Practices, Potential, and Perspectives for Detecting Predisease Using Raman Spectroscopy
Abstract
1. Introduction
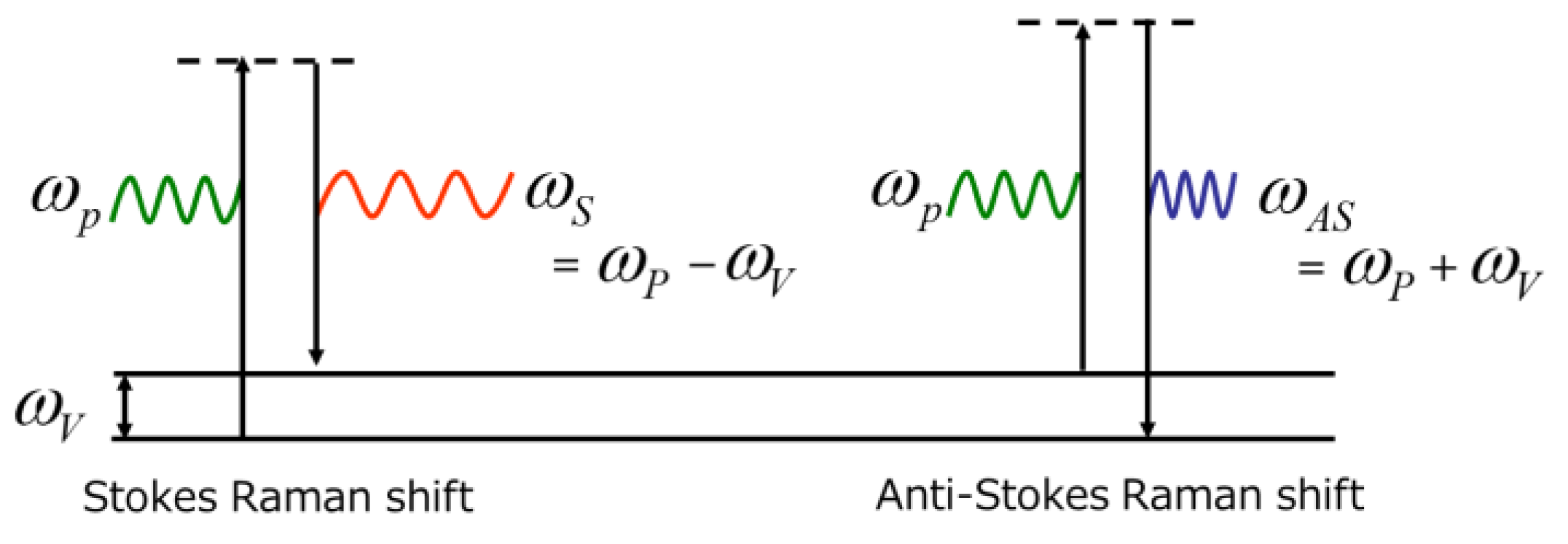
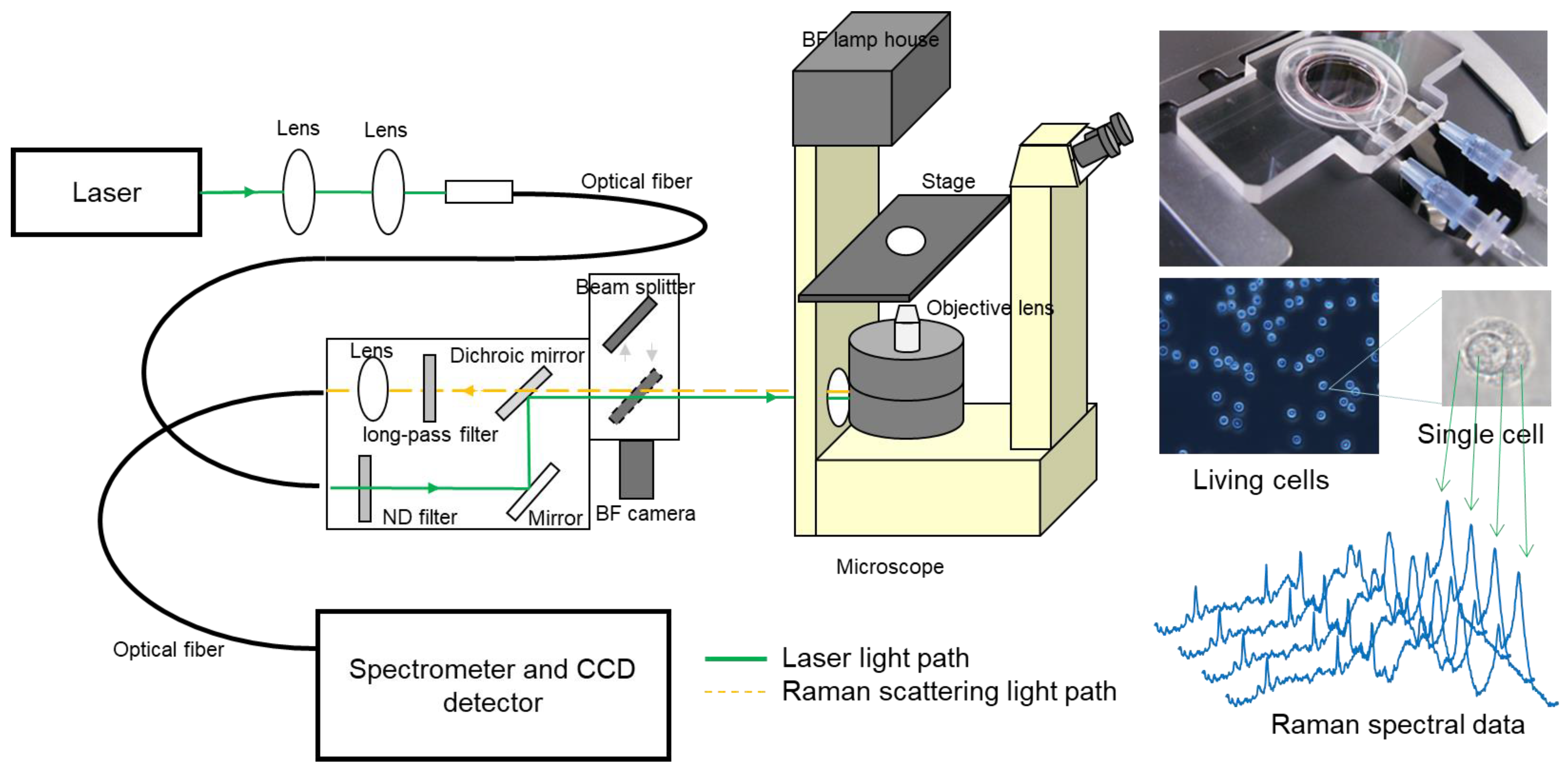
2. Principle of Raman Scattering and Instrumentation
3. Technical Breakthroughs toward Biomedical Applications
4. Molecular Fingerprints Possibly Associated with Diseases on the Raman Spectrum Obtained from Cells and Tissues
5. Recent Advances and Limitations in Clinical Applications of Raman Spectroscopy
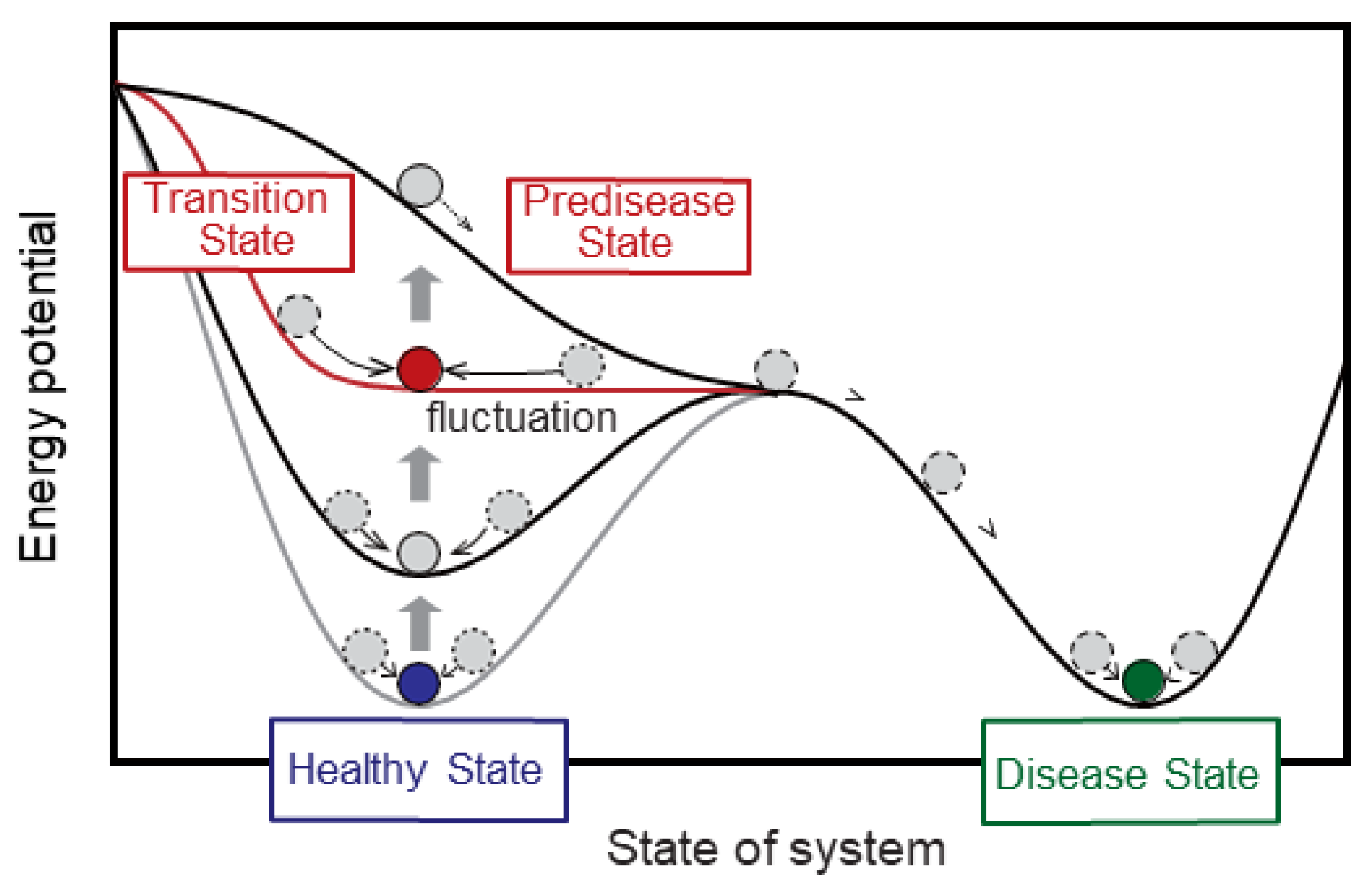
6. From “Discriminant Analysis” to “Transition-State Analysis”
7. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raman, C.V.; Krishnan, K.S. A New Type of Secondary Radiation. Nature 1928, 121, 501–502. [Google Scholar] [CrossRef]
- Landsberg, G.S.; Mandelstam, L.I. Über die Lichtzerstreuung in Kristallen. Z. Für Phys. 1928, 50, 769–780. [Google Scholar] [CrossRef]
- Shipp, D.W.; Sinjab, F.; Notingher, I. Raman spectroscopy: Techniques and applications in the life sciences. Adv. Opt. Photonics 2017, 9, 315–428. [Google Scholar] [CrossRef]
- Ozaki, Y.; Mizuno, A.; Itoh, K.; Iriyama, K. Inter- and intramolecular disulfide bond formation and related structural changes in the lens proteins. A Raman spectroscopic study in vivo of lens aging. J. Biol. Chem. 1987, 262, 15545–15551. [Google Scholar] [CrossRef]
- Bot, A.C.; Huizinga, A.; de Mul, F.F.; Vrensen, G.F.; Greve, J. Raman microspectroscopy of fixed rabbit and human lenses and lens slices: New potentialities. Exp. Eye Res. 1989, 49, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.H.; Isner, J.M.; Gauthier, T.; Nakagawa, K.; Cerio, F.; Hanlon, E.; Gaffney, E.; Rouse, E.; DeJesus, S. Spectroscopic characterization of cardiovascular tissue. Lasers Surg. Med. 1988, 8, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Bakker Schut, T.C.; Witjes, M.J.; Sterenborg, H.J.; Speelman, O.C.; Roodenburg, J.L.; Marple, E.T.; Bruining, H.A.; Puppels, G.J. In vivo detection of dysplastic tissue by Raman spectroscopy. Anal. Chem. 2000, 72, 6010–6018. [Google Scholar] [CrossRef]
- Haka, A.S.; Shafer-Peltier, K.E.; Fitzmaurice, M.; Crowe, J.; Dasari, R.R.; Feld, M.S. Identifying microcalcifications in benign and malignant breast lesions by probing differences in their chemical composition using Raman spectroscopy. Cancer Res. 2002, 62, 5375–5380. [Google Scholar] [PubMed]
- Yamazaki, H.; Kaminaka, S.; Kohda, E.; Mukai, M.; Hamaguchi, H.O. The diagnosis of lung cancer using 1064-nm excited near-infrared multichannel Raman spectroscopy. Radiat. Med. 2003, 21, 1–6. [Google Scholar]
- Huang, Z.; McWilliams, A.; Lui, H.; McLean, D.I.; Lam, S.; Zeng, H. Near-infrared Raman spectroscopy for optical diagnosis of lung cancer. Int. J. Cancer 2003, 107, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Koljenović, S.; Bakker Schut, T.C.; van Meerbeeck, J.P.; Maat, A.P.; Burgers, S.A.; Zondervan, P.E.; Kros, J.M.; Puppels, G.J. Raman microspectroscopic mapping studies of human bronchial tissue. J. Biomed. Opt. 2004, 9, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Chou, I.H.; Benford, M.; Beier, H.T.; Coté, G.L.; Wang, M.; Jing, N.; Kameoka, J.; Good, T.A. Nanofluidic biosensing for beta-amyloid detection using surface enhanced Raman spectroscopy. Nano Lett. 2008, 8, 1729–1735. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, E.B.; Manoharan, R.; Koo, T.W.; Shafer, K.E.; Motz, J.T.; Fitzmaurice, M.; Kramer, J.R.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Prospects for in vivo Raman spectroscopy. Phys. Med. Biol. 2000, 45, R1–R59. [Google Scholar] [CrossRef] [PubMed]
- Juszczak, L.J. Comparative vibrational spectroscopy of intracellular tau and extracellular collagen I reveals parallels of gelation and fibrillar structure. J. Biol. Chem. 2004, 279, 7395–7404. [Google Scholar] [CrossRef]
- Motz, J.T.; Gandhi, S.J.; Scepanovic, O.R.; Haka, A.S.; Kramer, J.R.; Dasari, R.R.; Feld, M.S. Real-time Raman system for in vivo disease diagnosis. J. Biomed. Opt. 2005, 10, 031113. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, G.V.; Silveira, L.; Martin, A.A.; Zângaro, R.A.; Pacheco, M.T.; Chavantes, M.C.; Pasqualucci, C.A. Raman spectroscopy study of atherosclerosis in human carotid artery. J. Biomed. Opt. 2005, 10, 031117. [Google Scholar] [CrossRef]
- Sćepanović, O.R.; Fitzmaurice, M.; Gardecki, J.A.; Angheloiu, G.O.; Awasthi, S.; Motz, J.T.; Kramer, J.R.; Dasari, R.R.; Feld, M.S. Detection of morphological markers of vulnerable atherosclerotic plaque using multimodal spectroscopy. J. Biomed. Opt. 2006, 11, 021007. [Google Scholar] [CrossRef]
- Egawa, M. Raman microscopy for skin evaluation. Analyst 2021, 146, 1142–1150. [Google Scholar] [CrossRef]
- Noothalapati, H.; Iwasaki, K.; Yamamoto, T. Non-invasive diagnosis of colorectal cancer by Raman spectroscopy: Recent developments in liquid biopsy and endoscopy approaches. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 258, 119818. [Google Scholar] [CrossRef] [PubMed]
- Balasundaram, G.; Krafft, C.; Zhang, R.; Dev, K.; Bi, R.; Moothanchery, M.; Popp, J.; Olivo, M. Biophotonic technologies for assessment of breast tumor surgical margins—A review. J. Biophotonics 2021, 14, e202000280. [Google Scholar] [CrossRef]
- Haruki, T.; Yonezawa, S.; Koizumi, K.; Yoshida, Y.; Watanabe, T.M.; Fujita, H.; Oshima, Y.; Oku, M.; Taketani, A.; Yamazaki, M.; et al. Application of the Dynamical Network Biomarker Theory to Raman Spectra. Biomolecules 2022, 12, 1730. [Google Scholar] [CrossRef]
- Sakaue, T.; Hamaguchi, M.; Aono, J.; Nakashiro, K.I.; Shikata, F.; Kawakami, N.; Oshima, Y.; Kurata, M.; Nanba, D.; Masumoto, J.; et al. Valve Interstitial Cell-Specific Cyclooxygenase-1 Associated with Calcification of Aortic Valves. Ann. Thorac. Surg. 2020, 110, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Akagi, Y.; Mori, N.; Kawamura, T.; Takayama, Y.; Kida, Y.S. Non-invasive cell classification using the Paint Raman Express Spectroscopy System (PRESS). Sci. Rep. 2021, 11, 8818. [Google Scholar] [CrossRef] [PubMed]
- Asaoka, R.; Kiyomatsu, H.; Miura, H.; Jono, A.; Kinoshita, T.; Takao, M.; Katagiri, T.; Oshima, Y. Prognostic potential and pathological validation of a diagnostic application using Raman spectroscopy in the characterization of degenerative changes in the cartilage of the humeral head. J. Biomed. Opt. 2022, 27, 115002. [Google Scholar] [CrossRef] [PubMed]
- Kneipp, K.; Kneipp, H. Single Molecule Raman Scattering. Appl. Spectrosc. 2006, 60, 322A–334A. [Google Scholar] [CrossRef]
- Puppels, G.J.; de Mul, F.F.; Otto, C.; Greve, J.; Robert-Nicoud, M.; Arndt-Jovin, D.J.; Jovin, T.M. Studying single living cells and chromosomes by confocal Raman microspectroscopy. Nature 1990, 347, 301–303. [Google Scholar] [CrossRef]
- Puppels, G.J.; Garritsen, H.S.; Segers-Nolten, G.M.; de Mul, F.F.; Greve, J. Raman microspectroscopic approach to the study of human granulocytes. Biophys. J. 1991, 60, 1046–1056. [Google Scholar] [CrossRef]
- Baraga, J.J.; Feld, M.S.; Rava, R.P. In situ optical histochemistry of human artery using near infrared Fourier transform Raman spectroscopy. Proc. Natl. Acad. Sci. USA 1992, 89, 3473–3477. [Google Scholar] [CrossRef]
- Shim, M.G.; Song, L.M.; Marcon, N.E.; Wilson, B.C. In vivo near-infrared Raman spectroscopy: Demonstration of feasibility during clinical gastrointestinal endoscopy. Photochem. Photobiol. 2000, 72, 146–150. [Google Scholar]
- Molckovsky, A.; Song, L.M.; Shim, M.G.; Marcon, N.E.; Wilson, B.C. Diagnostic potential of near-infrared Raman spectroscopy in the colon: Differentiating adenomatous from hyperplastic polyps. Gastrointest. Endosc. 2003, 57, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Haka, A.S.; Volynskaya, Z.; Gardecki, J.A.; Nazemi, J.; Lyons, J.; Hicks, D.; Fitzmaurice, M.; Dasari, R.R.; Crowe, J.P.; Feld, M.S. In vivo margin assessment during partial mastectomy breast surgery using raman spectroscopy. Cancer Res. 2006, 66, 3317–3322. [Google Scholar] [CrossRef] [PubMed]
- Oshima, Y.; Sato, H.; Zaghloul, A.; Foulks, G.N.; Yappert, M.C.; Borchman, D. Characterization of human meibum lipid using raman spectroscopy. Curr. Eye Res. 2009, 34, 824–835. [Google Scholar] [CrossRef] [PubMed]
- Hamada, K.; Fujita, K.; Smith, N.I.; Kobayashi, M.; Inouye, Y.; Kawata, S. Raman microscopy for dynamic molecular imaging of living cells. J. Biomed. Opt. 2008, 13, 044027. [Google Scholar] [CrossRef] [PubMed]
- Saar, B.G.; Freudiger, C.W.; Reichman, J.; Stanley, C.M.; Holtom, G.R.; Xie, X.S. Video-rate molecular imaging in vivo with stimulated Raman scattering. Science 2010, 330, 1368–1370. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, Y.; Kitagawa, Y.; Sumimura, K.; Nishizawa, N.; Umemura, W.; Kajiyama, S.; Fukui, K.; Itoh, K. Stimulated Raman scattering microscope with shot noise limited sensitivity using subharmonically synchronized laser pulses. Opt. Express 2010, 18, 13708–13719. [Google Scholar] [CrossRef]
- Satoh, S.; Otsuka, Y.; Ozeki, Y.; Itoh, K.; Hashiguchi, A.; Yamazaki, K.; Hashimoto, H.; Sakamoto, M. Label-free visualization of acetaminophen-induced liver injury by high-speed stimulated Raman scattering spectral microscopy and multivariate image analysis. Pathol. Int. 2014, 64, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Egawa, M.; Tokunaga, K.; Hosoi, J.; Iwanaga, S.; Ozeki, Y. In situ visualization of intracellular morphology of epidermal cells using stimulated Raman scattering microscopy. J. Biomed. Opt. 2016, 21, 86017. [Google Scholar] [CrossRef] [PubMed]
- Minamikawa, T.; Niioka, H.; Araki, T.; Hashimoto, M. Real-time imaging of laser-induced membrane disruption of a living cell observed with multifocus coherent anti-Stokes Raman scattering microscopy. J. Biomed. Opt. 2011, 16, 021111. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Segawa, H.; Okuno, M.; Kano, H.; Hamaguchi, H.O.; Haraguchi, T.; Hiraoka, Y.; Hasui, S.; Yamaguchi, T.; Hirose, F.; et al. Active involvement of micro-lipid droplets and lipid-droplet-associated proteins in hormone-stimulated lipolysis in adipocytes. J. Cell Sci. 2012, 125 Pt 24, 6127–6136. [Google Scholar] [CrossRef]
- Huff, T.B.; Shi, Y.; Sun, W.; Wu, W.; Shi, R.; Cheng, J.X. Real-time CARS imaging reveals a calpain-dependent pathway for paranodal myelin retraction during high-frequency stimulation. PLoS ONE 2011, 6, e17176. [Google Scholar] [CrossRef]
- Imitola, J.; Côté, D.; Rasmussen, S.; Xie, X.S.; Liu, Y.; Chitnis, T.; Sidman, R.L.; Lin, C.P.; Khoury, S.J. Multimodal coherent anti-Stokes Raman scattering microscopy reveals microglia-associated myelin and axonal dysfunction in multiple sclerosis-like lesions in mice. J. Biomed Opt. 2011, 16, 021109. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Fung, A.A.; Zhou, A. Advances in stimulated Raman scattering imaging for tissues and animals. Quant. Imaging Med. Surg. 2021, 11, 1078–1101. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Kobayashi, K.; Wakisaka, Y.; Deng, D.; Tanaka, S.; Huang, C.J.; Lei, C.; Sun, C.W.; Liu, H.; Fujiwaki, Y.; et al. Label-free chemical imaging flow cytometry by high-speed multicolor stimulated Raman scattering. Proc. Natl. Acad. Sci. USA 2019, 116, 15842–15848. [Google Scholar] [CrossRef]
- Nitta, N.; Iino, T.; Isozaki, A.; Yamagishi, M.; Kitahama, Y.; Sakuma, S.; Suzuki, Y.; Tezuka, H.; Oikawa, M.; Arai, F.; et al. Raman image-activated cell sorting. Nat. Commun. 2020, 11, 3452. [Google Scholar] [CrossRef] [PubMed]
- Oshima, Y.; Shinzawa, H.; Takenaka, T.; Furihata, C.; Sato, H. Discrimination analysis of human lung cancer cells associated with histological type and malignancy using Raman spectroscopy. J. Biomed. Opt. 2010, 15, 017009. [Google Scholar] [CrossRef]
- Bergholt, M.S.; Zheng, W.; Lin, K.; Ho, K.Y.; Teh, M.; Yeoh, K.G.; Yan So, J.B.; Huang, Z. In vivo diagnosis of gastric cancer using Raman endoscopy and ant colony optimization techniques. Int. J. Cancer 2011, 128, 2673–2680. [Google Scholar] [CrossRef] [PubMed]
- Duraipandian, S.; Sylvest Bergholt, M.; Zheng, W.; Yu Ho, K.; Teh, M.; Guan Yeoh, K.; Bok Yan So, J.; Shabbir, A.; Huang, Z. Real-time Raman spectroscopy for in vivo, online gastric cancer diagnosis during clinical endoscopic examination. J. Biomed. Opt. 2012, 17, 081418. [Google Scholar] [CrossRef] [PubMed]
- Lui, H.; Zhao, J.; McLean, D.; Zeng, H. Real-time Raman spectroscopy for in vivo skin cancer diagnosis. Cancer Res. 2012, 72, 2491–2500. [Google Scholar] [CrossRef]
- Ishigaki, M.; Maeda, Y.; Taketani, A.; Andriana, B.B.; Ishihara, R.; Wongravee, K.; Ozaki, Y.; Sato, H. Diagnosis of early-stage esophageal cancer by Raman spectroscopy and chemometric techniques. Analyst 2016, 141, 1027–1033. [Google Scholar] [CrossRef]
- Féré, M.; Gobinet, C.; Liu, L.H.; Beljebbar, A.; Untereiner, V.; Gheldof, D.; Chollat, M.; Klossa, J.; Chatelain, B.; Piot, O. Implementation of a classification strategy of Raman data collected in different clinical conditions: Application to the diagnosis of chronic lymphocytic leukemia. Anal. Bioanal Chem. 2020, 412, 949–962. [Google Scholar] [CrossRef]
- Ma, D.; Shang, L.; Tang, J.; Bao, Y.; Fu, J.; Yin, J. Classifying breast cancer tissue by Raman spectroscopy with one-dimensional convolutional neural network. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 256, 119732. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, Z.; Chen, Q.; Ramos, A.; Zhang, J.; Boudreaux, J.P.; Thiagarajan, R.; Bren-Mattison, Y.; Dunham, M.E.; McWhorter, A.J.; et al. Detection of pancreatic cancer by convolutional-neural-network-assisted spontaneous Raman spectroscopy with critical feature visualization. Neural Netw. 2021, 144, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Bouzy, P.; O’Grady, S.; Madupalli, H.; Tecklenburg, M.; Rogers, K.; Palombo, F.; Morgan, M.P.; Stone, N. A time-course Raman spectroscopic analysis of spontaneous in vitro microcalcifications in a breast cancer cell line. Lab. Investig. 2021, 101, 1267–1280. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Oshima, Y.; Etoh, T.; Kaisyakuji, Y.; Tojigamori, M.; Ohno, Y.; Shiraishi, N.; Inomata, M. Label-free detection of human enteric nerve system using Raman spectroscopy: A pilot study for diagnosis of Hirschsprung disease. J. Pediatr Surg. 2021, 56, 1150–1156. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, R.P.; Falcão, E.T.; Pasqualucci, C.A.; Silveira, L., Jr. Use of Raman spectroscopy to evaluate the biochemical composition of normal and tumoral human brain tissues for diagnosis. Lasers Med. Sci. 2022, 37, 121–133. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, P.; Wang, H.; Zheng, B.; Sun, L.; Zhang, D.; Fan, J. Raman Spectrum-Based Diagnosis Strategy for Bladder Tumor. Urol. Int. 2022, 106, 109–115. [Google Scholar] [CrossRef]
- Shaikh, R.; Daniel, A.; Lyng, F.M. Raman Spectroscopy for Early Detection of Cervical Cancer, a Global Women’s Health Issue-A Review. Molecules 2023, 28, 2502. [Google Scholar] [CrossRef]
- Nicolson, F.; Kircher, M.F.; Stone, N.; Matousek, P. Spatially offset Raman spectroscopy for biomedical applications. Chem. Soc. Rev. 2021, 50, 556–568. [Google Scholar] [CrossRef]
- Hanna, K.; Krzoska, E.; Shaaban, A.M.; Muirhead, D.; Abu-Eid, R.; Speirs, V. Raman spectroscopy: Current applications in breast cancer diagnosis, challenges and future prospects. Br. J. Cancer 2022, 126, 1125–1139. [Google Scholar]
- Zhang, Y.; Ren, L.; Wang, Q.; Wen, Z.; Liu, C.; Ding, Y. Raman Spectroscopy: A Potential Diagnostic Tool for Oral Diseases. Front. Cell Infect. Microbiol. 2022, 12, 775236. [Google Scholar]
- Liu, K.; Zhao, Q.; Li, B.; Zhao, X. Raman Spectroscopy: A Novel Technology for Gastric Cancer Diagnosis. Front. Bioeng. Biotechnol. 2022, 10, 856591. [Google Scholar] [PubMed]
- Huang, L.; Sun, H.; Sun, L.; Shi, K.; Chen, Y.; Ren, X.; Ge, Y.; Jiang, D.; Liu, X.; Knoll, W.; et al. Rapid, label-free histopathological diagnosis of liver cancer based on Raman spectroscopy and deep learning. Nat. Commun. 2023, 14, 48. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Y.; Li, P.; Zhang, Y.; Du, L.; Wang, Y.; Zhang, C.; Wang, C. Exosome detection via surface-enhanced Raman spectroscopy for cancer diagnosis. Acta Biomater. 2022, 144, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Gao, X.; Xu, C.; Liu, D. SERS Tags for Biomedical Detection and Bioimaging. Theranostics 2022, 12, 1870–1903. [Google Scholar] [CrossRef] [PubMed]
- Fornasaro, S.; Sergo, V.; Bonifacio, A. The key role of ergothioneine in label-free surface-enhanced Raman scattering spectra of biofluids: A retrospective re-assessment of the literature. FEBS Lett. 2022, 596, 1348–1355. [Google Scholar] [CrossRef] [PubMed]
- Dey, P.; Blakey, I.; Stone, N. Diagnostic prospects and preclinical development of optical technologies using gold nanostructure contrast agents to boost endogenous tissue contrast. Chem. Sci. 2020, 11, 8671–8685. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Dong, C.; Zhang, X.; Wu, W.; Chen, C.; Ma, B.; Chen, F.; Chen, C.; Lv, X. Rapid identification of papillary thyroid carcinoma and papillary microcarcinoma based on serum Raman spectroscopy combined with machine learning models. Photodiagn. Photodyn. Ther. 2022, 37, 102647. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Oh, J.; Lee, K.; Cho, M.; Paulson, B.; Kim, J.K. Diagnosis of Ischemic Renal Failure Using Surface-Enhanced Raman Spectroscopy and a Machine Learning Algorithm. Anal. Chem. 2022, 94, 17477–17484. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Zhang, G.; Yang, L.; Liu, B.; Zeng, H.; Xue, Q.; Liu, D.; Zheng, Q.; Liu, Y. High-Precision Intelligent Cancer Diagnosis Method: 2D Raman Figures Combined with Deep Learning. Anal. Chem. 2022, 94, 6491–6501. [Google Scholar] [CrossRef]
- Blake, N.; Gaifulina, R.; Griffin, L.D.; Bell, I.M.; Thomas, G.M.H. Machine Learning of Raman Spectroscopy Data for Classifying Cancers: A Review of the Recent Literature. Diagnostics 2022, 12, 1491. [Google Scholar] [CrossRef]
- Chen, M.; Feng, X.; Fox, M.C.; Reichenberg, J.S.; Lopes, F.C.P.S.; Sebastian, K.R.; Markey, M.K.; Tunnell, J.W. Deep learning on reflectance confocal microscopy improves Raman spectral diagnosis of basal cell carcinoma. J. Biomed. Opt. 2022, 27, 065004. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Liang, H.; Li, Q.; Wang, J.; Liu, J.; Zhang, Y.; Ru, Y.; Zhou, Y. Raman spectroscopy differ leukemic cells from their healthy counterparts and screen biomarkers in acute leukemia. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 281, 121558. [Google Scholar] [CrossRef] [PubMed]
- Cherkasova, O.P.; Nazarov, M.M.; Sapozhnikov, D.A.; Man’kova, A.A.; Fedulova, E.V.; Volodin, V.A.; Minaeva, V.A.; Minaev, B.F.; Baryshnikov, G.V. Vibrational spectra of corticosteroid hormones in the terahertz range. In Laser Applications in Life Sciences; SPIE Proceedings; SPIE: Oulu, Finland, 2010; Volume 7376, p. 73760P. [Google Scholar]
- Minaeva, V.A.; Minaev, B.F.; Baryshnikov, G.V.; Surovtsev, N.V.; Cherkasova, O.P.; Tkachenko, L.I.; Karaush, N.N.; Stromylo, E.V. Temperature effects in low-frequency Raman spectra of corticosteroid hormones. Opt. Spectrosc. 2015, 118, 214–223. [Google Scholar] [CrossRef]
- Minaeva, V.A.; Cherkasova, O.; Minaev, B.F.; Baryshnikov, G.V.; Khmara, A.V. Features of terahertz adsorption and Raman scattering of mineralocorticoid hormones. Bull. Russ. Acad. Sci. Phys. 2015, 79, 1196–1201. [Google Scholar] [CrossRef]
- Minaeva, V.A.; Minaev, B.F.; Hovorun, D.M. Vibrational spectra of the steroid hormones, estradiol and estriol, calculated by density functional theory: The role of low-frequency vibrations. Ukr. Biokhim. Zh. 2008, 80, 82–95. [Google Scholar]
- Swain, R.J.; Jell, G.; Stevens, M.M. Non-invasive analysis of cell cycle dynamics in single living cells with Raman micro-spectroscopy. J. Cell Biochem. 2008, 104, 1427–1438. [Google Scholar] [CrossRef]
- Neugebauer, U.; Bocklitz, T.; Clement, J.H.; Krafft, C.; Popp, J. Towards detection and identification of circulating tumor cells using Raman spectroscopy. Analyst 2010, 135, 3178–3182. [Google Scholar] [CrossRef]
- Kumamoto, Y.; Taguchi, A.; Smith, N.I.; Kawata, S. Deep ultraviolet resonant Raman imaging of a cell. J. Biomed. Opt. 2012, 17, 076001. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.H.; Li, Y.C.; Chang, C.H.; Liu, C.; Yu, A.L.; Chen, C.H. Single nuclei Raman spectroscopy for drug evaluation. Anal. Chem. 2012, 84, 113–120. [Google Scholar] [CrossRef]
- Movasaghi, Z.; Rehaman, S.; Rehman, I.U. Raman Spectroscopy of biological Tissues. Appl. Spectrosc. Rev. 2007, 42, 493–541. [Google Scholar] [CrossRef]
- Harvey, T.J.; Faria, E.C.; Henderson, A.; Gazi, E.; Ward, A.D.; Clarke, N.W.; Brown, M.D.; Snook, R.D.; Gardner, P. Spectral discrimination of live prostate and bladder cancer cell lines using Raman optical tweezers. J. Biomed. Opt. 2008, 13, 064004. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wood, B.R.; Hammer, L.; Davis, L.; McNaughton, D. Raman microspectroscopy and imaging provides insights into heme aggregation and denaturation within human erythrocytes. J. Biomed. Opt. 2005, 10, 14005. [Google Scholar] [CrossRef] [PubMed]
- Ishigaki, M.; Kashiwagi, S.; Wakabayashi, S.; Hoshino, Y. In situ assessment of mitochondrial respiratory activity and lipid metabolism of mouse oocytes using resonance Raman spectroscopy. Analyst 2021, 146, 7265–7273. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Smith, N.I.; Palonpon, A.F.; Endo, H.; Kawata, S.; Sodeoka, M.; Fujita, K. Label-free Raman observation of cytochrome c dynamics during apoptosis. Proc. Natl. Acad. Sci. USA 2012, 109, 28–32. [Google Scholar] [CrossRef]
- Abramczyk, H.; Brozek-Pluska, B.; Kopeć, M. Double face of cytochrome c in cancers by Raman imaging. Sci. Rep. 2022, 12, 2120. [Google Scholar] [CrossRef] [PubMed]
- Haka, A.S.; Shafer-Peltier, K.E.; Fitzmaurice, M.; Crowe, J.; Dasari, R.R.; Feld, M.S. Diagnosing breast cancer by using Raman spectroscopy. Proc. Natl. Acad. Sci. USA 2005, 102, 12371–12376. [Google Scholar] [CrossRef]
- You, A.Y.F.; Bergholt, M.S.; St-Pierre, J.P.; Kit-Anan, W.; Pence, I.J.; Chester, A.H.; Yacoub, M.H.; Bertazzo, S.; Stevens, M.M. Raman spectroscopy imaging reveals interplay between atherosclerosis and medial calcification in the human aorta. Sci. Adv. 2017, 3, e1701156. [Google Scholar] [CrossRef]
- Molino, G.; Dalpozzi, A.; Ciapetti, G.; Lorusso, M.; Novara, C.; Cavallo, M.; Baldini, N.; Giorgis, F.; Fiorilli, S.; Vitale-Brovarone, C. Osteoporosis-related variations of trabecular bone properties of proximal human humeral heads at different scale lengths. J. Mech. Behav. Biomed. Mater. 2019, 100, 103373. [Google Scholar] [CrossRef]
- Falgayrac, G.; Farlay, D.; Ponçon, C.; Béhal, H.; Gardegaront, M.; Ammann, P.; Boivin, G.; Cortet, B. Bone matrix quality in paired iliac bone biopsies from postmenopausal women treated for 12 months with strontium ranelate or alendronate. Bone 2021, 153, 116107. [Google Scholar] [CrossRef] [PubMed]
- Ishimaru, Y.; Oshima, Y.; Imai, Y.; Iimura, T.; Takanezawa, S.; Hino, K.; Miura, H. Raman Spectroscopic Analysis to Detect Reduced Bone Quality after Sciatic Neurectomy in Mice. Molecules 2018, 23, 3081. [Google Scholar] [CrossRef]
- Kumar, R.; Grønhaug, K.M.; Afseth, N.K.; Isaksen, V.; de Lange Davies, C.; Drogset, J.O.; Lilledahl, M.B. Optical investigation of osteoarthritic human cartilage (ICRS grade) by confocal Raman spectroscopy: A pilot study. Anal. Bioanal. Chem. 2015, 407, 8067–8077. [Google Scholar] [CrossRef] [PubMed]
- Kroupa, K.R.; Wu, M.I.; Zhang, J.; Jensen, M.; Wong, W.; Engiles, J.B.; Schaer, T.P.; Grinstaff, M.W.; Snyder, B.D.; Bergholt, M.S.; et al. Raman needle arthroscopy for in vivo molecular assessment of cartilage. J. Orthop. Res. 2022, 40, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Jaswandkar, S.V.; Katti, K.S.; Kang, J.W.; So, P.T.C.; Paulmurugan, R.; Liepmann, D.; Venkatesan, R.; Katti, D.R. Label-free discrimination of tumorigenesis stages using in vitro prostate cancer bone metastasis model by Raman imaging. Sci. Rep. 2022, 12, 8050. [Google Scholar] [CrossRef] [PubMed]
- Fousková, M.; Vališ, J.; Synytsya, A.; Habartová, L.; Petrtýl, J.; Petruželka, L.; Setnička, V. In vivo Raman spectroscopy in the diagnostics of colon cancer. Analyst 2023, 148, 2518–2526. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Li, Y.; Liu, J.; Chen, C.; Wu, W.; Chen, C.; Lv, X.; Liang, F. H-CNN combined with tissue Raman spectroscopy for cervical cancer detection. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 291, 122339. [Google Scholar] [CrossRef]
- David, S.; Tran, T.; Dallaire, F.; Sheehy, G.; Azzi, F.; Trudel, D.; Tremblay, F.; Omeroglu, A.; Leblond, F.; Meterissian, S. In situ Raman spectroscopy and machine learning unveil biomolecular alterations in invasive breast cancer. J. Biomed. Opt. 2023, 28, 036009. [Google Scholar] [CrossRef]
- Lima, A.M.F.; Daniel, C.R.; Pacheco, M.T.T.; de Brito, P.L.; Silveira, L., Jr. Discrimination of leukemias and non-leukemic cancers in blood serum samples of children and adolescents using a Raman spectral model. Lasers Med. Sci. 2022, 38, 22. [Google Scholar] [CrossRef]
- Aihara, K.; Liu, R.; Koizumi, K.; Liu, X.; Chen, L. Dynamical network biomarkers: Theory and applications. Gene 2022, 808, 145997. [Google Scholar] [CrossRef]
- Chen, L.; Liu, R.; Liu, Z.-P.; Li, M.; Aihara, K. Detecting early-warning signals for sudden deterioration of complex diseases by dynamical network biomarkers. Sci. Rep. 2021, 2, 342. [Google Scholar] [CrossRef]
- Liu, R.; Wang, X.; Aihara, K.; Chen, L. Early diagnosis of complex diseases by molecular biomarkers, network biomarkers, and dynamical network biomarkers. Med. Res. Rev. 2013, 34, 455–478. [Google Scholar] [CrossRef]
- Koizumi, K.; Oku, M.; Hayashi, S.; Inujima, A.; Shibahara, N.; Chen, L.; Igarashi, Y.; Tobe, K.; Saito, S.; Kadowaki, M.; et al. Identifying pre-disease signals before metabolic syndrome in mice by dynamical network biomarkers. Sci. Rep. 2019, 9, 8767. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Aihara, K.; Chen, L. Dynamics-based data science in biology. Natl. Sci. Rev. 2021, 8, nwab029. [Google Scholar] [CrossRef] [PubMed]

| Target Disease | Sample Type (Modality) | Analytical Method | Reference |
|---|---|---|---|
| colon cancer | in vivo (endoscopy) | PCA, neural network | Shim et al., 2000 [29] |
| ex vivo (fiber probe) | PCA-LDA | Molchovsky et al., 2003 [30] | |
| in vivo (endoscopy) | PLS-DA | Belgholt et al., 2010 [46] | |
| in vivo (endoscopy) | PCA, DT, AdaBoost | Fousková et al., 2023 [95] | |
| lung cancer | ex vivo (microscopy) | Histogram | Yamazaki et al., 2003 [9] |
| in vivo (fiber probe) | intensity ratio | Huang et al., 2003 [10] | |
| in vitro (microscopy) | PCA | Oshima et al., 2010 [45] | |
| ex vivo (microscopy) | CNN | Qi et al., 2022 [69] | |
| breast cancer | ex vivo (microscopy) | PCA | Haka et al., 2002 [8] |
| in vivo (fiber probe) | x2 analysis | Haka et al., 2006 [31] | |
| ex vivo (microscopy) | CNN | Ma et al., 2021 [51] | |
| ex vivo (fiber probe) | SVM, Lasso | David et al., 2023 [97] | |
| esophageal cancer | ex vivo (fiber probe) | PLSR, SOMs, LDA | Ishigaki et al., 2016 [49] |
| bladder cancer | in vivo (fiber probe) | PC-GDA | Lui et al., 2012 [56] |
| skin cancer | ex vivo (microscopy) | ResNet50 | Chen et al., 2022 [71] |
| gastric cancer | in vivo (endoscopy) | PCA, PLS-DA | Duraipandian et al., 2012 [47] |
| brain tumor | ex vivo (microscopy) | PCA, PLS, LDA | Aguiar et al., 2022 [55] |
| liver cancer | ex vivo (microscopy) | CNN | Huang et al., 2023 [62] |
| cervical cancer | ex vivo (microscopy) | CNN | Kang et al., 2023 [96] |
| thyroid cancer | blood serum (microscopy) | SMOTE | Song et al., 2021 [67] |
| leukemia | blood smear (microscopy) | PLS-DA, SVM | Féré et al., 2019 [50] |
| blood serum (microscopy) | PLS-DA | Lima et al., 2022 [98] | |
| bone marrow cells (microscopy) | OPLS-DA | Cheng et al., 2022 [72] | |
| prostate cancer bone metastasis | in vitro (microscopy) | PCA | Kar et al., 2022 [94] |
| atherosclerosis | in vitro (FT-Raman) | PCA | Nogueira et al., 2005 [16] |
| in vitro (fiber probe) | MCR | Sćepanović et al., 2006 [17] | |
| ex vivo (microscopy) | VCA image unmixing | You et al., 2017 [88] | |
| dry eye | meibum lipid (microscopy) | PCA | Oshima et al., 2009 [32] |
| osteoarthritis | ex vivo (microscopy) | PCA | Kumar et al., 2015 [92] |
| ex vivo (microscopy) | PCA, HCA | Asaoka et al., 2022 [24] | |
| in vivo (needle arthroscopy) | PLS-DA | Kroupa et al., 2021 [93] | |
| Hirschsprung disease | ex vivo (microscopy) | PCA | Ogawa et al., 2021 [54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oshima, Y.; Haruki, T.; Koizumi, K.; Yonezawa, S.; Taketani, A.; Kadowaki, M.; Saito, S. Practices, Potential, and Perspectives for Detecting Predisease Using Raman Spectroscopy. Int. J. Mol. Sci. 2023, 24, 12170. https://doi.org/10.3390/ijms241512170
Oshima Y, Haruki T, Koizumi K, Yonezawa S, Taketani A, Kadowaki M, Saito S. Practices, Potential, and Perspectives for Detecting Predisease Using Raman Spectroscopy. International Journal of Molecular Sciences. 2023; 24(15):12170. https://doi.org/10.3390/ijms241512170
Chicago/Turabian StyleOshima, Yusuke, Takayuki Haruki, Keiichi Koizumi, Shota Yonezawa, Akinori Taketani, Makoto Kadowaki, and Shigeru Saito. 2023. "Practices, Potential, and Perspectives for Detecting Predisease Using Raman Spectroscopy" International Journal of Molecular Sciences 24, no. 15: 12170. https://doi.org/10.3390/ijms241512170
APA StyleOshima, Y., Haruki, T., Koizumi, K., Yonezawa, S., Taketani, A., Kadowaki, M., & Saito, S. (2023). Practices, Potential, and Perspectives for Detecting Predisease Using Raman Spectroscopy. International Journal of Molecular Sciences, 24(15), 12170. https://doi.org/10.3390/ijms241512170