. Determination of the Main Phase Transition Temperature of Phospholipids by Nanoplasmonic Sensing. Sci. Rep. 2018, 8, 14815. [Google Scholar] [CrossRef]
- Zakharova, L.Y.; Kaupova, G.I.; Gabdrakhmanov, D.R.; Gaynanova, G.A.; Ermakova, E.A.; Mukhitov, A.R.; Galkina, I.V.; Cheresiz, S.V.; Pokrovsky, A.G.; Skvortsova, P.V.; et al. Alkyl Triphenylphosphonium Surfactants as Nucleic Acid Carriers: Complexation Efficacy toward DNA Decamers, Interaction with Lipid Bilayers and Cytotoxicity Studies. Phys. Chem. Chem. Phys. 2019, 21, 16706–16717. [Google Scholar] [CrossRef]
- Koirala, S.; Roy, B.; Guha, P.; Bhattarai, R.; Sapkota, M.; Nahak, P.; Karmakar, G.; Mandal, A.K.; Kumar, A.; Panda, A.K. Effect of Double Tailed Cationic Surfactants on the Physicochemical Behavior of Hybrid Vesicles. RSC Adv. 2016, 6, 13786–13796. [Google Scholar] [CrossRef]
- Amjadi, S.; Almasi, H.; Hamishehkar, H.; Alizadeh Khaledabad, M.; Lim, L.-T. Coating of Betanin and Carvone Co-Loaded Nanoliposomes with Synthesized Cationic Inulin: A Strategy for Enhancing the Stability and Bioavailability. Food Chem. 2022, 373, 131403. [Google Scholar] [CrossRef]
- Fang, J.-Y.; Hwang, T.-L.; Aljuffali, I.A.; Lin, C.-F.; Chang, C.-C. Cationic Additives in Nanosystems Activate Cytotoxicity and Inflammatory Response of Human Neutrophils: Lipid Nanoparticles versus Polymeric Nanoparticles. Int. J. Nanomed. 2015, 10, 371–385. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, H.; Wang, W.; Ma, Z.; Chi, Z.; Liu, S. A Cationic Amphiphilic AIE Polymer for Mitochondrial Targeting and Imaging. Pharmaceutics 2022, 15, 103. [Google Scholar] [CrossRef]
- Hammerer, F.; Poyer, F.; Fourmois, L.; Chen, S.; Garcia, G.; Teulade-Fichou, M.-P.; Maillard, P.; Mahuteau-Betzer, F. Mitochondria-Targeted Cationic Porphyrin-Triphenylamine Hybrids for Enhanced Two-Photon Photodynamic Therapy. Bioorg. Med. Chem. 2018, 26, 107–118. [Google Scholar] [CrossRef]
- Barbu, E.; Molnàr, É.; Tsibouklis, J.; Górecki, D.C. The Potential for Nanoparticle-Based Drug Delivery to the Brain: Overcoming the Blood–Brain Barrier. Expert Opin. Drug Deliv. 2009, 6, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Pandit, R.; Chen, L.; Götz, J. The Blood-Brain Barrier: Physiology and Strategies for Drug Delivery. Adv. Drug Deliv. Rev. 2020, 165–166, 1–14. [Google Scholar] [CrossRef]
- Pashirova, T.N.; Sapunova, A.S.; Lukashenko, S.S.; Burilova, E.A.; Lubina, A.P.; Shaihutdinova, Z.M.; Gerasimova, T.P.; Kovalenko, V.I.; Voloshina, A.D.; Souto, E.B.; et al. Synthesis, Structure-Activity Relationship and Biological Evaluation of Tetracationic Gemini Dabco-Surfactants for Transdermal Liposomal Formulations. Int. J. Pharm. 2020, 575, 118953. [Google Scholar] [CrossRef]
- Aleandri, S.; Bombelli, C.; Bonicelli, M.G.; Bordi, F.; Giansanti, L.; Mancini, G.; Ierino, M.; Sennato, S. Fusion of Gemini Based Cationic Liposomes with Cell Membrane Models: Implications for Their Biological Activity. Biochim. Biophys. Acta—Biomembr. 2013, 1828, 382–390. [Google Scholar] [CrossRef]
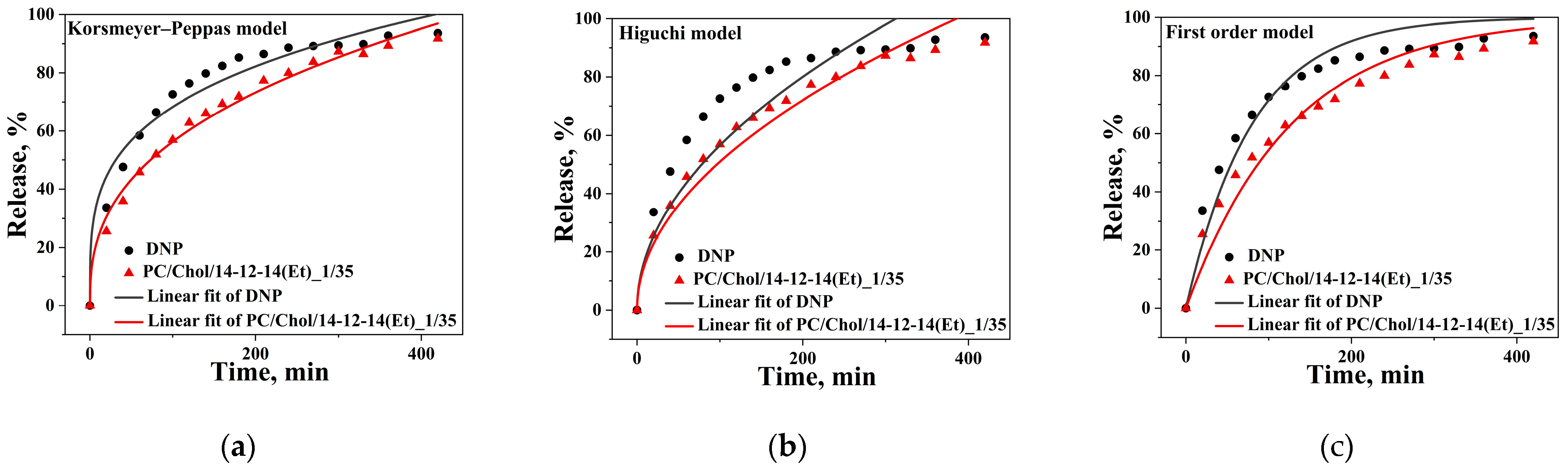
- Jain, A.; Jain, S.K. In Vitro Release Kinetics Model Fitting of Liposomes: An Insight. Chem. Phys. Lipids 2016, 201, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, Y.O.; Husain, M.; Patil, K.D.; Sodgir, V.; Patil, T.S.; Agnihotri, V.V.; Mahajan, H.S.; Sharma, C.; Ojha, S.; Goyal, S.N. Verapamil Hydrochloride Loaded Solid Lipid Nanoparticles: Preparation, Optimization, Characterisation, and Assessment of Cardioprotective Effect in Experimental Model of Myocardial Infarcted Rats. Biomed. Pharmacother. 2022, 154, 113429. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Akhtar, N. Development of Stable Tocopherol Succinate-loaded Ethosomes to Enhance Transdermal Permeation: In Vitro and in Vivo Characterizations. J. Cosmet. Dermatol. 2022, 21, 4942–4955. [Google Scholar] [CrossRef]
- Alruwaili, N.K.; Zafar, A.; Alsaidan, O.A.; Yasir, M.; Mostafa, E.M.; Alnomasy, S.F.; Rawaf, A.; Alquraini, A.; Alomar, F.A. Development of Surface Modified Bilosomes for the Oral Delivery of Quercetin: Optimization, Characterization in-Vitro Antioxidant, Antimicrobial, and Cytotoxicity Study. Drug Deliv. 2022, 29, 3035–3050. [Google Scholar] [CrossRef]
- De Souza, I.F.F.; Dos Santos, T.Q.; Placido, R.V.; Mangerona, B.A.; Carvalho, F.C.; Boralli, V.B.; Ruela, A.L.M.; Pereira, G.R. The Liquid Crystalline Phase Behaviour of a Nasal Formulation Modifies the Brain Disposition of Donepezil in Rats in the Treatment of Alzheimer’s Disease. Colloids Surf. B 2021, 203, 111721. [Google Scholar] [CrossRef]
- Schneider, L.S. A Critical Review of Cholinesterase Inhibitors as a Treatment Modality in Alzheimer’s Disease. Dialogues Clin. Neurosci. 2000, 2, 111–128. [Google Scholar] [CrossRef]
- Colovic, M.B.; Krstic, D.Z.; Lazarevic-Pasti, T.D.; Bondzic, A.M.; Vasic, V.M. Acetylcholinesterase Inhibitors: Pharmacology and Toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef] [PubMed]
- Darvesh, S.; Darvesh, K.V.; McDonald, R.S.; Mataija, D.; Walsh, R.; Mothana, S.; Lockridge, O.; Martin, E. Carbamates with Differential Mechanism of Inhibition toward Acetylcholinesterase and Butyrylcholinesterase. J. Med. Chem. 2008, 51, 4200–4212. [Google Scholar] [CrossRef] [PubMed]
- Burilova, E.A.; Pashirova, T.N.; Zueva, I.V.; Gibadullina, E.M.; Lushchekina, S.V.; Sapunova, A.S.; Kayumova, R.M.; Rogov, A.M.; Evtjugin, V.G.; Sudakov, I.A.; et al. Bi-Functional Sterically Hindered Phenol Lipid-Based Delivery Systems as Potential Multi-Target Agents against Alzheimer’s Disease via an Intranasal Route. Nanoscale 2020, 12, 13757–13770. [Google Scholar] [CrossRef]
- Correia, A.C.; Monteiro, A.R.; Silva, R.; Moreira, J.N.; Sousa Lobo, J.M.; Silva, A.C. Lipid Nanoparticles Strategies to Modify Pharmacokinetics of Central Nervous System Targeting Drugs: Crossing or Circumventing the Blood–Brain Barrier (BBB) to Manage Neurological Disorders. Adv. Drug Deliv. Rev. 2022, 189, 114485. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.; Meeks, C.; Vézina, A.; Robey, R.W.; Tanner, K.; Gottesman, M.M. Model Systems for Studying the Blood-Brain Barrier: Applications and Challenges. Biomaterials 2019, 214, 119217. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The Blood–Brain Barrier: Structure, Regulation, and Drug Delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef]
- Kaur, I.P.; Bhandari, R.; Bhandari, S.; Kakkar, V. Potential of Solid Lipid Nanoparticles in Brain Targeting. J. Control Release 2008, 127, 97–109. [Google Scholar] [CrossRef]
- Gordillo-Galeano, A.; Mora-Huertas, C.E. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: A Review Emphasizing on Particle Structure and Drug Release. Eur. J. Pharm. Biopharm. 2018, 133, 285–308. [Google Scholar] [CrossRef]
- Hernandez, C.; Shukla, S. Liposome Based Drug Delivery as a Potential Treatment Option for Alzheimer’s Disease. Neural Regen. Res. 2022, 17, 1190. [Google Scholar] [CrossRef]
- Sharma, S.; Dang, S. Nanocarrier-Based Drug Delivery to Brain: Interventions of Surface Modification. Curr. Neuropharmacol. 2023, 21, 517–535. [Google Scholar] [CrossRef]
- Hommoss, G.; Pyo, S.M.; Müller, R.H. Mucoadhesive Tetrahydrocannabinol-Loaded NLC—Formulation Optimization and Long-Term Physicochemical Stability. Eur. J. Pharm. Biopharm. 2017, 117, 408–417. [Google Scholar] [CrossRef]
- Leger, M.; Quiedeville, A.; Bouet, V.; Haelewyn, B.; Boulouard, M.; Schumann-Bard, P.; Freret, T. Object Recognition Test in Mice. Nat. Protoc. 2013, 8, 2531–2537. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.L.; Ganaraja, B.; Murlimanju, B.V.; Joy, T.; Krishnamurthy, A.; Agrawal, A. Hippocampus and Its Involvement in Alzheimer’s Disease: A Review. 3 Biotech 2022, 12, 55. [Google Scholar] [CrossRef] [PubMed]
- Valiullina, Y.A.; Ermakova, E.A.; Faizullin, D.A.; Mirgorodskaya, A.B.; Zuev, Y.F. Structure and Properties of Complexes of α-Chymotrypsin with Hydroxyl-Containing Gemini Dicationic Surfactants with a Spacer Moiety of Varying Length. J. Struct. Chem. 2014, 55, 1556–1564. [Google Scholar] [CrossRef]
- Matsuoka, K.; Yoshimura, T.; Shikimoto, T.; Hamada, J.; Yamawaki, M.; Honda, C.; Endo, K. Molecular Aggregates of Partially Fluorinated Quaternary Ammonium Salt Gemini Surfactants. Langmuir 2007, 23, 10990–10994. [Google Scholar] [CrossRef]
- Vasileva, L.A.; Eyupova, R.F.; Valeeva, F.G.; Gaynanova, G.A.; Zakharova, L.Y. Mixed Micellar Systems—Efficient Nanocontainers for the Delivery of Hydrophobic Substrates. Russ. Chem. Bull. 2022, 71, 1897–1906. [Google Scholar] [CrossRef]
- Mirgorodskaya, A.B.; Kuznetsova, D.A.; Kushnazarova, R.A.; Gabdrakhmanov, D.R.; Zhukova, N.A.; Lukashenko, S.S.; Sapunova, A.S.; Voloshina, A.D.; Sinyashin, O.G.; Mamedov, V.A.; et al. Soft Nanocarriers for New Poorly Soluble Conjugate of Pteridine and Benzimidazole: Synthesis and Cytotoxic Activity against Tumor Cells. J. Mol. Liq. 2020, 317, 114007. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
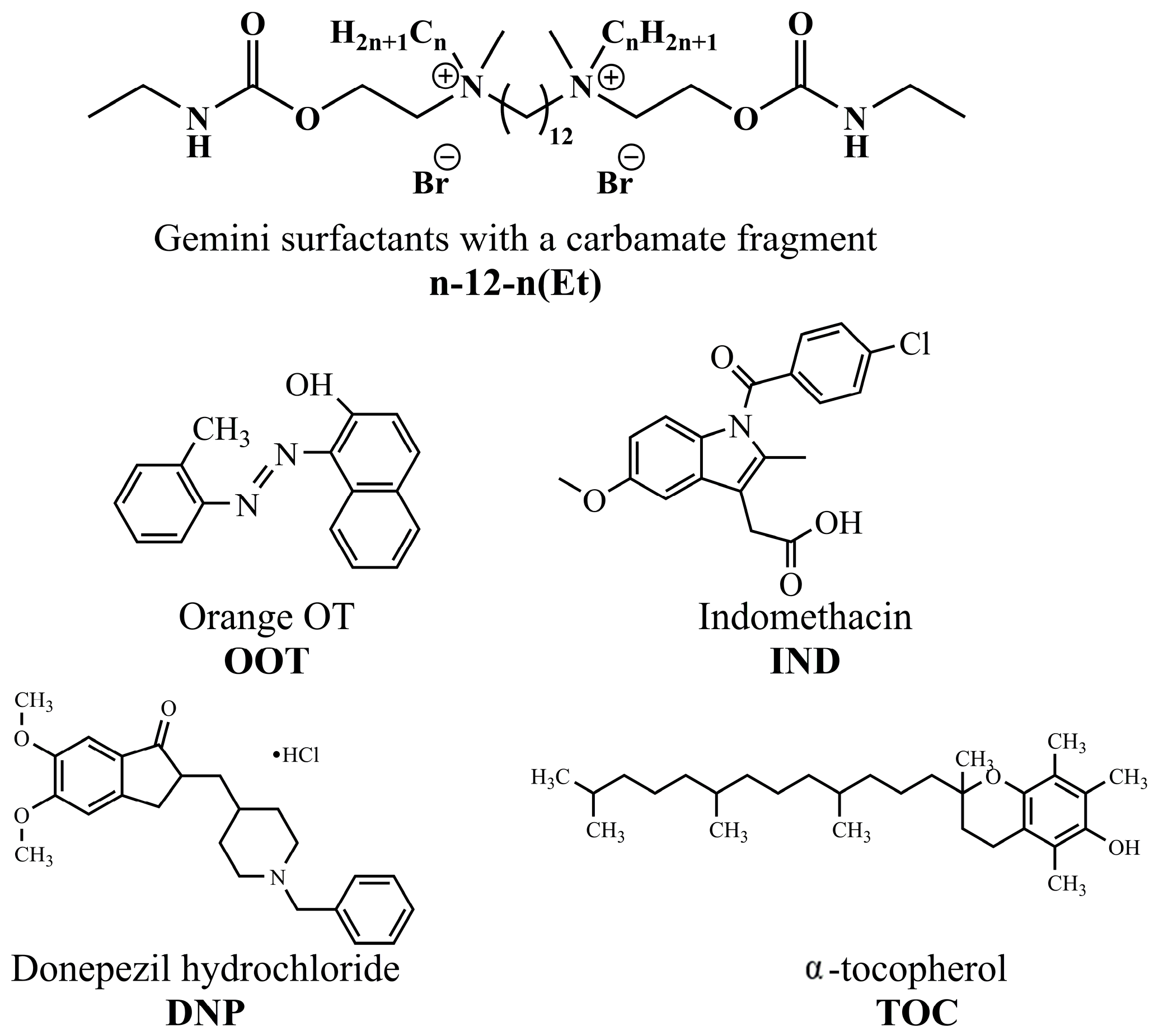
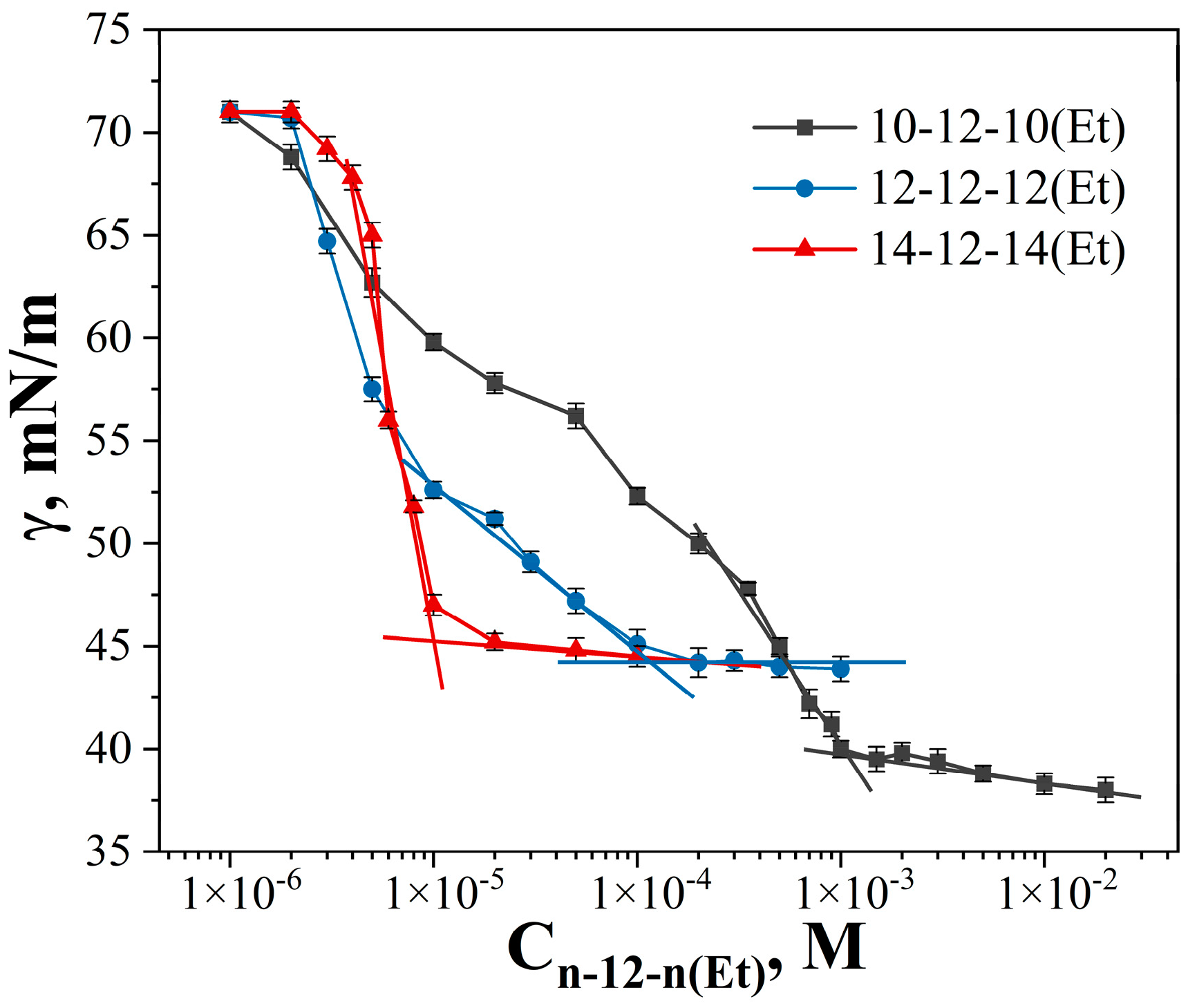

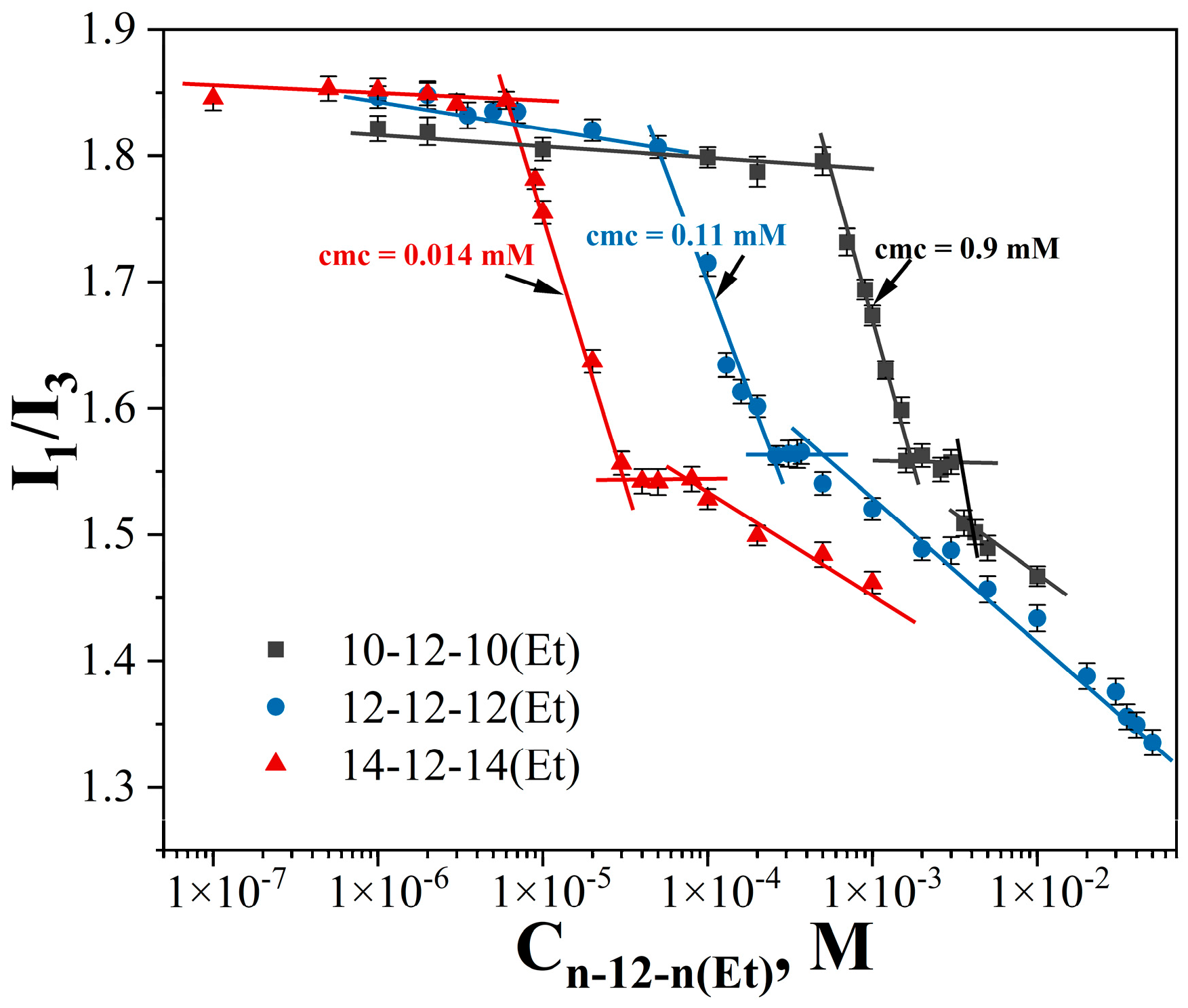
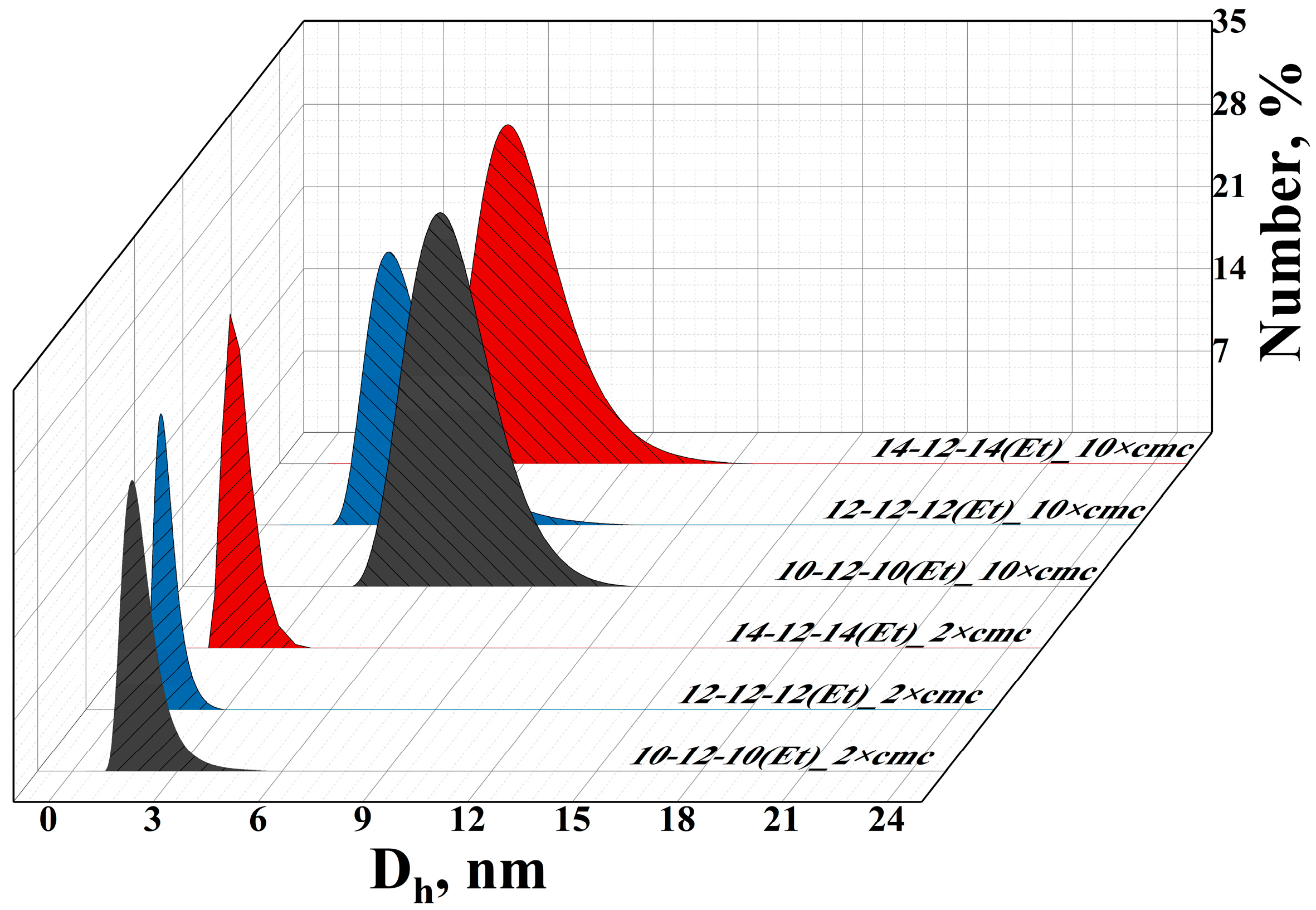

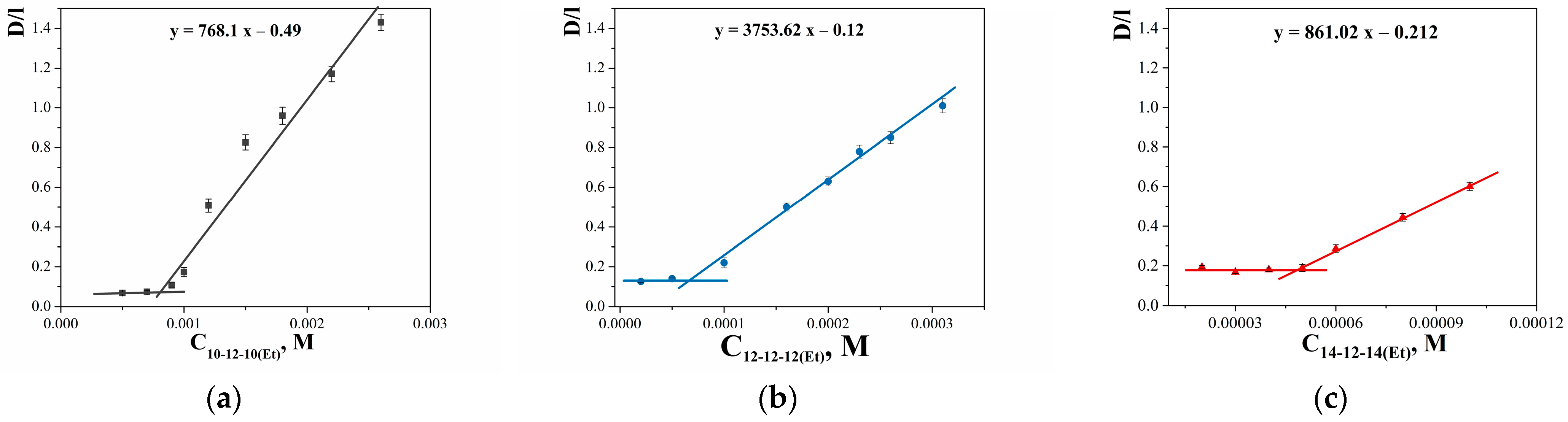
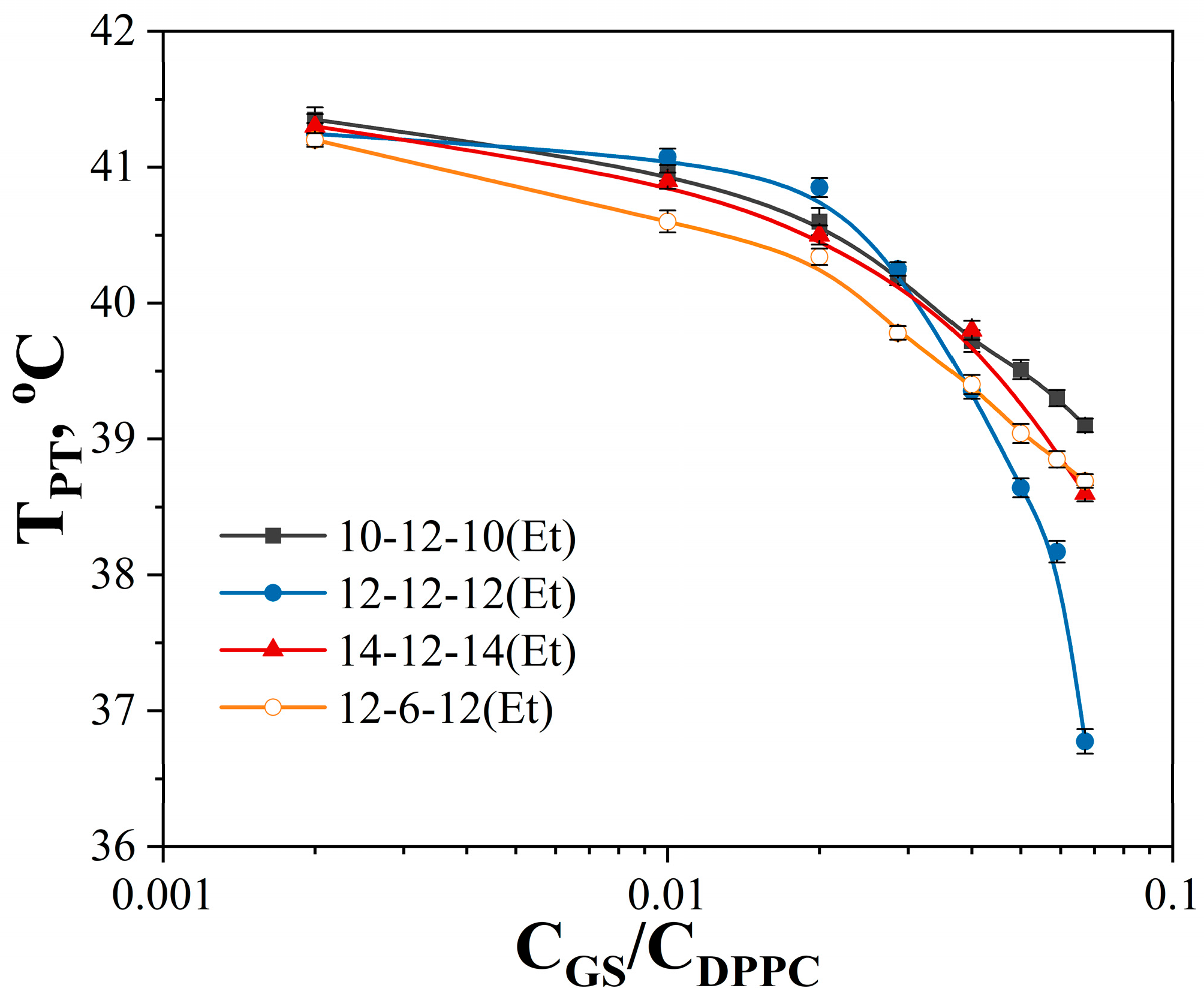

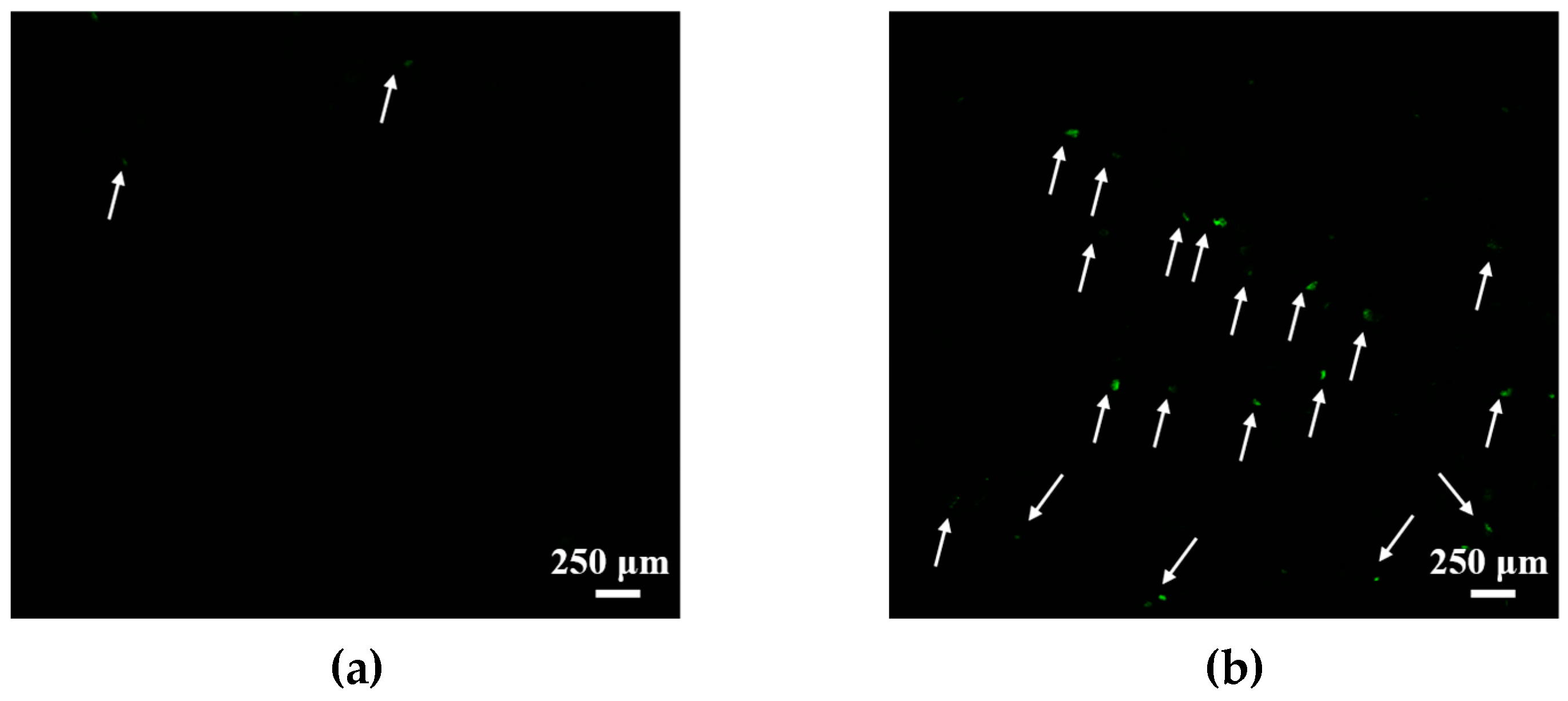
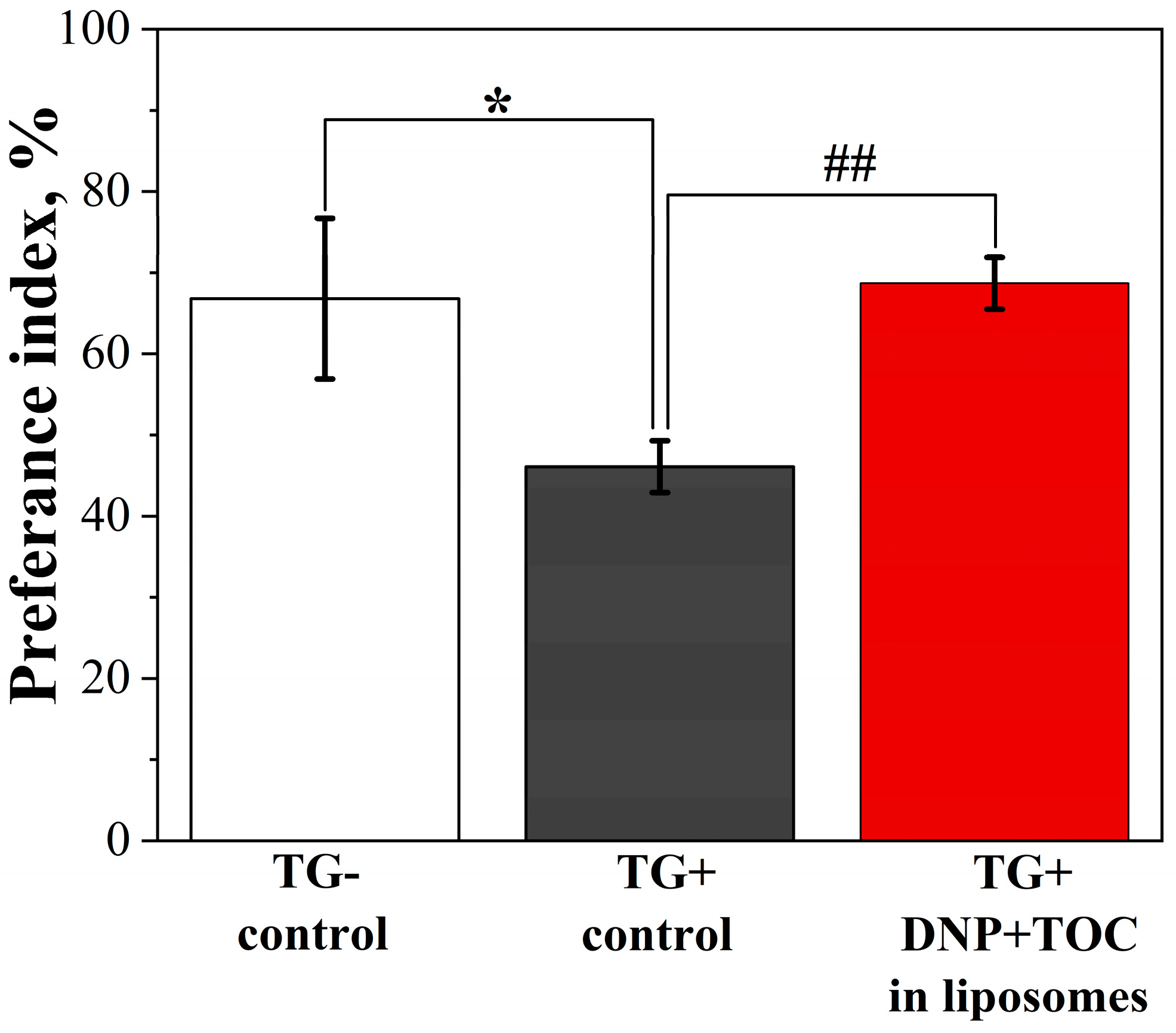

| GS | cmctens, mM | cmccond, mM | Гmax∙106, mol∙m−2 | Amin, nm2 | ∆Gmic, kJ∙mol−1 | βcond | βpot |
|---|---|---|---|---|---|---|---|
| 10-12-10(Et) | 1 ± 0.03 | 1.5 ± 0.04 | 0.71 | 2.34 | −25.5 | 0.41 | - |
| 12-12-12(Et) | 0.11 ± 0.022 | 0.33 ± 0.01 | 0.41 | 4.08 | −29.1 | 0.41 | 0.45 |
| 14-12-14(Et) | 0.013 ± 0.0007 | 0.05 ± 0.0013 | 3.21 | 0.52 | −37.0 | 0.44 | 0.3 |
| GS | cmc, mM | S, molprobe/molGS | Nagg * | ||
|---|---|---|---|---|---|
| Fluorimetry | Spectrophotometry | OOT | IND | ||
| 10-12-10(Et) | 0.9 ± 0.03 | 1.5 ± 0.1 | 0.021 | 0.13 | 50 |
| 12-12-12(Et) | 0.11 ± 0.002 | 0.16 ± 0.01 | 0.029 | 0.64 | 28 |
| 14-12-14(Et) | 0.014 ± 0.0004 | 0.045 ± 0.003 | 0.047 | 1.51 | 16 |
| GS | MIC, μg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gram-Positive Bacteria | Gram-Negative Bacteria | Fungi | ||||||||
| Sa | Bc | Ef | MRSA-1 | MRSA-2 | Ec | Pa | Ca | Tm | An | |
| 10-12-10(Et) | 0.5 ± 0.03 | 0.5 ± 0.04 | 0.5 ± 0.03 | 0.5 ± 0.03 | 0.5 ± 0.03 | 0.5 ± 0.03 | 1.9 ± 0.1 | 0.9 ± 0.07 | 0.9 ± 0.07 | 250 ± 19 |
| 12-12-12(Et) | 0.9 ± 0.07 | 7.8 ± 0.6 | 1.9 ± 0.1 | 1.9 ± 0.1 | 0.9 ± 0.07 | 15.6 ± 1.3 | 125 ± 9 | 3.9 ± 0.3 | 3.9 ± 0.3 | >250 |
| 14-12-14(Et) | 3.9 ± 0.2 | 125 ± 10 | 3.9 ± 0.3 | 7.8 ± 0.6 | 7.8 ± 0.7 | 31.3 ± 2.5 | 125 ± 10 | 250 ± 19 | 250 ± 19 | >250 |
| Ketoconazole | 3.9 ± 0.3 | 3.9 ± 0.3 | 15.6 ± 1.3 | |||||||
| MBC, μg/mL | MFC, μg/mL | |||||||||
| 10-12-10(Et) | 0.5 ± 0.04 | 0.5 ± 0.03 | 0.5 ± 0.04 | 1.9 ± 0.1 | 3.9 ± 0.3 | 0.5 ± 0.03 | 3.9 ± 0.3 | 15.6 ± 1.2 | 15.6 ± 1.3 | >250 |
| 12-12-12(Et) | 0.9 ± 0.07 | 15.6 ± 1.3 | 3.9 ± 0.2 | 1.9 ± 0.1 | 0.9 ± 0.06 | 15.6 ± 1.2 | 125 ± 10 | 7.8 ± 0.6 | 7.8 ± 0.6 | >250 |
| 14-12-14(Et) | 3.9 ± 0.3 | 125 ± 9 | 3.9 ± 0.2 | 7.8 ± 0.6 | 7.8 ± 0.7 | 31.3 ± 2.6 | 125 ± 10 | 250 ± 21 | 250 ± 18 | >250 |
| Ketoconazole | 3.9 ± 0.3 | 3.9 ± 0.3 | 125 ± 10 | |||||||
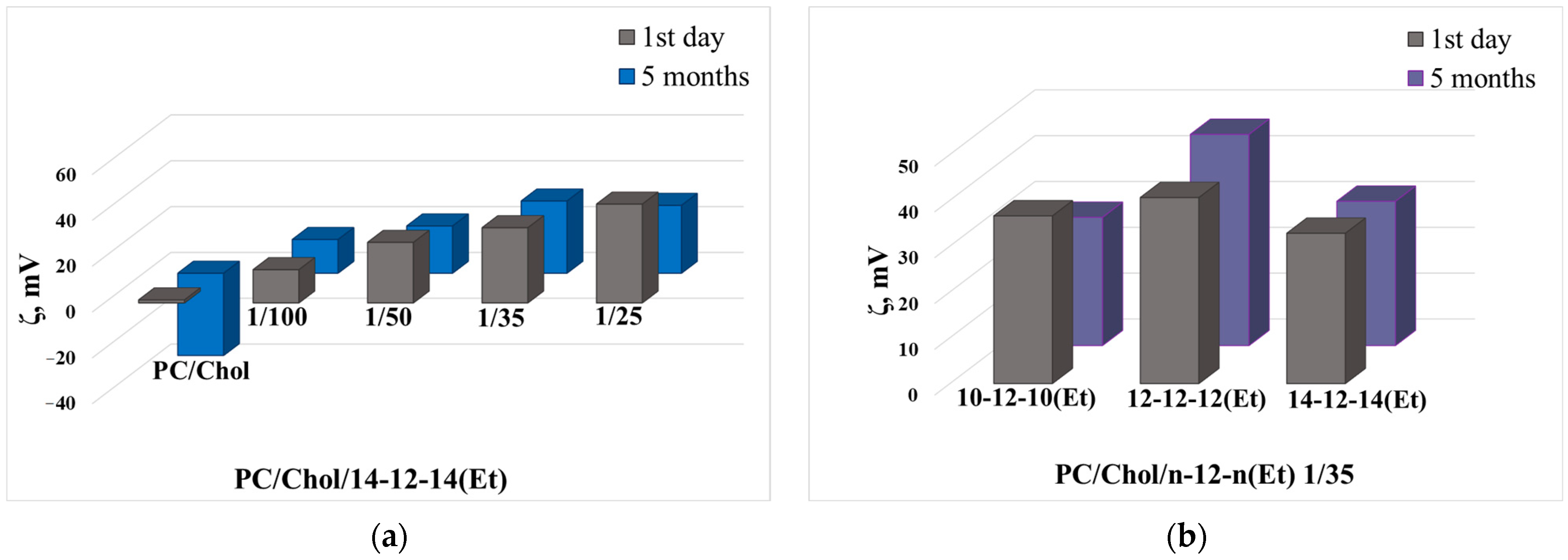
| Lipid Concentration, mM | 14-12-14(Et)/ Lipid Ratio | Dh, nm | PdI | ζ, mV | Dh, nm | PdI | ζ, mV |
|---|---|---|---|---|---|---|---|
| 1st Day | 5th Month | ||||||
| 5 | - | 128 ± 1 | 0.063 ± 0.024 | −1.6 ± 0.3 | 135 ± 17 | 0.414 ± 0.021 | −23 ± 2 |
| 1/100 | 120 ± 1 | 0.057 ± 0.029 | 13.8 ± 0.3 | 113 ± 4 | 0.346 ± 0.044 | −24 ± 2 | |
| 1/50 | 116 ± 1 | 0.070 ± 0.003 | 27 ± 1 | 129 ± 1 | 0.135 ± 0.013 | 15 ± 1 | |
| 1/35 | 114 ± 1 | 0.070 ± 0.012 | 36 ± 3 | 126 ± 2 | 0.098 ± 0.024 | 27 ± 2 | |
| 1/25 | 113 ± 1 | 0.060 ± 0.003 | 46 ± 2 | 127 ± 1 | 0.175 ± 0.029 | 22.3 ± 0.5 | |
| 10 | - | 129 ± 1 | 0.061 ± 0.024 | 0.8 ± 0.4 | 92 ± 1 | 0.216 ± 0.021 | −22 ± 2 |
| 1/100 | 112 ± 1 | 0.055 ± 0.019 | 13.7 ± 0.3 | 128 ± 1 | 0.127 ± 0.033 | 8 ± 1 | |
| 1/50 | 112 ± 1 | 0.070 ± 0.018 | 26.7 ± 0.8 | 119 ± 1 | 0.114 ± 0.017 | 25 ± 1 | |
| 1/35 | 112 ± 1 | 0.061 ± 0.013 | 34 ± 1 | 119 ± 1 | 0.095 ± 0.010 | 26 ± 1 | |
| 1/25 | 108 ± 1 | 0.069 ± 0.019 | 46 ± 2 | 114 ± 2 | 0.121 ± 0.034 | 22 ± 2 | |
| 15 | - | 131 ± 2 | 0.069 ± 0.027 | 1.3 ± 0.4 | 103 ± 1 | 0.236 ± 0.010 | −36 ± 6 |
| 1/100 | 113 ± 1 | 0.055 ± 0.015 | 14.5 ± 0.7 | 134 ± 1 | 0.101 ± 0.005 | 14.7 ± 0.2 | |
| 1/50 | 113 ± 1 | 0.050 ± 0.023 | 26.4 ± 0.3 | 127 ± 2 | 0.112 ± 0.004 | 21 ± 3 | |
| 1/35 | 113 ± 1 | 0.062 ± 0.014 | 33 ± 1 | 125 ± 2 | 0.102 ± 0.024 | 32 ± 3 | |
| 1/25 | 108 ± 1 | 0.045 ± 0.012 | 43 ± 3 | 122 ± 2 | 0.139 ± 0.004 | 30 ± 1 | |
| GS | GS/Lipid Ratio | Dh, nm | PdI | ζ, mV | Dh, nm | PdI | ζ, mV |
|---|---|---|---|---|---|---|---|
| 1st Day | 5th Month | ||||||
| (PC/Chol) | - | 131 ± 2 | 0.069 ± 0.027 | 1.3 ± 0.4 | 103 ± 1 | 0.236 ± 0.010 | −36 ± 6 |
| 10-12-10(Et) | 1/100 | 121 ± 1 | 0.049 ± 0.020 | 15 ± 1 | 131 ± 1 | 0.157 ± 0.017 | 8.1 ± 0.3 |
| 1/50 | 117 ± 1 | 0.060 ± 0.010 | 28 ± 1 | 121 ± 1 | 0.105 ± 0.013 | 24 ± 1 | |
| 1/35 | 114 ± 1 | 0.061 ± 0.018 | 37 ± 1 | 118 ± 2 | 0.079 ± 0.014 | 28 ± 2 | |
| 1/25 | 112 ± 1 | 0.066 ± 0.010 | 45 ± 2 | 120 ± 1 | 0.097 ± 0.023 | 42 ± 2 | |
| 12-12-12(Et) | 1/100 | 119 ± 1 | 0.053 ± 0.035 | 17.5 ± 0.3 | 199 ± 4 | 0.393 ± 0.012 | 20.6 ± 0.6 |
| 1/50 | 118 ± 1 | 0.072 ± 0.016 | 30 ± 2 | 185 ± 3 | 0.402 ± 0.050 | 33 ± 1 | |
| 1/35 | 119 ± 1 | 0.079 ± 0.016 | 40.6 ± 0.6 | 157 ± 1 | 0.328 ± 0.017 | 46 ± 1 | |
| 1/25 | 117 ± 1 | 0.081 ± 0.024 | 49 ± 2 | 149 ± 2 | 0.304 ± 0.041 | 49.8 ± 0.1 | |
| 14-12-14(Et) | 1/100 | 113 ± 1 | 0.055 ± 0.015 | 14.5 ± 0.7 | 134 ± 1 | 0.101 ± 0.005 | 14.7 ± 0.2 |
| 1/50 | 113 ± 1 | 0.050 ± 0.023 | 26.4 ± 0.4 | 127 ± 2 | 0.112 ± 0.004 | 21 ± 3 | |
| 1/35 | 113 ± 1 | 0.062 ± 0.014 | 33 ± 1 | 125 ± 2 | 0.102 ± 0.024 | 32 ± 3 | |
| 1/25 | 108 ± 1 | 0.045 ± 0.012 | 43 ± 3 | 122 ± 2 | 0.139 ± 0.004 | 30 ± 1 | |
| GS | PC/Chol Concentration, mM | EE, % | Dh, nm | PdI | ζ, mV |
|---|---|---|---|---|---|
| RhB | |||||
| 10-12-10(Et) | 15 | 72 ± 1 | 103 ± 1 | 0.068 ± 0.018 | 33 ± 3 |
| 12-12-12(Et) | 15 | 73.4 ± 0.8 | 105 ± 1 | 0.066 ± 0.009 | 36 ± 2 |
| 14-12-14(Et) | 5 | 46 ± 2 | 110 ± 1 | 0.070 ± 0.014 | 36 ± 3 |
| 10 | 66 ± 2 | 110 ± 1 | 0.079 ± 0.002 | 37 ± 2 | |
| 15 | 75 ± 3 | 110 ± 1 | 0.058 ± 0.015 | 35 ± 3 | |
| TOC | |||||
| 10-12-10(Et) | 15 | 95.8 ± 0.6 | 142 ± 2 | 0.124 ± 0.009 | 41 ± 2 |
| 12-12-12(Et) | 15 | 91 ± 1 | 141 ± 2 | 0.077 ± 0.027 | 38 ± 2 |
| 14-12-14(Et) | 5 | 94 ± 1 | 122 ± 1 | 0.074 ± 0.019 | 36 ± 2 |
| 10 | 95 ± 1 | 133 ± 1 | 0.072 ± 0.002 | 38 ± 2 | |
| 15 | 96.1 ± 0.5 | 132 ± 1 | 0.081 ± 0.006 | 37 ± 2 | |
| DNP | |||||
| 10-12-10(Et) | 15 | 97.8 ± 0.6 | 107 ± 1 | 0.089 ± 0.024 | 41 ± 3 |
| 12-12-12(Et) | 15 | 97.9 ± 0.4 | 109 ± 1 | 0.090 ± 0.013 | 50 ± 2 |
| 14-12-14(Et) | 5 | 97.3 ± 0.4 | 109 ± 2 | 0.079 ± 0.018 | 32 ± 2 |
| 10 | 97.6 ± 0.6 | 110 ± 2 | 0.105 ± 0.011 | 42 ± 1 | |
| 15 | 98.0 ± 0.5 | 110 ± 1 | 0.088 ± 0.005 | 40 ± 2 | |
| GS | IC50 (hAChE), nM | IC50 (hBChE), nM | SIhBChE/hAChE |
|---|---|---|---|
| 10-12-10(Et) | 43.1 ± 0.9 | 19 ± 5 | 0.44 |
| 12-12-12(Et) | 82 ± 13 | 61 ± 2 | 0.74 |
| 14-12-14(Et) | 11.0 ± 0.5 | 1440 ± 520 | 131 |
| Parameter | Equation |
|---|---|
| The surface excess (Гmax) | Гmax = 1/(2.3nRT) × limc→CMC − (dπ/dlgC) |
| The minimum area per molecule (Amin) | Amin = 1018/(NA × Гmax) |
| Free energy of micellization (ΔGmic) | ∆Gmic = RT (0.5 + β) lnXcmc − 0.5RTln2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasileva, L.; Gaynanova, G.; Valeeva, F.; Romanova, E.; Pavlov, R.; Kuznetsov, D.; Belyaev, G.; Zueva, I.; Lyubina, A.; Voloshina, A.; et al. Synthesis, Properties, and Biomedical Application of Dicationic Gemini Surfactants with Dodecane Spacer and Carbamate Fragments. Int. J. Mol. Sci. 2023, 24, 12312. https://doi.org/10.3390/ijms241512312
Vasileva L, Gaynanova G, Valeeva F, Romanova E, Pavlov R, Kuznetsov D, Belyaev G, Zueva I, Lyubina A, Voloshina A, et al. Synthesis, Properties, and Biomedical Application of Dicationic Gemini Surfactants with Dodecane Spacer and Carbamate Fragments. International Journal of Molecular Sciences. 2023; 24(15):12312. https://doi.org/10.3390/ijms241512312
Chicago/Turabian StyleVasileva, Leysan, Gulnara Gaynanova, Farida Valeeva, Elvira Romanova, Rais Pavlov, Denis Kuznetsov, Grigory Belyaev, Irina Zueva, Anna Lyubina, Alexandra Voloshina, and et al. 2023. "Synthesis, Properties, and Biomedical Application of Dicationic Gemini Surfactants with Dodecane Spacer and Carbamate Fragments" International Journal of Molecular Sciences 24, no. 15: 12312. https://doi.org/10.3390/ijms241512312
APA StyleVasileva, L., Gaynanova, G., Valeeva, F., Romanova, E., Pavlov, R., Kuznetsov, D., Belyaev, G., Zueva, I., Lyubina, A., Voloshina, A., Petrov, K., & Zakharova, L. (2023). Synthesis, Properties, and Biomedical Application of Dicationic Gemini Surfactants with Dodecane Spacer and Carbamate Fragments. International Journal of Molecular Sciences, 24(15), 12312. https://doi.org/10.3390/ijms241512312








