Abstract
Immune cells such as T cells and macrophages express α7 nicotinic acetylcholine receptors (α7 nAChRs), which contribute to the regulation of immune and inflammatory responses. Earlier findings suggest α7 nAChR activation promotes the development of regulatory T cells (Tregs) in mice. Using human CD4+ T cells, we investigated the mRNA expression of the α7 subunit and the human-specific dupα7 nAChR subunit, which functions as a dominant-negative regulator of ion channel function, under resting conditions and T cell receptor (TCR)-activation. We then explored the effects of the selective α7 nAChR agonist GTS-21 on proliferation of TCR-activated T cells and Treg development. Varied levels of mRNA for both the α7 and dupα7 nAChR subunits were detected in resting human CD4+ T cells. mRNA expression of the α7 nAChR subunit was profoundly suppressed on days 4 and 7 of TCR-activation as compared to day 1, whereas mRNA expression of the dupα7 nAChR subunit remained nearly constant. GTS-21 did not alter CD4+ T cell proliferation but significantly promoted Treg development. These results suggest the potential ex vivo utility of GTS-21 for preparing Tregs for adoptive immunotherapy, even with high expression of the dupα7 subunit.
1. Introduction
Immune cells such as T cells, B cells and monocytes express various subtypes of both muscarinic and nicotinic acetylcholine (ACh) receptors (mAChRs and nAChRs, respectively) [1,2,3,4,5]. Moreover, they also express mRNA for ACh synthase (choline acetyltransferase, ChAT) and synthesize ACh [1,2,3,4,5,6,7,8,9,10,11]. Activation of T-cell receptors (TCRs) by phytophemagglutinin (PHA) or anti-CD3/CD28 monoclonal antibodies or by activation of protein kinases A and C, enhances ChAT mRNA expression and increases ACh synthesis and release [8,12]. These findings indicate that ACh synthesized by immune cells acts in an autocrine and paracrine manner via AChRs on immune cells, especially T cells, and is involved in regulating immune function.
Among the various mAChRs and nAChRs subtypes expressed by immune cells, α7 nAChR has received much attention. This is because α7 nAChR activation in lipopolysaccharide (LPS)-treated mice prevents septic shock by inhibiting the synthesis and release of the pro-inflammatory cytokine tumor necrosis factor-α (TNF-α) from macrophages [13]. In addition, α7 nAChR gene-deficient (α7-KO) mice immunized with ovalbumin had higher serum concentrations of anti-ovalbumin-specific IgG1 than identically treated wild-type mice. At the same time, synthesis of the pro-inflammatory cytokines TNF-α, interferon-γ (IFN-γ) and IL-6 was up-regulated in spleen cells from ovalbumin-immunized α7-KO mice [14]. On the other hand, ACh produced in T cells and α7 nAChRs expressed on macrophages are known to play key roles in various cholinergic anti-inflammatory pathways [15,16,17,18,19,20]. Together, these findings indicate the involvement of α7 nAChRs on immune cells in the regulation of inflammatory and immune functions.
Recently, Mashimo et al. (2019) found that GTS-21, a partial α7 nAChR agonist [21], promotes the differentiation of TCR-activated mouse CD4+ T cells into regulatory T cells (Tregs) and effector helper T (Th1, Th2, and Th17) cells [22]. However, GTS-21 suppresses antigen-processing and antigen-presenting cell (APC)-dependent activation of mouse CD4+ T cell differentiation. This suggests that α7 nAChRs play a variety of roles affecting immune function, and that their effects depending on the cells in which they are expressed (CD4+ T cells or APCs) [22,23].
The gene encoding the human neuronal α7 nAChR subunit CHRNA7 is located on chromosome 15 and contains 10 exons. Exons 1–6 encode the extracellular N-terminal region of the receptor, including the ligand-binding domain, and exons 7–10 encode the channel region [24]. Notably, chromosome 15 also contains a human-specific partial duplicate α7 nAChR subunit-like gene with exons 5–10. This gene rearranges with the kinase gene FAM7A on chromosome 3 to form a hybrid, CHRFAM7A [25]. The CHRFAM7A gene product, dupα7, lacks a ligand-binding region and assembles with intact α7 subunits to form α7 nAChRs composed of a total of five α7 and dupα7 subunits in various ratios [26]. Because dupα7 acts as a dominant negative regulator of ion channel function, α7 nAChRs with a large dupα7 component do not function well as ion channels, despite retaining of channel structure [26,27,28,29,30,31]. Neuronally expressed α7 nAChRs, which function mainly as ligand-gated ion channels play key roles in cellular signaling, and their dysfunction due to widespread expression of dupα7 is thought to be associated with several central nervous system disorders, including schizophrenia and certain forms of cognitive deficits (see reviews by Bertrand et al. (2015) and Bertrand and Terry (2017)) [32,33]. Multiple clinical trials with α7 nAChR agonists failed to demonstrate efficacy in patients with cognitive deficits or schizophrenia, suggesting decreased expression of functional α7 nAChRs in these patients’ neuronal cells [32,33]. However, most α7 nAChR agonists, including GTS-21, have been shown clinically to be safe [32,34].
Evidence now suggests that α7 nAChRs have dual functions as canonical ionotropic channels and as non-canonical metabolic signaling receptors in both neuronal and non-neuronal cells [35]. α7 nAChRs with metabotropic function are coupled to heterotrimeric G proteins such as Gαq and activate a cascade of signals leading to the release of Ca2+ from intracellular stores [35,36,37,38]. In immune cells, α7 nAChRs appear to function as metabotropic receptors rather than as ionotropic receptors [22,39,40,41]. The functional effects of dupα7 contained within metabotropic α7 nAChRs are not yet known. Considering the potential utility of α7 nAChR agonists as immunomodulatory agents [13,14,19,22,23], it is noteworthy that human peripheral blood leukocytes express more CHRFAM7A than CHRNA7 [31,42,43]. We therefore investigated the mRNA expression of both α7 and dupα7 subunits in human CD4+ T cells and the effect of GTS-21 on Treg development.
2. Results
2.1. mRNA Expression of α7 and Dupα7 Subunits
2.1.1. Under the Resting Conditions
Figure 1 shows a scatterplot of CHRNA7 and CHRFAM7A expression for each individual divided by the values closest to their respective medians to compare the magnitude of interindividual variability in CHRNA7 and CHRFAM7A expression. Varied expression of both CHRNA7 and CHRFAM7A were detected in all cryopreserved human peripheral blood CD4+ T cells examined under the resting conditions. Interindividual variation was greater for CHRNA7 expression than for CHRFAM7A expression (note the log scale of the vertical axis).
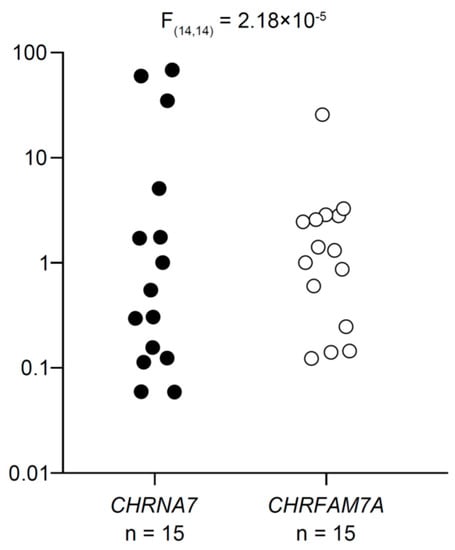
Figure 1.
Expression of α7 and dupα7 subunit mRNAs in resting human CD4+ T cells. CHRNA7 and CHRFAM7A (α7 and dupα7 subunit mRNA, respectively) expression levels were first normalized to GAPDH mRNA in each individual. Then to compare the magnitude of interindividual variability in CHRNA7 and CHRFAM7A expression, levels of CHRNA7 and CHRFAM7A mRNA were divided by the values closest to their respective medians and plotted on a logarithmic scale. The interindividual variability of CHRNA7 expression was statistically greater than that of CHRFAM7A expression. The difference between the standard deviations was assessed with the F-test.
2.1.2. Changes during TCR-Activation
Expression of CHRNA7 in cells cultured in RPMI medium containing 20 μg/mL IL-2 (Control) was nearly unchanged over the 7-day observation period (Figure 2A). TCR activation with Human T-activator CD3/CD28 Dynabeads did not alter CHRNA7 expression on day 1, but the expression levels were significantly suppressed on days 4 and 7. Addition of 30 μM GTS-21 to the culture did not affect CHRNA7 expression in the TCR-activated group over the 7-day observation period.
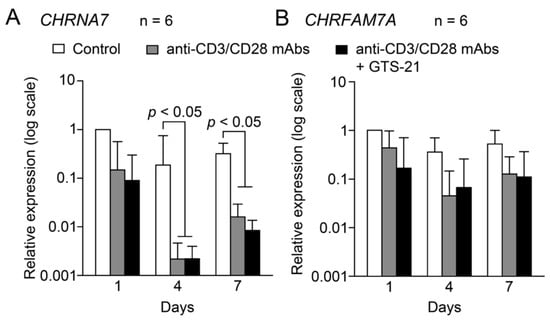
Figure 2.
Fluctuations in mRNA expression of the α7 and dupα7 subunits during TCR activation. Human CD4+ T cells were cultured for up to 7 days in the standard culture medium in the presence or absence of Human T-activator CD3/CD28 Dynabeads at a beads-to-cell ratio of 1:1 with or without 30 μM GTS-21. Levels of CHRNA7 and CHRFAM7A expression in cells from each individual was first normalized to GAPDH expression. Then to detect fluctuations over time induced by TCR activation, CHRNA7 (A) and CHRFAM7A (B) mRNA levels were further divided by the respective levels in controls observed on day 1. Bars are the geomean ± S.E.M. (n = 6). Statistical significance was assessed with two-way ANOVA and post-hoc Tukey tests.
Under control conditions, levels of CHRFAM7A expression did not fluctuate over the 7-day observation period (Figure 2B). Moreover, TCR activation in the absence or presence of 30 μM GTS-21 did not affect CHRFAM7A expression at any time during the 7-day observation period.
2.2. Effects of GTS-21 on CD4+ T Cell Proliferation and Treg Development
2.2.1. Proliferation
Numbers of CD4+ T cells remained nearly constant over the 7-day culture period in RPMI medium containing 20 ng/mL IL-2 (Figure 3). With TCR activation, however, CD4+ T cell proliferation was significantly enhanced on culture days 5 and 7, as indicated by staining with CFSE (Figure 3A) and by increasing cell number (Figure 3B). Addition of 30 μM GTS-21 caused no further enhancement of proliferation compared to TCR activation alone.
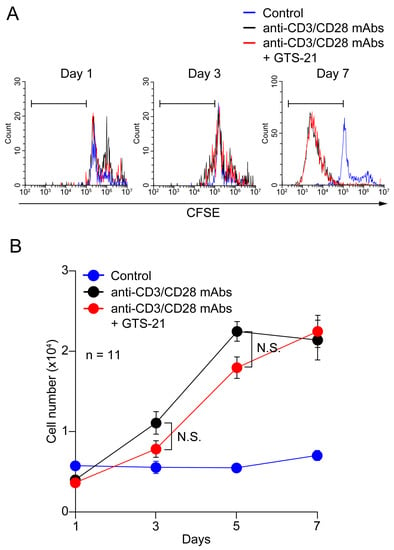
Figure 3.
Effects of GTS-21 on CD4+ T cell proliferation. (A) Representative flow cytometric histograms for CFSE-labeled CD4+ T cells. Human CD4+ T cells were cultured for up to 7 days in the standard medium in the presence of Human T-activator CD3/CD28 Dynabeads at a beads-to-cell ratio of 1:1 with or without 30 μM GTS-21. The gates (horizontal bars) indicate proliferating cells. (B) Cell numbers were counted after staining with trypan blue. Data are shown as the mean ± S.E.M. (n = 11). N.S.; not significant (two-way ANOVA with post-hoc Tukey test).
2.2.2. Treg Development
Figure 4A shows flow cytometric plots for CD4+CD25+FoxP3+ cells (Tregs) from a representative individual in the absence and presence of 30 μM GTS-21 on day 5 of culture. The data show that GTS-21 promoted Treg development from CD4+ T cells.
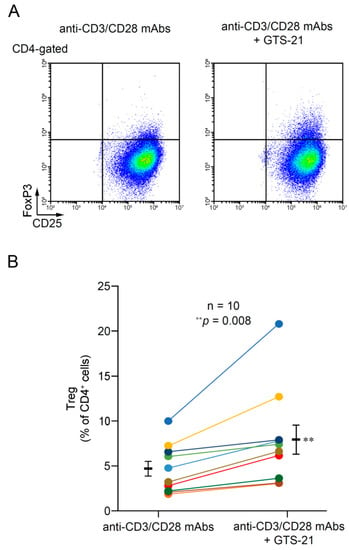
Figure 4.
Effects of GTS-21 on Treg development. (A) Representative flow cytometric plots for Treg development among TCR-activated human CD4+ T cells in the presence or absence of 30 μM GTS-21. (B) GTS-21 enhanced Treg development from TCR-activated human CD4+ T cells on day 5 of culture. Gates were used to calculate the percentages of CD4+CD25+FoxP3+ cells. For comparison, the percentages of Tregs in the absence and presence of 30 μM GTS-21 in TCR-activated CD4+ T cells were tied by dots of the same color between the same individuals. Bars are means ± S.E.M. (n = 10). Statistical significance was assessed using paired t-tests (** p < 0.01).
GTS-21 promoted Treg development to varying degrees in samples from all individuals on day 5 of culture in the presence of 20 ng/mL IL-2 and 5 ng/mL transforming growth factor-β (TGF-β). The average number of Tregs was significantly higher in cultures with GTS-21 than without it (p = 0.008) (Figure 4B).
3. Discussion
3.1. Expression of mRNAs for α7 and Dupα7 Subunits in Resting Human CD4+ T Cells
Varied levels of both α7 and dupα7 subunits mRNAs were detected in cryopreserved CD4+ T cells from normal volunteers. However, it is not yet clear whether the large interindividual variation in α7 and dupα7 subunit expression is due to genetic disposition, immunological regulation or both. The greater interindividual variation in α7 subunit expression suggests α7 nAChRs in CD4+ T cells are likely more susceptible to immune stimulation in daily life. Expression of α7 and dupα7 subunit mRNAs showed no clear trends across gender, age or ethnicity.
3.2. Expression of α7 and Dupα7 Subunit mRNAs during TCR Activation
Changes in the mRNA expression of the α7 and dupα7 subunits during TCR activation were explored for the first time and provide valuable information about the dynamics of α7 nAChRs in human CD4+ T cells. Activation of TCRs on T cells up-regulates ChAT mRNA expression and ACh synthesis [8,9,10,12,44,45,46]. PHA induces ChAT mRNA expression in human mononuclear cells; induction is first evident after 48 h of exposure, and the effect persists for at least 72 h [8]. PHA-activated MOLT-3 cells, a human leukemic T cell line, secrete sufficient amounts of ACh to activate AChRs in an autocrine and paracrine manner [47]. It is therefore suggested that sustained exposure to elevated concentrations of ACh released from activated T cells attenuates CHRNA7 expression. Indeed, the plasticity of CHRNA7 expression has been confirmed in TCR-activated T cells [46,47,48]. The marked down-regulation of CHRNA7 expression in TCR-activated CD4+ T cells after 4 days in culture observed in the present study can be attributed to negative regulation of CHRNA7 expression mediated by sustained α7 nAChR activation induced by ACh synthesized and released from CD4+ T cells. This in turn suggests α7 nAChRs are involved in regulating CD4+ T cell function. The absence of significant variation in CHRFAM7A expression during TCR activation indicates dupα7 nAChRs are less involved in ACh-mediated regulation of CD4+ T cell function.
In contrast to the dynamic fluctuation in CHRNA7 expression, the nearly constant CHRFAM7A expression in TCR-activated CD4+ T cells is consistent with the finding that CHRNA7 and CHRFAM7A are independently regulated by their respective promoters driving their differential expression [31,42,49]. Our finding that GTS-21 did not affect CHRFAM7A expression in human CD4+ T cells differed from that in human monocyte-derived macrophages, where nicotine suppresses CHRFAM7A expression [26]. This suggests that regulation of CHRFAM7A is cell type-specific and that dupα7 nAChRs are less involved in ACh-mediated regulation of CD4+ T cell function.
Recent studies revealed that the α7 nAChRs have both ionotropic and metabotropic functions in neurons [36,37,50]. Moreover, most studies indicate that α7 nAChRs in macrophages and T cells function as metabotropic receptors independent of ionotropic signaling [23,39,40,41,51,52,53,54]. In oocytes injected with various combinations of dupα7 and α7 mRNAs, decreased ionotropic function of α7 nAChRs was observed with increasing dupα7/α7 composition ratios [26]. Under the present experimental conditions, α7 nAChRs in TCR-activated CD4+ T cells may have elevated dupα7/α7 subunit ratios after 4 days in culture and little ionotropic function. There is currently little evidence to suggest that the dupα7 subunit plays a specific physiological role in T cells, and the present findings indicate there is little if any involvement of ionotropic signaling in the regulation of CD4+ T cell function.
3.3. Effects of GTS-21 on Proliferation
In J774A.1 cells (a mouse macrophage cell line), GTS-21 mitigates LPS-induced proliferation arrest for up to 9 h [55]. In mice, proliferation of activated T cells is reportedly inhibited by stimulation of a α7 nAChR-mediated pathway [56]. In the present study, however, GTS-21 did not affect the proliferation of highly purified TCR-activated human CD4+ T cells. It is unclear why α7 nAChR agonists have different effects on mouse and human T cell proliferation; little information is currently available on the effects of α7 nAChR agonists, including GTS-21, on human T cell proliferation.
3.4. The Effect of GTS-21 on Treg Development in TCR-Activated CD4+ T Cells
As in our earlier study of TCR-activated CD4+ T cells from mice [22], GTS-21 (30 μM) promoted Treg development from human TCR-activated CD4+ T cells, even in the presence of appreciable levels of CHRFAM7A expression, thereby increasing the proportion of Tregs to about 1.8-fold over the control in 5 days. This suggests ex vivo utilization of GTS-21 could potentially help to reduce the time required to prepare sufficient numbers of Tregs for adoptive immunotherapy and reduce the associated costs. It remains possible to continue to improve Treg development through further optimization of culture conditions, including the GTS-21 concentration, beads-to-cells ratio (currently 1:1) and culture duration.
An earlier study [26] indicated that under conditions where CHRNA7 expression is profoundly suppressed, as observed in TCR-activated CD4+ T cells on days 4 and 7 of the present experiment, most of the subunits that make up α7 nAChRs may be occupied by dupα7 subunits, which lack a ligand-binding site. Nevertheless, GTS-21 promoted Treg development from CD4+ T cell (Figure 4). Based on the observation that GTS-21 suppresses TNF-α and IL-6 secretion in LPS-stimulated macrophages from α7-KO mice, Garg and Loring (2019) reported that GTS-21 may inhibit proinflammatory cytokine production by acting at sites unrelated to α7 nAChRs [57]. However, because Mashimo et al. (2019) observed that GTS-21 promotes Treg development from wild-type CD4+ T cells but not in α7-KO mice [22], it seems most likely that GTS-21 promotes Treg development by acting on the α7 subunit of α7 nAChRs. However, to further confirm the involvement of α7 nAChRs in Treg development, the effects of α7 nAChR agonists other than GTS-21 should be assessed.
Metabotropic α7 nAChR function induces the release of G-proteins bound to M3-M4 loop of the channel, activating signaling cascades leading to the release of Ca2+ from intracellular stores [58]. The resulting increase in intracellular free Ca2+ ion concentration activates protein kinase C, which in turn activates PI3K/Akt signaling pathway, leading to promotion of nuclear translocation of nuclear factor erythroid 2-related factor 2 to the nucleus and overexpression of heme oxygenase-1, and finally resulting in inhibition of pro-inflammatory cytokine production, in macrophages [59,60]. Both α7 and dupα7 subunits retain G-protein binding site in their intracellular M3-M4 loop [35,37,53]. Therefore, the above process should work even if α7 subunit makes up a small fraction of the α7 nAChR. However, it remains unclear how the PI3K/Akt signaling pathway in T cells is involved in promoting Treg development.
TCR activation induces de novo IL-2 synthesis and initiates a signaling cascade that leads to activation of Janus kinase 1 (JAK1) and JAK3 [61]. IL-6 signaling is essential for optimal T cell differentiation and is transduced via JAK family proteins, culminating in STAT3 activation [62,63,64]. STAT3 is a critical positive regulator of T cell differentiation and functions in several CD4+ T cell subsets, including Th2 and Th17 cells and Tregs [64,65,66]. In our earlier study using TCR-activated CD4+ T cells from mice, GTS-21 dose-dependently enhanced IL-6 production and significantly promoted Treg development without affecting IL-2 production [22]. In the presence of IL-2 and TGF-β, GTS-21 significantly enhanced Treg development from TCR-activated human CD4+ T cells. These findings suggest that enhanced IL-6 production contributes to the up-regulation of Treg development by GTS-21.
In summary, results of the present study demonstrate that TCR-activation of human peripheral blood CD4+ T cells expressing variable levels of CHRNA7 and CHRFAM7A profoundly suppressed CHRNA7 expression with time by day 4 of the experiments, without affecting CHRFAM7A expression. GTS-21 did not affect the proliferation induced by TCR-activation. Despite low expression of CHRNA7 and high expression of CHRFAM7A, GTS-21 promoted Treg development in human TCR-activated T cells, increasing the number of Tregs to about 1.8-fold over control in 5 days of culture. In conclusion, these findings support the ex vivo utilization of GTS-21 to reduce the time and associated costs of preparing sufficient numbers of Tregs for adoptive immunotherapy.
4. Materials and Methods
4.1. Cell Culture
Cryopreserved human peripheral blood CD4+ T cells from donors with various backgrounds (15 males and 3 females; 18–80 years old; Ethnicity, 4 African Americans, 4 Asians, 6 Caucasians, 3 Hispanics and 1 mixed ethnicity) (STEMCELL Technologies, Vancouver, Canada and Zen-Bio Laboratories, Durham, NC, USA) were used for this study. Vials of CD4+ T cells were thawed rapidly with vigorous agitation in a 37 °C water bath and washed once with RPMI 1640 (Nacalai tesque, Kyoto, Japan) supplemented with 10% fetal bovine serum Thermo Fisher Scientific (Waltham, MA, USA), 100 units/mL penicillin (Nacalai tesque), 100 μg/mL streptomycin (Nacalai tesque), 50 μM 2-mercaptoethanol (Fuji film, Tokyo, Japan) and 20 ng/mL IL-2 (BioLegend, San Diego, CA, USA) (the standard medium) at 37 °C under a humidified atmosphere with 5% CO2.
4.1.1. CHRNA7 and CHRFAM7A Expression under the Resting Conditions
Portions of the cells from 15 samples were used for investigation of CHRNA7 and CHRFAM7A expression levels under the resting conditions.
4.1.2. CHRNA7 and CHRFAM7A Expression during T Cell Activation
Cells from six specimens with sufficient cell numbers were used to investigate changes in CHRNA7 and CHRFAM7A expression levels during TCR-activation. The cells (1.5 × 105 cells) were cultured for 7 days in triplicate in a 24-well plate containing 2 mL of the standard medium with or without 30 μM GTS-21 (Cayman Chemical Company, Ann Arbor, MI, USA). The cells were activated using Human T-activator CD3/CD28 Dynabeads (Veritas, Tokyo, Japan) at a beads-to-cell ratio of 1:1.
4.1.3. Effects of GTS-21 on TCR-Activated T Cell Proliferation
To assess the effect of GTS-21 on cell proliferation, portions of the cells (2 × 104 cells) from 11 samples were cultured for 7 days in duplicate in a 48-well plate containing 200 μL of the standard medium and Human T-activator CD3/CD28 Dynabeads at a beads-to-cell ratio of 1:1. After staining with 4% trypan blue, cell numbers were counted using a Countess II automated cell counter (Thermo Fisher Scientific).
For cell proliferation assay, cells were stained with 1 μM CFSE (Nacalai tesque) in PBS for 10 min and cultured under the same experimental conditions as described above for indicated times. After washing, the prepared cells were subjected to flow cytometry (CytoFLEX, Beckman Coulter, Brea, CA, USA).
4.1.4. Effects of GTS-21 on Treg Development in TCR-Activated T Cells
To investigate the effect of GTS-21 on Treg development, portions of the cells (3 × 104 cells) from 10 specimens were cultured for 5 days in triplicate in a 24-well plate containing 2 mL of the standard medium with 5 ng/mL TGF-β (BioLegend) and Human T-activator CD3/CD28 Dynabeads at a beads-to-cell ratio of 1:1 with or without 30 μM GTS-21.
4.2. Real-Time PCR
Total mRNA was extracted from human CD4+ T cells using Sepasol RNA II Super (Nacalai Tesque), after which cDNAs were prepared by reverse transcription using a Prime Script RT reagent Kit (Takara Bio., Shiga, Japan) in a S1000 Thermal Cycler (Bio-rad, Hercules, CA, USA). Real-time PCR analysis was conducted using TB Green Premix Ex Taq II, and predesigned primers (Takara Bio.) with a Thermal Cycler Dice Real Time System (Takara Bio.). The sequences and catalog numbers of the predesigned primers were as follows: for CHRNA7 (HA164722), 5′-TGGCCAGATTTGGAAACCAGA-3′ (sense) and 5′-AGTGTGGAATGTGGCGTCAAAG-3 (anti-sense); for CHRFAM7A (HA137753), 5′-GGTTCAAGGCCAAACCGAAG-3′ (sense) and 5′-TCCTGCTGACTCAGGTGTCCA-3′ (anti-sense); and for GAPDH (HA067812), 5′-GCACCGTCAAGGCTGAGAAC-3′ (sense) and 5′-TGGTGAAGACGCCAGTGGA-3′ (anti-sense).
No amplification was observed without primers or cDNA samples. Melting curve analysis confirmed the amplicons were single at the expected melting temperature and did not form primer dimers.
CHRNA7 and CHRFAM7A expression in each individual was normalized by GAPDH expression. Then to compare the magnitude of interindividual variability in CHRNA7 and CHRFAM7A expression in resting CD4+ T cells, the levels of CHRNA7 and CHRFAM7A expression in each individual were divided by the values closest to their respective medians and plotted in Figure 1.
Firstly, the expression levels of CHRNA7 and CHRFAM7A under control conditions and under TCR activation in the presence or absence of GTS-21 over time were normalized by the GAPDH expression. Next, to detect changes over time in the expression levels of CHRNA7 (A) and CHRFAM7A (B) in each individual under control conditions or under TCR activation in the presence or absence of GTS-21, the ratios of GAPDH-normalized CHRNA7 and CHRFAM7A expression levels in each individual were calculated by dividing by respective GAPDH-normalized expression levels found under control conditions on day 1 when frozen/thawed samples had adapted to culture medium [67]. The ratios are shown as relative expression in Figure 2.
4.3. Flow Cytometry for Treg Development
To detect Tregs on day 5 of culture, cells were first washed with Hanks’ balanced salt solution supplemented with 0.1% bovine serum albumin and 0.1% NaN3, then stained using FITC-conjugated anti-CD4 antibody (RM4.5, Thermo Fisher Scientific) and PE-conjugated anti-CD25 antibody (PC61.5, Thermo Fisher Scientific). After subsequent fixation and permeabilization using BD Cytofix/Cytoperm solution (BD Biosciences, Franklin Lakes, NJ, USA), the cells were further stained with APC-conjugated anti-FoxP3 antibody (3G3, Thermo Fisher Scientific) and subjected to flow cytometric analysis. A gate was set on the lymphocytes using characteristic forward scatter (FSC) and side scatter (SSC) parameters. Isotype-matched FITC-, PE- and APC-conjugated mouse IgG1 Abs were used as controls. The acquired data was analyzed using CytExpert (Beckman Coulter). Treg development was determined by calculating the percentage of CD4+CD25+FoxP3+ cells cleared the gates.
4.4. Statistical Analysis
Data are presented as means ± S.E.M. Statistical analyses were performed using SPSS (IBM, Armonk, NY, USA). When performing parametric tests, the normality tests were performed on the data for each group. Difference of the standard deviation was assessed using F-test. Differences between two groups were evaluated using paired t-test, and between three or more groups using two-way analysis of variance (ANOVA) with post-hoc Tukey’s test, respectively. Values of p < 0.05 were considered as significant.
Author Contributions
K.K., M.M., T.F. and T.K. were involved in study design, interpretation of the results, writing and revising the manuscript; M.M. and T.F. performed experiments; S.O., H.M., Y.M. and T.A. were participated in revising the manuscript; K.K., M.M., T.F., S.O., Y.M., H.M. and T.A. reviewed and approved the final version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The research described in this manuscript was supported in part by funding from the SRF, Tokyo, Japan (K.K., T.F., M.M., S.O., H.M. and Y.M.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from M.M. (mmashimo@dwc.doshisha.ac.jp) if and when such request is deemed appropriate and justified.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sato, K.Z.; Fujii, T.; Watanabe, Y.; Yamada, S.; Ando, T.; Kazuko, F.; Kawashima, K. Diversity of mRNA expression for muscarinic acetylcholine receptor subtypes and neuronal nicotinic acetylcholine receptor subunits in human mononuclear leukocytes and leukemic cell lines. Neurosci. Lett. 1999, 266, 17–20. [Google Scholar] [CrossRef]
- Kawashima, K.; Fujii, T. Extraneuronal cholinergic system in lymphocytes. Pharmacol. Ther. 2000, 86, 29–48. [Google Scholar] [CrossRef]
- Kawashima, K.; Fujii, T. The lymphocytic cholinergic system and its biological function. Life Sci. 2003, 72, 2101–2109. [Google Scholar] [CrossRef]
- Kawashima, K.; Fujii, T. Expression of non-neuronal acetylcholine in lymphocytes and its contribution to the regulation of immune function. Front. Biosci. 2004, 9, 2063–2085. [Google Scholar] [CrossRef]
- Kawashima, K.; Yoshikawa, K.; Fujii, Y.X.; Moriwaki, Y.; Misawa, H. Expression and function of genes encoding cholinergic components in murine immune cells. Life Sci. 2007, 80, 2314–2319. [Google Scholar] [CrossRef]
- Fujii, T.; Tajima, S.; Yamada, S.; Watanabe, Y.; Sato, K.Z.; Matsui, M.; Misawa, H.; Kasahara, T.; Kawashima, K. Constitutive expression of mRNA for the same choline acetyltransferase as that in the nervous system, an acetylcholine-synthesizing enzyme, in human leukemic T-cell lines. Neurosci. Lett. 1999, 259, 71–74. [Google Scholar] [CrossRef]
- Fujii, T.; Yamada, S.; Misawa, H.; Tajima, S.; Fujimoto, K.; Suzuki, T.; Kawashima, K. Expression of choline acetyltransferase mRNA and protein in t-lymphocytes. Proc. Jpn. Acad. 1995, 71B, 231–235. [Google Scholar] [CrossRef][Green Version]
- Fujii, T.; Yamada, S.; Watanabe, Y.; Misawa, H.; Tajima, S.; Fujimoto, K.; Kasahara, T.; Kawashima, K. Induction of choline acetyltransferase mRNA in human mononuclear leukocytes stimulated by phytohemagglutinin, a T-cell activator. J. Neuroimmunol. 1998, 82, 101–107. [Google Scholar] [CrossRef]
- Fujii, T.; Kawashima, K. An independent non-neuronal cholinergic system in lymphocytes. Jpn. J. Pharmacol. 2001, 85, 11–15. [Google Scholar] [CrossRef]
- Rinner, I.; Kawashima, K.; Schauenstein, K. Rat lymphocytes produce and secrete acetylcholine in dependence of differentiation and activation. J. Neuroimmunol. 1998, 81, 31–37. [Google Scholar] [CrossRef]
- Tarnawski, L.; Shavva, V.S.; Kort, E.J.; Zhuge, Z.; Nilsson, I.; Gallina, A.L.; Martinez-Enguita, D.; Heller Sahlgren, B.; Weiland, M.; Caravaca, A.S.; et al. Cholinergic regulation of vascular endothelial function by human ChAT(+) T cells. Proc. Natl. Acad. Sci. USA 2023, 120, e2212476120. [Google Scholar] [CrossRef]
- Fujii, T.; Takada-Takatori, Y.; Kawashima, K. Regulatory mechanisms of acetylcholine synthesis and release by T cells. Life Sci. 2012, 91, 981–985. [Google Scholar] [CrossRef]
- Wang, H.; Yu, M.; Ochani, M.; Amella, C.A.; Tanovic, M.; Susarla, S.; Li, J.H.; Wang, H.; Yang, H.; Ulloa, L.; et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 2003, 421, 384–388. [Google Scholar] [CrossRef]
- Fujii, Y.X.; Fujigaya, H.; Moriwaki, Y.; Misawa, H.; Kasahara, T.; Grando, S.A.; Kawashima, K. Enhanced serum antigen-specific IgG1 and proinflammatory cytokine production in nicotinic acetylcholine receptor alpha7 subunit gene knockout mice. J. Neuroimmunol. 2007, 189, 69–74. [Google Scholar] [CrossRef]
- Rosas-Ballina, M.; Olofsson, P.S.; Ochani, M.; Valdes-Ferrer, S.I.; Levine, Y.A.; Reardon, C.; Tusche, M.W.; Pavlov, V.A.; Andersson, U.; Chavan, S.; et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 2011, 334, 98–101. [Google Scholar] [CrossRef]
- Hoover, D.B.; Poston, M.D.; Brown, S.; Lawson, S.E.; Bond, C.E.; Downs, A.M.; Williams, D.L.; Ozment, T.R. Cholinergic leukocytes in sepsis and at the neuroimmune junction in the spleen. Int. Immunopharmacol. 2020, 81, 106359. [Google Scholar] [CrossRef]
- Fujii, T.; Mashimo, M.; Moriwaki, Y.; Misawa, H.; Ono, S.; Horiguchi, K.; Kawashima, K. Expression and Function of the Cholinergic System in Immune Cells. Front. Immunol. 2017, 8, 1085. [Google Scholar] [CrossRef]
- Fujii, T.; Mashimo, M.; Moriwaki, Y.; Misawa, H.; Ono, S.; Horiguchi, K.; Kawashima, K. Physiological functions of the cholinergic system in immune cells. J. Pharmacol. Sci. 2017, 134, 1–21. [Google Scholar] [CrossRef]
- Kawashima, K.; Fujii, T.; Moriwaki, Y.; Misawa, H.; Horiguchi, K. Non-neuronal cholinergic system in regulation of immune function with a focus on alpha7 nAChRs. Int. Immunopharmacol. 2015, 29, 127–134. [Google Scholar] [CrossRef]
- Galitovskiy, V.; Qian, J.; Chernyavsky, A.I.; Marchenko, S.; Gindi, V.; Edwards, R.A.; Grando, S.A. Cytokine-induced alterations of alpha7 nicotinic receptor in colonic CD4 T cells mediate dichotomous response to nicotine in murine models of Th1/Th17- versus Th2-mediated colitis. J. Immunol. 2011, 187, 2677–2687. [Google Scholar] [CrossRef]
- Defiebre, C.M.; Meyer, E.M.; Henry, J.C.; Muraskin, S.I.; Kem, W.R.; Papke, R.L. Characterization of a Series of Anabaseine-Derived Compounds Reveals That the 3-(4)-Dimethylaminocinnamylidine Derivative Is a Selective Agonist at Neuronal Nicotinic Alpha-7/I-125-Alpha-Bungarotoxin Receptor Subtypes. Mol. Pharmacol. 1995, 47, 164–171. [Google Scholar]
- Mashimo, M.; Komori, M.; Matsui, Y.Y.; Murase, M.X.; Fujii, T.; Takeshima, S.; Okuyama, H.; Ono, S.; Moriwaki, Y.; Misawa, H.; et al. Distinct roles of alpha7 nAChRs in antigen-presenting cells and CD4(+) T cells in the regulation of T cell differentiation. Front. Immunol. 2019, 10, 1102. [Google Scholar] [CrossRef]
- Mashimo, M.; Fujii, T.; Ono, S.; Moriwaki, Y.; Misawa, H.; Kawashima, K. Minireview: Divergent roles of alpha7 nicotinic acetylcholine receptors expressed on antigen-presenting cells and CD4(+) T cells in the regulation of T cell differentiation. Int. Immunopharmacol. 2020, 82, 106306. [Google Scholar] [CrossRef]
- Gault, J.; Robinson, M.; Berger, R.; Drebing, C.; Logel, J.; Hopkins, J.; Moore, T.; Jacobs, S.; Meriwether, J.; Choi, M.J.; et al. Genomic organization and partial duplication of the human alpha7 neuronal nicotinic acetylcholine receptor gene (CHRNA7). Genomics 1998, 52, 173–185. [Google Scholar] [CrossRef]
- Riley, B.; Williamson, M.; Collier, D.; Wilkie, H.; Makoff, A. A 3-Mb map of a large Segmental duplication overlapping the alpha7-nicotinic acetylcholine receptor gene (CHRNA7) at human 15q13-q14. Genomics 2002, 79, 197–209. [Google Scholar] [CrossRef]
- de Lucas-Cerrillo, A.M.; Maldifassi, M.C.; Arnalich, F.; Renart, J.; Atienza, G.; Serantes, R.; Cruces, J.; Sanchez-Pacheco, A.; Andres-Mateos, E.; Montiel, C. Function of partially duplicated human alpha7 nicotinic receptor subunit CHRFAM7A gene: Potential implications for the cholinergic anti-inflammatory response. J. Biol. Chem. 2011, 286, 594–606. [Google Scholar] [CrossRef]
- Araud, T.; Graw, S.; Berger, R.; Lee, M.; Neveu, E.; Bertrand, D.; Leonard, S. The chimeric gene CHRFAM7A, a partial duplication of the CHRNA7 gene, is a dominant negative regulator of alpha7*nAChR function. Biochem. Pharmacol. 2011, 82, 904–914. [Google Scholar] [CrossRef]
- Lasala, M.; Corradi, J.; Bruzzone, A.; Esandi, M.D.; Bouzat, C. A human-specific, truncated 7 nicotinic receptor subunit assembles with full-length 7 and forms functional receptors with different stoichiometries. J. Biol. Chem. 2018, 293, 10707–10717. [Google Scholar] [CrossRef]
- Sinkus, M.L.; Graw, S.; Freedman, R.; Ross, R.G.; Lester, H.A.; Leonard, S. The human CHRNA7 and CHRFAM7A genes: A review of the genetics, regulation, and function. Neuropharmacology 2015, 96, 274–288. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, C.; Indersmitten, T.; Freedman, R.; Leonard, S.; Lester, H.A. The Duplicated alpha 7 Subunits Assemble and Form Functional Nicotinic Receptors with the Full-length alpha 7. J. Biol. Chem. 2014, 289, 26451–26463. [Google Scholar] [CrossRef]
- Costantini, T.W.; Dang, X.; Yurchyshyna, M.V.; Coimbra, R.; Eliceiri, B.P.; Baird, A. A Human-Specific alpha7-Nicotinic Acetylcholine Receptor Gene in Human Leukocytes: Identification, Regulation and the Consequences of CHRFAM7A Expression. Mol. Med. 2015, 21, 323–336. [Google Scholar] [CrossRef]
- Bertrand, D.; Lee, C.H.; Flood, D.; Marger, F.; Donnelly-Roberts, D. Therapeutic Potential of alpha7 Nicotinic Acetylcholine Receptors. Pharmacol. Rev. 2015, 67, 1025–1073. [Google Scholar] [CrossRef]
- Bertrand, D.; Terry, A.V., Jr. The wonderland of neuronal nicotinic acetylcholine receptors. Biochem. Pharmacol. 2018, 151, 214–225. [Google Scholar] [CrossRef]
- Kitagawa, H.; Takenouchi, T.; Azuma, R.; Wesnes, K.A.; Kramer, W.G.; Clody, D.E.; Burnett, A.L. Safety, pharmacokinetics, and effects on cognitive function of multiple doses of GTS-21 in healthy, male volunteers. Neuropsychopharmacology 2003, 28, 542–551. [Google Scholar] [CrossRef]
- Kabbani, N.; Nordman, J.C.; Corgiat, B.A.; Veltri, D.P.; Shehu, A.; Seymour, V.A.; Adams, D.J. Are nicotinic acetylcholine receptors coupled to G proteins? Bioessays 2013, 35, 1025–1034. [Google Scholar] [CrossRef]
- King, J.R.; Kabbani, N. Alpha 7 nicotinic receptor coupling to heterotrimeric G proteins modulates RhoA activation, cytoskeletal motility, and structural growth. J. Neurochem. 2016, 138, 532–545. [Google Scholar] [CrossRef]
- King, J.R.; Nordman, J.C.; Bridges, S.P.; Lin, M.K.; Kabbani, N. Identification and characterization of a G protein-binding cluster in alpha7 nicotinic acetylcholine receptors. J. Biol. Chem. 2015, 290, 20060–20070. [Google Scholar] [CrossRef]
- Nordman, J.C.; Kabbani, N. An interaction between alpha7 nicotinic receptors and a G-protein pathway complex regulates neurite growth in neural cells. J. Cell Sci. 2012, 125, 5502–5513. [Google Scholar] [CrossRef]
- de Jonge, W.J.; Ulloa, L. The alpha7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. Br. J. Pharmacol. 2007, 151, 915–929. [Google Scholar] [CrossRef]
- Mashimo, M.; Takeshima, S.; Okuyama, H.; Matsurida, A.; Murase, M.; Ono, S.; Kawashima, K.; Fujii, T. alpha7 nAChRs expressed on antigen presenting cells are insensitive to the conventional antagonists alpha-bungarotoxin and methyllycaconitine. Int. Immunopharmacol. 2020, 81, 106276. [Google Scholar] [CrossRef]
- Razani-Boroujerdi, S.; Boyd, R.T.; Davila-Garcia, M.I.; Nandi, J.S.; Mishra, N.C.; Singh, S.P.; Pena-Philippides, J.C.; Langley, R.; Sopori, M.L. T cells express alpha7-nicotinic acetylcholine receptor subunits that require a functional TCR and leukocyte-specific protein tyrosine kinase for nicotine-induced Ca2+ response. J. Immunol. 2007, 179, 2889–2898. [Google Scholar] [CrossRef]
- Benfante, R.; Antonini, R.A.; De Pizzol, M.; Gotti, C.; Clementi, F.; Locati, M.; Fornasari, D. Expression of the alpha7 nAChR subunit duplicate form (CHRFAM7A) is down-regulated in the monocytic cell line THP-1 on treatment with LPS. J. Neuroimmunol. 2011, 230, 74–84. [Google Scholar] [CrossRef]
- Villiger, Y.; Szanto, I.; Jaconi, S.; Blanchet, C.; Buisson, B.; Krause, K.H.; Bertrand, D.; Romand, J.A. Expression of an alpha7 duplicate nicotinic acetylcholine receptor-related protein in human leukocytes. J. Neuroimmunol. 2002, 126, 86–98. [Google Scholar] [CrossRef]
- Fujii, T.; Tsuchiya, T.; Yamada, S.; Fujimoto, K.; Suzuki, T.; Kasahara, T.; Kawashima, K. Localization and synthesis of acetylcholine in human leukemic T cell lines. J. Neurosci. Res. 1996, 44, 66–72. [Google Scholar] [CrossRef]
- Fujii, T.; Watanabe, Y.; Fujimoto, K.; Kawashima, K. Expression of acetylcholine in lymphocytes and modulation of an independent lymphocytic cholinergic activity by immunological stimulation. Biog. Amine 2003, 17, 373–386. [Google Scholar] [CrossRef]
- Fujii, T.; Watanabe, Y.; Inoue, T.; Kawashima, K. Upregulation of mRNA encoding the M5 muscarinic acetylcholine receptor in human T- and B-lymphocytes during immunological responses. Neurochem. Res. 2003, 28, 423–429. [Google Scholar] [CrossRef]
- Mashimo, M.; Iwasaki, Y.; Inoue, S.; Saito, S.; Kawashima, K.; Fujii, T. Acetylcholine released from T cells regulates intracellular Ca2+, IL-2 secretion and T cell proliferation through nicotinic acetylcholine receptor. Life Sci. 2017, 172, 13–18. [Google Scholar] [CrossRef]
- Qian, J.; Galitovskiy, V.; Chernyavsky, A.I.; Marchenko, S.; Grando, S.A. Plasticity of the murine spleen T-cell cholinergic receptors and their role in in vitro differentiation of naive CD4 T cells toward the Th1, Th2 and Th17 lineages. Genes. Immun. 2011, 12, 222–230. [Google Scholar] [CrossRef]
- Dang, X.; Eliceiri, B.P.; Baird, A.; Costantini, T.W. CHRFAM7A: A human-specific alpha7-nicotinic acetylcholine receptor gene shows differential responsiveness of human intestinal epithelial cells to LPS. FASEB J. 2015, 29, 2292–2302. [Google Scholar] [CrossRef][Green Version]
- King, J.R.; Ullah, A.; Bak, E.; Jafri, M.S.; Kabbani, N. Ionotropic and metabotropic mechanisms of allosteric modulation of alpha7 nicotinic receptor intracellular calcium. Mol. Pharmacol. 2018, 93, 601–611. [Google Scholar] [CrossRef]
- de Jonge, W.J.; van der Zanden, E.P.; The, F.O.; Bijlsma, M.F.; van Westerloo, D.J.; Bennink, R.J.; Berthoud, H.R.; Uematsu, S.; Akira, S.; van den Wijngaard, R.M.; et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat. Immunol. 2005, 6, 844–851. [Google Scholar] [CrossRef]
- Grau, V.; Richter, K.; Hone, A.J.; McIntosh, J.M. Conopeptides [V11L;V16D]ArIB and RgIA4: Powerful tools for the identification of novel nicotinic acetylcholine receptors in monocytes. Front. Pharmacol. 2018, 9, 1499. [Google Scholar] [CrossRef]
- Kabbani, N.; Nichols, R.A. Beyond the channel: Metabotropic signaling by nicotinic receptors. Trends Pharmacol. Sci. 2018, 39, 354–366. [Google Scholar] [CrossRef]
- Treinin, M.; Papke, R.L.; Nizri, E.; Ben-David, Y.; Mizrachi, T.; Brenner, T. Role of the alpha7 Nicotinic Acetylcholine Receptor and RIC-3 in the Cholinergic Anti-inflammatory Pathway. Cent. Nerv. Syst. Agents Med. Chem. 2017, 17, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Kan, H.; Ruan, Y.; Malik, K.U. Localization and characterization of the subtypes(s) of muscarinic receptor involved in prostacyclin synthesis in rabbit heart. J. Pharmacol. Exp. Ther. 1996, 276, 934–941. [Google Scholar]
- Nizri, E.; Irony-Tur-Sinai, M.; Lory, O.; Orr-Urtreger, A.; Lavi, E.; Brenner, T. Activation of the cholinergic anti-inflammatory system by nicotine attenuates neuroinflammation via suppression of Th1 and Th17 responses. J. Immunol. 2009, 183, 6681–6688. [Google Scholar] [CrossRef]
- Garg, B.K.; Loring, R.H. GTS-21 has cell-specific anti-inflammatory effects independent of alpha7 nicotinic acetylcholine receptors. PLoS ONE 2019, 14, e0214942. [Google Scholar] [CrossRef]
- Papke, R.L.; Lindstrom, J.M. Nicotinic acetylcholine receptors: Conventional and unconventional ligands and signaling. Neuropharmacology 2020, 168, 108021. [Google Scholar] [CrossRef] [PubMed]
- Báez-Pagán, C.A.; Delgado-Vélez, M.; Lasalde-Dominicci, J.A. Activation of the Macrophage alpha7 Nicotinic Acetylcholine Receptor and Control of Inflammation. J. Neuroimmune Pharmacol. 2015, 10, 468–476. [Google Scholar] [CrossRef]
- Corradi, J.; Bouzat, C. Understanding the bases of function and modulation of alpha7 nicotinic receptors: Implications for drug discovery. Mol. Pharmacol. 2016, 90, 288–299. [Google Scholar] [CrossRef]
- Miyazaki, T.; Kawahara, A.; Fujii, H.; Nakagawa, Y.; Minami, Y.; Liu, Z.J.; Oishi, I.; Silvennoinen, O.; Witthuhn, B.A.; Ihle, J.N.; et al. Functional activation of Jak1 and Jak3 by selective association with IL-2 receptor subunits. Science 1994, 266, 1045–1047. [Google Scholar] [CrossRef]
- Eto, D.; Lao, C.; DiToro, D.; Barnett, B.; Escobar, T.C.; Kageyama, R.; Yusuf, I.; Crotty, S. IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (Tfh) differentiation. PLoS ONE 2011, 6, e17739. [Google Scholar] [CrossRef] [PubMed]
- Austin, J.W.; Lu, P.; Majumder, P.; Ahmed, R.; Boss, J.M. STAT3, STAT4, NFATc1, and CTCF regulate PD-1 through multiple novel regulatory regions in murine T cells. J. Immunol. 2014, 192, 4876–4886. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, J.J.; Murray, P.J. Cytokine signaling modules in inflammatory responses. Immunity 2008, 28, 477–487. [Google Scholar] [CrossRef]
- Stritesky, G.L.; Muthukrishnan, R.; Sehra, S.; Goswami, R.; Pham, D.; Travers, J.; Nguyen, E.T.; Levy, D.E.; Kaplan, M.H. The transcription factor STAT3 is required for T helper 2 cell development. Immunity 2011, 34, 39–49. [Google Scholar] [CrossRef]
- Yang, X.O.; Panopoulos, A.D.; Nurieva, R.; Chang, S.H.; Wang, D.; Watowich, S.S.; Dong, C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J. Biol. Chem. 2007, 282, 9358–9363. [Google Scholar] [CrossRef]
- Cossarizza, A.; Chang, H.D.; Radbruch, A.; Acs, A.; Adam, D.; Adam-Klages, S.; Agace, W.W.; Aghaeepour, N.; Akdis, M.; Allez, M.; et al. Guidelines for the use of flow cytometry and cell sorting in immunological studies (second edition). Eur. J. Immunol. 2019, 49, 1457–1973. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).