Evaluating the Oxidation Rate of Reduced Ferredoxin in Arabidopsis thaliana Independent of Photosynthetic Linear Electron Flow: Plausible Activity of Ferredoxin-Dependent Cyclic Electron Flow around Photosystem I
Abstract
1. Introduction
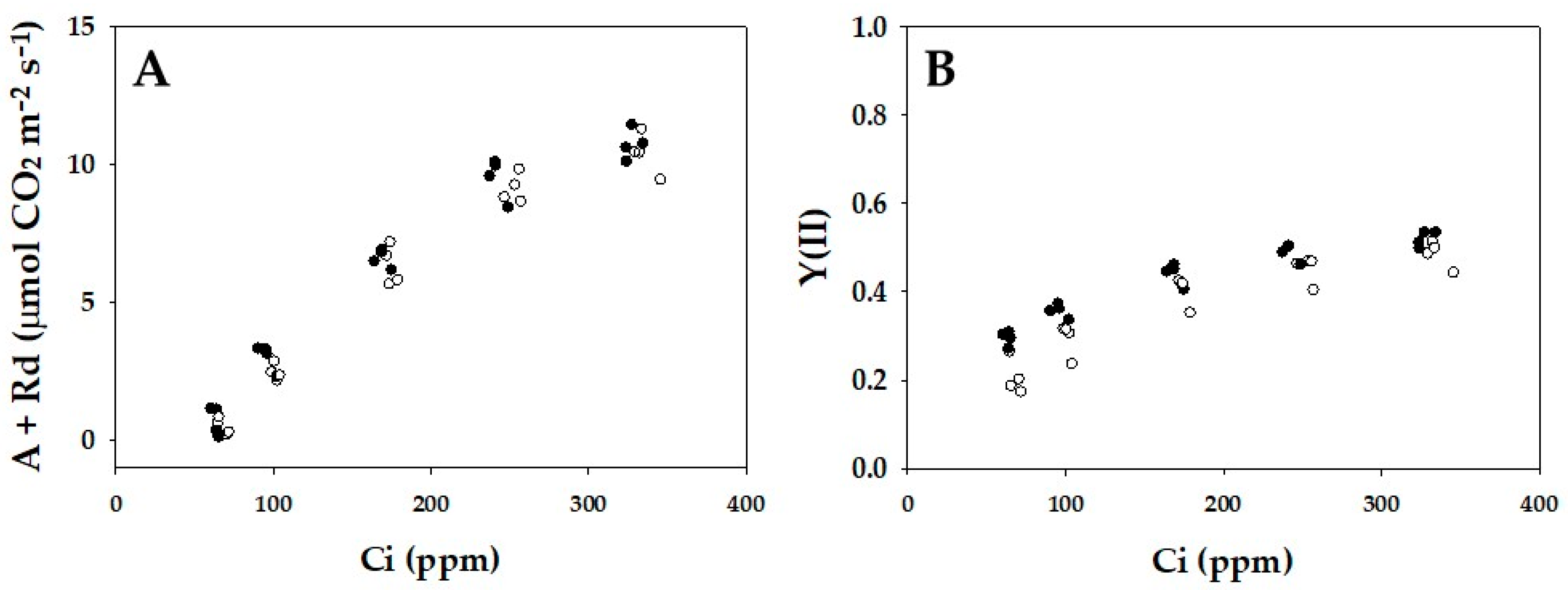
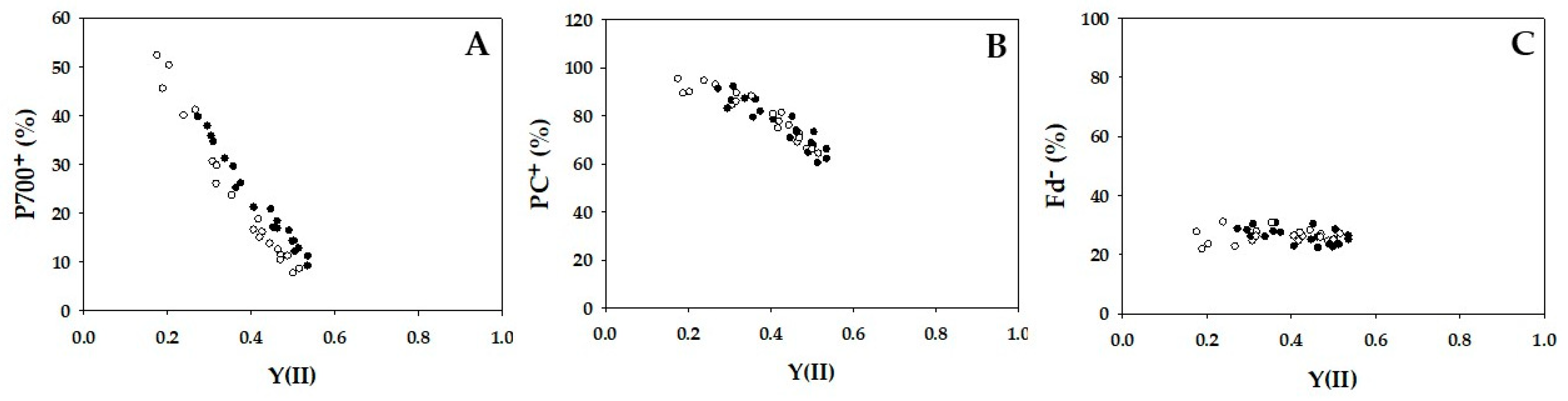
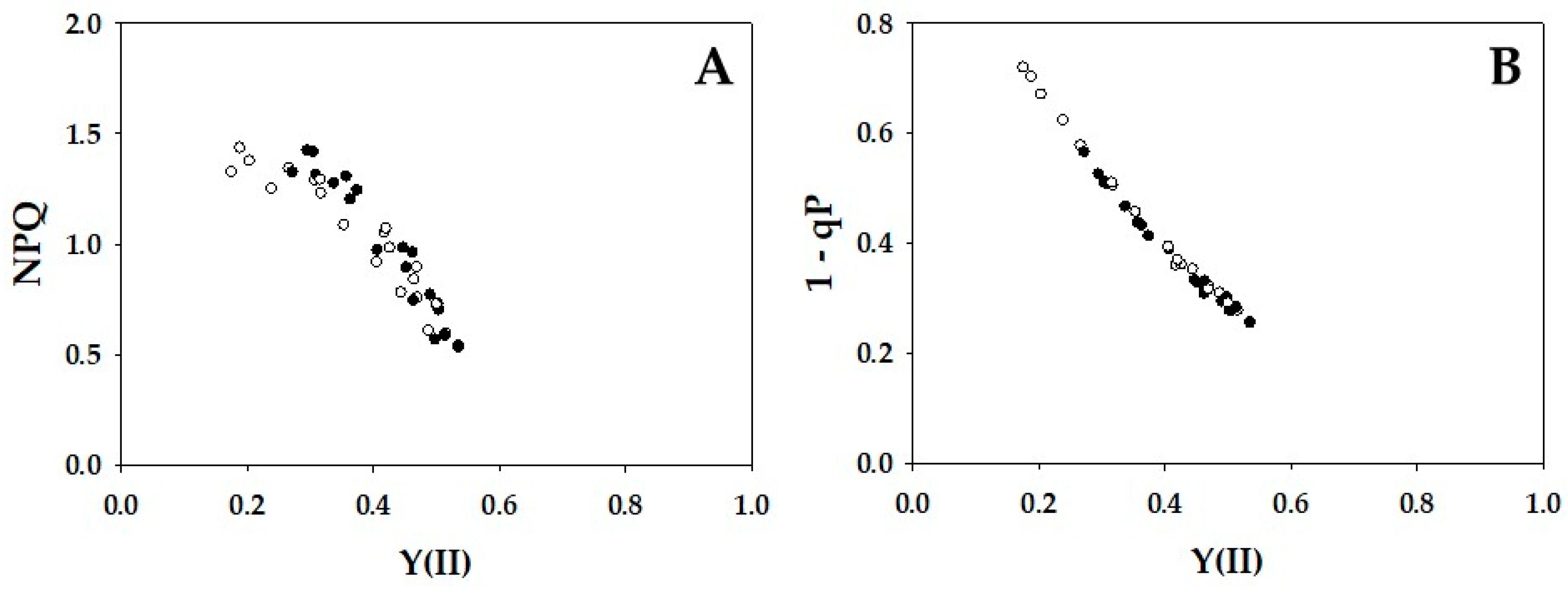
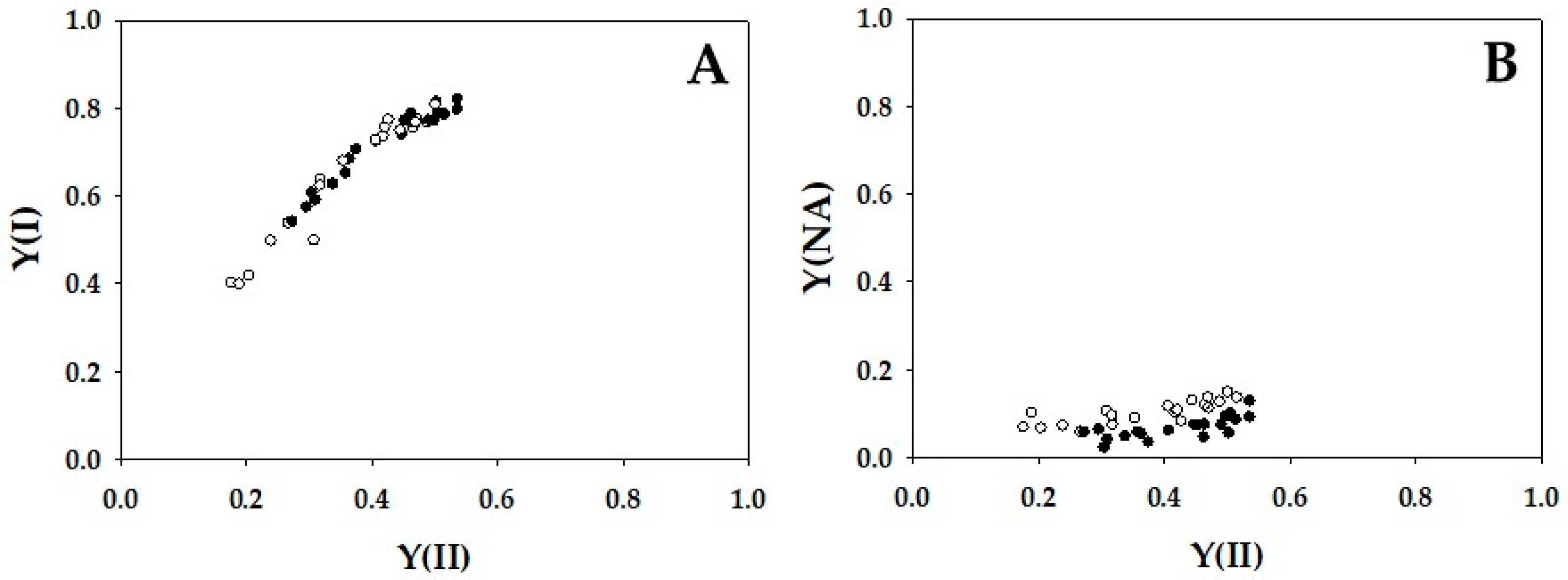
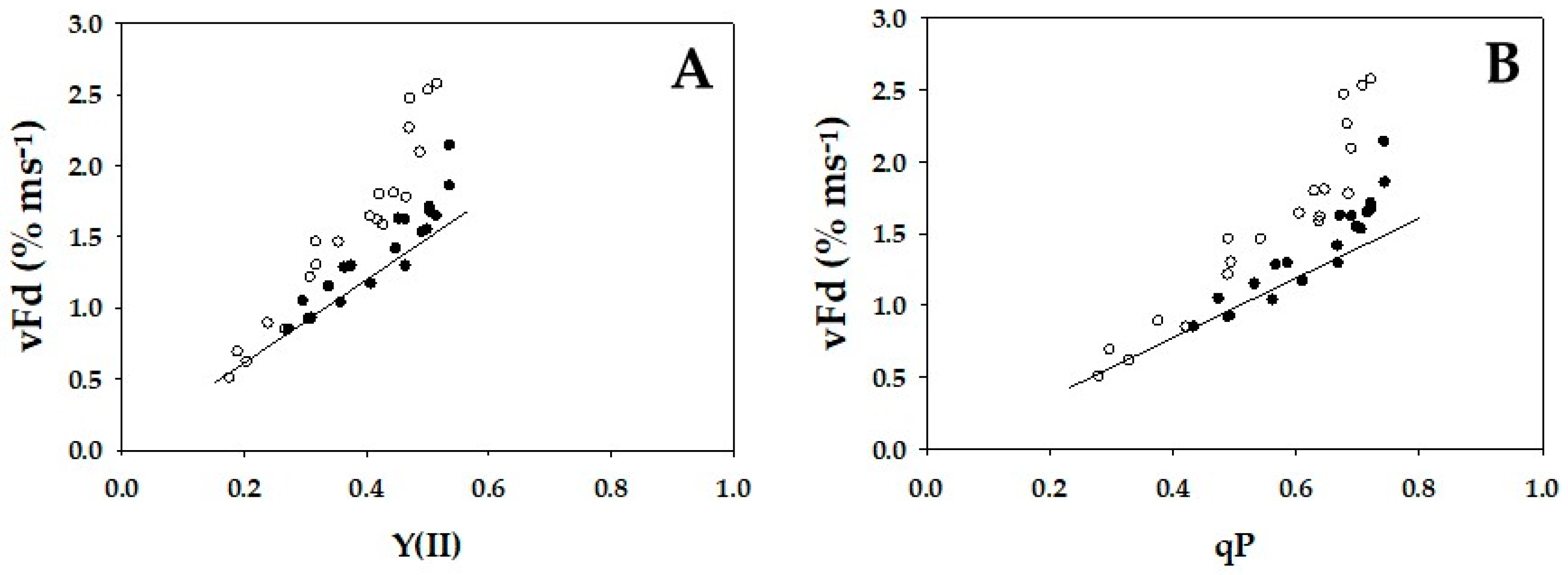
2. Results
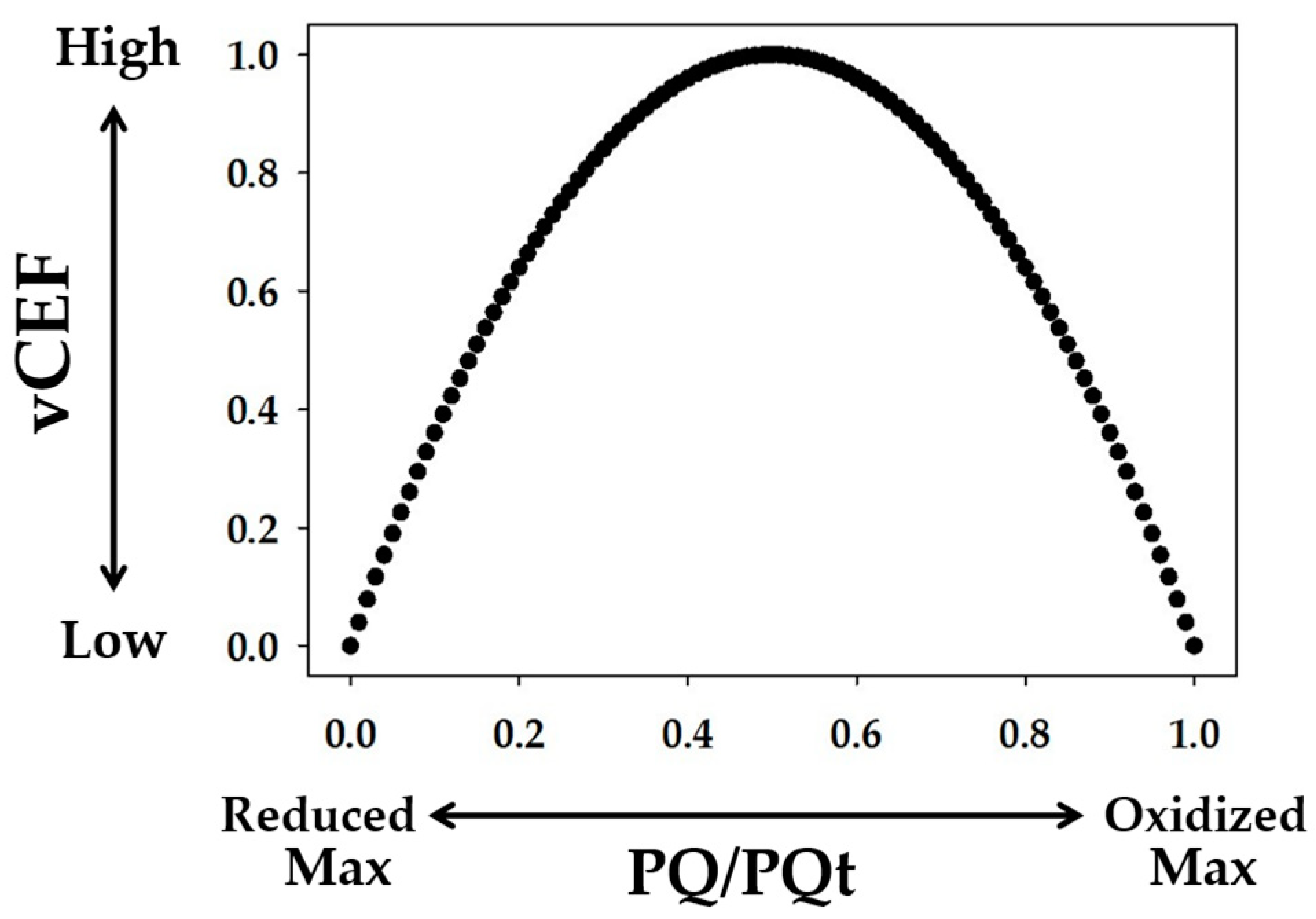
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Determination of Chlorophyll and Nitrogen
4.3. Simultaneous Measurements of Chlorophyll Fluorescence, P700, and Fd-Signals with Gas Exchange
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allen, J.F. Cyclic, pseudocyclic and noncyclic photophosphorylation: New links in the chain. Trend. Plant Sci. 2003, 8, 15–19. [Google Scholar] [CrossRef]
- Heber, U.; Walker, D. Concerning a dual function of coupled cyclic electron transport in leaves. Plant Physiol. 1992, 100, 1621–1626. [Google Scholar] [CrossRef]
- Munekage, Y.; Hojo, M.; Meurer, J.; Endo, T.; Tasaka, M.; Shikanai, T. PGR5 is involved in cyclic electron flow around photosystem I and is essential for photoprotection in Arabidopsis. Cell 2002, 110, 361–371. [Google Scholar] [CrossRef]
- Laisk, A.; Talts, E.; Oja, V.; Eichelmann, H.; Peterson, R.B. Fast cyclic electron transport around photosystem I in leaves under far-red light: A proton-uncoupled pathway? Photosyn. Res. 2010, 103, 79–95. [Google Scholar] [CrossRef]
- Miyake, C. Alternative electron flows (water-water cycle and cyclic electron flow around PSI) in photosynthesis: Molecular mechanisms and physiological functions. Plant Cell Physiol. 2010, 51, 1951–1963. [Google Scholar] [CrossRef]
- Yamamoto, H.; Takahashi, S.; Badger, M.R.; Shikanai, T. Artificial remodelling of alternative electron flow by flavodiiron proteins in Arabidopsis. Nat. Plants 2016, 2, 16012. [Google Scholar] [CrossRef] [PubMed]
- Wada, S.; Yamamoto, H.; Suzuki, Y.; Yamori, W.; Shikanai, T.; Makino, A. Flavodiiron protein substitutes for cyclic electron flow without competing CO2 assimilation in rice. Plant Physiol. 2018, 176, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Miyake, C.; Miyata, M.; Shinzaki, Y.; Tomizawa, K. CO2 response of cyclic electron flow around PSI (CEF-PSI) in tobacco leaves--relative electron fluxes through PSI and PSII determine the magnitude of non-photochemical quenching (NPQ) of Chl fluorescence. Plant Cell Physiol. 2005, 46, 629–637. [Google Scholar] [CrossRef]
- Furutani, R.; Ohnishi, M.; Mori, Y.; Wada, S.; Miyake, C. The difficulty of estimating the electron transport rate at photosystem I. J. Plant Res. 2022, 135, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Klughammer, C.; Schreiber, U. An improved method, using saturating light pulses, for the determination of photosystem I quantum yield via P700-absorbance changes at 830 nm. Planta 1994, 192, 261–268. [Google Scholar] [CrossRef]
- Fisher, N.; Kramer, D.M. Non-photochemical reduction of thylakoid photosynthetic redox carriers in vitro: Relevance to cyclic electron flow around photosystem I? Biochim. Biophys. Acta 2014, 1837, 1944–1954. [Google Scholar] [CrossRef] [PubMed]
- Miyake, C.; Schreiber, U.; Asada, K. Ferredoxin-dependent and antimycin A-sensitive reduction of cytochrome b-559 by far-red light in maize thylakoids; Participation of a menadiol-reducible cytochrome b-559 in cyclic electron flow. Plant Cell Physiol. 1995, 36, 743–748. [Google Scholar] [CrossRef]
- Kadota, K.; Furutani, R.; Makino, A.; Suzuki, Y.; Wada, S.; Miyake, C. Oxidation of P700 induces alternative electron flow in photosystem I in wheat leaves. Plants 2019, 8, 152. [Google Scholar] [CrossRef] [PubMed]
- Yamori, W.; Shikanai, T. Physiological functions of cyclic electron transport around photosystem I in sustaining photosynthesis and plant growth. Ann. Rev. Plant Physiol. 2016, 67, 81–106. [Google Scholar] [CrossRef]
- Hashimoto, M.; Endo, T.; Peltier, G.; Tasaka, M.; Shikanai, T. A nucleus-encoded factor, CRR2, is essential for the expression of chloroplast ndhB in Arabidopsis. Plant J. 2003, 36, 541–549. [Google Scholar] [CrossRef]
- Furutani, R.; Wada, S.; Ifuku, K.; Maekawa, S.; Miyake, C. Higher reduced state of Fe/S-signals, with the suppressed oxidation of P700, causes PSI inactivation in Arabidopsis thaliana. Antioxidants 2022, 12, 21. [Google Scholar] [CrossRef]
- Miyake, C. Molecular mechanism of oxidation of P700 and suppression of ROS production in photosystem I in response to electron-sink limitations in C3 Plants. Antioxidants 2020, 9, 230. [Google Scholar] [CrossRef] [PubMed]
- Furutani, R.; Ifuku, K.; Suzuki, Y.; Noguchi, K.; Shimakawa, G.; Wada, S.; Makino, A.; Sohtome, T.; Miyake, C. P700 Oxidation Suppresses the Production of Reactive Oxygen Species in Photosystem I; Toru, H., Ed.; Acad Press: Cambridge, MA, USA, 2020; Volume 96, p. 26. [Google Scholar]
- Asada, K.; Kiso, K.; Yoshikawa, K. Univalent reduction of molecular oxygen by spinach chloroplasts on illumination. J. Biochem. Biol. 1974, 249, 2175–2181. [Google Scholar]
- Kozuleva, M.; Petrova, A.; Milrad, Y.; Semenov, A.; Ivanov, B.; Redding, K.E.; Yacoby, I. Phylloquinone is the principal Mehler reaction site within photosystem I in high light. Plant Physiol. 2021, 186, 1848–1858. [Google Scholar] [CrossRef]
- Havaux, M.; Davaud, A. Photoinhibition of photosynthesis in chilled potato leaves is not correlated with a loss of Photosystem-II activity: Preferential inactivation of photosystem I. Photosyn. Res. 1994, 40, 75–92. [Google Scholar] [CrossRef]
- Inoue, K.; Fujie, T.; Yokoyama, E.; Matsuura, K.; Hiyama, T.; Sakurai, H. The photoinhibition sites of photosystem I in isolated chloroplasts under extremely reducing conditions. Plant Cell Physiol. 1989, 30, 7. [Google Scholar] [CrossRef]
- Satoh, K. Mechanism of photoinactivation in photosynthetic systems. III. The site and mode of photoinactivation in photosystem I. Plant Cell Physiol. 1970, 11, 187. [Google Scholar] [CrossRef]
- Sonoike, K.; Terashima, I.; Iwaki, M.; Itoh, S. Destruction of photosystem I iron-sulfur centers in leaves of Cucumis sativus L. by weak illumination at chilling temperatures. FEBS Lett. 1995, 362, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Terashima, I.; Funayama, S.; Sonoike, K. The site of photoinhibition in leaves of Cucumis sativus L. at low temperatures in photosystem I, not photosystem II. Planta 1994, 193, 7. [Google Scholar] [CrossRef]
- Foyer, C.; Furbank, R.; Harbinson, J.; Horton, P. The mechanisms contributing to photosynthetic control of electron transport by carbon assimilation in leaves. Photosyn. Res. 1990, 25, 83–100. [Google Scholar] [CrossRef]
- Tikhonov, A.N. The cytochrome b6f complex at the crossroad of photosynthetic electron transport pathways. Plant Physiol. Biochem. PPB 2014, 81, 163–183. [Google Scholar] [CrossRef]
- Furutani, R.; Makino, A.; Suzuki, Y.; Wada, S.; Shimakawa, G.; Miyake, C. Intrinsic fluctuations in transpiration induce photorespiration to oxidize P700 in photosystem I. Plants 2020, 9, 1761. [Google Scholar] [CrossRef]
- Wada, S.; Suzuki, Y.; Miyake, C. Photorespiration enhances acidification of the thylakoid lumen, reduces the plastoquinone pool, and contributes to the oxidation of P700 at a lower partial pressure of CO2 in wheat leaves. Plants 2020, 9, 319. [Google Scholar] [CrossRef]
- Hanawa, H.; Ishizaki, K.; Nohira, K.; Takagi, D.; Shimakawa, G.; Sejima, T.; Shaku, K.; Makino, A.; Miyake, C. Land plants drive photorespiration as higher electron-sink: Comparative study of post-illumination transient O2-uptake rates from liverworts to angiosperms through ferns and gymnosperms. Physiol. Plant. 2017, 161, 138–149. [Google Scholar] [CrossRef]
- Sejima, T.; Hanawa, H.; Shimakawa, G.; Takagi, D.; Suzuki, Y.; Fukayama, H.; Makino, A.; Miyake, C. Post-illumination transient O2-uptake is driven by photorespiration in tobacco leaves. Physiol. Plant. 2016, 156, 227–238. [Google Scholar] [CrossRef]
- Miyake, C.; Suzuki, Y.; Yamamoto, H.; Amako, K.; Makino, A. O2-enhanced induction of photosynthesis in rice leaves: The Mehler-ascorbate peroxidase (MAP) pathway drives cyclic electron flow within PSII and cyclic electron flow around PSI. Soil Sci. Plant Nutri. 2012, 58, 718–727. [Google Scholar] [CrossRef]
- Yamamoto, H.; Shikanai, T. PGR5-dependent cyclic electron flow protects photosystem I under fluctuating light at donor and acceptor sides. Plant Physiol. 2019, 179, 588–600. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Shikanai, T. Does the Arabidopsis proton gradient regulation 5 mutant leak protons from the thylakoid membrane? Plant Physiol. 2020, 184, 421–427. [Google Scholar] [CrossRef]
- Suganami, M.; Konno, S.; Maruhashi, R.; Takagi, D.; Tazoe, Y.; Wada, S.; Yamamoto, H.; Shikanai, T.; Ishida, H.; Suzuki, Y.; et al. Expression of flavodiiron protein rescues defects in electron transport around PSI resulting from overproduction of Rubisco activase in rice. J. Exp. Bot. 2022, 73, 2589–2600. [Google Scholar] [CrossRef]
- Rantala, S.; Lempiäinen, T.; Gerotto, C.; Tiwari, A.; Aro, E.M.; Tikkanen, M. PGR5 and NDH-1 systems do not function as protective electron acceptors but mitigate the consequences of PSI inhibition. Biochim. Biophys. Acta Bioenerg. 2020, 1861, 148154. [Google Scholar] [CrossRef] [PubMed]
- Wada, S.; Amako, K.; Miyake, C. Identification of a novel mutation exacerbated the PSI photoinhibition in pgr5/pgrl1 mutants; Caution for overestimation of the phenotypes in Arabidopsis pgr5-1 Mutant. Cells 2021, 10, 2884. [Google Scholar] [CrossRef]
- Ohnishi, M.; Furutani, R.; Sohtome, T.; Suzuki, T.; Wada, S.; Tanaka, S.; Ifuku, K.; Ueno, D.; Miyake, C. Photosynthetic parameters show specific responses to essential mineral deficiencies. Antioxidants 2021, 10, 996. [Google Scholar] [CrossRef]
- Porra, R.J.; Scheer, H. Towards a more accurate future for chlorophyll a and b determinations: The inaccuracies of Daniel Arnon’s assay. Photosyn. Res. 2019, 140, 215–219. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef]
- Genty, B.; Harbinson, J.; Briantais, J.M.; Baker, N.R. The relationship between non-photochemical quenching of chlorophyll fluorescence and the rate of photosystem 2 photochemistry in leaves. Photosyn. Res. 1990, 25, 249–257. [Google Scholar] [CrossRef]
- Bilger, W.; Björkman, O. Relationships among violaxanthin deepoxidation, thylakoid membrane conformation, and nonphotochemical chlorophyll fluorescence quenching in leaves of cotton (Gossypium hirsutum L.). Planta 1994, 193, 238–246. [Google Scholar] [CrossRef]
- Oxborough, K.; Baker, N.R. An evaluation of the potential triggers of photoinactivation of photosystem II in the context of a Stern-Volmer model for downregulation and the reversible radical pair equilibrium model. Phil. Trans. R. Soc. Lond. B Biol. Sci. 2000, 355, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Klughammer, C.; Schreiber, U. Deconvolution of ferredoxin, plastocyanin, and P700 transmittance changes in intact leaves with a new type of kinetic LED array spectrophotometer. Photosyn. Res. 2016, 128, 195–214. [Google Scholar] [CrossRef]
- Sacksteder, C.A.; Kramer, D.M. Dark-interval relaxation kinetics (DIRK) of absorbance changes as a quantitative probe of steady-state electron transfer. Photosyn. Res. 2000, 66, 145–158. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohnishi, M.; Maekawa, S.; Wada, S.; Ifuku, K.; Miyake, C. Evaluating the Oxidation Rate of Reduced Ferredoxin in Arabidopsis thaliana Independent of Photosynthetic Linear Electron Flow: Plausible Activity of Ferredoxin-Dependent Cyclic Electron Flow around Photosystem I. Int. J. Mol. Sci. 2023, 24, 12145. https://doi.org/10.3390/ijms241512145
Ohnishi M, Maekawa S, Wada S, Ifuku K, Miyake C. Evaluating the Oxidation Rate of Reduced Ferredoxin in Arabidopsis thaliana Independent of Photosynthetic Linear Electron Flow: Plausible Activity of Ferredoxin-Dependent Cyclic Electron Flow around Photosystem I. International Journal of Molecular Sciences. 2023; 24(15):12145. https://doi.org/10.3390/ijms241512145
Chicago/Turabian StyleOhnishi, Miho, Shu Maekawa, Shinya Wada, Kentaro Ifuku, and Chikahiro Miyake. 2023. "Evaluating the Oxidation Rate of Reduced Ferredoxin in Arabidopsis thaliana Independent of Photosynthetic Linear Electron Flow: Plausible Activity of Ferredoxin-Dependent Cyclic Electron Flow around Photosystem I" International Journal of Molecular Sciences 24, no. 15: 12145. https://doi.org/10.3390/ijms241512145
APA StyleOhnishi, M., Maekawa, S., Wada, S., Ifuku, K., & Miyake, C. (2023). Evaluating the Oxidation Rate of Reduced Ferredoxin in Arabidopsis thaliana Independent of Photosynthetic Linear Electron Flow: Plausible Activity of Ferredoxin-Dependent Cyclic Electron Flow around Photosystem I. International Journal of Molecular Sciences, 24(15), 12145. https://doi.org/10.3390/ijms241512145






