New Insights into the Reparative Angiogenesis after Myocardial Infarction
Abstract
1. Introduction
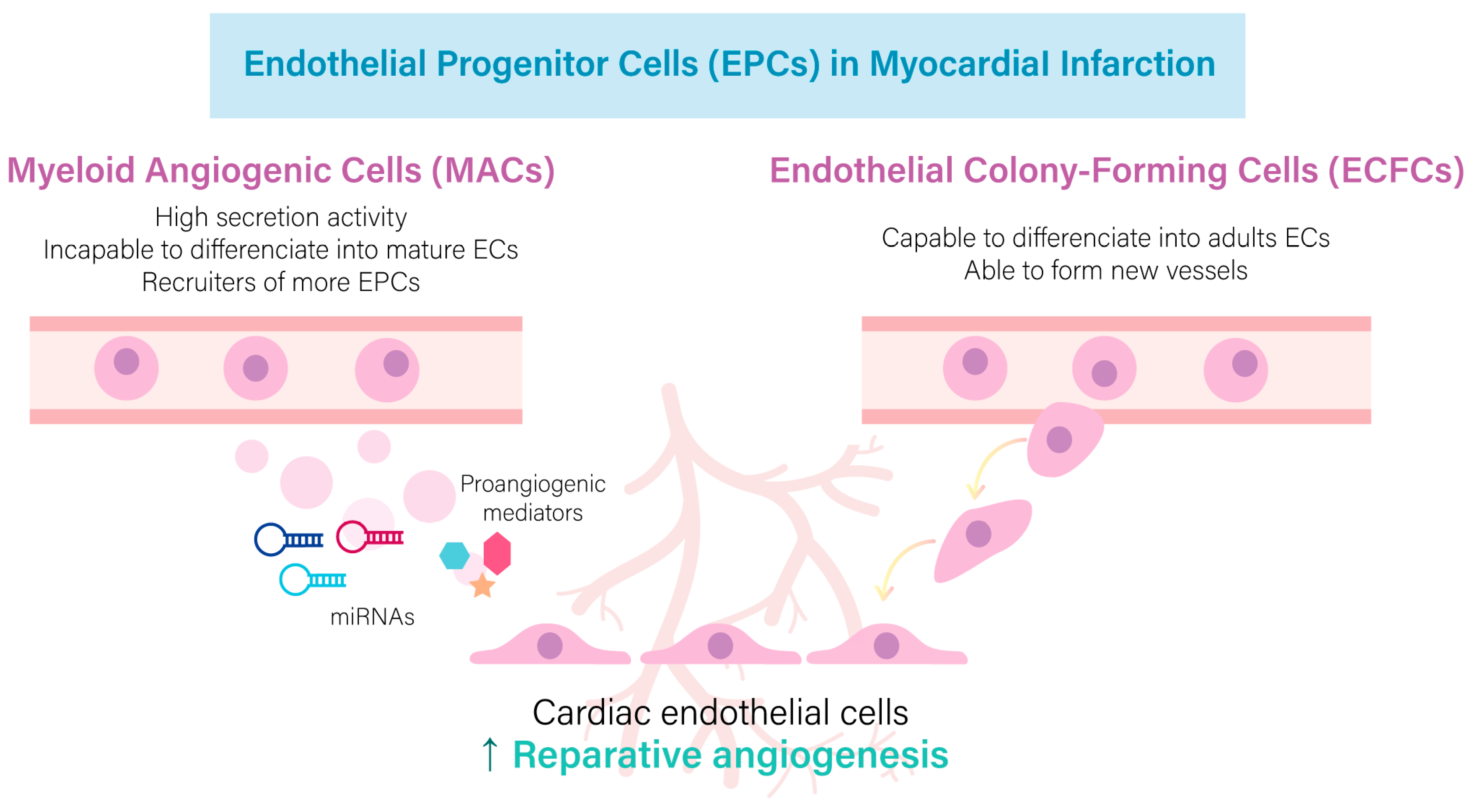
2. Reparative Role of Mature and Endothelial Progenitor Cells in the Infarcted Heart
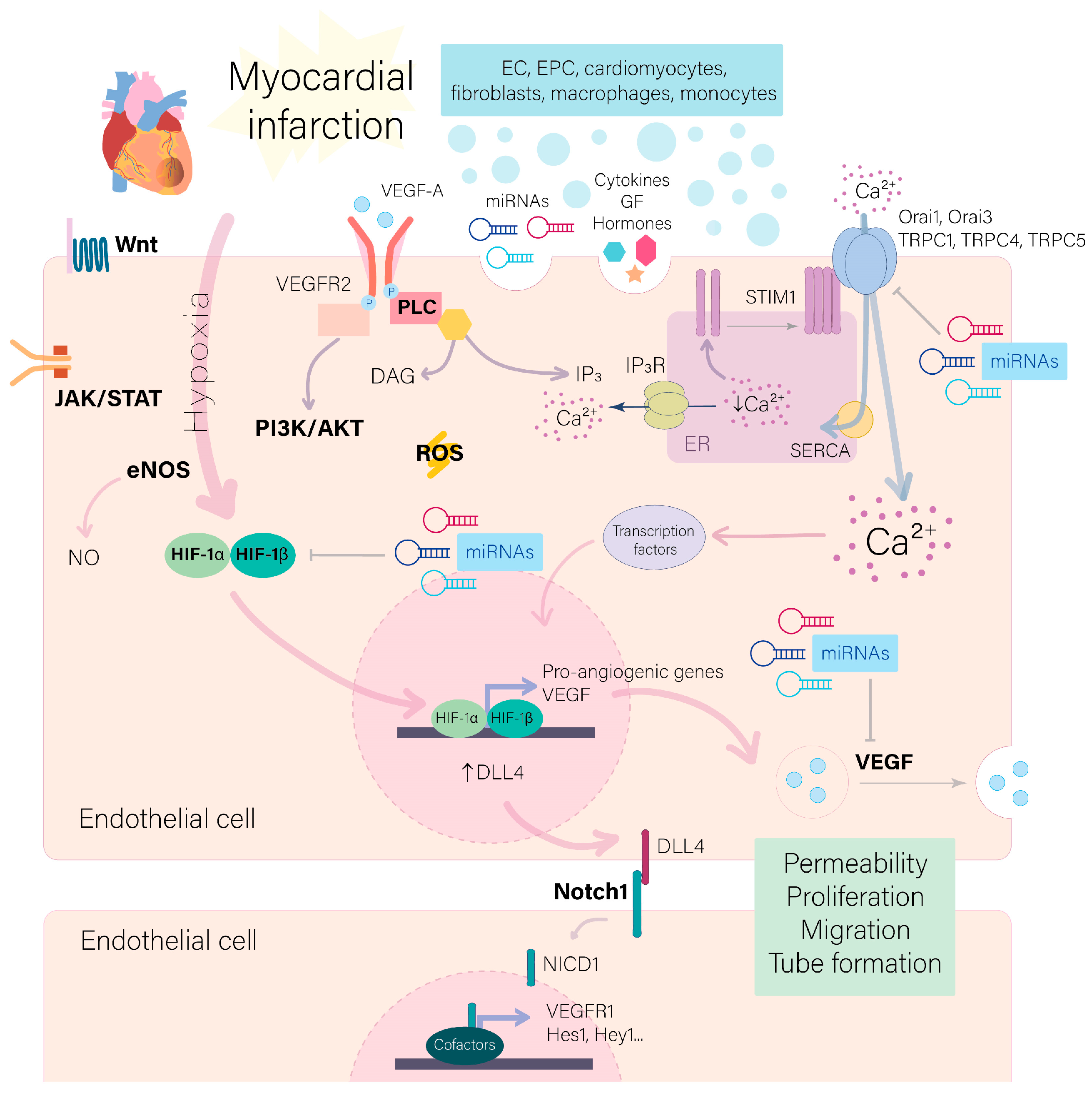
3. Signaling Pathways Participating in the Post-Ischemic Angiogenesis
4. Reactive Oxygen Species Regulation of Angiogenesis
5. Ca2+ Signaling in Angiogenesis
6. miRNAs as Regulators of Post-Ischemic Angiogenesis
| miRNA | Released or Delivered by | Target Genes | Promotes/ Prevents | References |
|---|---|---|---|---|
| miR-322/miR-424 | ECs | CUL2/VEGF/GLUT1/EPO/HIF-1α | ↑ | [94] |
| miR-19a-3p | Cardiomyocytes | HIF-1α | ↓ | [95] |
| miR-126-3p | MSCs/exosomes | PIK3R2/SPRED1/VEGF/bFGF/DLL4/ mTORC1/HIF-1α | ↑ | [97,98] |
| EPCs | VEGF-A/IL-10/IL-3/IGF-1/angiogenin | ↑ | [99,115] | |
| miR126/miR-130a | MACs | SPRED1 | ↑ | [100] |
| miR-130a | Lentivirus | VEGF/HoxA5/AKT | ↑ | [101] |
| PTEN | ↓ | |||
| miR-375 | LNA anti-miR-375 | PDK-1/AKT | ↓ | [102] |
| miR-21-5p | Cardiac stromal cells | PTEN/AKT | ↓/↑ | [103] |
| miR-499-5p | Tanshinone IIA administration | PTEN/VEGF/angiotensin-1 | ↑ | [104] |
| miR-210 | MSC-EVs; transgenic mice | Efna3/VEGF | ↑ | [118,119] |
| miR-378 | CD34+ progenitor cells | SuFu/Fus-1 | ↑ | [105] |
| miR-185-5p | ECs | CatK | ↓ | [107] |
| miR-143 | Cardiomyocytes | IGF-1R | ↓ | [108] |
| miR-92a | ECs | ITGA5 | ↓ | [109] |
| miR-24 | encapsuled antagomiR | PAK4, GATA, eNOS | ↓ | [110,111] |
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Brown, J.C.; Gerhardt, T.E.; Kwon, E. Risk Factors for Coronary Artery Disease. Risk Factors in Coronary Artery Disease; CRC Press: Boca Raton, FL, USA, 2006; pp. 1–219. [Google Scholar] [CrossRef]
- Cardiovascular Diseases. Available online: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1 (accessed on 8 December 2022).
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report from the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, Y.; Zhu, W. Targeting Angiogenesis in Myocardial Infarction: Novel Therapeutics (Review). Exp. Ther. Med. 2021, 23, 64. [Google Scholar] [CrossRef]
- Zou, J.; Fei, Q.; Xiao, H.; Wang, H.; Liu, K.; Liu, M.; Zhang, H.; Xiao, X.; Wang, K.; Wang, N. VEGF-A Promotes Angiogenesis after Acute Myocardial Infarction through Increasing ROS Production and Enhancing ER Stress-Mediated Autophagy. J. Cell Physiol. 2019, 234, 17690–17703. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Rodríguez, A.; Mayoral-Gonzalez, I.; Avila-Medina, J.; de Rojas-de Pedro, E.S.R.; Calderón-Sánchez, E.; Díaz, I.; Hmadcha, A.; Castellano, A.; Rosado, J.A.; Benitah, J.P.; et al. Urocortin-2 Prevents Dysregulation of Ca2+ Homeostasis and Improves Early Cardiac Remodeling After Ischemia and Reperfusion. Front. Physiol. 2018, 9, 813. [Google Scholar] [CrossRef] [PubMed]
- Nauli, S.M. Endothelial Nitric Oxide Synthase (ENOS) and the Cardiovascular System: In Physiology and in Disease States. Am. J. Biomed. Sci. Res. 2022, 15, 155–179. [Google Scholar] [CrossRef]
- Gonzalez-King, H.; García, N.A.; Ontoria-Oviedo, I.; Ciria, M.; Montero, J.A.; Sepúlveda, P. Hypoxia Inducible Factor-1α Potentiates Jagged 1-Mediated Angiogenesis by Mesenchymal Stem Cell-Derived Exosomes. Stem Cells 2017, 35, 1747–1759. [Google Scholar] [CrossRef]
- Ferraro, B.; Leoni, G.; Hinkel, R.; Ormanns, S.; Paulin, N.; Ortega-Gomez, A.; Viola, J.R.; de Jong, R.; Bongiovanni, D.; Bozoglu, T.; et al. Pro-Angiogenic Macrophage Phenotype to Promote Myocardial Repair. J. Am. Coll. Cardiol. 2019, 73, 2990–3002. [Google Scholar] [CrossRef]
- Kobayashi, K.; Maeda, K.; Takefuji, M.; Kikuchi, R.; Morishita, Y.; Hirashima, M.; Murohara, T. Dynamics of Angiogenesis in Ischemic Areas of the Infarcted Heart. Sci. Rep. 2017, 7, 7156. [Google Scholar] [CrossRef]
- Loyer, X.; Zlatanova, I.; Devue, C.; Yin, M.; Howangyin, K.Y.; Klaihmon, P.; Guerin, C.L.; Khelouf, M.; Vilar, J.; Zannis, K.; et al. Intra-Cardiac Release of Extracellular Vesicles Shapes Inflammation Following Myocardial Infarction Short Communication. Circ. Res. 2018, 123, 100–106. [Google Scholar] [CrossRef]
- Virag, J.A.I.; Rolle, M.L.; Reece, J.; Hardouin, S.; Feigl, E.O.; Murry, C.E. Fibroblast Growth Factor-2 Regulates Myocardial Infarct Repair: Effects on Cell Proliferation, Scar Contraction, and Ventricular Function. Am. J. Pathol. 2007, 171, 1431–1440. [Google Scholar] [CrossRef]
- Ono, K.; Matsumori, A.; Shioi, T.; Furakawa, Y.; Sasayama, S. Enhanced Expression of Hepatocyte Growth Factor/c-Met by Myocardial Ischemia and Reperfusion in a Rat Model. Circulation 1997, 95, 2552–2558. [Google Scholar] [CrossRef] [PubMed]
- Tomita, N.; Morishita, R.; Taniyama, Y.; Koike, H.; Aoki, M.; Shimizu, H.; Matsumoto, K.; Nakamura, T.; Kaneda, Y.; Ogihara, T. Angiogenic Property of Hepatocyte Growth Factor Is Dependent on Upregulation of Essential Transcription Factor for Angiogenesis, Ets-1. Circulation 2003, 107, 1411–1417. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhao, T.; Huang, V.; Chen, Y.; Ahokas, R.A.; Sun, Y. Platelet-Derived Growth Factor Involvement in Myocardial Remodeling Following Infarction. J. Mol. Cell Cardiol. 2011, 51, 830. [Google Scholar] [CrossRef] [PubMed]
- Dobrucki, L.W.; Tsutsumi, Y.; Kalinowski, L.; Dean, J.; Gavin, M.; Sen, S.; Mendizabal, M.; Sinusas, A.J.; Aikawa, R. Analysis of Angiogenesis Induced by Local IGF-1 Expression after Myocardial Infarction Using MicroSPECT-CT Imaging. J. Mol. Cell Cardiol. 2011, 48, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Iwakura, A.; Shastry, S.; Luedemann, C.; Hamada, H.; Kawamoto, A.; Kishore, R.; Zhu, Y.; Qin, G.; Silver, M.; Thorne, T.; et al. Estradiol Enhances Recovery After Myocardial Infarction by Augmenting Incorporation of Bone Marrow–Derived Endothelial Progenitor Cells into Sites of Ischemia-Induced Neovascularization via Endothelial Nitric Oxide Synthase–Mediated Activation of Matrix Metalloproteinase-9. Circulation 2006, 113, 1605–1614. [Google Scholar] [CrossRef] [PubMed]
- Barnabas, O.; Wang, H.; Gao, X.M. Role of Estrogen in Angiogenesis in Cardiovascular Diseases. J. Geriatr. Cardiol. 2013, 10, 377–382. [Google Scholar] [CrossRef]
- Bouchentouf, M.; Williams, P.; Forner, K.A.; Cuerquis, J.; Michaud, V.; Paradis, P.; Schiffrin, E.L.; Galipeau, J. Interleukin-2 Enhances Angiogenesis and Preserves Cardiac Function Following Myocardial Infarction. Cytokine 2011, 56, 732–738. [Google Scholar] [CrossRef]
- Mayfield, A.E.; Kanda, P.; Nantsios, A.; Parent, S.; Mount, S.; Dixit, S.; Ye, B.; Seymour, R.; Stewart, D.J.; Davis, D.R. Interleukin-6 Mediates Post-Infarct Repair by Cardiac Explant-Derived Stem Cells. Theranostics 2017, 7, 4850–4861. [Google Scholar] [CrossRef]
- Kishore, R.; Tkebuchava, T.; Sasi, S.P.; Silver, M.; Gilbert, H.Y.; Yoon, Y.S.; Park, H.Y.; Thorne, T.; Losordo, D.W.; Goukassian, D.A. Tumor Necrosis Factor-α Signaling via TNFR1/P55 Is Deleterious Whereas TNFR2/P75 Signaling Is Protective in Adult Infarct Myocardium. Adv. Exp. Med. Biol. 2011, 691, 433–448. [Google Scholar] [CrossRef]
- Xia, Y.; Frangogiannis, N.G. MCP-1/CCL2 as a Therapeutic Target in Myocardial Infarction and Ischemic Cardiomyopathy. Inflamm. Allergy Drug Targets 2007, 6, 101–107. [Google Scholar] [CrossRef]
- Wang, W.; Zheng, Y.; Wang, M.; Yan, M.; Jiang, J.; Li, Z. Exosomes Derived MiR-126 Attenuates Oxidative Stress and Apoptosis from Ischemia and Reperfusion Injury by Targeting ERRFI1. Gene 2019, 690, 75–80. [Google Scholar] [CrossRef]
- Zhu, L.P.; Tian, T.; Wang, J.Y.; He, J.N.; Chen, T.; Pan, M.; Xu, L.; Zhang, H.X.; Qiu, X.T.; Li, C.C.; et al. Hypoxia-Elicited Mesenchymal Stem Cell-Derived Exosomes Facilitates Cardiac Repair through MiR-125b-Mediated Prevention of Cell Death in Myocardial Infarction. Theranostics 2018, 8, 6163–6177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, L.; Wang, S.; Cheng, H.; Xu, L.; Pei, G.; Wang, Y.; Fu, C.; Jiang, Y.; He, C.; et al. Signaling Pathways and Targeted Therapy for Myocardial Infarction. Signal Transduct. Target. Ther. 2022, 7, 78. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.; Zhao, L.; Manuel, G.; Taylor, H.; Liu, D. Approaches to Therapeutic Angiogenesis for Ischemic Heart Disease. J. Mol. Med. 2019, 97, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Chen, P.; Zhong, J.; Cheng, Y.; Chen, H.; He, Y.; Chen, C. HIF-1α in Myocardial Ischemia-Reperfusion Injury. Mol. Med. Rep. 2021, 23, 352. [Google Scholar] [CrossRef]
- Blanco, R.; Gerhardt, H. VEGF and Notch in Tip and Stalk Cell Selection. Cold Spring Harb. Perspect. Med. 2013, 3, a006569. [Google Scholar] [CrossRef]
- Wu, X.; Reboll, M.R.; Korf-Klingebiel, M.; Wollert, K.C. Angiogenesis after Acute Myocardial Infarction. Cardiovasc. Res. 2021, 117, 1257–1273. [Google Scholar] [CrossRef]
- Seidel, T.; Edelmann, J.-C.; Sachse, F.B. Analyzing Remodeling of Cardiac Tissue: A Comprehensive Approach Based on Confocal Microscopy and 3D Reconstructions. Annu. Biomed. Eng. 2016, 44, 1436–1448. [Google Scholar] [CrossRef]
- Jujo, K.; Ii, M.; Losordo, D.W. Endothelial Progenitor Cells in Neovascularization of Infarcted Myocardium. J. Mol. Cell Cardiol. 2008, 45, 530–544. [Google Scholar] [CrossRef]
- Ratajczak, J.; Kucia, M.; Mierzejewska, K.; Marlicz, W.; Pietrzkowski, Z.; Wojakowski, W.; Greco, N.J.; Tendera, M.; Ratajczak, M.Z. Paracrine Proangiopoietic Effects of Human Umbilical Cord Blood-Derived Purified CD133+ Cells-Implications for Stem Cell Therapies in Regenerative Medicine. Stem Cells Dev. 2013, 22, 422–430. [Google Scholar] [CrossRef]
- Huang, H.; Huang, W. Regulation of Endothelial Progenitor Cell Functions in Ischemic Heart Disease: New Therapeutic Targets for Cardiac Remodeling and Repair. Front. Cardiovasc. Med. 2022, 9, 896782. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Huang, K.; Zhou, J.; Yan, D.; Tang, Y.L.; Zhao, T.C.; Miller, R.J.; Kishore, R.; Losordo, D.W.; Qin, G. A Critical Role of Src Family Kinase in SDF-1/CXCR4-Mediated Bone-Marrow Progenitor Cell Recruitment to the Ischemic Heart. J. Mol. Cell Cardiol. 2015, 81, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Abbott, J.D.; Huang, Y.; Liu, D.; Hickey, R.; Krause, D.S.; Giordano, F.J. Stromal Cell-Derived Factor-1α Plays a Critical Role in Stem Cell Recruitment to the Heart after Myocardial Infarction but Is Not Sufficient to Induce Homing in the Absence of Injury. Circulation 2004, 110, 3300–3305. [Google Scholar] [CrossRef] [PubMed]
- Medina, R.J.; Barber, C.L.; Sabatier, F.; Dignat-George, F.; Melero-Martin, J.M.; Khosrotehrani, K.; Ohneda, O.; Randi, A.M.; Chan, J.K.Y.; Yamaguchi, T.; et al. Endothelial Progenitors: A Consensus Statement on Nomenclature. Stem Cells Transl. Med. 2017, 6, 1316–1320. [Google Scholar] [CrossRef]
- Balducci, V.; Faris, P.; Balbi, C.; Costa, A.; Negri, S.; Rosti, V.; Bollini, S.; Moccia, F. The Human Amniotic Fluid Stem Cell Secretome Triggers Intracellular Ca2+ Oscillations, NF-ΚB Nuclear Translocation and Tube Formation in Human Endothelial Colony-Forming Cells. J. Cell Mol. Med. 2021, 25, 8074–8086. [Google Scholar] [CrossRef]
- Dubois, C.; Liu, X.; Claus, P.; Marsboom, G.; Pokreisz, P.; Vandenwijngaert, S.; Dépelteau, H.; Streb, W.; Chaothawee, L.; Maes, F.; et al. Differential Effects of Progenitor Cell Populations on Left Ventricular Remodeling and Myocardial Neovascularization After Myocardial Infarction. J. Am. Coll. Cardiol. 2010, 55, 2232–2243. [Google Scholar] [CrossRef]
- Tripathi, H.; Domingues, A.; Donahue, R.; Cras, A.; Guerin, C.L.; Gao, E.; Levitan, B.; Ratajczak, M.Z.; Smadja, D.M.; Abdel-Latif, A.; et al. Combined Transplantation of Human MSCs and ECFCs Improves Cardiac Function and Decrease Cardiomyocyte Apoptosis After Acute Myocardial Infarction. Stem Cell Rev. Rep. 2023, 19, 573–577. [Google Scholar] [CrossRef]
- Kim, S.W.; Jin, H.L.; Kang, S.M.; Kim, S.; Yoo, K.J.; Jang, Y.; Kim, H.O.; Yoon, Y.S. Therapeutic Effects of Late Outgrowth Endothelial Progenitor Cells or Mesenchymal Stem Cells Derived from Human Umbilical Cord Blood on Infarct Repair. Int. J. Cardiol. 2016, 203, 498–507. [Google Scholar] [CrossRef][Green Version]
- Li, Z.; Solomonidis, E.G.; Meloni, M.; Taylor, R.S.; Duffin, R.; Dobie, R.; Magalhaes, M.S.; Henderson, B.E.P.; Louwe, P.A.; D’Amico, G.; et al. Single-Cell Transcriptome Analyses Reveal Novel Targets Modulating Cardiac Neovascularization by Resident Endothelial Cells Following Myocardial Infarction. Eur. Heart J. 2019, 40, 2507–2520. [Google Scholar] [CrossRef]
- Fujisawa, T.; Tura-Ceide, O.; Hunter, A.; Mitchell, A.; Vesey, A.; Medine, C.; Gallogly, S.; Hadoke, P.W.F.; Keith, C.; Sproul, A.; et al. Endothelial Progenitor Cells Do Not Originate from the Bone Marrow. Circulation 2019, 140, 1524–1526. [Google Scholar] [CrossRef]
- Lin, Y.; Weisdorf, D.J.; Solovey, A.; Hebbel, R.P. Origins of Circulating Endothelial Cells and Endothelial Outgrowth from Blood. J. Clin. Investig. 2000, 105, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, L.; Feng, Z.; Chen, W.; Yan, S.; Yang, R.; Xiao, J.; Gao, J.; Zhang, D.; Ke, X. EPC-Derived Exosomal MiR-1246 and MiR-1290 Regulate Phenotypic Changes of Fibroblasts to Endothelial Cells to Exert Protective Effects on Myocardial Infarction by Targeting ELF5 and SP1. Front. Cell Dev. Biol. 2021, 9, 647763. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Yang, R.; Wu, F.; Wang, X.; Liang, J.; Hu, X.; Hu, C. Exosomal MiR-218-5p/MiR-363-3p from Endothelial Progenitor Cells Ameliorate Myocardial Infarction by Targeting the P53/JMY Signaling Pathway. Oxid. Med. Cell Longev. 2021, 2021, 5529430. [Google Scholar] [CrossRef]
- Yu, B.; Li, H.; Zhang, Z.; Chen, P.; Wang, L.; Fan, X.; Ning, X.; Pan, Y.; Zhou, F.; Hu, X.; et al. Extracellular Vesicles Engineering by Silicates-Activated Endothelial Progenitor Cells for Myocardial Infarction Treatment in Male Mice. Nat. Commun. 2023, 14, 2094. [Google Scholar] [CrossRef]
- Mack, J.J.; Mosqueiro, T.S.; Archer, B.J.; Jones, W.M.; Sunshine, H.; Faas, G.C.; Briot, A.; Aragón, R.L.; Su, T.; Romay, M.C.; et al. NOTCH1 Is a Mechanosensor in Adult Arteries. Nat. Commun. 2017, 8, 1620. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Rivas, B.; Agoulnik, A.I. NOTCH1 Gain of Function in Germ Cells Causes Failure of Spermatogenesis in Male Mice. PLoS ONE 2013, 8, e71213. [Google Scholar] [CrossRef] [PubMed]
- Xuan, W.; Khan, M.; Ashraf, M. Extracellular Vesicles from Notch Activated Cardiac Mesenchymal Stem Cells Promote Myocyte Proliferation and Neovasculogenesis. Front. Cell Dev. Biol. 2020, 8, 11. [Google Scholar] [CrossRef]
- Gude, N.A.; Emmanuel, G.; Wu, W.; Cottage, C.T.; Fischer, K.; Quijada, P.; Muraski, J.A.; Alvarez, R.; Rubio, M.; Schaefer, E.; et al. Activation of Notch-Mediated Protective Signaling in the Myocardium. Circ. Res. 2008, 102, 1025–1035. [Google Scholar] [CrossRef]
- He, Y.; Pang, S.; Huang, J.; Zhu, K.; Tong, J.; Tang, Y.; Ma, G.; Chen, L. Blockade of RBP-J-Mediated Notch Signaling Pathway Exacerbates Cardiac Remodeling after Infarction by Increasing Apoptosis in Mice. Biomed. Res. Int. 2018, 2018, 5207031. [Google Scholar] [CrossRef]
- Zhou, X.-L.; Zhu, R.-R.; Liu, S.; Xu, H.; Xu, X.; Wu, Q.-C.; Liu, J.-C. Notch Signaling Promotes Angiogenesis and Improves Cardiac Function after Myocardial Infarction. J. Cell Biochem. 2018, 119, 7105–7112. [Google Scholar] [CrossRef]
- Li, Y.; Hiroi, Y.; Liao, J.K. Notch Signaling Negatively Regulates Cardiac Differentiation (Nemir, 2006). Trends Cardiovasc. Med. 2010, 20, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Shen, W.; Lee, S.R.; Mathai, A.E.; Zhang, R.; Xu, G.; Gillies, M.C. Targeting the Notch and TGF-β Signaling Pathways to Prevent Retinal Fibrosis in Vitro and in Vivo. Theranostics 2020, 10, 7956–7973. [Google Scholar] [CrossRef]
- Crovella, S.; Ticarico, P.M.; Kachanova, O.; Lobov, A.; Malashicheva, A. The Role of the Notch Signaling Pathway in Recovery of Cardiac Function after Myocardial Infarction. Int. J. Mol. Sci. 2022, 23, 12509. [Google Scholar] [CrossRef]
- Wang, K.; Ding, R.; Ha, Y.; Jia, Y.; Liao, X.; Wang, S.; Li, R.; Shen, Z.; Xiong, H.; Guo, J.; et al. Hypoxia-Stressed Cardiomyocytes Promote Early Cardiac Differentiation of Cardiac Stem Cells through HIF-1 α/Jagged1/Notch1 Signaling. Acta Pharm. Sin. B 2018, 8, 795–804. [Google Scholar] [CrossRef]
- Sciarretta, S.; Forte, M.; Frati, G.; Sadoshima, J. New Insights into the Role of MTOR Signaling in the Cardiovascular System. Circ. Res. 2018, 122, 489–505. [Google Scholar] [CrossRef] [PubMed]
- Kazakov, A.; Hall, R.; Jagoda, P.; Bachelier, K.; Müller-Best, P.; Semenov, A.; Lammert, F.; Böhm, M.; Laufs, U. Inhibition of Endothelial Nitric Oxide Synthase Induces and Enhances Myocardial Fibrosis. Cardiovasc. Res. 2013, 100, 211–221. [Google Scholar] [CrossRef]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef] [PubMed]
- Meloni, M.; Caporali, A.; Graiani, G.; Lagrasta, C.; Katare, R.; van Linthout, S.; Spillmann, F.; Campesi, I.; Madeddu, P.; Quaini, F.; et al. Nerve Growth Factor Promotes Cardiac Repair Following Myocardial Infarction. Circ. Res. 2010, 106, 1275–1284. [Google Scholar] [CrossRef] [PubMed]
- Ronnebaum, S.M.; Patterson, C. The FoxO Family in Cardiac Function and Dysfunction. Annu. Rev. Physiol. 2009, 72, 81–94. [Google Scholar] [CrossRef]
- Fu, W.-B.; Wang, W.E.; Zeng, C.-Y. Wnt Signaling Pathways in Myocardial Infarction and the Therapeutic Effects of Wnt Pathway Inhibitors. Acta Pharmacol. Sin. 2019, 40, 9–12. [Google Scholar] [CrossRef]
- Aisagbonhi, O.; Rai, M.; Ryzhov, S.; Atria, N.; Feoktistov, I.; Hatzopoulos, A.K. Experimental Myocardial Infarction Triggers Canonical Wnt Signaling and Endothelial-to-Mesenchymal Transition. DMM Dis. Models Mech. 2011, 4, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Oerlemans, M.I.F.J.; Goumans, M.J.; van Middelaar, B.; Clevers, H.; Doevendans, P.A.; Sluijter, J.P.G. Active Wnt Signaling in Response to Cardiac Injury. Basic. Res. Cardiol. 2010, 105, 631–641. [Google Scholar] [CrossRef]
- Matteucci, M.; Casieri, V.; Gabisonia, K.; Aquaro, G.D.; Agostini, S.; Pollio, G.; Diamanti, D.; Rossi, M.; Travagli, M.; Porcari, V.; et al. Magnetic Resonance Imaging of Infarct-Induced Canonical Wingless/Integrated (Wnt)/β-Catenin/T-Cell Factor Pathway Activation, In Vivo. Cardiovasc. Res. 2016, 112, 645–655. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Paik, D.T.; Rai, M.; Ryzhov, S.; Sanders, L.N.; Aisagbonhi, O.; Funke, M.J.; Feoktistov, I.; Hatzopoulos, A.K. Wnt10b Gain-of-Function Improves Cardiac Repair by Arteriole Formation and Attenuation of Fibrosis. Circ. Res. 2015, 117, 804–816. [Google Scholar] [CrossRef] [PubMed]
- Hilfiker-Kleiner, D.; Hilfiker, A.; Fuchs, M.; Kaminski, K.; Schaefer, A.; Schieffer, B.; Hillmer, A.; Schmiedl, A.; Ding, Z.; Podewski, E.; et al. Signal Transducer and Activator of Transcription 3 Is Required for Myocardial Capillary Growth, Control of Interstitial Matrix Deposition, and Heart Protection from Ischemic Injury. Circ. Res. 2004, 95, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.B.; Wang, Y.; Liu, L.; Liu, F.; Zhang, Y.Q. Astragaloside IV Alleviates Heart Failure by Promoting Angiogenesis through the JAK-STAT3 Pathway. Pharm. Biol. 2019, 57, 48–54. [Google Scholar] [CrossRef]
- Wang, N.; Liu, C.; Wang, X.; He, T.; Li, L.; Liang, X.; Wang, L.; Song, L.; Wei, Y.; Wu, Q.; et al. Hyaluronic Acid Oligosaccharides Improve Myocardial Function Reconstruction and Angiogenesis against Myocardial Infarction by Regulation of Macrophages. Theranostics 2019, 9, 1980–1992. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhao, T.; Chen, Y.; Ahokas, R.A.; Sun, Y. Reactive Oxygen Species Promote Angiogenesis in the Infarcted Rat Heart. Int. J. Exp. Pathol. 2009, 90, 621–629. [Google Scholar] [CrossRef]
- Kim, Y.W.; Byzova, T.V. Oxidative Stress in Angiogenesis and Vascular Disease. Blood 2014, 123, 625–631. [Google Scholar] [CrossRef]
- Teixeira, R.B.; Pfeiffer, M.; Zhang, P.; Shafique, E.; Rayta, B.; Karbasiafshar, C.; Ahsan, N.; Sellke, F.W.; Abid, M.R. Reduction in Mitochondrial ROS Improves Oxidative Phosphorylation and Provides Resilience to Coronary Endothelium in Non-Reperfused Myocardial Infarction. Basic. Res. Cardiol. 2023, 118, 3. [Google Scholar] [CrossRef]
- Thirunavukkarasu, M.; Adluri, R.S.; Juhasz, B.; Samuel, S.M.; Zhan, L.; Kaur, A.; Maulik, G.; Sanchez, J.A.; Hager, J.; Maulik, N. Novel Role of NADPH Oxidase in Ischemic Myocardium: A Study with Nox2 Knockout Mice. Funct. Integr. Genomics 2012, 12, 501–514. [Google Scholar] [CrossRef]
- Dalal, P.J.; Muller, W.A.; Sullivan, D.P. Endothelial Cell Calcium Signaling during Barrier Function and Inflammation. Am. J. Pathol. 2020, 190, 535–542. [Google Scholar] [CrossRef]
- Tran, Q.K.; Ohashi, K.; Watanabe, H. Calcium Signalling in Endothelial Cells. Cardiovasc. Res. 2000, 48, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Filippini, A.; D’Amore, A.; D’Alessio, A. Calcium Mobilization in Endothelial Cell Functions. Int. J. Mol. Sci. 2019, 20, 4525. [Google Scholar] [CrossRef]
- Wen, X.; Peng, Y.; Gao, M.; Zhu, Y.; Zhu, Y.; Yu, F.; Zhou, T.; Shao, J.; Feng, L.; Ma, X. Endothelial Transient Receptor Potential Canonical Channel Regulates Angiogenesis and Promotes Recovery After Myocardial Infarction. J. Am. Heart Assoc. 2022, 11, e023678. [Google Scholar] [CrossRef] [PubMed]
- Galeano-Otero, I.; Del Toro, R.; Khatib, A.M.; Rosado, J.A.; Ordóñez-Fernández, A.; Smani, T. SARAF and Orai1 Contribute to Endothelial Cell Activation and Angiogenesis. Front. Cell Dev. Biol. 2021, 9, 639952. [Google Scholar] [CrossRef]
- Moccia, F.; Tanzi, F.; Munaron, L. Endothelial Remodelling and Intracellular Calcium Machinery. Curr. Mol. Med. 2014, 14, 457–480. [Google Scholar] [CrossRef] [PubMed]
- Andrikopoulos, P.; Eccles, S.A.; Yaqoob, M.M. Coupling between the TRPC3 Ion Channel and the NCX1 Transporter Contributed to VEGF-Induced ERK1/2 Activation and Angiogenesis in Human Primary Endothelial Cells. Cell Signal 2017, 37, 12–30. [Google Scholar] [CrossRef]
- Smani, T.; Shapovalov, G.; Skryma, R.; Prevarskaya, N.; Rosado, J.A. Functional and Physiopathological Implications of TRP Channels. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2015, 1853, 1772–1782. [Google Scholar] [CrossRef]
- Abdullaev, I.F.; Bisaillon, J.M.; Potier, M.; Gonzalez, J.C.; Motiani, R.K.; Trebak, M. Stim1 and Orai1 Mediate CRAC Currents and Store-Operated Calcium Entry Important for Endothelial Cell Proliferation. Circ. Res. 2008, 103, 1289–1299. [Google Scholar] [CrossRef]
- Li, J.; Bruns, A.F.; Hou, B.; Rode, B.; Webster, P.J.; Bailey, M.A.; Appleby, H.L.; Moss, N.K.; Ritchie, J.E.; Yuldasheva, N.Y.; et al. Orai3 Surface Accumulation and Calcium Entry Evoked by Vascular Endothelial Growth Factor. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1987–1994. [Google Scholar] [CrossRef]
- Ye, J.; Wei, J.; Luo, Y.; Deng, Y.; Que, T.; Zhang, X.; Liu, F.; Zhang, J.; Luo, X. Epstein-Barr Virus Promotes Tumor Angiogenesis by Activating STIM1-Dependent Ca2+ Signaling in Nasopharyngeal Carcinoma. Pathogens 2021, 10, 1275. [Google Scholar] [CrossRef]
- Simons, M.; Gordon, E.; Claesson-Welsh, L. Mechanisms and Regulation of Endothelial VEGF Receptor Signalling. Nat. Rev. Mol. Cell Biol. 2016, 17, 611–625. [Google Scholar] [CrossRef]
- Falcón, D.; Galeano-Otero, I.; Calderón-Sánchez, E.; del Toro, R.; Martín-Bórnez, M.; Rosado, J.A.; Hmadcha, A.; Smani, T. TRP Channels: Current Perspectives in the Adverse Cardiac Remodeling. Front. Physiol. 2019, 10, 159. [Google Scholar] [CrossRef]
- Du, S.L.; Jia, Z.Q.; Zhong, J.C.; Wang, L.F. TRPC5 in Cardiovascular Diseases. Rev. Cardiovasc. Med. 2021, 22, 127–135. [Google Scholar] [CrossRef]
- Zhu, Y.; Gao, M.; Zhou, T.; Xie, M.; Mao, A.; Feng, L.; Yao, X.; Wong, W.T.; Ma, X. The TRPC5 Channel Regulates Angiogenesis and Promotes Recovery from Ischemic Injury in Mice. J. Biol. Chem. 2019, 294, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Wang, L.; Wu, Y.; Liu, Y.; Pei, H.; Xiang, H. Adeno-Associated Virus Packaged TRPC5 Gene Therapy Alleviated Spinal Cord Ischemic Reperfusion Injury in Rats. Neuroreport 2020, 31, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Fadaei, S.; Zarepour, F.; Parvaresh, M.; Motamedzadeh, A.; Tamehri Zadeh, S.S.; Sheida, A.; Shabani, M.; Hamblin, M.R.; Rezaee, M.; Zarei, M.; et al. Epigenetic Regulation in Myocardial Infarction: Non-Coding RNAs and Exosomal Non-Coding RNAs. Front. Cardiovasc. Med. 2022, 9, 1014961. [Google Scholar] [CrossRef]
- Smani, T.; Mayoral-González, I.; Galeano-Otero, I.; Gallardo-Castillo, I.; Rosado, J.A.; Ordoñez, A.; Hmadcha, A. Chapter 15: Non-Coding RNAs and Ischemic Cardiovascular Diseases. In Non-Coding RNAs in Cardiovascular Diseases, Advances in Experimental Medicine and Biology; Xiao, J., Ed.; Springer: Singapore, 2020. [Google Scholar]
- Kesidou, D.; da Costa Martins, P.A.; de Windt, L.J.; Brittan, M.; Beqqali, A.; Baker, A.H. Extracellular Vesicle MiRNAs in the Promotion of Cardiac Neovascularisation. Front. Physiol. 2020, 11, 579892. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Yang, J.; Zhao, X.; Zhang, E.; Zeng, Q.; Yu, Y.; Yang, L.; Wu, B.; Yi, G.; Mao, X.; et al. Circulating Myocardial MicroRNAs from Infarcted Hearts Are Carried in Exosomes and Mobilise Bone Marrow Progenitor Cells. Nat. Commun. 2019, 10, 959. [Google Scholar] [CrossRef]
- Ghosh, G.; Subramanian, I.V.; Adhikari, N.; Zhang, X.; Joshi, H.P.; Basi, D.; Chandrashekhar, Y.S.; Hall, J.L.; Roy, S.; Zeng, Y.; et al. Hypoxia-Induced MicroRNA-424 Expression in Human Endothelial Cells Regulates HIF-α Isoforms and Promotes Angiogenesis. J. Clin. Investig. 2010, 120, 4141–4154. [Google Scholar] [CrossRef]
- Gou, L.; Xue, C.; Tang, X.; Fang, Z. Inhibition of Exo-MiR-19a-3p Derived from Cardiomyocytes Promotes Angiogenesis and Improves Heart Function in Mice with Myocardial Infarction via Targeting HIF-1α. Aging 2020, 12, 23609–23618. [Google Scholar] [CrossRef]
- Fernandes, T.; Baraúna, V.G.; Negrão, C.E.; Ian Phillips, M.; Oliveira, E.M. Aerobic Exercise Training Promotes Physiological Cardiac Remodeling Involving a Set of MicroRNAs. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H543–H552. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Aurora, A.B.; Johnson, B.A.; Qi, X.; McAnally, J.; Hill, J.A.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. The Endothelial-Specific MicroRNA MiR-126 Governs Vascular Integrity and Angiogenesis. Dev. Cell 2008, 15, 261–271. [Google Scholar] [CrossRef]
- Huang, F.; Zhu, X.; Hu, X.Q.; Fang, Z.F.; Tang, L.; Lu, X.L.; Zhou, S.H. Mesenchymal Stem Cells Modified with MiR-126 Release Angiogenic Factors and Activate Notch Ligand Delta-like-4, Enhancing Ischemic Angiogenesis and Cell Survival. Int. J. Mol. Med. 2013, 31, 484–492. [Google Scholar] [CrossRef]
- Li, H.; Liu, Q.; Wang, N.; Xu, Y.; Kang, L.; Ren, Y.; Zhu, G. Transplantation of Endothelial Progenitor Cells Overexpressing MiR-126-3p Improves Heart Function in Ischemic Cardiomyopathy. Circ. J. 2018, 82, 2332–2341. [Google Scholar] [CrossRef]
- Jakob, P.; Doerries, C.; Briand, S.; Mocharla, P.; Kränkel, N.; Besler, C.; Mueller, M.; Manes, C.; Templin, C.; Baltes, C.; et al. Loss of AngiomiR-126 and 130a in Angiogenic Early Outgrowth Cells from Patients with Chronic Heart Failure: Role for Impaired in Vivo Neovascularization and Cardiac Repair Capacity. Circulation 2012, 126, 2962–2975. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Wang, X.; Ha, T.; Hu, Y.; Liu, L.; Zhang, X.; Yu, H.; Miao, J.; Kao, R.; Kalbfleisch, J.; et al. Attenuation of Cardiac Dysfunction and Remodeling of Myocardial Infarction by MicroRNA-130a Is Mediated by Suppression of PTEN and Activation of PI3K Dependent Signaling. J. Mol. Cell Cardiol. 2015, 89, 87–97. [Google Scholar] [CrossRef]
- Garikipati, V.N.S.; Verma, S.K.; Jolardarashi, D.; Cheng, Z.; Ibetti, J.; Cimini, M.; Tang, Y.; Khan, M.; Yue, Y.; Benedict, C.; et al. Therapeutic Inhibition of MiR-375 Attenuates Post-Myocardial Infarction Inflammatory Response and Left Ventricular Dysfunction via PDK-1-AKT Signalling Axis. Cardiovasc. Res. 2017, 113, 938–949. [Google Scholar] [CrossRef]
- Qiao, L.; Hu, S.; Liu, S.; Zhang, H.; Ma, H.; Huang, K.; Li, Z.; Su, T.; Vandergriff, A.; Tang, J.; et al. MicroRNA-21-5p Dysregulation in Exosomes Derived from Heart Failure Patients Impairs Regenerative Potential. J. Clin. Investig. 2019, 129, 2237–2250. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, C. Tanshinone IIA Improves Cardiac Function via Regulating MiR-499-5p Dependent Angiogenesis in Myocardial Ischemic Mice. Microvasc. Res. 2022, 143, 104399. [Google Scholar] [CrossRef]
- Templin, C.; Volkmann, J.; Emmert, M.Y.; Mocharla, P.; Müller, M.; Kraenkel, N.; Ghadri, J.R.; Meyer, M.; Styp-Rekowska, B.; Briand, S.; et al. Increased Proangiogenic Activity of Mobilized CD34+ Progenitor Cells of Patients with Acute ST-Segment-Elevation Myocardial Infarction: Role of Differential MicroRNA-378 Expression. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 341–349. [Google Scholar] [CrossRef]
- Bellera, N.; Barba, I.; Rodriguez-Sinovas, A.; Ferret, E.; Asín, M.A.; Gonzalez-Alujas, M.T.; Pérez-Rodon, J.; Esteves, M.; Fonseca, C.; Toran, N.; et al. Single Intracoronary Injection of Encapsulated Antagomir-92a Promotes Angiogenesis and Prevents Adverse Infarct Remodeling. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 2014, 3, e000946. [Google Scholar] [CrossRef]
- Li, C.C.; Qiu, X.T.; Sun, Q.; Zhou, J.P.; Yang, H.J.; Wu, W.Z.; He, L.F.; Tang, C.E.; Zhang, G.G.; Bai, Y.P. Endogenous Reduction of MiR-185 Accelerates Cardiac Function Recovery in Mice Following Myocardial Infarction via Targeting of Cathepsin K. J. Cell Mol. Med. 2019, 23, 1164–1173. [Google Scholar] [CrossRef] [PubMed]
- Geng, T.; Song, Z.Y.; Xing, J.X.; Wang, B.X.; Dai, S.P.; Xu, Z.S. Exosome Derived from Coronary Serum of Patients with Myocardial Infarction Promotes Angiogenesis Through the MiRNA-143/IGF-IR Pathway. Int. J. Nanomed. 2020, 15, 2647–2658. [Google Scholar] [CrossRef]
- Daniel, J.M.; Penzkofer, D.; Teske, R.; Dutzmann, J.; Koch, A.; Bielenberg, W.; Bonauer, A.; Boon, R.A.; Fischer, A.; Bauersachs, J.; et al. Editor’s Choice: Inhibition of MiR-92a Improves Re-Endothelialization and Prevents Neointima Formation Following Vascular Injury. Cardiovasc. Res. 2014, 103, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Bang, C.; Fiedler, J.; Thum, T. Cardiovascular Importance of the MicroRNA-23/27/24 Family. Microcirculation 2012, 19, 208–214. [Google Scholar] [CrossRef]
- Meloni, M.; Marchetti, M.; Garner, K.; Littlejohns, B.; Sala-Newby, G.; Xenophontos, N.; Floris, I.; Suleiman, M.S.; Madeddu, P.; Caporali, A.; et al. Local Inhibition of MicroRNA-24 Improves Reparative Angiogenesis and Left Ventricle Remodeling and Function in Mice with Myocardial Infarction. Mol. Ther. 2013, 21, 1390–1402. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zhao, J.L.; Peng, Z.Y.; Xu, W.F.; Yu, G.L. Exosomal MiR-25-3p from Mesenchymal Stem Cells Alleviates Myocardial Infarction by Targeting pro-Apoptotic Proteins and EZH2. Cell Death Dis. 2020, 11, 317. [Google Scholar] [CrossRef]
- Wen, Z.; Mai, Z.; Zhu, X.; Wu, T.; Chen, Y.; Geng, D.; Wang, J. Mesenchymal Stem Cell-Derived Exosomes Ameliorate Cardiomyocyte Apoptosis in Hypoxic Conditions through MicroRNA144 by Targeting the PTEN/AKT Pathway. Stem Cell Res. Ther. 2020, 11, 36. [Google Scholar] [CrossRef]
- Pan, J.; Alimujiang, M.; Chen, Q.; Shi, H.; Luo, X. Exosomes Derived from MiR-146a-Modified Adipose-Derived Stem Cells Attenuate Acute Myocardial Infarction−induced Myocardial Damage via Downregulation of Early Growth Response Factor 1. J. Cell Biochem. 2019, 120, 4433–4443. [Google Scholar] [CrossRef]
- Duan, S.; Wang, C.; Xu, X.; Zhang, X.; Su, G.; Li, Y.; Fu, S.; Sun, P.; Tian, J. Peripheral Serum Exosomes Isolated from Patients with Acute Myocardial Infarction Promote Endothelial Cell Angiogenesis via the MiR-126-3p/TSC1/MTORC1/HIF-1α Pathway. Int. J. Nanomed. 2022, 17, 1577–1592. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Yuan, J.; Gao, W.; Zhong, X.; Yao, K.; Lin, L.; Ge, J. Dendritic Cell-Derived Exosomal MiR-494-3p Promotes Angiogenesis Following Myocardial Infarction. Int. J. Mol. Med. 2021, 47, 315–325. [Google Scholar] [CrossRef]
- Youn, S.W.; Li, Y.; Kim, Y.M.; Sudhahar, V.; Abdelsaid, K.; Kim, H.W.; Liu, Y.; Fulton, D.J.R.; Ashraf, M.; Tang, Y.; et al. Modification of Cardiac Progenitor Cell-Derived Exosomes by MiR-322 Provides Protection against Myocardial Infarction through Nox2-Dependent Angiogenesis. Antioxidants 2019, 8, 18. [Google Scholar] [CrossRef]
- Wang, N.; Chen, C.; Yang, D.; Liao, Q.; Luo, H.; Wang, X.; Zhou, F.; Yang, X.; Yang, J.; Zeng, C.; et al. Mesenchymal Stem Cells-Derived Extracellular Vesicles, via MiR-210, Improve Infarcted Cardiac Function by Promotion of Angiogenesis. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2085–2092. [Google Scholar] [CrossRef] [PubMed]
- Arif, M.; Pandey, R.; Alam, P.; Jiang, S.; Sadayappan, S.; Paul, A.; Ahmed, R.P.H. MicroRNA-210-Mediated Proliferation, Survival, and Angiogenesis Promote Cardiac Repair Post Myocardial Infarction in Rodents. J. Mol. Med. 2017, 95, 1369–1385. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Chen, Y.; Duan, C.; Zhu, K.; Huang, R.; Zhao, H.; Hintze, M.; Pu, Q.; Yuan, Z.; Lv, L.; et al. Cardiac Telocytes Inhibit Cardiac Microvascular Endothelial Cell Apoptosis through Exosomal MiRNA-21-5p-Targeted Cdip1 Silencing to Improve Angiogenesis Following Myocardial Infarction. Theranostics 2021, 11, 268–291. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Bórnez, M.; Falcón, D.; Morrugares, R.; Siegfried, G.; Khatib, A.-M.; Rosado, J.A.; Galeano-Otero, I.; Smani, T. New Insights into the Reparative Angiogenesis after Myocardial Infarction. Int. J. Mol. Sci. 2023, 24, 12298. https://doi.org/10.3390/ijms241512298
Martín-Bórnez M, Falcón D, Morrugares R, Siegfried G, Khatib A-M, Rosado JA, Galeano-Otero I, Smani T. New Insights into the Reparative Angiogenesis after Myocardial Infarction. International Journal of Molecular Sciences. 2023; 24(15):12298. https://doi.org/10.3390/ijms241512298
Chicago/Turabian StyleMartín-Bórnez, Marta, Débora Falcón, Rosario Morrugares, Geraldine Siegfried, Abdel-Majid Khatib, Juan A. Rosado, Isabel Galeano-Otero, and Tarik Smani. 2023. "New Insights into the Reparative Angiogenesis after Myocardial Infarction" International Journal of Molecular Sciences 24, no. 15: 12298. https://doi.org/10.3390/ijms241512298
APA StyleMartín-Bórnez, M., Falcón, D., Morrugares, R., Siegfried, G., Khatib, A.-M., Rosado, J. A., Galeano-Otero, I., & Smani, T. (2023). New Insights into the Reparative Angiogenesis after Myocardial Infarction. International Journal of Molecular Sciences, 24(15), 12298. https://doi.org/10.3390/ijms241512298







