The Bladder Tumor Microenvironment Components That Modulate the Tumor and Impact Therapy
Abstract
1. Introduction
2. Bladder Cancer Cells
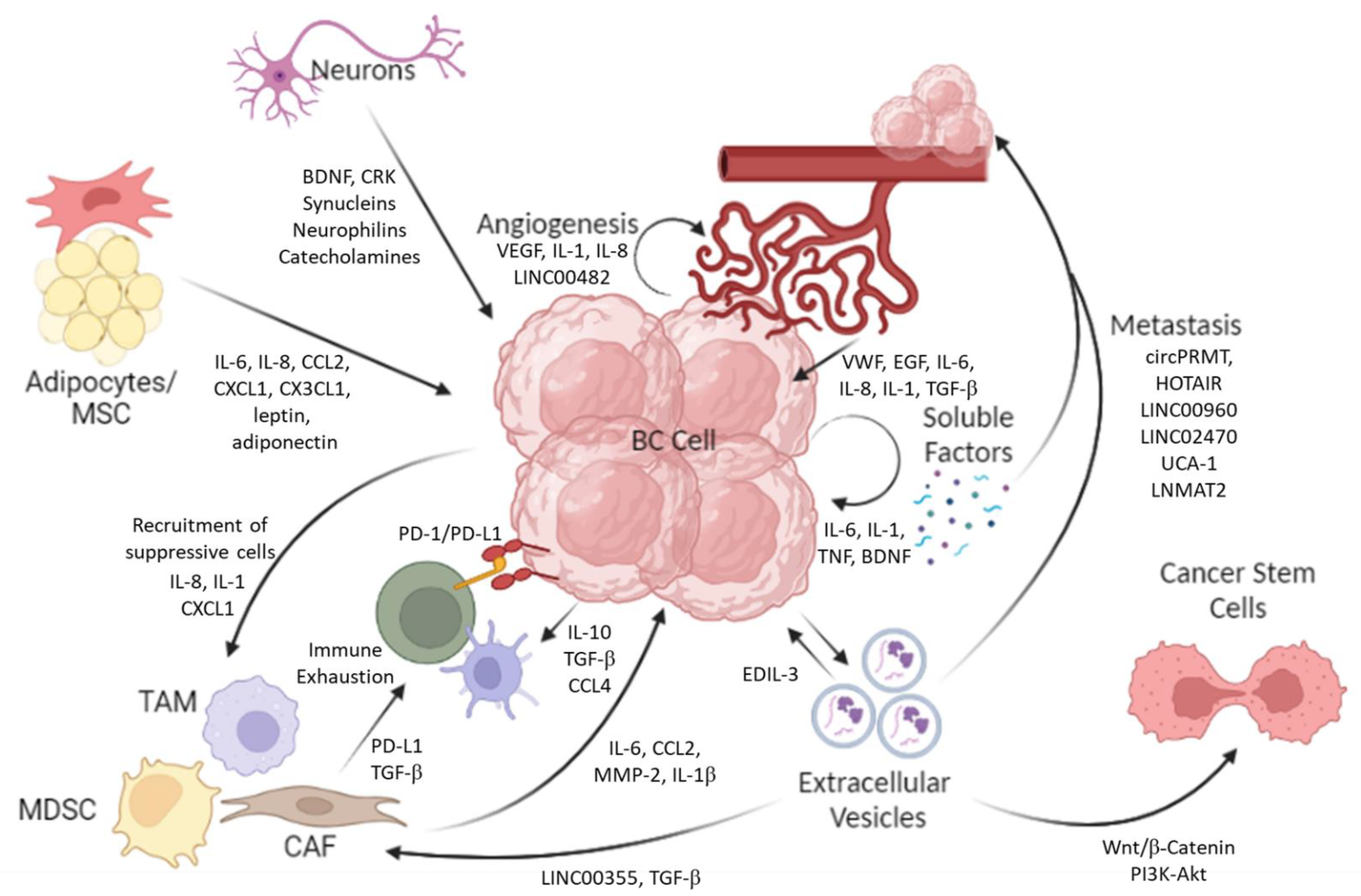
2.1. Soluble Factors
| Study | Cell Line | Factors | Impact | Reference |
|---|---|---|---|---|
| Mouse models | ||||
| In vivo mouse model | MB49 | IL10 |
| [9] |
| In vitro allograft | MB49 | CCL2 |
| [10] |
| In vivo mouse model | - | Pyruvate kinase M2 (PKM2) controlled vascular endothelial growth factor (VEGF) |
| [8] |
| In vivo mouse model | MBT-2 | Prostaglandin E 2 (PGE2)/cyclooxygenase 2 (COX2) |
| [14] |
| Human cell lines and cancer databases | ||||
| In vitro assays (Boyden Chamber) | RT112, Cal-29 | IL-8, IL-1 |
| [12] |
| In vitro assay | T24 | IL-1 and IL-1α |
| [4] |
| In vitro assay | Various Cell Lines | BMP4 secretion |
| [11] |
| In vitro and microfluidic assays | RT4, RT112, and T24/83 | VEGF-A, IL-8, TIMP1 |
| [5] |
| Human Database | The Cancer Genome Atlas (TCGA) | CCL4 |
| [16] |
| In vitro assay | Patient derived Cell line | VEGF, cylin dependant kinase 4 (CDK4) |
| [6] |
| In vitro (transwell) assays | T24 253J | VEGF, CXC motif chemokine ligand 1 (CXCL1), CXCL5, CXCL8 |
| [17] |
| In vitro assays | HT-1376, T24,5637(HTB-9), and J82 | LINC00482 |
| [7] |
| 3D cell culture and in vitro assays | J82, UMUC3, T24 | CXCL1 |
| [13] |
| In vitro assays | T24 and UMUC3 | Brain-derived neurotrophic factor (BDNF) |
| [18] |
2.2. Extracellular Vesicles (EVs)
| Study | Cell Line | Factors | Impact | Reference |
|---|---|---|---|---|
| Mouse | ||||
| In vitro (sphere formation, migration and invasion assays) | MB49 Cells | Unidentified factor |
| [27] |
| In vivo implantation in nude mice | T24 | Cathepsin B |
| [34] |
| In vivo implantation in BALB/c nude mice | T24/5637 | Circular RNA PRMT5 (circPRMT5) |
| [35] |
| Human | ||||
| In vitro cell co-culture | T24 and RT4 | TGF-β |
| [28] |
| In vitro assays (wound-healing and tube-formation assays) | TCCSUP, T24 | EDIL-3 |
| [20] |
| In vitro assays (transwell and migration) | TCCSUP, T24 | HOTAIR |
| [24] |
| Various in vitro assays | TSGH-8301, T24, J82 | LINC00960 and LINC02470 |
| [26] |
| In vitro co-culture and assays | T24, SV-HUC-1 | miR-221-5p and miR-186-5p |
| [29] |
| In vitro assays | T24 | MiR-217 |
| [36] |
| In vitro co-culture and assays | 5637 | lncRNA-UCA1 |
| [21] |
| In vitro assays | T24 | Unidentified factor |
| [37] |
| In vitro co-culture and assays | T24 and UMUC3 | Unidentified factor |
| [38] |
| In vitro assays | T24, TCCSUP, 5637 and UMUC3 | circPRMT5 |
| [35] |
| In vitro co-culture and assays | 5637 and UMUC3 | LNMAT2 |
| [25] |
| In vitro migration and tube-formation assays | 5637 and UMUC3 | Brain cytoplasmic RNA 1 (BCYRN1) |
| [39] |
2.3. Bladder Cancer Stem Cells
2.4. Targeting CSCs
3. Cancer-Associated Fibroblasts (CAFs)
4. Endothelial Cells/Pericytes
5. Other Stromal Cells (Adipose Cells/Adipose Stem Cells/Mesenchymal Stem Cells)
6. Do Neurons Support Cancer Growth and/or Inhibit It?
7. Impact of Anti-Hypertensive Drugs and Anesthetics on Bladder Cancer
8. Conclusions
9. Future Direction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Joyce, D.D.; Sharma, V.; Williams, S.B. Cost-Effectiveness and Economic Impact of Bladder Cancer Management: An Updated Review of the Literature. Pharmacoeconomics 2023, 41, 751–769. [Google Scholar] [CrossRef] [PubMed]
- Compérat, E.; Amin, M.B.; Cathomas, R.; Choudhury, A.; De Santis, M.; Kamat, A.; Stenzl, A.; Thoeny, H.C.; Witjes, J.A. Current best practice for bladder cancer: A narrative review of diagnostics and treatments. Lancet 2022, 400, 1712–1721. [Google Scholar] [CrossRef] [PubMed]
- Kartikasari, A.E.R.; Huertas, C.S.; Mitchell, A.; Plebanski, M. Tumor-Induced Inflammatory Cytokines and the Emerging Diagnostic Devices for Cancer Detection and Prognosis. Front. Oncol. 2021, 11. [Google Scholar] [CrossRef]
- John, A.; Günes, C.; Bolenz, C.; Vidal-Y-Sy, S.; Bauer, A.T.; Schneider, S.W.; Gorzelanny, C. Bladder cancer-derived interleukin-1 converts the vascular endothelium into a pro-inflammatory and pro-coagulatory surface. BMC Cancer 2020, 20, 1178. [Google Scholar] [CrossRef] [PubMed]
- John, A.; Robador, J.R.; Vidal-Y-Sy, S.; Houdek, P.; Wladykowski, E.; Guenes, C.; Bolenz, C.; Schneider, S.W.; Bauer, A.T.; Gorzelanny, C. Urothelial Carcinoma of the Bladder Induces Endothelial Cell Activation and Hypercoagulation. Mol. Cancer Res. 2020, 18, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; An, M.; Zhang, H.; Tan, D.; Chen, X.; Wu, P.; Qin, W.; Zhang, C.; Shi, C. Patient-derived bladder cancer xenograft models reveal VEGF and CDK4 enhancing tumor metastasis behavior. RSC Adv. 2019, 9, 17877–17884. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Wei, N.; Sun, Y.; Pan, W.; Chen, Y. Silencing LINC00482 inhibits tumor-associated inflammation and angiogenesis through down-regulation of MMP-15 via FOXA1 in bladder cancer. Aging 2020, 13, 2264–2278. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, X.; Liu, Y.; Shapiro, E.; Lepor, H.; Tang, M.-S.; Sun, T.-T.; Wu, X.-R. Data from PKM2 Is Essential for Bladder Cancer Growth and Maintenance. Cancer Res. 2023, 82, 571–585. [Google Scholar] [CrossRef]
- Yang, A.S.; Lattime, E.C. Tumor-induced interleukin 10 suppresses the ability of splenic dendritic cells to stimulate CD4 and CD8 T-cell responses. Cancer Res. 2003, 63, 2150–2157. [Google Scholar]
- Chiang, Y.; Tsai, Y.-C.; Wang, C.-C.; Hsueh, F.-J.; Huang, C.-Y.; Chung, S.-D.; Chen, C.-H.; Pu, Y.-S.; Cheng, J.C.-H. Tumor-Derived C-C Motif Ligand 2 Induces the Recruitment and Polarization of Tumor-Associated Macrophages and Increases the Metastatic Potential of Bladder Cancer Cells in the Postirradiated Microenvironment. Int. J. Radiat. Oncol. 2022, 114, 321–333. [Google Scholar] [CrossRef]
- Martínez, V.G.; Rubio, M.C.; Martínez-Fernández, C.; Segovia, F.; López-Calderón, M.I.; Garín, A.; Teijeira, E.; Munera-Maravilla, A.; Varas, R.; Sacedón, F.; et al. Bmp4 Induces M2 Macrophage Polarization and Favors Tumor Progression in Bladder Cancer. Clin. Cancer Res. 2017, 23, 7388–7399. [Google Scholar] [CrossRef]
- Grimm, S.; Jennek, S.; Singh, R.; Enkelmann, A.; Junker, K.; Rippaus, N.; Berndt, A.; Friedrich, K. Malignancy of bladder cancer cells is enhanced by tumor-associated fibroblasts through a multifaceted cytokine-chemokine loop. Exp. Cell Res. 2015, 335, 1–11. [Google Scholar] [CrossRef]
- Miyake, M.; Hori, S.; Morizawa, Y.; Tatsumi, Y.; Nakai, Y.; Anai, S.; Torimoto, K.; Aoki, K.; Tanaka, N.; Shimada, K.; et al. CXCL1-Mediated Interaction of Cancer Cells with Tumor-Associated Macrophages and Cancer-Associated Fibroblasts Promotes Tumor Progression in Human Bladder Cancer. Neoplasia 2016, 18, 636–646. [Google Scholar] [CrossRef]
- Prima, V.; Kaliberova, L.N.; Kaliberov, S.; Curiel, D.T.; Kusmartsev, S. COX2/mPGES1/PGE2 pathway regulates PD-L1 ex-pression in tumor-associated macrophages and myeloid-derived suppressor cells. Proc. Natl. Acad. Sci. USA 2017, 114, 1117–1122. [Google Scholar] [CrossRef]
- Abdel-Hafiz, H.A.; Schafer, J.M.; Chen, X.; Xiao, T.; Gauntner, T.D.; Li, Z.; Theodorescu, D. Y chromosome loss in cancer drives growth by evasion of adaptive immunity. Nature 2023, 619, 624–631. [Google Scholar] [CrossRef]
- Sweis, R.F.; Spranger, S.; Bao, R.; Paner, G.P.; Stadler, W.M.; Steinberg, G.; Gajewski, T.F. Molecular Drivers of the Non-T-Cell-Inflamed Tumor Microenvironment in Urothelial Bladder Cancer. Cancer Immunol. Res. 2016, 4, 563–568. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, M.; Chen, G.; Wang, W.; Zhang, P.; Yue, Y.; Guan, Z.; Wang, X.; Fan, J. Bladder cancer cells interact with vascular endothelial cells triggering EGFR signals to promote tumor progression. Int. J. Oncol. 2019, 54, 1555–1566. [Google Scholar] [CrossRef]
- Gao, L.; Yan, P.; Guo, F.F.; Liu, H.J.; Zhao, Z.F. MiR-1-3p inhibits cell proliferation and invasion by regulating BDNF-TrkB signaling pathway in bladder cancer. Neoplasma 2018, 65, 89–96. [Google Scholar] [CrossRef]
- Liu, Y.-R.; Ortiz-Bonilla, C.J.; Lee, Y.-F. Extracellular Vesicles in Bladder Cancer: Biomarkers and Beyond. Int. J. Mol. Sci. 2018, 19, 2822. [Google Scholar] [CrossRef]
- Beckham, C.J.; Olsen, J.; Yin, P.N.; Wu, C.H.; Ting, H.J.; Hagen, F.K.; Scosyrev, E.; Messing, E.M.; Lee, Y.F. Bladder cancer exosomes contain EDIL-3/Del1 and facilitate cancer progression. J. Urol. 2014, 192, 583–592. [Google Scholar] [CrossRef]
- Xue, M.; Chen, W.; Xiang, A.; Wang, R.; Chen, H.; Pan, J.; Pang, H.; An, H.; Wang, X.; Hou, H.; et al. Hypoxic exosomes facilitate bladder tumor growth and development through transferring long non-coding RNA-UCA1. Mol. Cancer 2017, 16, 1–13. [Google Scholar] [CrossRef]
- Kalluri, R. EMT: When epithelial cells decide to become mesenchymal-like cells. J. Clin. Investig. 2009, 119, 1417–1419. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Yuan, L.; Ma, B.; Wang, G.; Qiu, W.; Tian, Y. An EMT-related gene signature for the prognosis of human bladder cancer. J. Cell. Mol. Med. 2019, 24, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Berrondo, C.; Flax, J.; Kucherov, V.; Siebert, A.; Osinski, T.; Rosenberg, A.; Fucile, C.; Richheimer, S.; Beckham, C.J. Expression of the long non-coding RNA HOTAIR correlates with disease progression in bladder cancer and is contained in bladder cancer patient urinary exosomes. PLoS ONE 2016, 11, e0147236. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Luo, Y.; He, W.; Zhao, Y.; Kong, Y.; Liu, H.; Zhong, G.; Li, Y.; Li, J.; Huang, J.; et al. Exosomal long noncoding RNA LNMAT2 promotes lymphatic metastasis in bladder cancer. J. Clin. Investig. 2019, 130, 404–421. [Google Scholar] [CrossRef]
- Huang, C.-S.; Ho, J.-Y.; Chiang, J.-H.; Yu, C.-P.; Yu, D.-S. Exosome-Derived LINC00960 and LINC02470 Promote the Epithelial-Mesenchymal Transition and Aggressiveness of Bladder Cancer Cells. Cells 2020, 9, 1419. [Google Scholar] [CrossRef]
- Chung, W.-M.; Molony, R.D.; Lee, Y.-F. Non-stem bladder cancer cell-derived extracellular vesicles promote cancer stem cell survival in response to chemotherapy. Stem Cell Res. Ther. 2021, 12, 1–12. [Google Scholar] [CrossRef]
- Ringuette Goulet, C.; Bernard, G.; Tremblay, S.; Chabaud, S.; Bolduc, S.; Pouliot, F. Exosomes Induce Fibroblast Differentiation into Cancer-Associated Fibroblasts through TGFβ Signaling. Mol. Cancer Res. 2018, 16, 1196–1204. [Google Scholar] [CrossRef]
- Huyan, T.; Gao, L.; Gao, N.; Wang, C.; Guo, W.; Zhou, X.; Li, Q. miR-221-5p and miR-186-5p Are the Critical Bladder Cancer Derived Exosomal miRNAs in Natural Killer Cell Dysfunction. Int. J. Mol. Sci. 2022, 23, 15177. [Google Scholar] [CrossRef]
- Lobb, R.J.; Lima, L.G.; Möller, A. Exosomes: Key mediators of metastasis and pre-metastatic niche formation. Semin. Cell Dev. Biol. 2017, 67, 3–10. [Google Scholar] [CrossRef]
- Silvers, C.R.; Messing, E.M.; Miyamoto, H.; Lee, Y.-F. Tenascin-C expression in the lymph node pre-metastatic niche in muscle-invasive bladder cancer. Br. J. Cancer 2021, 125, 1399–1407. [Google Scholar] [CrossRef]
- Madeo, M.; Colbert, P.L.; Vermeer, D.W.; Lucido, C.T.; Cain, J.T.; Vichaya, E.G.; Grossberg, A.J.; Muirhead, D.; Rickel, A.P.; Hong, Z.; et al. Cancer exosomes induce tumor innervation. Nat. Commun. 2018, 9, 4284. [Google Scholar] [CrossRef]
- Mencucci, M.V.; Lapyckyj, L.; Rosso, M.; Besso, M.J.; Belgorosky, D.; Isola, M.; Vanzulli, S.; Lodillinsky, C.; Eiján, A.M.; Tejerizo, J.C.; et al. Ephrin-B1 Is a Novel Biomarker of Bladder Cancer Aggressiveness. Studies in Murine Models and in Human Samples. Front. Oncol. 2020, 10, 283. [Google Scholar] [CrossRef]
- Li, X.; Wei, Z.; Yu, H.; Xu, Y.; He, W.; Zhou, X.; Gou, X. Secretory autophagy-induced bladder tumour-derived extracellular vesicle secretion promotes angiogenesis by activating the TPX2-mediated phosphorylation of the AURKA-PI3K-AKT axis. Cancer Lett. 2021, 523, 10–28. [Google Scholar] [CrossRef]
- Chen, X.; Chen, R.X.; Wei, W.S.; Li, Y.H.; Feng, Z.H.; Tan, L.; Chen, J.W.; Yuan, G.J.; Chen, S.L.; Guo, S.J.; et al. Prmt5 Circular Rna Promotes Metastasis of Urothelial Carcinoma of the Bladder through Sponging Mir-30c to Induce Epithelial-Mesenchymal Transition. Clin. Cancer Res. 2018, 24, 6319–6330. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, H.; Ji, Z. Bladder cancer tissue-derived exosomes suppress ferroptosis of T24 bladder cancer cells by transporting miR -217. Environ. Mol. Mutagen. 2022, 64, 39–49. [Google Scholar] [CrossRef]
- Yang, L.; Wu, X.-H.; Wang, D.; Luo, C.-L.; Chen, L.-X. Bladder cancer cell-derived exosomes inhibit tumor cell apoptosis and induce cell proliferation in vitro. Mol. Med. Rep. 2013, 8, 1272–1278. [Google Scholar] [CrossRef]
- Franzen, C.A.; Blackwell, R.H.; Todorovic, V.; Greco, K.A.; Foreman, K.E.; Flanigan, R.C.; Kuo, P.C.; Gupta, G.N. Urothelial cells undergo epithelial-to-mesenchymal transition after exposure to muscle invasive bladder cancer exosomes. Oncogenesis 2015, 4, e163. [Google Scholar] [CrossRef]
- Zheng, H.; Chen, C.; Luo, Y.; Yu, M.; He, W.; An, M.; Gao, B.; Kong, Y.; Ya, Y.; Lin, Y.; et al. Tumor-Derived Exosomal Bcyrn1 Activates Wnt5a/Vegf-C/Vegfr3 Feedforward Loop to Drive Lymphatic Metastasis of Bladder Cancer. Clin. Transl. Med. 2021, 11, e497. [Google Scholar] [CrossRef]
- Yadav, A.K.; Desai, N.S. Cancer Stem Cells: Acquisition, Characteristics, Therapeutic Implications, Targeting Strategies and Future Prospects. Stem Cell Rev. Rep. 2019, 15, 331–355. [Google Scholar] [CrossRef]
- Das, B.; Tsuchida, R.; Malkin, D.; Koren, G.; Baruchel, S.; Yeger, H. Hypoxia Enhances Tumor Stemness by Increasing the Invasive and Tumorigenic Side Population Fraction. Stem Cells 2008, 26, 1818–1830. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, T.; Koga, F.; Migita, T. Bladder Cancer Stem-Like Cells: Their Origin and Therapeutic Perspectives. Int. J. Mol. Sci. 2015, 17, 43. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.S.; Espinosa, I.; Chao, M.; Wong, D.; Ailles, L.; Diehn, M.; Gill, H.; Presti, J., Jr.; Chang, H.Y.; van de Rijn, M.; et al. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proc. Natl. Acad. Sci. USA 2009, 106, 14016–14021. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; Kitamura, H. Cancer stem cells and epithelial-mesenchymal transition in urothelial carcinoma: Possible pathways and potential therapeutic approaches. Int. J. Urol. 2017, 25, 7–17. [Google Scholar] [CrossRef]
- Yang, Z.; Li, C.; Fan, Z.; Liu, H.; Zhang, X.; Cai, Z.; Xu, L.; Luo, J.; Huang, Y.; He, L.; et al. Single-cell Sequencing Reveals Variants in ARID1A, GPRC5A and MLL2 Driving Self-renewal of Human Bladder Cancer Stem Cells. Eur. Urol. 2016, 71, 8–12. [Google Scholar] [CrossRef]
- Ojha, R.; Singh, S.K.; Bhattacharyya, S. JAK-mediated autophagy regulates stemness and cell survival in cisplatin resistant bladder cancer cells. Biochim. Biophys. Acta BBA-Gen. Subj. 2016, 1860, 2484–2497. [Google Scholar] [CrossRef]
- de Andrés, J.L.; Griñán-Lisón, C.; Jiménez, G.; Marchal, J.A. Cancer stem cell secretome in the tumor microenvironment: A key point for an effective personalized cancer treatment. J. Hematol. Oncol. 2020, 13, 1–22. [Google Scholar] [CrossRef]
- Wang, H.; Mei, Y.; Luo, C.; Huang, Q.; Wang, Z.; Lu, G.M.; Qin, L.; Sun, Z.; Huang, C.W.; Yang, Z.W.; et al. Single-Cell Analyses Reveal Mechanisms of Cancer Stem Cell Maintenance and Epithelial-Mesenchymal Transition in Recurrent Bladder Cancer. Clin. Cancer Res. 2021, 27, 6265–6278. [Google Scholar] [CrossRef]
- Hayami, S.; Yoshimatsu, M.; Veerakumarasivam, A.; Unoki, M.; Iwai, Y.; Tsunoda, T.; Field, H.I.; Kelly, J.D.; Neal, D.E.; Yamaue, H.; et al. Overexpression of the JmjC histone demethylase KDM5B in human carcinogenesis: Involvement in the proliferation of cancer cells through the E2F/RB pathway. Mol. Cancer 2010, 9, 59. [Google Scholar] [CrossRef]
- Yao, J.; Liu, Y.; Yang, J.; Li, M.; Li, S.; Zhang, B.; Yang, R.; Zhang, Y.; Cui, X.; Feng, C. Single-Cell Sequencing Reveals that DBI is the Key Gene and Potential Therapeutic Target in Quiescent Bladder Cancer Stem Cells. Front. Genet. 2022, 13. [Google Scholar] [CrossRef]
- Qiu, Y.; Qiu, S.; Deng, L.; Nie, L.; Gong, L.; Liao, X.; Zheng, X.; Jin, K.; Li, J.; Tu, X.; et al. Biomaterial 3D collagen I gel culture model: A novel approach to investigate tumorigenesis and dormancy of bladder cancer cells induced by tumor microenvironment. Biomaterials 2020, 256, 120217. [Google Scholar] [CrossRef]
- Aghaalikhani, N.; Rashtchizadeh, N.; Shadpour, P.; Allameh, A.; Mahmoodi, M. Cancer stem cells as a therapeutic target in bladder cancer. J. Cell. Physiol. 2018, 234, 3197–3206. [Google Scholar] [CrossRef]
- Zhu, Y.-T.; Zhao, Z.; Fu, X.-Y.; Luo, Y.; Lei, C.-Y.; Chen, W.; Li, F.; Pang, S.-Y.; Chen, S.-S.; Tan, W.-L. The granulocyte macrophage–colony stimulating factor surface modified MB49 bladder cancer stem cells vaccine against metastatic bladder cancer. Stem Cell Res. 2014, 13, 111–122. [Google Scholar] [CrossRef][Green Version]
- Tatokoro, M.; Koga, F.; Yoshida, S.; Kawakami, S.; Fujii, Y.; Neckers, L.; Kihara, K. Potential role of Hsp90 inhibitors in overcoming cisplatin resistance of bladder cancer-initiating cells. Int. J. Cancer 2011, 131, 987–996. [Google Scholar] [CrossRef]
- Liu, Q.; Yuan, W.; Tong, D.; Liu, G.; Lan, W.; Zhang, D.; Xiao, H.; Zhang, Y.; Huang, Z.; Yang, J.; et al. Metformin represses bladder cancer progression by inhibiting stem cell repopulation via COX2/PGE2/STAT3 axis. Oncotarget 2016, 7, 28235–28246. [Google Scholar] [CrossRef]
- Kurtova, A.V.; Xiao, J.; Mo, Q.; Pazhanisamy, S.; Krasnow, R.; Lerner, S.P.; Chen, F.; Roh, T.T.; Lay, E.; Ho, P.L.; et al. Blocking PGE2-induced tumour repopulation abrogates bladder cancer chemoresistance. Nature 2015, 517, 209–213. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, L.; Liu, L.; Hou, Y.; Xiong, M.; Yang, Y.; Hu, J.; Chen, K. Single-cell RNA sequencing highlights the role of inflammatory cancer-associated fibroblasts in bladder urothelial carcinoma. Nat. Commun. 2020, 11, 5077. [Google Scholar] [CrossRef]
- Yang, F.; Guo, Z.; He, C.; Qing, L.; Wang, H.; Wu, J.; Lu, X. Cancer-associated fibroblasts promote cell proliferation and invasion via paracrine Wnt/IL1β signaling pathway in human bladder cancer. Neoplasma 2021, 68, 79–86. [Google Scholar] [CrossRef]
- Wu, G.; Weng, W.; Xia, P.; Yan, S.; Zhong, C.; Xie, L.; Xie, Y.; Fan, G. Wnt signalling pathway in bladder cancer. Cell. Signal. 2020, 79, 109886. [Google Scholar] [CrossRef]
- Garg, M.; Maurya, N. WNT/β-catenin signaling in urothelial carcinoma of bladder. World J. Nephrol. 2019, 8, 83–94. [Google Scholar] [CrossRef]
- Ma, Z.; Li, X.; Mao, Y.; Wei, C.; Huang, Z.; Li, G.; Yin, J.; Liang, X.; Liu, Z. Interferon-dependent SLC14A1+ cancer-associated fibroblasts promote cancer stemness via WNT5A in bladder cancer. Cancer Cell 2022, 40, 1550–1565.e7. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.-R.; Hsu, I.; Song, W.; Chang, H.; Miyamoto, H.; Xiao, G.-Q.; Li, L.; Yeh, S. Fibroblast ERα promotes bladder cancer invasion via increasing the CCL1 and IL-6 signals in the tumor microenvironment. Am. J. Cancer Res. 2015, 5, 1146–1157. [Google Scholar] [PubMed]
- Goulet, C.R.; Champagne, A.; Bernard, G.; Vandal, D.; Chabaud, S.; Pouliot, F.; Bolduc, S. Cancer-associated fibroblasts induce epithelial–mesenchymal transition of bladder cancer cells through paracrine IL-6 signalling. BMC Cancer 2019, 19, 137. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Lu, Q.; Shen, B.; Huang, X.; Shen, L.; Zheng, X.; Huang, R.; Yan, J.; Guo, H. TGFβ1 secreted by cancer-associated fibroblasts induces epithelial-mesenchymal transition of bladder cancer cells through lncRNA-ZEB2NAT. Sci. Rep. 2015, 5, 11924. [Google Scholar] [CrossRef]
- Zhuang, J.; Shen, L.; Li, M.; Sun, J.; Hao, J.; Li, J.; Zhu, Z.; Ge, S.; Zhang, D.; Guo, H.; et al. Cancer-Associated Fibroblast–Derived miR-146a-5p Generates a Niche That Promotes Bladder Cancer Stemness and Chemoresistance. Cancer Res. 2023, 83, OF1–OF17. [Google Scholar] [CrossRef]
- Yan, L.; Wang, P.; Fang, W.; Liang, C. Cancer-associated fibroblasts–derived exosomes-mediated transfer of LINC00355 regulates bladder cancer cell proliferation and invasion. Cell Biochem. Funct. 2019, 38, 257–265. [Google Scholar] [CrossRef]
- Luo, G.; Zhang, Y.; Wu, Z.; Zhang, L.; Liang, C.; Chen, X. Exosomal LINC00355 derived from cancer-associated fibroblasts promotes bladder cancer cell resistance to cisplatin by regulating miR-34b-5p/ABCB1 axis. Acta Biochim. Biophys. Sin. 2021, 53, 558–566. [Google Scholar] [CrossRef]
- Feng, R.; Li, Z.; Ge, G.; Wang, C.; Jia, Y.; Ouyang, J. Cancer-associated fibroblast-derived extracellular vesicles mediate immune escape of bladder cancer via PD-L1/PD-1 expression. Endocr. Metab. Immune Disord. Drug Targets 2023. [Google Scholar] [CrossRef]
- Ziani, L.; Chouaib, S.; Thiery, J. Alteration of the Antitumor Immune Response by Cancer-Associated Fibroblasts. Front. Immunol. 2018, 9, 414. [Google Scholar] [CrossRef]
- Gu, Y.; Zhuo, H. Identification of a cancer-associated fibroblast signature for predicting prognosis and immunotherapeutic responses in bladder urothelial carcinoma. Aging Male 2023, 26, 2233609. [Google Scholar] [CrossRef]
- Chen, H.; Yang, W.; Xue, X.; Li, Y.; Jin, Z.; Ji, Z. Integrated Analysis Revealed an Inflammatory Cancer-Associated Fibroblast-Based Subtypes with Promising Implications in Predicting the Prognosis and Immunotherapeutic Response of Bladder Cancer Patients. Int. J. Mol. Sci. 2022, 23, 15970. [Google Scholar] [CrossRef]
- Du, Y.; Jiang, X.; Wang, B.; Cao, J.; Wang, Y.; Yu, J.; Wang, X.; Liu, H. The cancer-associated fibroblasts related gene CALD1 is a prognostic biomarker and correlated with immune infiltration in bladder cancer. Cancer Cell Int. 2021, 21, 1–15. [Google Scholar] [CrossRef]
- Belhabib, I.; Zaghdoudi, S.; Lac, C.; Bousquet, C.; Jean, C. Extracellular Matrices and Cancer-Associated Fibroblasts: Targets for Cancer Diagnosis and Therapy? Cancers 2021, 13, 3466. [Google Scholar] [CrossRef]
- Brunner, A.; Tzankov, A. The Role of Structural Extracellular Matrix Proteins in Urothelial Bladder Cancer (Review). Biomark. Insights 2007, 2, 418–427. [Google Scholar] [CrossRef]
- Szarvas, T.; Dorp, F.V.; Ergün, S.; Rübben, H. Matrix metalloproteinases and their clinical relevance in urinary bladder cancer. Nat. Rev. Urol. 2011, 8, 241–254. [Google Scholar] [CrossRef]
- Brunner, A.; Mayerl, C.; Tzankov, A.; Verdorfer, I.; Tschörner, I.; Rogatsch, H.; Mikuz, G. Prognostic significance of tenascin-C expression in superficial and invasive bladder cancer. J. Clin. Pathol. 2004, 57, 927–931. [Google Scholar] [CrossRef]
- Booth, C.; Harnden, P.; Selby, P.J.; Southgate, J. Towards defining roles and relationships for tenascin-C and TGFbeta-1 in the normal and neoplastic urinary bladder. J. Pathol. 2002, 198, 359–368. [Google Scholar] [CrossRef]
- Guan, Z.; Sun, Y.; Mu, L.; Jiang, Y.; Fan, J. Tenascin-C promotes bladder cancer progression and its action depends on syndecan-4 and involves NF-κB signaling activation. BMC Cancer 2022, 22, 240. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, J.; Yang, X.; Jiao, W.; Shen, C.; Zhao, X.; Wang, Y. High expression of COL6A1 predicts poor prognosis and response to immunotherapy in bladder cancer. Cell Cycle 2022, 22, 610–618. [Google Scholar] [CrossRef]
- Ratliff, T.L.; Palmer, J.O.; McGarr, J.A.; Brown, E.J. Intravesical Bacillus Calmette-Guérin therapy for murine bladder tumors: Initiation of the response by fibronectin-mediated attachment of Bacillus Calmette-Guérin. Cancer Res. 1987, 47, 1762–1766. [Google Scholar]
- Höbarth, K.; Maier, U.; Marberger, M. Topical Chemoprophylaxis of Superficial Bladder Cancer with Mitomycin C and Adjuvant Hyaluronidase. Eur. Urol. 1992, 21, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Jiang, H.; Wang, L.; Cao, Y.; Liu, P.; Xu, X.; Wang, Y.; Sun, L.; Niu, H. Overexpression of monocarboxylate anion transporter 1 and 4 in T24-induced cancer-associated fibroblasts regulates the progression of bladder cancer cells in a 3D microfluidic device. Cell Cycle 2015, 14, 3058–3065. [Google Scholar] [CrossRef] [PubMed]
- Sobierajska, K.; Ciszewski, W.M.; Sacewicz-Hofman, I.; Niewiarowska, J. Endothelial Cells in the Tumor Microenvironment. Tumor Microenviron. Non-Hematop. Cells 2020, 1234, 71–86. [Google Scholar] [CrossRef]
- Jin, X.; Wang, Q.; Luo, F.; Pan, J.; Lu, T.; Zhao, Y.; Zhang, X.; Xiang, E.; Zhou, C.; Huang, B.; et al. Single-cell transcriptomic analysis of tumor heterogeneity and intercellular networks in human urothelial carcinoma. Chin. Med. J. 2023, 136, 690–706. [Google Scholar] [CrossRef]
- Li, D.-X.; Feng, D.-C.; Shi, X.; Wu, R.-C.; Chen, K.; Han, P. Identification of endothelial-related molecular subtypes for bladder cancer patients. Front. Oncol. 2023, 13, 1101055. [Google Scholar] [CrossRef]
- Zhang, Z.; Dong, Z.; Lauxen, I.S.; Filho, M.S.; Nör, J.E. Endothelial Cell-Secreted EGF Induces Epithelial to Mesenchymal Transition and Endows Head and Neck Cancer Cells with Stem-like Phenotype. Cancer Res. 2014, 74, 2869–2881. [Google Scholar] [CrossRef]
- Pirtskhalaishvili, G.; Nelson, J.B. Endothelium-derived factors as paracrine mediators of prostate cancer progression. Prostate 2000, 44, 77–87. [Google Scholar] [CrossRef]
- Warner, K.A.; Miyazawa, M.; Cordeiro, M.M.; Love, W.J.; Pinsky, M.S.; Neiva, K.G.; Spalding, A.C.; Jacques E, N. Endothelial Cells Enhance Tumor Cell Invasion through a Crosstalk Mediated by CXC Chemokine Signaling. Neoplasia 2008, 10, 131–139. [Google Scholar] [CrossRef]
- Freshour, S.L.; Chen, T.H.P.; Fisk, B.; Shen, H.; Mosior, M.; Skidmore, Z.L.; Fronick, C.; Bolzenius, J.K.; Griffith, O.L.; Arora, V.K.; et al. Endothelial cells are a key target of IFN-g during response to combined PD-1/CTLA-4 ICB treatment in a mouse model of bladder cancer. bioRxiv 2023. [Google Scholar] [CrossRef]
- Rajan, V.S.; Laurent, V.M.; Verdier, C.; Duperray, A. Unraveling the Receptor-Ligand Interactions between Bladder Cancer Cells and the Endothelium Using AFM. Biophys. J. 2017, 112, 1246–1257. [Google Scholar] [CrossRef]
- Li, D.; Jiao, W.; Liang, Z.; Wang, L.; Chen, Y.; Wang, Y.; Liang, Y.; Niu, H. Variation in energy metabolism arising from the effect of the tumor microenvironment on cell biological behaviors of bladder cancer cells and endothelial cells. Biofactors 2019, 46, 64–75. [Google Scholar] [CrossRef]
- Sun, R.; Kong, X.; Qiu, X.; Huang, C.; Wong, P.-P. The Emerging Roles of Pericytes in Modulating Tumor Microenvironment. Front. Cell Dev. Biol. 2021, 9, 676342. [Google Scholar] [CrossRef]
- Bose, A.; Barik, S.; Banerjee, S.; Ghosh, T.; Mallick, A.; Majumdar, S.B.; Goswami, K.K.; Bhuniya, A.; Banerjee, S.; Baral, R.; et al. Tumor-Derived Vascular Pericytes Anergize Th Cells. J. Immunol. 2013, 191, 971–981. [Google Scholar] [CrossRef]
- Zhang, X.-N.; Yang, K.-D.; Chen, C.; He, Z.-C.; Wang, Q.-H.; Feng, H.; Lv, S.-Q.; Wang, Y.; Mao, M.; Liu, Q.; et al. Pericytes augment glioblastoma cell resistance to temozolomide through CCL5-CCR5 paracrine signaling. Cell Res. 2021, 31, 1072–1087. [Google Scholar] [CrossRef]
- Hariharan, N.; Ashcraft, K.A.; Svatek, R.S.; Livi, C.B.; Wilson, D.; Kaushik, D.; Leach, R.J.; Johnson-Pais, T.L. Adipose Tissue-Secreted Factors Alter Bladder Cancer Cell Migration. J. Obes. 2018, 2018, 9247864. [Google Scholar] [CrossRef]
- Kashiwagi, E.; Abe, T.; Kinoshita, F.; Ushijima, M.; Masaoka, H.; Shiota, M.; Netto, G.J.; Eto, M.; Miyamoto, H. The role of adipocytokines and their receptors in bladder cancer: Expression of adiponectin or leptin is an independent prognosticator. Am. J. Transl. Res. 2020, 12, 3033–3045. [Google Scholar] [CrossRef]
- Kawasaki-Nanri, M.; Aoki, S.; Uchihashi, K.; Yamamoto, M.; Udo, K.; Nishijima-Matsunobu, A.; Kakihara, N.; Noguchi, M.; Uozumi, J.; Toda, S. Differential effects of adipose tissue stromal cells on the apoptosis, growth and invasion of bladder urothelial carcinoma between the superficial and invasive types. Int. J. Urol. 2016, 23, 510–519. [Google Scholar] [CrossRef][Green Version]
- Wu, Q.; Li, B.; Li, J.; Sun, S.; Yuan, J.; Sun, S. Cancer-associated adipocytes as immunomodulators in cancer. Biomark. Res. 2021, 9, 2. [Google Scholar] [CrossRef]
- Wang, T.; Yu, X.; Lin, J.; Qin, C.; Bai, T.; Xu, T.; Wang, L.; Liu, X.; Li, S. Adipose-Derived Stem Cells Inhibited the Proliferation of Bladder Tumor Cells by S Phase Arrest and Wnt/β-Catenin Pathway. Cell. Reprogramming 2019, 21, 331–338. [Google Scholar] [CrossRef]
- Yu, X.; Su, B.; Ge, P.; Wang, Z.; Li, S.; Huang, B.; Gong, Y.; Lin, J. Human adipose derived stem cells induced cell apoptosis and s phase arrest in bladder tumor. Stem Cells Int. 2015, 2015, 619290. [Google Scholar] [CrossRef]
- Maj, M.; Kokocha, A.; Bajek, A.; Drewa, T. The effects of adipose-derived stem cells on CD133-expressing bladder cancer cells. J. Cell. Biochem. 2019, 120, 11562–11572. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Chen, H.; Yang, W.; Ji, Z. Crosstalk between Mesenchymal Stem Cells and Cancer Stem Cells Reveals a Novel Stemness-Related Signature to Predict Prognosis and Immunotherapy Responses for Bladder Cancer Patients. Int. J. Mol. Sci. 2023, 24, 4760. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Shi, B.; Chen, J.; Zhang, D.; Zhu, Y.; Zhou, C.; Zhao, H.; Jiang, X.; Xu, Z. Bone Marrow Mesenchymal Stem Cells Induce Angiogenesis and Promote Bladder Cancer Growth in a Rabbit Model. Urol. Int. 2010, 84, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ma, L.; Zhang, N.; Zhu, Y.; Zhang, K.; Xu, Z.; Wang, Q. Mesenchymal Stem Cells Promote Tumor Progression via Inducing Stroma Remodeling on Rabbit VX2 Bladder Tumor Model. Int. J. Biol. Sci. 2018, 14, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Chen, J.; Zhu, Y.; Xu, Z. Mesenchymal Stem Cells Accelerate the Remodeling of Bladder VX2 Tumor Interstitial Microenvironment by TGFβ1-Smad Pathway. J. Cancer 2019, 10, 4532–4539. [Google Scholar] [CrossRef]
- Rezaeian, A.; Khatami, F.; Heidari Keshel, S.; Akbari, M.R.; Mirzaei, A.; Gholami, K.; Mohammadi Farsani, R.; Aghamir, S.M.K. The effect of mesenchymal stem cells-derived exosomes on the prostate, bladder, and renal cancer cell lines. Sci. Rep. 2022, 12, 20924. [Google Scholar] [CrossRef]
- Weddell, A.P.a.G. Induction of Tumours in Denervated Skin. Nature 1967, 213, 1234–12236. [Google Scholar]
- Romeo, H.E.; Colombo, L.L.; Esquifino, A.I.; Rosenstein, R.E.; Chuluyan, H.E.; Cardinali, D.P. Slower growth of tumours in sympathetically denervated murine skin. J. Auton. Nerv. Syst. 1991, 32, 159–164. [Google Scholar] [CrossRef]
- Aghion, D.M.; Capek, S.; Howe, B.M.; Hepel, J.T.; Sambandam, S.; Oyelese, A.A.; Spinner, R.J. Perineural tumor spread of bladder cancer causing lumbosacral plexopathy: An anatomic explanation. Acta Neurochir. 2014, 156, 2331–2336. [Google Scholar] [CrossRef]
- Hutchings, C.; Phillips, J.A.; Djamgoz, M.B. Nerve input to tumours: Pathophysiological consequences of a dynamic relationship. Biochim. Biophys. Acta (BBA) Rev. Cancer 2020, 1874, 188411. [Google Scholar] [CrossRef]
- Mauffrey, P.; Tchitchek, N.; Barroca, V.; Bemelmans, A.-P.; Firlej, V.; Allory, Y.; Roméo, P.-H.; Magnon, C. Progenitors from the central nervous system drive neurogenesis in cancer. Nature 2019, 569, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Mhawech-Fauceglia, P.; Ali, L.; Cheney, R.T.; Groth, J.; Herrmann, F.R. Prognostic significance of neuron-associated protein expression in non-muscle-invasive urothelial bladder cancer. J. Clin. Pathol. 2009, 62, 710–714. [Google Scholar] [CrossRef] [PubMed]
- Huang; Lai, P.C.; Chiu, T.H.; Huang, Y.T. Overexpression of BDNF and TrkB in human bladder cancer specimens. Oncol. Rep. 2010, 24, 1265–1270. [Google Scholar] [CrossRef] [PubMed]
- Chiu; Huang, Y.T.; Lai, P.C.; Wu, C.C.; Hsu, S.H.; Cheng, C.C.; Lan, Y.F.; Chiu, T.H. BDNF mediated TrkB activation is a survival signal for transitional cell carcinoma cells. Int. J. Oncol. 2010, 36, 1469–1476. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jiang, C.C.; Marsland, M.; Wang, Y.; Dowdell, A.; Eden, E.; Gao, F.; Faulkner, S.; Jobling, P.; Li, X.; Liu, L.; et al. Tumor innervation is triggered by endoplasmic reticulum stress. Oncogene 2021, 41, 586–599. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, A.; Nagaya, H.; Kanno, T.; Nishizaki, T. Antitumor action of α(1)-adrenoceptor blockers on human bladder, prostate and renal cancer cells. Pharmacology 2012, 90, 242–246. [Google Scholar] [CrossRef]
- Nakagawa, Y.U.; Nagaya, H.; Miyata, T.; Wada, Y.; Oyama, T.; Gotoh, A. Piperazine-based Alpha-1 AR Blocker, Naftopidil, Selectively Suppresses Malignant Human Bladder Cells via Induction of Apoptosis. Anticancer. Res. 2016, 36, 1563–1570. [Google Scholar]
- Pan, L.; Liu, C.; Kong, Y.; Piao, Z.; Cheng, B. Phentolamine inhibits angiogenesis in vitro: Suppression of proliferation migration and differentiation of human endothelial cells. Clin. Hemorheol. Microcirc. 2017, 65, 31–41. [Google Scholar] [CrossRef]
- Martin, F.M.; Harris, A.M.; Rowland, R.G.; Conner, W.; Lane, M.; Durbin, E.; Baron, A.T.; Kyprianou, N. Decreased risk of bladder cancer in men treated with quinazoline-based α1-adrenoceptor antagonists. Gene Ther. Mol. Biol. 2008, 12, 253–258. [Google Scholar]
- Tahmatzopoulos, A.; Lagrange, C.A.; Zeng, L.; Mitchell, B.L.; Conner, W.T.; Kyprianou, N. Effect of terazosin on tissue vascularity and apoptosis in transitional cell carcinoma of bladder. Urology 2005, 65, 1019–1023. [Google Scholar] [CrossRef]
- Nagata, Y.; Kawahara, T.; Goto, T.; Inoue, S.; Teramoto, Y.; Jiang, G.; Fujimoto, N.; Miyamoto, H. Effects of α1-adrenergic receptor antagonists on the development and progression of urothelial cancer. Am. J. Cancer Res. 2020, 10, 4386–4398. [Google Scholar]
- Flint, M.S.; Baum, A.; Chambers, W.H.; Jenkins, F.J. Induction of DNA damage, alteration of DNA repair and transcriptional activation by stress hormones. Psychoneuroendocrinology 2007, 32, 470–479. [Google Scholar] [CrossRef]
- Mravec, B.; Horvathova, L.; Hunakova, L. Neurobiology of Cancer: The Role of β-Adrenergic Receptor Signaling in Various Tumor Environments. Int. J. Mol. Sci. 2020, 21, 7958. [Google Scholar] [CrossRef]
- Udumyan, R.; Botteri, E.; Jerlstrom, T.; Montgomery, S.; Smedby, K.E.; Fall, K. Beta-blocker use and urothelial bladder cancer survival: A Swedish register-based cohort study. Acta Oncol. 2022, 61, 922–930. [Google Scholar] [CrossRef]
- Hu, Q.; Hu, J.; Chen, C.; Wang, Y.; Zhang, Y.; Wan, J.; Jing, O.; Yi, H.; Wang, S.; Huang, W.; et al. Propranolol suppresses bladder cancer by manipulating intracellular pH via NHE1. Transl. Androl. Urol. 2022, 11, 1083–1095. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, P.; Wang, M.; Zheng, Y.; Tian, T.; Yang, S.; Deng, Y.; Wu, Y.; Zhai, Z.; Hao, Q.; et al. Antihypertensive medications are associated with the risk of kidney and bladder cancer: A systematic review and meta-analysis. Aging 2020, 12, 1545–1562. [Google Scholar] [CrossRef]
- Kim, S.J.; Nam, W.; You, D.; Jeong, I.G.; Song, C.; Hong, B.; Kim, C.-S.; Ahn, H.; Hong, J.H. Prognostic Factors Related to Recurrence-Free Survival for Primary Carcinoma in situ of the Bladder after Bacillus Calmette-Guérin: A Retrospective Study. Urol. Int. 2018, 101, 269–276. [Google Scholar] [CrossRef]
- Santala, E.E.E.; Kotsar, A.; Veitonmäki, T.; Tammela, T.L.J.; Murtola, T.J. Risk of urothelial cancer death among people using antihypertensive drugs—a cohort study from Finland. Scand. J. Urol. 2019, 53, 185–192. [Google Scholar] [CrossRef]
- Yoshida, T.; Kinoshita, H.; Fukui, K.; Matsuzaki, T.; Yoshida, K.; Mishima, T.; Yanishi, M.; Komai, Y.; Sugi, M.; Inoue, T.; et al. Prognostic Impact of Renin-Angiotensin Inhibitors in Patients with Bladder Cancer Undergoing Radical Cystectomy. Ann. Surg. Oncol. 2016, 24, 823–831. [Google Scholar] [CrossRef]
- Motterle, G.; Morlacco, A.; Giovannini, G.; Vecchiato, E.; Iafrate, M.; Calpista, A.; Prayer-Galetti, T.; Martino, F.; Dal Moro, F.; Novara, G. Role of Renin-Angiotensin System Blockers on BCG Response in Nonmuscle Invasive, High Risk Bladder Cancer. Clin. Genitourin. Cancer 2022, 20, e303–e309. [Google Scholar] [CrossRef]
- Blute, M.L.; Rushmer, T.J.; Shi, F.; Fuller, B.J.; Abel, E.J.; Jarrard, D.F.; Downs, T.M. Renin-Angiotensin Inhibitors Decrease Recurrence after Transurethral Resection of Bladder Tumor in Patients with Nonmuscle Invasive Bladder Cancer. J. Urol. 2015, 194, 1214–1219. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Hou, H.; Liang, B.; Guo, X.; Chen, L.; Yang, Y.; Wang, Y. Effect of renin-angiotensin-aldosterone system inhibitors on survival outcomes in cancer patients treated with immune checkpoint inhibitors: A systematic review and meta-analysis. Front. Immunol. 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K.; Skelton Iv, W.P.; Pond, G.R.; Naqvi, M.; Kim, Y.; Curran, C.; Freeman, D.; Nuzzo, P.V.; Alaiwi, S.A.; Nassar, A.H.; et al. Angiotensin Blockade Modulates the Activity of PD1/L1 Inhibitors in Metastatic Urothelial Carcinoma. Clin. Genitourin. Cancer 2021, 19, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Dubowitz, J.A.; Sloan, E.K.; Riedel, B.J. Implicating anaesthesia and the perioperative period in cancer recurrence and metastasis. Clin. Exp. Metastasis 2017, 35, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Looney, M.; Doran, P.; Buggy, D.J. Effect of Anesthetic Technique on Serum Vascular Endothelial Growth Factor C and Transforming Growth Factor β in Women Undergoing Anesthesia and Surgery for Breast Cancer. Anesthesiology 2010, 113, 1118–1125. [Google Scholar] [CrossRef]
- Angele, M.K.; Faist, E. Clinical review: Immunodepression in the surgical patient and increased susceptibility to infection. Crit. Care 2002, 6, 298–305. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, S.; Jin, D.; Luo, J.; Shi, Y.; Zhang, Y.; Wu, L.; Song, Y.; Su, D.; Pan, Z.; et al. μ-opioid receptor agonist facilitates circulating tumor cell formation in bladder cancer via the MOR/AKT/Slug pathway: A comprehensive study including randomized controlled trial. Cancer Commun. 2023, 43, 365–386. [Google Scholar] [CrossRef]
- Liu, H.; Dilger, J.P.; Lin, J. Effects of local anesthetics on cancer cells. Pharmacol. Ther. 2020, 212, 107558. [Google Scholar] [CrossRef]
- Li, G.; Zhang, X.; Guo, X.; Li, Y.; Li, C. Propofol Inhibits the Proliferation, Migration, and Stem-like Properties of Bladder Cancer Mainly by Suppressing the Hedgehog Pathway. Cell Transplant. 2021, 30, 0963689720985113. [Google Scholar] [CrossRef]
- Hao, J.; Zhang, W.; Huang, Z. Bupivacaine modulates the apoptosis and ferroptosis in bladder cancer via phosphatidylinositol 3-kinase (PI3K)/AKT pathway. Bioengineered 2022, 13, 6794–6806. [Google Scholar] [CrossRef]
- Choi, W.-J.; Baek, S.; Joo, E.-Y.; Yoon, S.-H.; Kim, E.; Hong, B.; Hwang, J.-H.; Kim, Y.-K. Comparison of the effect of spinal anesthesia and general anesthesia on 5-year tumor recurrence rates after transurethral resection of bladder tumors. Oncotarget 2017, 8, 87667–87674. [Google Scholar] [CrossRef]
- Koumpan, Y.; Jaeger, M.; Mizubuti, G.B.; Tanzola, R.; Jain, K.; Hosier, G.; Hopman, W.; Siemens, D.R. Spinal Anesthesia is Associated with Lower Recurrence Rates after Resection of Nonmuscle Invasive Bladder Cancer. J. Urol. 2018, 199, 940–946. [Google Scholar] [CrossRef]
- Lee, S.W.; Tae, B.S.; Choi, Y.J.; Yoon, S.M.; Lee, Y.S.; Kim, J.H.; Shin, H.W.; Park, J.Y.; Bae, J.H. A Comparison of the Anesthetic Methods for Recurrence Rates of Bladder Cancer after Transurethral Resection of Bladder Tumors Using National Health Insurance Claims Data of South Korea. J. Clin. Med. 2022, 11, 1143. [Google Scholar] [CrossRef]
- Wang, Y.; Song, Y.; Qin, C.; Zhang, C.; Du, Y.; Xu, T. Effect of regional versus general anesthesia on recurrence of non-muscle invasive bladder cancer: A systematic review and meta-analysis of eight retrospective cohort studies. BMC Anesthesiol. 2023, 23, 201. [Google Scholar] [CrossRef]
- Pfail, J.L.; Katims, A.B.; Gul, Z.; Rosenzweig, S.J.; Razdan, S.; Nathaniel, S.; Martini, A.; Mehrazin, R.; Wiklund, P.N.; Loftus, K.; et al. Can anesthetics affect bladder cancer recurrence? Total intravenous versus volatile anesthesia in patients undergoing robot-assisted radical cystectomy: A single institution retrospective analysis. Urol. Oncol. Semin. Orig. Investig. 2020, 39, 233.e1–233.e8. [Google Scholar] [CrossRef]
- Chipollini, J.; Alford, B.; Boulware, D.C.; Forget, P.; Gilbert, S.M.; Lockhart, J.L.; Pow-Sang, J.M.; Sexton, W.J.; Spiess, P.E.; Poch, M.A.; et al. Epidural anesthesia and cancer outcomes in bladder cancer patients: Is it the technique or the medication? A matched-cohort analysis from a tertiary referral center. BMC Anesthesiol. 2018, 18, 157. [Google Scholar] [CrossRef]
- Xue, R.; Zhao, C.; Chen, D.; Wang, P.; Xing, W.; Zeng, W.; Li, Q. Potential influence of anaesthesia techniques on the recurrence and progression after resection of non-muscle-invasive bladder cancer: A propensity score-matched analysis. BMC Anesthesiol. 2022, 22, 263. [Google Scholar] [CrossRef] [PubMed]
- Orriach, J.L.G.; Ponferrada, A.R.; Manso, A.M.; Imbroda, B.H.; Belmonte, J.J.E.; Aliaga, M.R.; Fernandez, A.R.; Crespo, J.D.; Perez, A.M.S.; Heredia, A.F.; et al. Anesthesia in Combination with Propofol Increases Disease-Free Survival in Bladder Cancer Patients Who Undergo Radical Tumor Cystectomy as Compared to Inhalational Anesthetics and Opiate-Based Analgesia. Oncology 2020, 98, 161–167. [Google Scholar] [CrossRef]
- Sato, M.; Yanagisawa, T.; Minamino, S.; Arai, T. Hypotension caused by oral administration of 5-aminolevulinic acid persists after surgery in patients undergoing transurethral resection of bladder tumor under spinal anesthesia. JA Clin. Rep. 2020, 6, 93. [Google Scholar] [CrossRef]
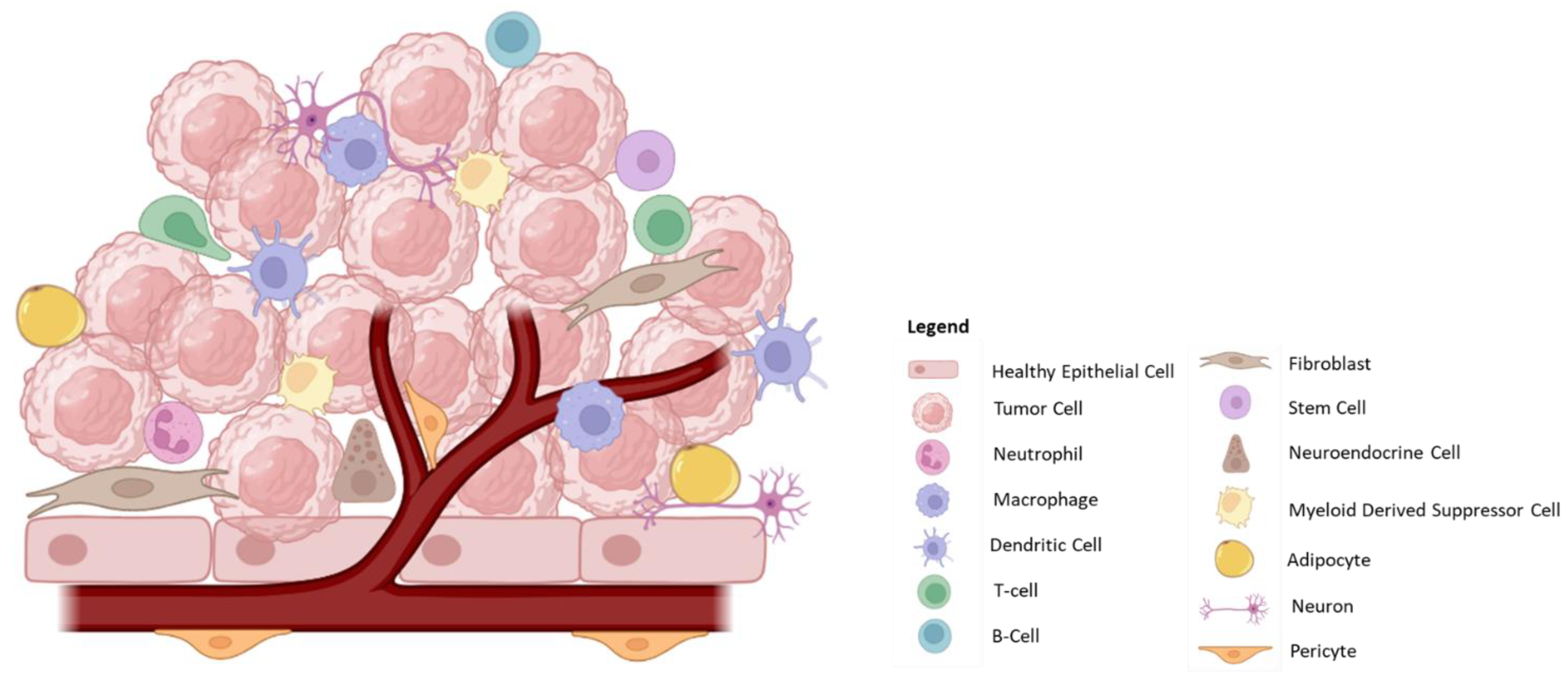
| Cell Type | Cell | Tumorigenic Potential | Description/Properties |
|---|---|---|---|
| Healthy Tissue | Epithelial Cells | Healthy Cells | |
| Tumor | Cancer cells | + | Dysregulated, rapidly dividing cells that establish the tumor microenvironment to allow for growth and metastasis. |
| Cancer Stem Cells | + | Stem-like cells with the potential to rapidly divide in order to restore the cancer cell population. These cells have been implicated in tumor recurrence. | |
| Immune | Neutrophils | +/− | Pro-inflammatory response involving the generation of cytokines/chemokines and reactive oxygen species (ROS), which promotes tumor cell death; also important in recruitment of T-cells. However, in late-stage tumors, neutrophils promote angiogenesis and metastasis. |
| Tumor-Associated Macrophages (TAM) | +/− | Can be either pro-(M2) or anti-tumorigenic (M1). Tumor-resident macrophages are highly important in regulating the surrounding environment by the generation of inflammatory molecules, cytokines/chemokines, ligands, and through interaction with infiltrating immune cells. | |
| Dendritic Cells (DC) | − | Important in antigen presentation and activation of T-cells within the tumor microenvironment. However, DCs are susceptible to immunosuppressive environments. | |
| NK Cells | − | Low penetration into the tumor microenvironment but are potent in lysing tumor cells. | |
| Myeloid-Derived Suppressor Cells (MDSCs) | + | Exert an immunosuppressive effect through the production of cytokines/chemokines. | |
| T-cells | − | Highly important component of the anti-tumorigenic response. Mediate tumor cell death. | |
| Regulatory T-cells | + | Contribute to an immunosuppressive environment resulting in T-cell inactivation/exhaustion, which promotes tumor growth. | |
| B-cells | +/− | Important in antigen presentation and antibody generation; however, the presence of regulatory B-cells in some tumors contributes to immune suppression. | |
| Stromal Cells | Endothelial Cells | + | Important for angiogenesis and nutrient supply to the tumor. Tumor-derived endothelial cells also present with altered morphology and function, forming leaky and disorganized vasculature. |
| Pericytes | +/− | Support angiogenesis and may promote tumor metastasis. However, pericytes may also aid in the recruitment and activation of immune cells. | |
| Cancer-associated Fibroblasts (CAFs) | +/− | Largely studied for their pro-tumorigenic functions due to their involvement in creating an immunosuppressive environment and further supporting tumor cells by supplying nutrients. Also heavily involved in the generation of the extracellular matrix. | |
| Adipose Cells | + | Regulate the tumor microenvironment by secreting nutrients, growth factors, hormones, peptides, enzymes and cytokines. | |
| Innervation | Neurons/Neuro-endocrine Cells | + | Secrete signaling molecules, ligands, and peptides, which promote tumor growth and proliferation and intra-tumoral signaling. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patwardhan, M.V.; Mahendran, R. The Bladder Tumor Microenvironment Components That Modulate the Tumor and Impact Therapy. Int. J. Mol. Sci. 2023, 24, 12311. https://doi.org/10.3390/ijms241512311
Patwardhan MV, Mahendran R. The Bladder Tumor Microenvironment Components That Modulate the Tumor and Impact Therapy. International Journal of Molecular Sciences. 2023; 24(15):12311. https://doi.org/10.3390/ijms241512311
Chicago/Turabian StylePatwardhan, Mugdha Vijay, and Ratha Mahendran. 2023. "The Bladder Tumor Microenvironment Components That Modulate the Tumor and Impact Therapy" International Journal of Molecular Sciences 24, no. 15: 12311. https://doi.org/10.3390/ijms241512311
APA StylePatwardhan, M. V., & Mahendran, R. (2023). The Bladder Tumor Microenvironment Components That Modulate the Tumor and Impact Therapy. International Journal of Molecular Sciences, 24(15), 12311. https://doi.org/10.3390/ijms241512311





