Antioxidant Activity of a Sicilian Almond Skin Extract Using In Vitro and In Vivo Models
Abstract
1. Introduction
2. Results
2.1. Results of the Polyphenol Profile of the ASE
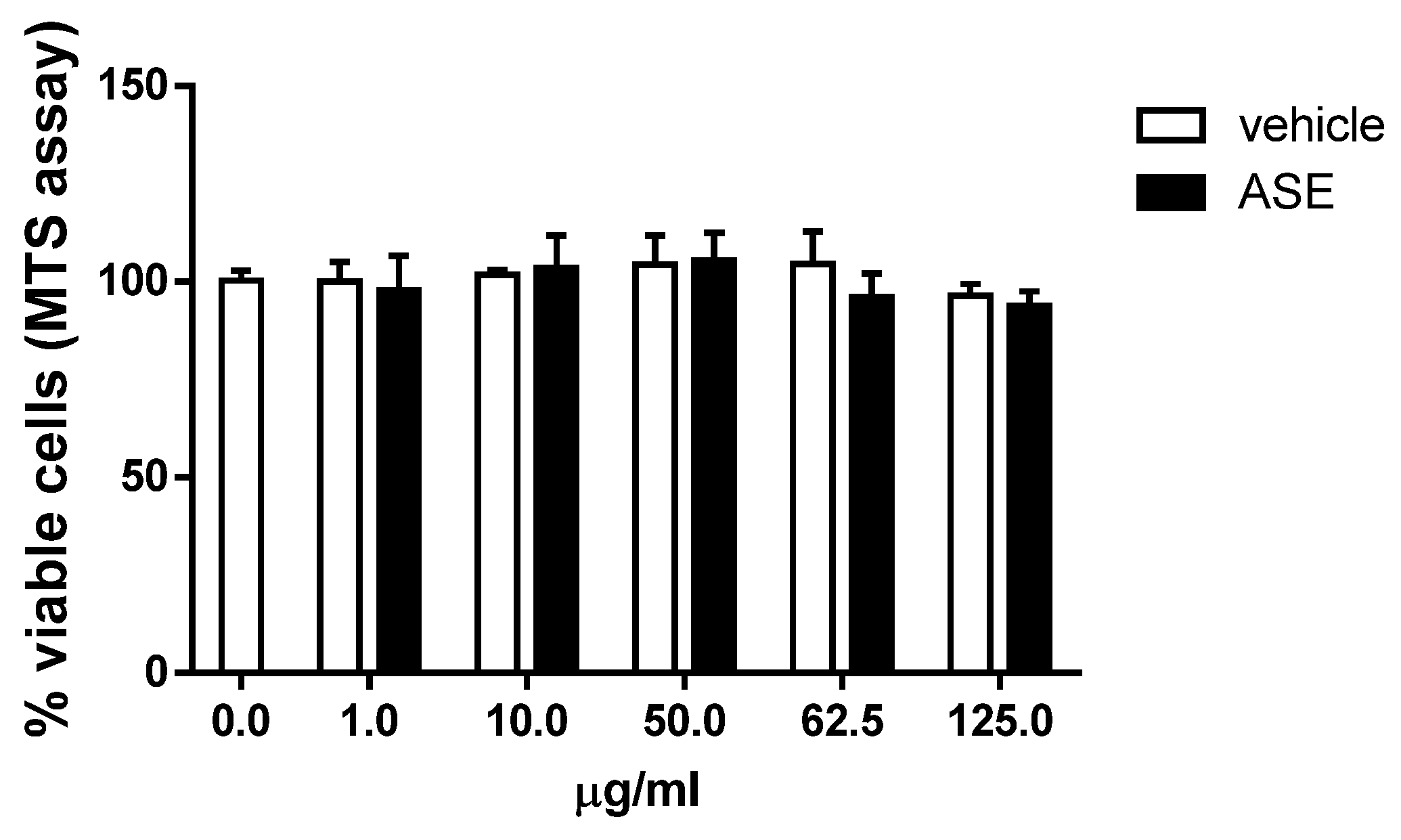
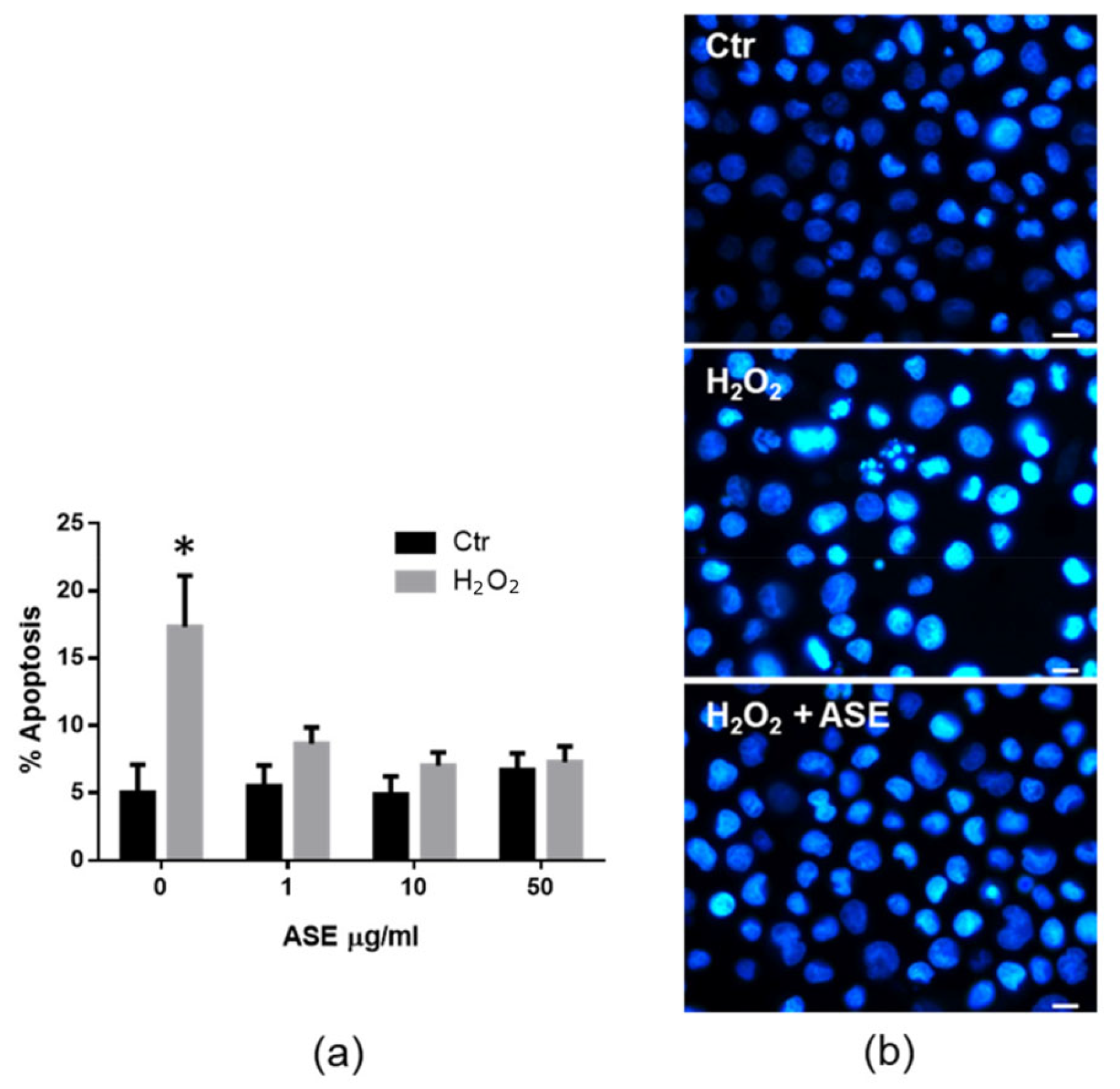
2.2. In Vitro Assessment of the Cytotoxicity and Antioxidant Properties of the ASE
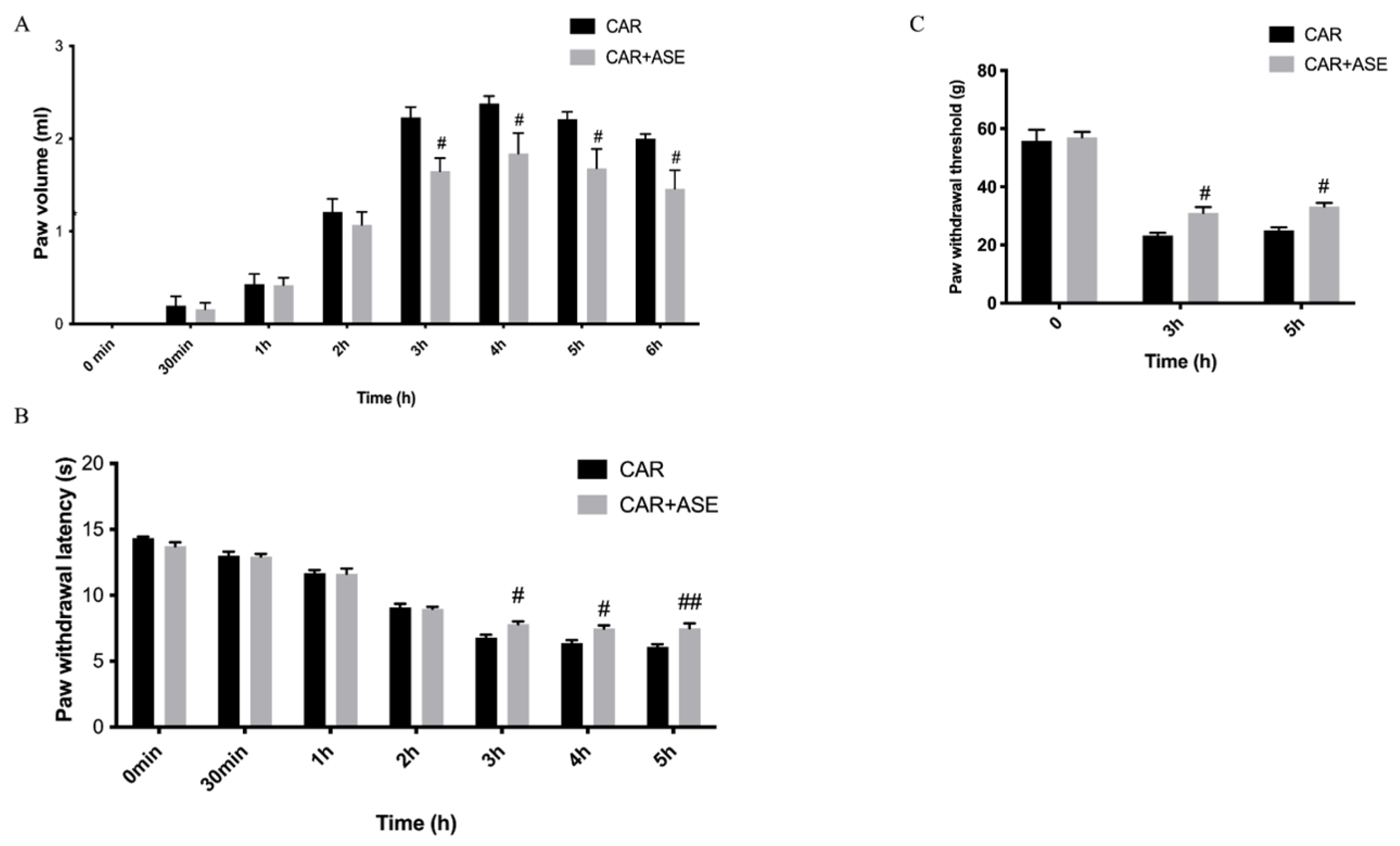
2.3. In Vivo Studies: Effect of the ASE on CAR-Induced Inflammation and Pain
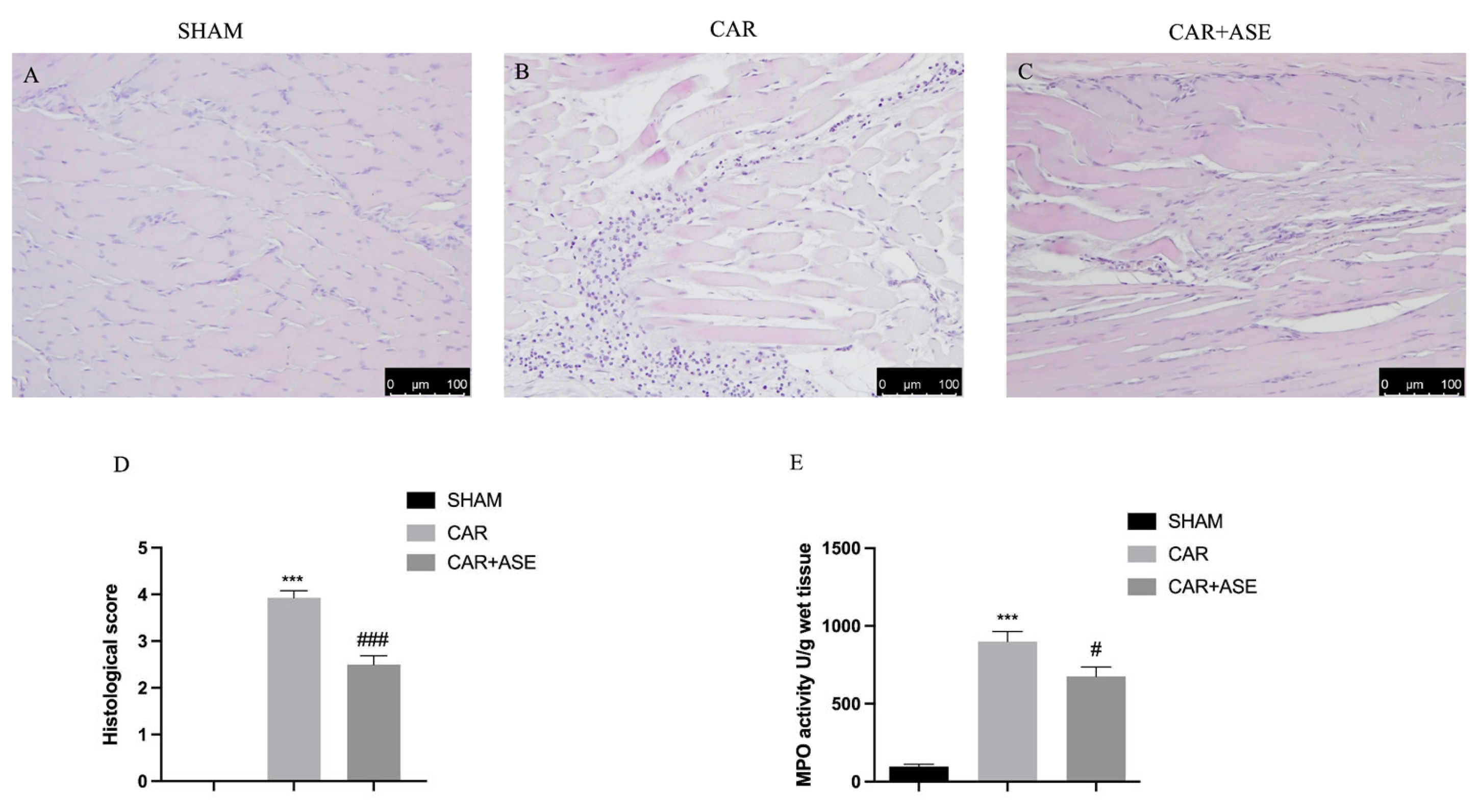
2.4. Effects of the ASE on Histological Alteration after CAR Injection
2.5. Effect of the ASE on Inducible NO Synthase and Cyclooxygenase-2 Enzyme Expression
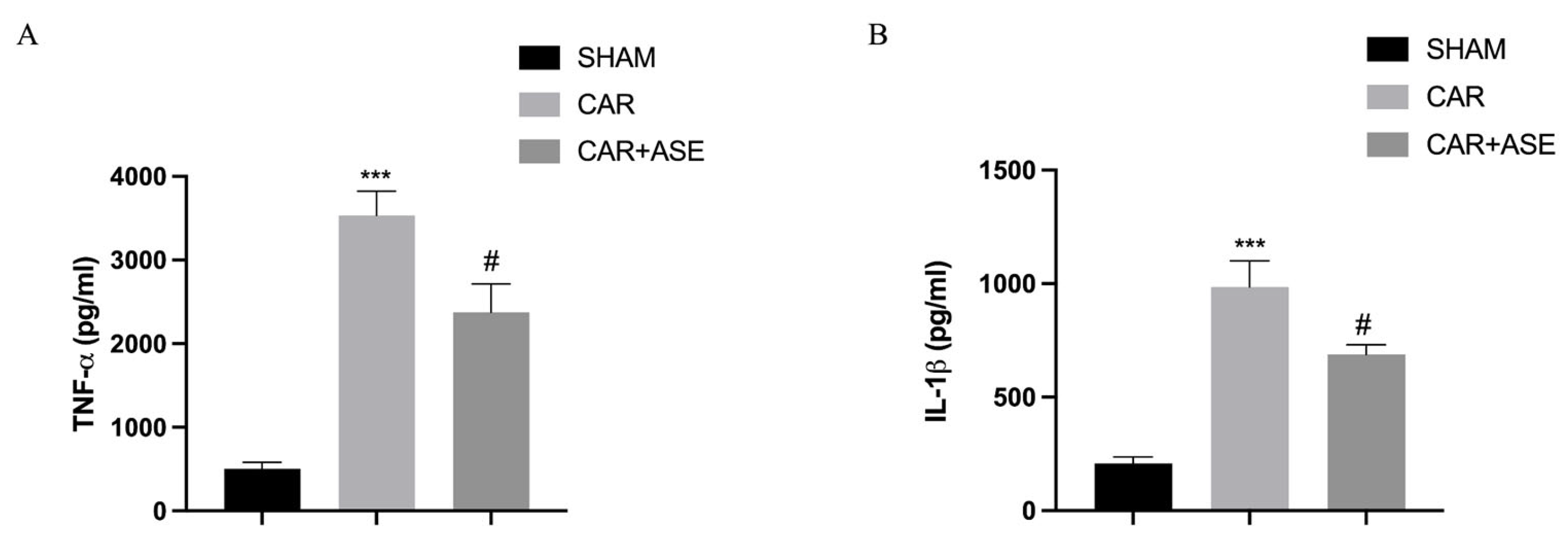
2.6. Effects of the ASE on Cytokine Production
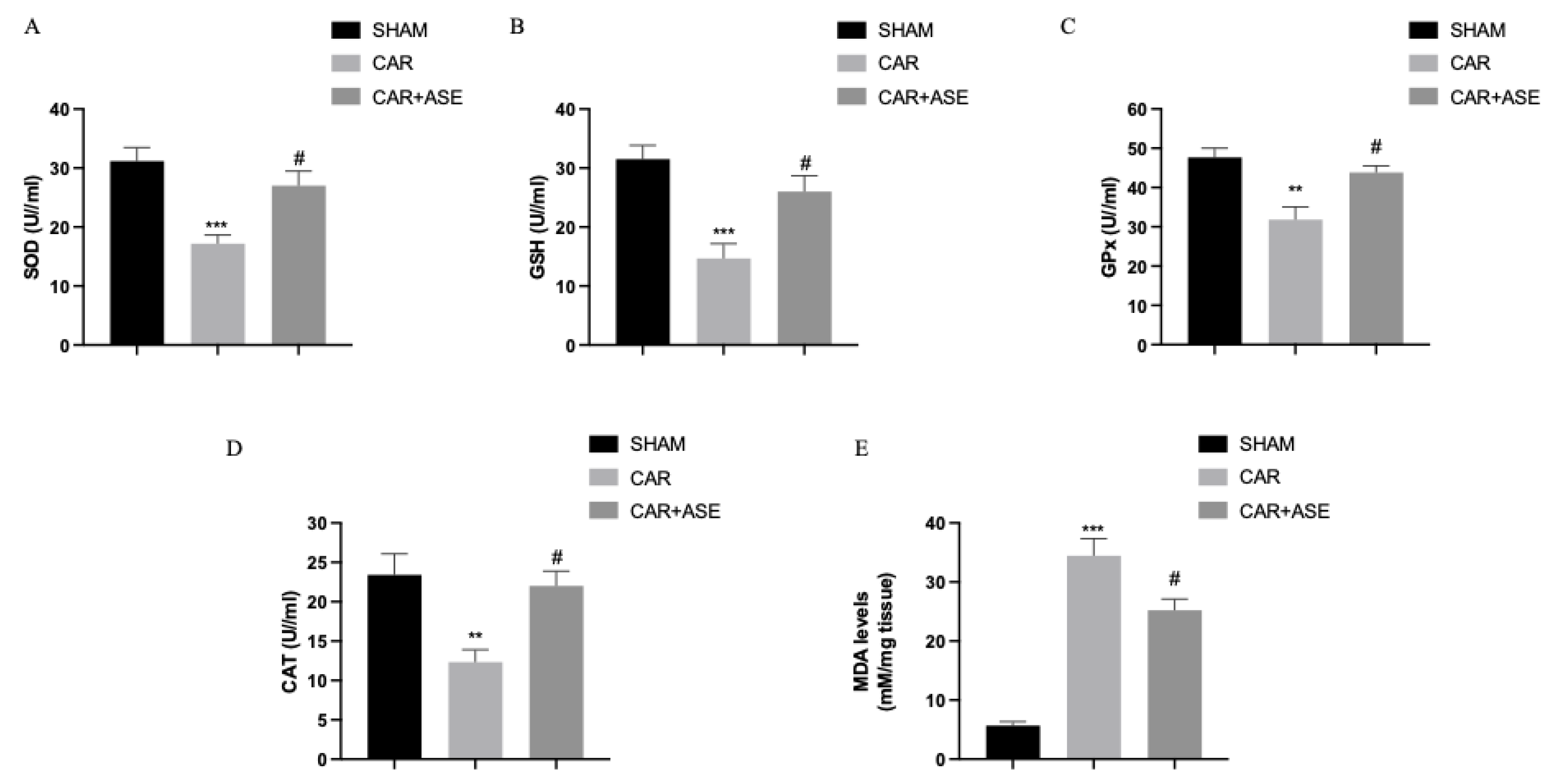
2.7. Effects of the ASE on CAR-Induced Oxidative Stress
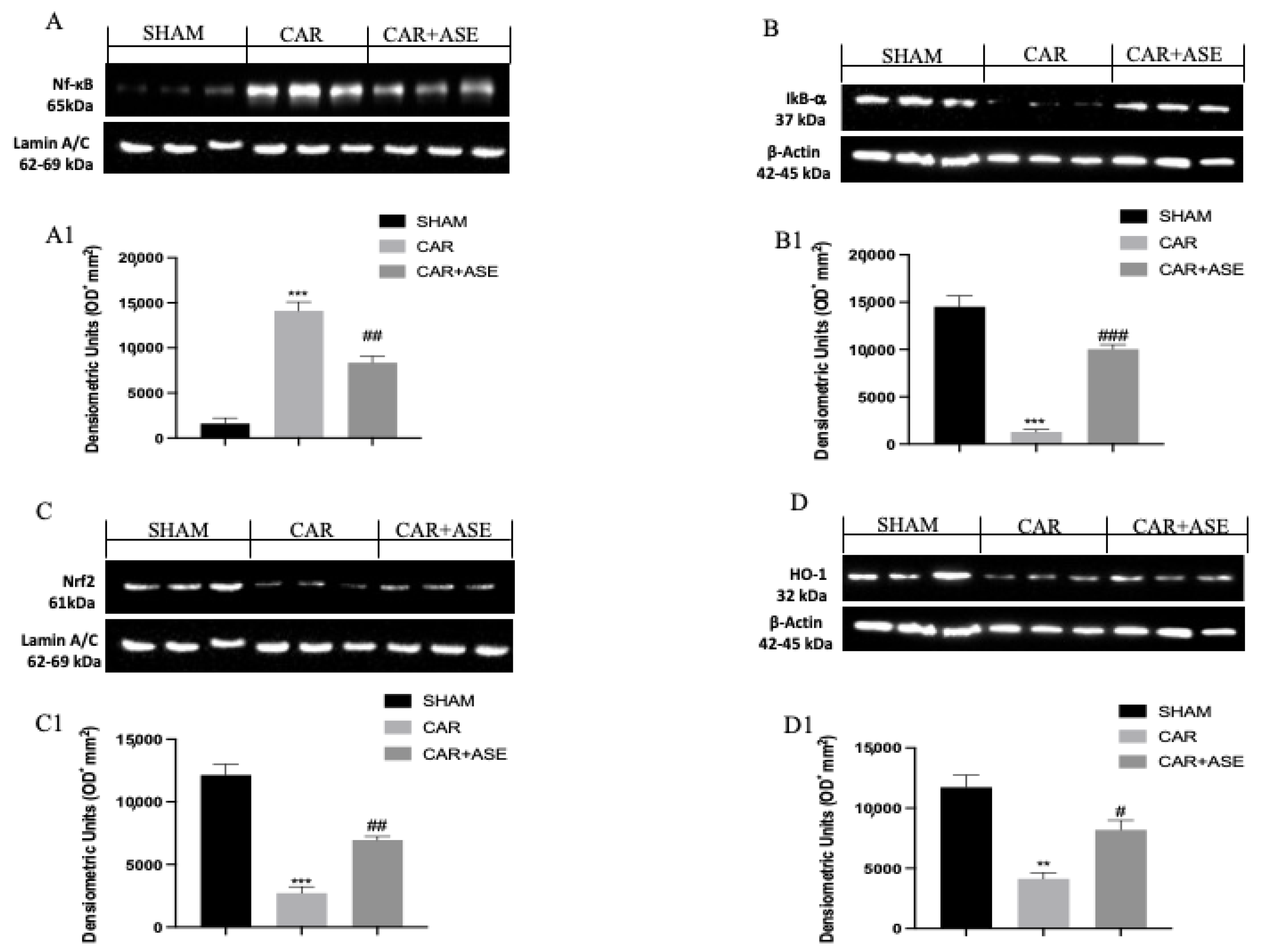
2.8. Effect of the ASE on CAR-Induced NF-κB, Nrf2, Heme Oxygenase-1 (HO-1), and IkB-α Expression
3. Discussion
4. Materials and Methods
4.1. Almond Skin Extract
4.1.1. Sample Preparation
4.1.2. Qualitative and Quantitative Analysis of Polyphenols
4.2. Preliminary In Vitro Studies
4.2.1. Cells
4.2.2. Treatments and Reagents
4.2.3. Viability Assay
4.2.4. ROS Detection
4.2.5. Apoptosis Assay
4.3. In Vivo Studies
4.3.1. Animals
4.3.2. CAR-Induced Paw Edema
4.3.3. Experimental Groups
- (1)
- CAR + vehicle (saline): rats were subjected to CAR-induced paw edema;
- (2)
- CAR + ASE (100 mg/kg): rats were subjected to CAR-induced paw edema, and almond skin extract (100 mg/kg) was administered 30 min before CAR;
- (3)
- The sham-operated group underwent the same surgical procedures as the CAR group, with the exception that saline or an alternative compound was administered instead of CAR.
4.3.4. Assessment of CAR-Induced Paw Edema
4.3.5. Behavioral Analysis
4.3.6. MPO Activity
4.3.7. MDA Levels
4.3.8. Evaluation of Cytokines
4.3.9. Histological Examination of the CAR-Inflamed Hind Paw
4.3.10. Immunohistochemistry for iNOS and COX-2
4.3.11. Western Blot Analysis
4.3.12. Reagents
4.3.13. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patil, K.R.; Mahajan, U.B.; Unger, B.S.; Goyal, S.N.; Belemkar, S.; Surana, S.J.; Ojha, S.; Patil, C.R. Animal Models of Inflammation for Screening of Anti-inflammatory Drugs: Implications for the Discovery and Development of Phytopharmaceuticals. Int. J. Mol. Sci. 2019, 20, 4367. [Google Scholar] [CrossRef] [PubMed]
- Omoboyowa, D.A.; Nwodo, O.F.C.; Joshua, P.E. Anti-diarrhoeal activity of chloroform-ethanol extracts of cashew (Anacardium occidentale) kernel. J. Nat. Prod. 2013, 6, 109–117. [Google Scholar]
- Dewanjee, S.; Dua, T.K.; Sahu, R. Potential anti-inflammatory effect of Leea macrophylla Roxb. leaves: A wild edible plant. Food Chem. Toxicol. 2013, 59, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Meagher, E.A.; Barry, O.P.; Lawson, J.A.; Rokach, J.; FitzGerald, G.A. Effects of Vitamin E on Lipid Peroxidation in Healthy Persons. JAMA 2001, 285, 1178–1182. [Google Scholar] [CrossRef]
- Vincent, H.K.; Bourguignon, C.M.; Vincent, K.R.; Weltman, A.L.; Bryant, M.; Taylor, A.G. Antioxidant Supplementation Lowers Exercise-Induced Oxidative Stress in Young Overweight Adults. Obesity 2006, 14, 2224–2235. [Google Scholar] [CrossRef]
- Hogan, S.; Canning, C.; Sun, S.; Sun, X.; Zhou, K. Effects of Grape Pomace Antioxidant Extract on Oxidative Stress and Inflammation in Diet Induced Obese Mice. J. Agric. Food Chem. 2010, 58, 11250–11256. [Google Scholar] [CrossRef]
- Sacchet, C.; Mocelin, R.; Sachett, A.; Bevilaqua, F.; Chitolina, R.; Kuhn, F.; Boligon, A.A.; Athayde, M.L.; Roman Junior, W.A.; Rosemberg, D.B.; et al. Antidepressant-Like and Antioxidant Effects of Plinia trunciflora in Mice. Evid. Based Complement. Altern. Med. 2015, 2015, 601503. [Google Scholar] [CrossRef]
- Rahman, I.; Biswas, S.K.; Kirkham, P.A. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem. Pharmacol. 2006, 72, 1439–1452. [Google Scholar] [CrossRef]
- Cordaro, M.; Fusco, R.; D’Amico, R.; Siracusa, R.; Peritore, A.F.; Gugliandolo, E.; Genovese, T.; Crupi, R.; Mandalari, G.; Cuzzocrea, S.; et al. Cashew (Anacardium occidentale L.) Nuts Modulate the Nrf2 and NLRP3 Pathways in Pancreas and Lung after Induction of Acute Pancreatitis by Cerulein. Antioxidants 2020, 9, 992. [Google Scholar] [CrossRef]
- Arola-Arnal, A.; Cruz-Carrión, Á.; Torres-Fuentes, C.; Ávila-Román, J.; Aragonès, G.; Mulero, M.; Bravo, F.I.; Muguerza, B.; Arola, L.; Suárez, M. Chrononutrition and Polyphenols: Roles and Diseases. Nutrients 2019, 11, 2602. [Google Scholar] [CrossRef]
- Mandalari, G.; Nueno-Palop, C.; Bisignano, G.; Wickham, M.S.; Narbad, A. Potential prebiotic properties of almond (Amygdalus communis L.) seeds. Appl. Environ. Microbiol. 2008, 74, 4264–4270. [Google Scholar] [CrossRef] [PubMed]
- Mandalari, G.; Tomaino, A.; Arcoraci, T.; Martorana, M.; Turco, V.L.; Cacciola, F.; Rich, G.T.; Bisignano, C.; Saija, A.; Dugo, P.; et al. Characterization of polyphenols, lipids and dietary fibre from almond skins (Amygdalus communis L.). J. Food Compos. Anal. 2010, 23, 166–174. [Google Scholar] [CrossRef]
- Mandalari, G.; Tomaino, A.; Rich, G.T.; Lo Curto, R.; Arcoraci, T.; Martorana, M.; Bisignano, C.; Saija, A.; Parker, M.L.; Waldron, K.W.; et al. Polyphenol and nutrient release from skin of almonds during simulated human digestion. Food Chem. 2010, 122, 1083–1088. [Google Scholar] [CrossRef]
- Prgomet, I.; Gonçalves, B.; Domínguez-Perles, R.; Pascual-Seva, N.; Barros, A. Valorization Challenges to Almond Residues: Phytochemical Composition and Functional Application. Molecules 2017, 22, 1774. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, I.; Meyer, A.S.; Afonso, S.; Sequeira, A.; Vilela, A.; Goufo, P.; Trindade, H.; Gonçalves, B. Effects of Different Processing Treatments on Almond (Prunus dulcis) Bioactive Compounds, Antioxidant Activities, Fatty Acids, and Sensorial Characteristics. Plants 2020, 9, 1627. [Google Scholar] [CrossRef]
- Ali, Z.K.; Sahib, H.B. Antiangiogenic Activity of Sweet Almond (Prunus dulcis) Oil Alone and in Combination with Aspirin in both in vivo and in vitro Assays. Asian Pac. J. Cancer Prev. 2022, 23, 1405–1413. [Google Scholar] [CrossRef]
- Barreira, J.C.; Ferreira, I.C.; Oliveira, M.B.; Pereira, J.A. Antioxidant potential of chestnut (Castanea sativa L.) and almond (Prunus dulcis L.) by-products. Food Sci. Technol. Int. 2010, 16, 209–216. [Google Scholar] [CrossRef]
- Barreca, D.; Nabavi, S.M.; Sureda, A.; Rasekhian, M.; Raciti, R.; Silva, A.S.; Annunziata, G.; Arnone, A.; Tenore, G.C.; Süntar, İ.; et al. Almonds (Prunus Dulcis Mill. D. A. Webb): A Source of Nutrients and Health-Promoting Compounds. Nutrients 2020, 12, 672. [Google Scholar] [CrossRef]
- Olatunde, O.G.; Abidemi, O.R.; Solomon, B.A.; Bawa, J.a.; Ibukun, O.E.; Oluwatimilehin, I.I.; Ayinke, A.B. Assessment of Anti-inflammatory Activity of Prunus dulcis [Miller D.A. Webb (ALMOND)] SEED Aqueous Extract and Fractions. Egypt. Acad. J. Biol. Sci. H. Bot. 2022, 13, 113–120. [Google Scholar] [CrossRef]
- Qureshi, M.N.; Numonov, S.; Aisa, H.A. Chemical and Pharmacological Evaluation of Hulls of Prunus dulcis Nuts. Int. J. Anal. Chem. 2019, 2019, 5861692. [Google Scholar] [CrossRef]
- Ingegneri, M.; Smeriglio, A.; Rando, R.; Gervasi, T.; Tamburello, M.P.; Ginestra, G.; La Camera, E.; Pennisi, R.; Sciortino, M.T.; Mandalari, G.; et al. Composition and Biological Properties of Blanched Skin and Blanch Water Belonging to Three Sicilian Almond Cultivars. Nutrients 2023, 15, 1545. [Google Scholar] [CrossRef] [PubMed]
- Bottone, A.; Montoro, P.; Masullo, M.; Pizza, C.; Piacente, S. Metabolomics and antioxidant activity of the leaves of Prunus dulcis Mill. (Italian cvs. Toritto and Avola). J. Pharm. Biomed. Anal. 2018, 158, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Badalamenti, N.; Bruno, M.; Loizzo, M.R.; Puccio, V.; Gaglio, R.; Francesca, N.; Settanni, L.; Sottile, F. Antibacterial activity and chemical characterization of almond (Prunus dulcis L.) peel extract. Nat. Prod. Res. 2023, 37, 1680–1686. [Google Scholar] [CrossRef] [PubMed]
- Kahlaoui, M.; Borotto Dalla Vecchia, S.; Giovine, F.; Ben Haj Kbaier, H.; Bouzouita, N.; Barbosa Pereira, L.; Zeppa, G. Characterization of Polyphenolic Compounds Extracted from Different Varieties of Almond Hulls (Prunus dulcis L.). Antioxidants 2019, 8, 647. [Google Scholar] [CrossRef] [PubMed]
- Musarra-Pizzo, M.; Ginestra, G.; Smeriglio, A.; Pennisi, R.; Sciortino, M.T.; Mandalari, G. The Antimicrobial and Antiviral Activity of Polyphenols from Almond (Prunus dulcis L.) Skin. Nutrients 2019, 11, 2355. [Google Scholar] [CrossRef]
- Barreira, J.; Ferreira, I.; Oliveira, M.; Pereira, J. Effects of different phenols extraction conditions on antioxidant activity of almond (Prunus dulcis) fruits. J. Food Biochem. 2009, 33, 763–776. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Abid, M.; Elamrani, A.; Drouet, S.; Addi, M.; Hano, C. Almond Skin Extracts and Chlorogenic Acid Delay Chronological Aging and Enhanced Oxidative Stress Response in Yeast. Life 2020, 10, 80. [Google Scholar] [CrossRef]
- Ben Khedir, S.; Mzid, M.; Bardaa, S.; Moalla, D.; Sahnoun, Z.; Rebai, T. In Vivo Evaluation of the Anti-Inflammatory Effect of Pistacia lentiscus Fruit Oil and Its Effects on Oxidative Stress. Evid. Based Complement. Alternat. Med. 2016, 2016, 6108203. [Google Scholar] [CrossRef]
- Mansouri, M.T.; Hemmati, A.A.; Naghizadeh, B.; Mard, S.A.; Rezaie, A.; Ghorbanzadeh, B. A study of the mechanisms underlying the anti-inflammatory effect of ellagic acid in carrageenan-induced paw edema in rats. Indian J. Pharmacol. 2015, 47, 292–298. [Google Scholar] [CrossRef]
- Smeriglio, A.; Mandalari, G.; Bisignano, C.; Filocamo, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Polyphenolic content and biological properties of Avola almond (Prunus dulcis Mill. D.A. Webb) skin and its industrial byproducts. Ind. Crops Prod. 2016, 83, 283–293. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- Gardner, A.M.; Xu, F.H.; Fady, C.; Jacoby, F.J.; Duffey, D.C.; Tu, Y.; Lichtenstein, A. Apoptotic vs. nonapoptotic cytotoxicity induced by hydrogen peroxide. Free Radic. Biol. Med. 1997, 22, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Matteucci, C.; Grelli, S.; De Smaele, E.; Fontana, C.; Mastino, A. Identification of nuclei from apoptotic, necrotic, and viable lymphoid cells by using multiparameter flow cytometry. Cytometry 1999, 35, 145–153. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in inflammatory disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef] [PubMed]
- Paterniti, I.; Impellizzeri, D.; Cordaro, M.; Siracusa, R.; Bisignano, C.; Gugliandolo, E.; Carughi, A.; Esposito, E.; Mandalari, G.; Cuzzocrea, S. The Anti-Inflammatory and Antioxidant Potential of Pistachios (Pistacia vera L.) In Vitro and In Vivo. Nutrients 2017, 9, 915. [Google Scholar] [CrossRef]
- Mandalari, G.; Genovese, T.; Bisignano, C.; Mazzon, E.; Wickham, M.S.; Di Paola, R.; Bisignano, G.; Cuzzocrea, S. Neuroprotective effects of almond skins in experimental spinal cord injury. Clin. Nutr. 2011, 30, 221–233. [Google Scholar] [CrossRef]
- Mandalari, G.; Bisignano, C.; Genovese, T.; Mazzon, E.; Wickham, M.S.; Paterniti, I.; Cuzzocrea, S. Natural almond skin reduced oxidative stress and inflammation in an experimental model of inflammatory bowel disease. Int. Immunopharmacol. 2011, 11, 915–924. [Google Scholar] [CrossRef]
- Cordaro, M.; Siracusa, R.; Fusco, R.; D’Amico, R.; Peritore, A.F.; Gugliandolo, E.; Genovese, T.; Scuto, M.; Crupi, R.; Mandalari, G.; et al. Cashew (Anacardium occidentale L.) Nuts Counteract Oxidative Stress and Inflammation in an Acute Experimental Model of Carrageenan-Induced Paw Edema. Antioxidants 2020, 9, 660. [Google Scholar] [CrossRef]
- Suzuki, J.; Ogawa, M.; Muto, S.; Itai, A.; Isobe, M.; Hirata, Y.; Nagai, R. Novel IkB kinase inhibitors for treatment of nuclear factor-kB-related diseases. Expert. Opin. Investig. Drugs 2011, 20, 395–405. [Google Scholar] [CrossRef]
- Goldstein, B.I.; Kemp, D.E.; Soczynska, J.K.; McIntyre, R.S. Inflammation and the phenomenology, pathophysiology, comorbidity, and treatment of bipolar disorder: A systematic review of the literature. J. Clin. Psychiatry 2009, 70, 1078–1090. [Google Scholar] [CrossRef]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta 2014, 1843, 2563–2582. [Google Scholar] [CrossRef]
- Di Rosa, M.; Giroud, J.P.; Willoughby, D.A. Studies of the mediators of the acute inflammatory response induced in rats in different sites by carrageenan and turpentine. J. Pathol. 1971, 104, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Lauro, M.R.; Marzocco, S.; Rapa, S.F.; Musumeci, T.; Giannone, V.; Picerno, P.; Aquino, R.P.; Puglisi, G. Recycling of Almond By-Products for Intestinal Inflammation: Improvement of Physical-Chemical, Technological and Biological Characteristics of a Dried Almond Skins Extract. Pharmaceutics 2020, 12, 884. [Google Scholar] [CrossRef] [PubMed]
- Udenigwe, C.C.; Je, J.Y.; Cho, Y.S.; Yada, R.Y. Almond protein hydrolysate fraction modulates the expression of proinflammatory cytokines and enzymes in activated macrophages. Food Funct. 2013, 4, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Luo, B.; Mohammad, W.T.; Jalil, A.T.; Saleh, M.M.; Al-Taee, M.M.; Alshahrani, M.Y.; Mohammed, N.M.; Heydani, A. Effects of almond intake on oxidative stress parameters: A systematic review and meta-analysis of clinical trials. Complement. Ther. Med. 2023, 73, 102935. [Google Scholar] [CrossRef]
- Chen, C.O.; Milbury, P.E.; Blumberg, J.B. Polyphenols in Almond Skins after Blanching Modulate Plasma Biomarkers of Oxidative Stress in Healthy Humans. Antioxidants 2019, 8, 95. [Google Scholar] [CrossRef]
- Takeoka, G.R.; Dao, L.T. Antioxidant Constituents of Almond [Prunus dulcis (Mill.) D.A. Webb] Hulls. J. Agric. Food Chem. 2003, 51, 496–501. [Google Scholar] [CrossRef]
- Grotto, D.; Maria, L.S.; Valentini, J.; Paniz, C.; Schmitt, G.; Garcia, S.C.; Pomblum, V.J.; Rocha, J.B.T.; Farina, M. Importance of the lipid peroxidation biomarkers and methodological aspects for malondialdehyde quantification. Quim. Nova 2009, 32, 169–174. [Google Scholar] [CrossRef]
- Vandemoortele, A.; Babat, P.; Yakubu, M.; De Meulenaer, B. Reactivity of Free Malondialdehyde during In Vitro Simulated Gastrointestinal Digestion. J. Agric. Food Chem. 2017, 65, 2198–2204. [Google Scholar] [CrossRef]
- Truong, V.-L.; Bak, M.-J.; Jun, M.; Kong, A.-N.T.; Ho, C.-T.; Jeong, W.-S. Antioxidant Defense and Hepatoprotection by Procyanidins from Almond (Prunus amygdalus) Skins. J. Agric. Food Chem. 2014, 62, 8668–8678. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, J.; Duan, H.; Li, R.; Peng, W.; Wu, C. Activation of Nrf2/HO-1 signaling: An important molecular mechanism of herbal medicine in the treatment of atherosclerosis via the protection of vascular endothelial cells from oxidative stress. J. Adv. Res. 2021, 34, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef] [PubMed]
- Adebayo, A.A.; Oboh, G.; Ademosun, A.O. Nutraceutical potential of almond fruits in managing diabetes-related erectile dysfunction: Effect on Nrf-2 level and smooth muscle/collagen ratio. Andrologia 2022, 54, e14636. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Du, Z.-X.; Zhang, H.-Y.; Meng, X.; Guan, Y.; Wang, H.-Q. Role of oxidative stress and intracellular glutathione in the sensitivity to apoptosis induced by proteasome inhibitor in thyroid cancer cells. BMC Cancer 2009, 9, 56. [Google Scholar] [CrossRef]
- Barral-Martinez, M.; Fraga-Corral, M.; Garcia-Perez, P.; Simal-Gandara, J.; Prieto, M.A. Almond By-Products: Valorization for Sustainability and Competitiveness of the Industry. Foods 2021, 10, 1793. [Google Scholar] [CrossRef]
- Bodoira, R.; Maestri, D. Phenolic Compounds from Nuts: Extraction, Chemical Profiles, and Bioactivity. J. Agric. Food Chem. 2020, 68, 927–942. [Google Scholar] [CrossRef]
- Smeriglio, A.; Denaro, M.; D’Angelo, V.; Germanò, M.P.; Trombetta, D. Antioxidant, Anti-Inflammatory and Anti-Angiogenic Properties of Citrus lumia Juice. Front. Pharmacol. 2020, 11, 593506. [Google Scholar] [CrossRef]
- Marino-Merlo, F.; Klett, A.; Papaianni, E.; Drago, S.F.A.; Macchi, B.; Rincón, M.G.; Andreola, F.; Serafino, A.; Grelli, S.; Mastino, A.; et al. Caspase-8 is required for HSV-1-induced apoptosis and promotes effective viral particle release via autophagy inhibition. Cell Death Differ. 2023, 30, 885–896. [Google Scholar] [CrossRef]
- Marino-Merlo, F.; Papaianni, E.; Frezza, C.; Pedatella, S.; De Nisco, M.; Macchi, B.; Grelli, S.; Mastino, A. NF-κB-Dependent Production of ROS and Restriction of HSV-1 Infection in U937 Monocytic Cells. Viruses 2019, 11, 428. [Google Scholar] [CrossRef] [PubMed]
- Marino-Merlo, F.; Papaianni, E.; Medici, M.A.; Macchi, B.; Grelli, S.; Mosca, C.; Borner, C.; Mastino, A. HSV-1-induced activation of NF-κB protects U937 monocytic cells against both virus replication and apoptosis. Cell Death Dis. 2016, 7, e2354. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.J. Carrageenan-induced paw edema in the rat and mouse. Inflamm. Protoc. 2003, 225, 115–121. [Google Scholar]
- Britti, D.; Crupi, R.; Impellizzeri, D.; Gugliandolo, E.; Fusco, R.; Schievano, C.; Morittu, V.M.; Evangelista, M.; Di Paola, R.; Cuzzocrea, S. A novel composite formulation of palmitoylethanolamide and quercetin decreases inflammation and relieves pain in inflammatory and osteoarthritic pain models. BMC Vet. Res. 2017, 13, 229. [Google Scholar] [CrossRef]
- Salvemini, D.; Wang, Z.Q.; Wyatt, P.S.; Bourdon, D.M.; Marino, M.H.; Manning, P.T.; Currie, M.G. Nitric oxide: A key mediator in the early and late phase of carrageenan-induced rat paw inflammation. Br. J. Pharmacol. 1996, 118, 829–838. [Google Scholar] [CrossRef]
- Hargreaves, K.; Dubner, R.; Brown, F.; Flores, C.; Joris, J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988, 32, 77–88. [Google Scholar] [CrossRef]
- Janes, K.; Little, J.W.; Li, C.; Bryant, L.; Chen, C.; Chen, Z.; Kamocki, K.; Doyle, T.; Snider, A.; Esposito, E.; et al. The development and maintenance of paclitaxel-induced neuropathic pain require activation of the sphingosine 1-phosphate receptor subtype 1. J. Biol. Chem. 2014, 289, 21082–21097. [Google Scholar] [CrossRef]
- Peritore, A.F.; Siracusa, R.; Fusco, R.; Gugliandolo, E.; D’Amico, R.; Cordaro, M.; Crupi, R.; Genovese, T.; Impellizzeri, D.; Cuzzocrea, S.; et al. Ultramicronized Palmitoylethanolamide and Paracetamol, a New Association to Relieve Hyperalgesia and Pain in a Sciatic Nerve Injury Model in Rat. Int. J. Mol. Sci. 2020, 21, 3509. [Google Scholar] [CrossRef]
- Fusco, R.; Siracusa, R.; D’Amico, R.; Peritore, A.F.; Cordaro, M.; Gugliandolo, E.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; Di Paola, R. Melatonin Plus Folic Acid Treatment Ameliorates Reserpine-Induced Fibromyalgia: An Evaluation of Pain, Oxidative Stress, and Inflammation. Antioxidants 2019, 8, 628. [Google Scholar] [CrossRef]
- Ferrier, J.; Marchand, F.; Balayssac, D. Assessment of mechanical allodynia in rats using the electronic von frey test. Bio-Protocol 2016, 6, e1933. [Google Scholar] [CrossRef]
- Cuzzocrea, S.; Mazzon, E.; Esposito, E.; Muià, C.; Abdelrahman, M.; Di Paola, R.; Crisafulli, C.; Bramanti, P.; Thiemermann, C. Glycogen synthase kinase-3β inhibition attenuates the development of ischaemia/reperfusion injury of the gut. Intensive Care Med. 2007, 33, 880–893. [Google Scholar] [CrossRef]
- Costantino, G.; Cuzzocrea, S.; Mazzon, E.; Caputi, A.P. Protective effects of melatonin in zymosan-activated plasma-induced paw inflammation. Eur. J. Pharmacol. 1998, 363, 57–63. [Google Scholar] [CrossRef]
- Cuzzocrea, S.; Mazzon, E.; Bevilaqua, C.; Costantino, G.; Britti, D.; Mazzullo, G.; De Sarro, A.; Caputi, A.P. Cloricromene, a coumarine derivative, protects against collagen-induced arthritis in Lewis rats. Br. J. Pharmacol. 2000, 131, 1399–1407. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Esposito, E.; Di Paola, R.; Ahmad, A.; Campolo, M.; Peli, A.; Morittu, V.M.; Britti, D.; Cuzzocrea, S. Palmitoylethanolamide and luteolin ameliorate development of arthritis caused by injection of collagen type II in mice. Arthritis Res. Ther. 2013, 15, R192. [Google Scholar] [CrossRef]
- Fusco, R.; Salinaro, A.T.; Siracusa, R.; D’Amico, R.; Impellizzeri, D.; Scuto, M.; Ontario, M.L.; Crea, R.; Cordaro, M.; Cuzzocrea, S.; et al. Hidrox(®) Counteracts Cyclophosphamide-Induced Male Infertility through NRF2 Pathways in a Mouse Model. Antioxidants 2021, 10, 778. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Cordaro, M.; Siracusa, R.; Peritore, A.F.; Gugliandolo, E.; Genovese, T.; D’Amico, R.; Crupi, R.; Smeriglio, A.; Mandalari, G.; et al. Consumption of Anacardium occidentale L. (Cashew Nuts) Inhibits Oxidative Stress through Modulation of the Nrf2/HO−1 and NF-kB Pathways. Molecules 2020, 25, 4426. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, R.; Monaco, F.; Fusco, R.; Peritore, A.F.; Genovese, T.; Impellizzeri, D.; Crupi, R.; Interdonato, L.; Sforza, A.M.; Gugliandolo, E.; et al. Exposure to Atrazine Induces Lung Inflammation through Nrf2-HO1 and Beclin 1/LC3 Pathways. Cell Physiol. Biochem. 2021, 55, 413–427. [Google Scholar] [CrossRef]
- Nithya, S. Anti-inflammatory effect of Elettaria cardamom oil on carrageenan-induced paw edema using rats based on tumor necrosis factor α, interleukin 6, and interleukin 1 levels in serum. Asian J. Pharm. Clin. Res 2018, 11, 207. [Google Scholar]
- Cuzzocrea, S.; Nocentini, G.; Di Paola, R.; Agostini, M.; Mazzon, E.; Ronchetti, S.; Crisafulli, C.; Esposito, E.; Caputi, A.P.; Riccardi, C. Proinflammatory Role of Glucocorticoid-Induced TNF Receptor-Related Gene in Acute Lung Inflammation1. J. Immunol. 2006, 177, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Parlar, A.; Arslan, S.O.; Doğan, M.F.; Çam, S.A.; Yalçin, A.; Elibol, E.; Özer, M.K.; Üçkardeş, F.; Kara, H. The exogenous administration of CB2 specific agonist, GW405833, inhibits inflammation by reducing cytokine production and oxidative stress. Exp. Ther. Med. 2018, 16, 4900–4908. [Google Scholar] [CrossRef]
- El-Sayed, E.S.M.; Mansour, A.M.; Nady, M.E. Protective effects of pterostilbene against acetaminophen-induced hepatotoxicity in rats. J. Biochem. Mol. Toxicol. 2015, 29, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Vajic, U.-J.; Grujic-Milanovic, J.; Miloradovic, Z.; Jovovic, D.; Ivanov, M.; Karanovic, D.; Savikin, K.; Bugarski, B.; Mihailovic-Stanojevic, N. Urtica dioica L. leaf extract modulates blood pressure and oxidative stress in spontaneously hypertensive rats. Phytomedicine 2018, 46, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Gugliandolo, E.; Peritore, A.F.; D’Amico, R.; Licata, P.; Crupi, R. Evaluation of Neuroprotective Effects of Quercetin against Aflatoxin B1-Intoxicated Mice. Animals 2020, 10, 898. [Google Scholar] [CrossRef] [PubMed]
- Bruschetta, G.; Di Pietro, P.; Miano, M.; Zanghì, G.; Fazio, E.; Ferlazzo, A.M. Daily variations of plasma serotonin levels in 2-year-old horses. J. Vet. Behav. 2013, 8, 95–99. [Google Scholar] [CrossRef]
- Medica, P.; Bruschetta, G.; Cravana, C.; Ferlazzo, A.; Fazio, E. Effect of transportation on the sympatho-adrenal system responses in horses. Res. Vet. Sci. 2019, 125, 401–404. [Google Scholar] [CrossRef]
- Fusco, R.; Siracusa, R.; Peritore, A.F.; Gugliandolo, E.; Genovese, T.; D’Amico, R.; Cordaro, M.; Crupi, R.; Mandalari, G.; Impellizzeri, D.; et al. The Role of Cashew (Anacardium occidentale L.) Nuts on an Experimental Model of Painful Degenerative Joint Disease. Antioxidants 2020, 9, 511. [Google Scholar] [CrossRef]
- Ye, Y.; Guo, Y.; Luo, Y.T. Anti-inflammatory and analgesic activities of a novel biflavonoid from shells of Camellia oleifera. Int. J. Mol. Sci. 2012, 13, 12401–12411. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Di Paola, R.; Cordaro, M.; Gugliandolo, E.; Casili, G.; Morittu, V.M.; Britti, D.; Esposito, E.; Cuzzocrea, S. Adelmidrol, a palmitoylethanolamide analogue, as a new pharmacological treatment for the management of acute and chronic inflammation. Biochem. Pharmacol. 2016, 119, 27–41. [Google Scholar] [CrossRef]
- Bang, J.S.; Oh, D.H.; Choi, H.M.; Sur, B.-J.; Lim, S.-J.; Kim, J.Y.; Yang, H.-I.; Yoo, M.C.; Hahm, D.-H.; Kim, K.S. Anti-inflammatory and antiarthritic effects of piperine in human interleukin 1β-stimulated fibroblast-like synoviocytes and in rat arthritis models. Arthritis Res. Ther. 2009, 11, R49. [Google Scholar] [CrossRef]
- Coura, C.O.; Souza, R.B.; Rodrigues, J.A.; Vanderlei Ede, S.; de Araújo, I.W.; Ribeiro, N.A.; Frota, A.F.; Ribeiro, K.A.; Chaves, H.V.; Pereira, K.M.; et al. Mechanisms involved in the anti-inflammatory action of a polysulfated fraction from Gracilaria cornea in rats. PLoS ONE 2015, 10, e0119319. [Google Scholar] [CrossRef]
- Cordaro, M.; Impellizzeri, D.; Gugliandolo, E.; Siracusa, R.; Crupi, R.; Esposito, E.; Cuzzocrea, S. Adelmidrol, a Palmitoylethanolamide Analogue, as a New Pharmacological Treatment for the Management of Inflammatory Bowel Disease. Mol. Pharmacol. 2016, 90, 549–561. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, R.; Fusco, R.; Cordaro, M.; Siracusa, R.; Peritore, A.F.; Gugliandolo, E.; Crupi, R.; Scuto, M.; Cuzzocrea, S.; Di Paola, R. Modulation of NLRP3 inflammasome through formyl peptide receptor 1 (Fpr-1) pathway as a new therapeutic target in bronchiolitis obliterans syndrome. Int. J. Mol. Sci. 2020, 21, 2144. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Campolo, M.; Casili, G.; Lanza, M.; Franco, D.; Filippone, A.; Peritore, A.F.; Cuzzocrea, S. Protective effects of xyloglucan in association with the polysaccharide gelose in an experimental model of gastroenteritis and urinary tract infections. Int. J. Mol. Sci. 2018, 19, 1844. [Google Scholar] [CrossRef] [PubMed]
- Impellizzeri, D.; Siracusa, R.; Cordaro, M.; Peritore, A.F.; Gugliandolo, E.; D’amico, R.; Fusco, R.; Crupi, R.; Rizzarelli, E.; Cuzzocrea, S.; et al. Protective effect of a new hyaluronic acid -carnosine conjugate on the modulation of the inflammatory response in mice subjected to collagen-induced arthritis. Biomed. Pharmacother. 2020, 125, 110023. [Google Scholar] [CrossRef] [PubMed]
- Cordaro, M.; Impellizzeri, D.; Siracusa, R.; Gugliandolo, E.; Fusco, R.; Inferrera, A.; Esposito, E.; Di Paola, R.; Cuzzocrea, S. Effects of a co-micronized composite containing palmitoylethanolamide and polydatin in an experimental model of benign prostatic hyperplasia. Toxicol. Appl. Pharmacol. 2017, 329, 231–240. [Google Scholar] [CrossRef]
- Siracusa, R.; Fusco, R.; Peritore, A.F.; Cordaro, M.; D’Amico, R.; Genovese, T.; Gugliandolo, E.; Crupi, R.; Smeriglio, A.; Mandalari, G.; et al. The Antioxidant and Anti-Inflammatory Properties of Anacardium occidentale L. Cashew Nuts in a Mouse Model of Colitis. Nutrients 2020, 12, 834. [Google Scholar] [CrossRef]
- Sawant, S.; Gokulan, R.; Dongre, H.; Vaidya, M.; Chaukar, D.; Prabhash, K.; Ingle, A.; Joshi, S.; Dange, P.; Joshi, S. Prognostic role of Oct4, CD44 and c-Myc in radio–chemo-resistant oral cancer patients and their tumourigenic potential in immunodeficient mice. Clin. Oral Investig. 2016, 20, 43–56. [Google Scholar] [CrossRef]
- Russo, E.; Citraro, R.; Donato, G.; Camastra, C.; Iuliano, R.; Cuzzocrea, S.; Constanti, A.; De Sarro, G. mTOR inhibition modulates epileptogenesis, seizures and depressive behavior in a genetic rat model of absence epilepsy. Neuropharmacology 2013, 69, 25–36. [Google Scholar] [CrossRef]
- Gugliandolo, E.; Fusco, R.; D’Amico, R.; Militi, A.; Oteri, G.; Wallace, J.L.; Di Paola, R.; Cuzzocrea, S. Anti-inflammatory effect of ATB-352, a H2S− releasing ketoprofen derivative, on lipopolysaccharide-induced periodontitis in rats. Pharmacol. Res. 2018, 132, 220–231. [Google Scholar] [CrossRef]
- Petrosino, S.; Cordaro, M.; Verde, R.; Schiano Moriello, A.; Marcolongo, G.; Schievano, C.; Siracusa, R.; Piscitelli, F.; Peritore, A.F.; Crupi, R.; et al. Oral Ultramicronized Palmitoylethanolamide: Plasma and Tissue Levels and Spinal Anti-hyperalgesic Effect. Front. Pharmacol. 2018, 9, 249. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Siracusa, R.; Cordaro, M.; Crupi, R.; Peritore, A.F.; Gugliandolo, E.; D’Amico, R.; Petrosino, S.; Evangelista, M.; Di Paola, R.; et al. N-Palmitoylethanolamine-oxazoline (PEA-OXA): A new therapeutic strategy to reduce neuroinflammation, oxidative stress associated to vascular dementia in an experimental model of repeated bilateral common carotid arteries occlusion. Neurobiol. Dis. 2019, 125, 77–91. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, R.; Monaco, F.; Siracusa, R.; Cordaro, M.; Fusco, R.; Peritore, A.F.; Gugliandolo, E.; Crupi, R.; Cuzzocrea, S.; Di Paola, R.; et al. Ultramicronized Palmitoylethanolamide in the Management of Sepsis-Induced Coagulopathy and Disseminated Intravascular Coagulation. Int. J. Mol. Sci. 2021, 22, 11388. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, R.; Monaco, F.; Fusco, R.; Siracusa, R.; Impellizzeri, D.; Peritore, A.F.; Crupi, R.; Gugliandolo, E.; Cuzzocrea, S.; Di Paola, R.; et al. Atrazine Inhalation Worsen Pulmonary Fibrosis Regulating the Nuclear Factor-Erythroid 2-Related Factor (Nrf2) Pathways Inducing Brain Comorbidities. Cell Physiol. Biochem. 2021, 55, 704–725. [Google Scholar] [CrossRef]
- Siracusa, R.; Monaco, F.; D’Amico, R.; Genovese, T.; Cordaro, M.; Interdonato, L.; Gugliandolo, E.; Peritore, A.F.; Crupi, R.; Cuzzocrea, S.; et al. Epigallocatechin-3-Gallate Modulates Postoperative Pain by Regulating Biochemical and Molecular Pathways. Int. J. Mol. Sci. 2021, 22, 6879. [Google Scholar] [CrossRef] [PubMed]


| Polyphenols | RT a (min) | λmax (nm) | [M − H]− | mg/100 g DE b |
|---|---|---|---|---|
| Hydroxybenzoic acids | ||||
| Protocatechuic acid | 7.04 | 258; 293 | 153 | 38.01 ± 0.55 |
| 4-Hydroxybenzoic acid | 12.00 | 253 | 137 | 0.29 ± 0.01 |
| Vanillic acid | 16.00 | 262; 291 | 167 | 30.88 ± 0.42 |
| Hydroxycinnamic acids | ||||
| Chlorogenic acid | 20.50 | 291; 319 | 353 | 28.28 ± 0.28 |
| trans-p-coumaric acid | 22.80 | 309 | 163 | - |
| Flavanones | ||||
| Eriodictyol-7-O-glucoside | 29.71 | 283 | 449 | 3.90 ± 0.08 |
| Naringenin-7-O-glucoside | 32.43 | 282 | 433 | 39.36 ± 0.67 |
| Eriodyctiol | 35.66 | 287 | 287 | 0.01 ± 0.00 |
| Naringenin | 40.26 | 289 | 271 | 0.02 ± 0.00 |
| Flavonols | ||||
| Quercetin-3-O-galactoside | 32.36 | 253; 354 | 463 | 0.52 ± 0.01 |
| Quercetin-3-O-rutinoside | 32.41 | 254; 354 | 609 | 0.08 ± 0.00 |
| Quercetin-3-O-glucoside | 32.64 | 254; 354 | 463 | 2.81 ± 0.02 |
| Kaempferol-3-O-rutinoside | 33.96 | 265; 348 | 593 | 9.20 ± 0.03 |
| Kaempferol-3-O-glucoside | 34.34 | 264; 347 | 447 | 3.33 ± 0.02 |
| Quercetin-3-O-rhamnoside | 34.36 | 257; 358 | 447 | 2.04 ± 0.01 |
| Isorhamnetin-3-O-glucoside | 34.88 | 254; 353 | 477 | 377.29 ± 1.55 |
| Quercetin | 39.39 | 255; 370 | 301 | 0.09 ± 0.00 |
| Kaempferol | 43.66 | 264; 365 | 285 | 1.02 ± 0.02 |
| Isorhamnetin | 44.47 | 253; 368 | 315 | 0.11 ± 0.00 |
| Flavanols | ||||
| Catechin | 18.65 | 279 | 289 | 388.91 ± 2.21 |
| Epicatechin | 23.64 | 279 | 289 | 178.92 ± 1.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arangia, A.; Ragno, A.; Cordaro, M.; D’Amico, R.; Siracusa, R.; Fusco, R.; Marino Merlo, F.; Smeriglio, A.; Impellizzeri, D.; Cuzzocrea, S.; et al. Antioxidant Activity of a Sicilian Almond Skin Extract Using In Vitro and In Vivo Models. Int. J. Mol. Sci. 2023, 24, 12115. https://doi.org/10.3390/ijms241512115
Arangia A, Ragno A, Cordaro M, D’Amico R, Siracusa R, Fusco R, Marino Merlo F, Smeriglio A, Impellizzeri D, Cuzzocrea S, et al. Antioxidant Activity of a Sicilian Almond Skin Extract Using In Vitro and In Vivo Models. International Journal of Molecular Sciences. 2023; 24(15):12115. https://doi.org/10.3390/ijms241512115
Chicago/Turabian StyleArangia, Alessia, Agnese Ragno, Marika Cordaro, Ramona D’Amico, Rosalba Siracusa, Roberta Fusco, Francesca Marino Merlo, Antonella Smeriglio, Daniela Impellizzeri, Salvatore Cuzzocrea, and et al. 2023. "Antioxidant Activity of a Sicilian Almond Skin Extract Using In Vitro and In Vivo Models" International Journal of Molecular Sciences 24, no. 15: 12115. https://doi.org/10.3390/ijms241512115
APA StyleArangia, A., Ragno, A., Cordaro, M., D’Amico, R., Siracusa, R., Fusco, R., Marino Merlo, F., Smeriglio, A., Impellizzeri, D., Cuzzocrea, S., Mandalari, G., & Di Paola, R. (2023). Antioxidant Activity of a Sicilian Almond Skin Extract Using In Vitro and In Vivo Models. International Journal of Molecular Sciences, 24(15), 12115. https://doi.org/10.3390/ijms241512115












