Equol and Resveratrol Improve Bone Turnover Biomarkers in Postmenopausal Women: A Clinical Trial
Abstract
1. Introduction
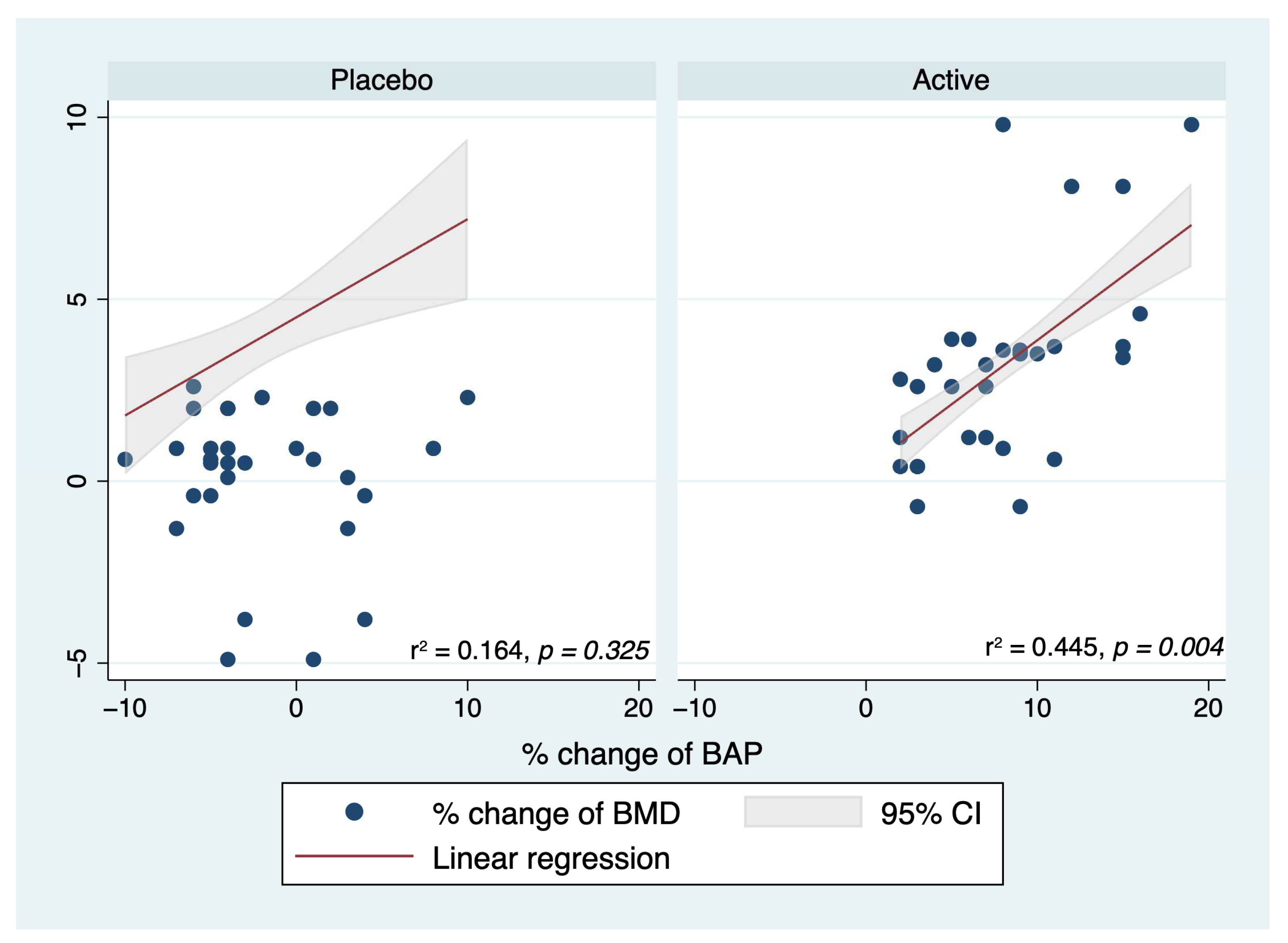
2. Results
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. Study Design
4.3. Intervention and Randomization
4.4. Measurement of Bone Markers
4.5. Measurement of BMD
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singer, A.; Exuzides, A.; Spangler, L.; O’Malley, C.; Colby, C.; Johnston, K.; Agodoa, I.; Baker, J.; Kagan, R. Burden of illness for osteoporotic fractures compared with other serious diseases among postmenopausal women in the United States. Mayo Clin. Proc. 2015, 90, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Clynes, M.A.; Harvey, N.C.; Curtis, E.M.; Fuggle, N.R.; Dennison, E.M.; Cooper, C. The epidemiology of osteoporosis. Br. Med. Bull. 2020, 133, 105–117. [Google Scholar] [CrossRef]
- Cheng, C.H.; Chen, L.R.; Chen, K.H. Osteoporosis Due to Hormone Imbalance: An Overview of the Effects of Estrogen Deficiency and Glucocorticoid Overuse on Bone Turnover. Int. J. Mol. Sci. 2022, 23, 1376. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.R.; Chen, C.H. Bone biomarker for the clinical assessment of osteoporosis: Recent developments and future perspectives. Biomark. Res. 2017, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, Y.; Miura, M.; Ichimura, S.; Inaba, M.; Imanishi, Y.; Shiraki, M.; Takada, J.; Chaki, O.; Hagino, H.; Fukunaga, M.; et al. From the Japan Osteoporosis Society Bone Turnover Marker Investigation Committee. Executive summary of the Japan Osteoporosis Society Guide for the Use of Bone Turnover Markers in the Diagnosis and Treatment of Osteoporosis (2018 Edition). Clin. Chim. Acta 2019, 498, 101–107. [Google Scholar] [CrossRef]
- Lumachi, F.; Ermani, M.; Camozzi, V.; Tombolan, V.; Luisetto, G. Changes of bone formation markers osteocalcin and bone-specific alkaline phosphatase in postmenopausal women with osteoporosis. Ann. N. Y. Acad. Sci. 2009, 1173 (Suppl. S1), E60–E63. [Google Scholar] [CrossRef]
- Hlaing, T.T.; Compston, J.E. Biochemical markers of bone turnover—Uses and limitations. Ann. Clin. Biochem. 2014, 51 Pt 2, 189–202. [Google Scholar] [CrossRef]
- Sansai, K.; Na Takuathung, M.; Khatsri, R.; Teekachunhatean, S.; Hanprasertpong, N.; Koonrungsesomboon, N. Effects of isoflavone interventions on bone mineral density in postmenopausal women: A systematic review and meta-analysis of randomized controlled trials. Osteoporos. Int. 2020, 31, 1853–1864. [Google Scholar] [CrossRef]
- Fatima, A.; Khan, M.S.; Ahmad, M.W. Therapeutic Potential of Equol: A Comprehensive Review. Curr. Pharm. Des. 2020, 26, 5837–5843. [Google Scholar] [CrossRef]
- Tousen, Y.; Ezaki, J.; Fujii, Y.; Ueno, T.; Nishimuta, M.; Ishimi, Y. Natural S-equol decreases bone resorption in postmenopausal, non-equol-producing Japanese women: A pilot randomized, placebo-controlled trial. Menopause 2011, 18, 563–574. [Google Scholar] [CrossRef]
- Yoshikata, R.; Myint, K.Z.Y.; Ohta, H. Effects of Equol Supplement on Bone and Cardiovascular Parameters in Middle-Aged Japanese Women: A Prospective Observational Study. J. Altern. Complement. Med. 2018, 24, 701–708. [Google Scholar] [CrossRef]
- Davinelli, S.; Scapagnini, G.; Marzatico, F.; Nobile, V.; Ferrara, N.; Corbi, G. Influence of equol and resveratrol supplementation on health-related quality of life in menopausal women: A randomized, placebo-controlled study. Maturitas 2017, 96, 77–83. [Google Scholar] [CrossRef]
- Davinelli, S.; Sapere, N.; Visentin, M.; Zella, D.; Scapagnini, G. Enhancement of mitochondrial biogenesis with polyphenols: Combined effects of resveratrol and equol in human endothelial cells. Immun. Ageing 2013, 10, 28. [Google Scholar] [CrossRef]
- Qasem, R.J. The estrogenic activity of resveratrol: A comprehensive review of in vitro and in vivo evidence and the potential for endocrine disruption. Crit. Rev. Toxicol. 2020, 50, 439–462. [Google Scholar] [CrossRef]
- Tou, J.C. Evaluating resveratrol as a therapeutic bone agent: Preclinical evidence from rat models of osteoporosis. Ann. N. Y. Acad. Sci. 2015, 1348, 75–85. [Google Scholar] [CrossRef]
- Majumder, M.I.; Harun, M.A.S.I. Alveolar Bone Changes in Post-menopausal Osteopenic and Osteoporosis Women: An Original Research. Int. J. Dent. Med. Spec. 2015, 2, 9–14. [Google Scholar] [CrossRef]
- Lambert, M.N.T.; Thybo, C.B.; Lykkeboe, S.; Rasmussen, L.M.; Frette, X.; Christensen, L.P.; Jeppesen, P.B. Combined bioavailable isoflavones and probiotics improve bone status and estrogen metabolism in postmenopausal osteopenic women: A randomized controlled trial. Am. J. Clin. Nutr. 2017, 106, 909–920. [Google Scholar] [CrossRef]
- Arooj, A.; Rabail, R.; Naeem, M.; Goksen, G.; Xu, B.; Aadil, R.M. A comprehensive review of the bioactive components of sesame seeds and their impact on bone health issues in postmenopausal women. Food Funct. 2023, 14, 4966–4980. [Google Scholar] [CrossRef]
- Jackson, R.L.; Greiwe, J.S.; Schwen, R.J. Emerging evidence of the health benefits of S-equol, an estrogen receptor β agonist. Nutr. Rev. 2011, 69, 432–448. [Google Scholar] [CrossRef]
- Wei, P.; Liu, M.; Chen, Y.; Chen, D.D. Systematic review of soy isoflavone supplements on osteoporosis in women. Asian Pac. J. Trop. Med. 2012, 5, 243–248. [Google Scholar] [CrossRef]
- Pawlowski, J.W.; Martin, B.R.; McCabe, G.P.; McCabe, L.; Jackson, G.S.; Peacock, M.; Barnes, S.; Weaver, C.M. Impact of equol-producing capacity and soy-isoflavone profiles of supplements on bone calcium retention in postmenopausal women: A randomized crossover trial. Am. J. Clin. Nutr. 2015, 102, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Chow, H.H.; Garland, L.L.; Heckman-Stoddard, B.M.; Hsu, C.H.; Butler, V.D.; Cordova, C.A.; Chew, W.M.; Cornelison, T.L. A pilot clinical study of resveratrol in postmenopausal women with high body mass index: Effects on systemic sex steroid hormones. J. Transl. Med. 2014, 12, 223. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.H.; Thaung Zaw, J.J.; Xian, C.J.; Howe, P.R. Regular Supplementation with Resveratrol Improves Bone Mineral Density in Postmenopausal Women: A Randomized, Placebo-Controlled Trial. J. Bone Miner. Res. 2020, 35, 2121–2131. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, X.; Li, N.; Liu, T.; Liu, J.; Li, Z.; Xiao, H.; Li, J. Long-term resveratrol treatment prevents ovariectomy-induced osteopenia in rats without hyperplastic effects on the uterus. Br. J. Nutr. 2014, 111, 836–846. [Google Scholar] [CrossRef]
- Arjmandi, B.H.; Smith, B.J. Soy isoflavones’ osteoprotective role in postmenopausal women: Mechanism of action. J. Nutr. Biochem. 2002, 13, 130–137. [Google Scholar] [CrossRef]
- Hooshiar, S.H.; Tobeiha, M.; Jafarnejad, S. Soy Isoflavones and Bone Health: Focus on the RANKL/RANK/OPG Pathway. Biomed. Res. Int. 2022, 2022, 8862278. [Google Scholar] [CrossRef]
- Tousen, Y.; Ichimaru, R.; Kondo, T.; Inada, M.; Miyaura, C.; Ishimi, Y. The Combination of Soy Isoflavones and Resveratrol Preserve Bone Mineral Density in Hindlimb-Unloaded Mice. Nutrients 2020, 12, 2043. [Google Scholar] [CrossRef]
- Tai, T.Y.; Tsai, K.S.; Tu, S.T.; Wu, J.S.; Chang, C.I.; Chen, C.L.; Shaw, N.S.; Peng, H.Y.; Wang, S.Y.; Wu, C.H. The effect of soy isoflavone on bone mineral density in postmenopausal Taiwanese women with bone loss: A 2-year randomized double-blind placebo-controlled study. Osteoporos. Int. 2012, 23, 1571–1580. [Google Scholar] [CrossRef]
- Kreijkamp-Kaspers, S.; Kok, L.; Grobbee, D.E.; de Haan, E.H.; Aleman, A.; Lampe, J.W.; van der Schouw, Y.T. Effect of soy protein containing isoflavones on cognitive function, bone mineral density, and plasma lipids in postmenopausal women: A randomized controlled trial. JAMA 2004, 292, 65–74. [Google Scholar] [CrossRef]
- Wu, J.; Oka, J.; Ezaki, J.; Ohtomo, T.; Ueno, T.; Uchiyama, S.; Toda, T.; Uehara, M.; Ishimi, Y. Possible role of equol status in the effects of isoflavone on bone and fat mass in postmenopausal Japanese women: A double-blind, randomized, controlled trial. Menopause 2007, 14, 866–874. [Google Scholar] [CrossRef]
- WHO. Menopause. Available online: https://www.who.int/news-room/fact-sheets/detail/menopause (accessed on 10 January 2023).
- Ishiwata, N.; Melby, M.K.; Mizuno, S.; Watanabe, S. New equol supplement for relieving menopausal symptoms: Randomized, placebo-controlled trial of Japanese women. Menopause 2009, 16, 141–148. [Google Scholar] [CrossRef]
- Oyama, A.; Ueno, T.; Uchiyama, S.; Aihara, T.; Miyake, A.; Kondo, S.; Matsunaga, K. The effects of natural S-equol supplementation on skin aging in postmenopausal women: A pilot randomized placebo-controlled trial. Menopause 2012, 19, 202–210. [Google Scholar] [CrossRef]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E., Jr.; Walle, U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef]
- Almeida, L.; Vaz-da-Silva, M.; Falcão, A.; Soares, E.; Costa, R.; Loureiro, A.I.; Fernandes-Lopes, C.; Rocha, J.F.; Nunes, T.; Wright, L.; et al. Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Mol. Nutr. Food Res. 2009, 53 (Suppl. S1), S7–S15. [Google Scholar] [CrossRef]
- Cottart, C.H.; Nivet-Antoine, V.; Laguillier-Morizot, C.; Beaudeux, J.L. Resveratrol bioavailability and toxicity in humans. Mol. Nutr. Food Res. 2010, 54, 7–16. [Google Scholar] [CrossRef]


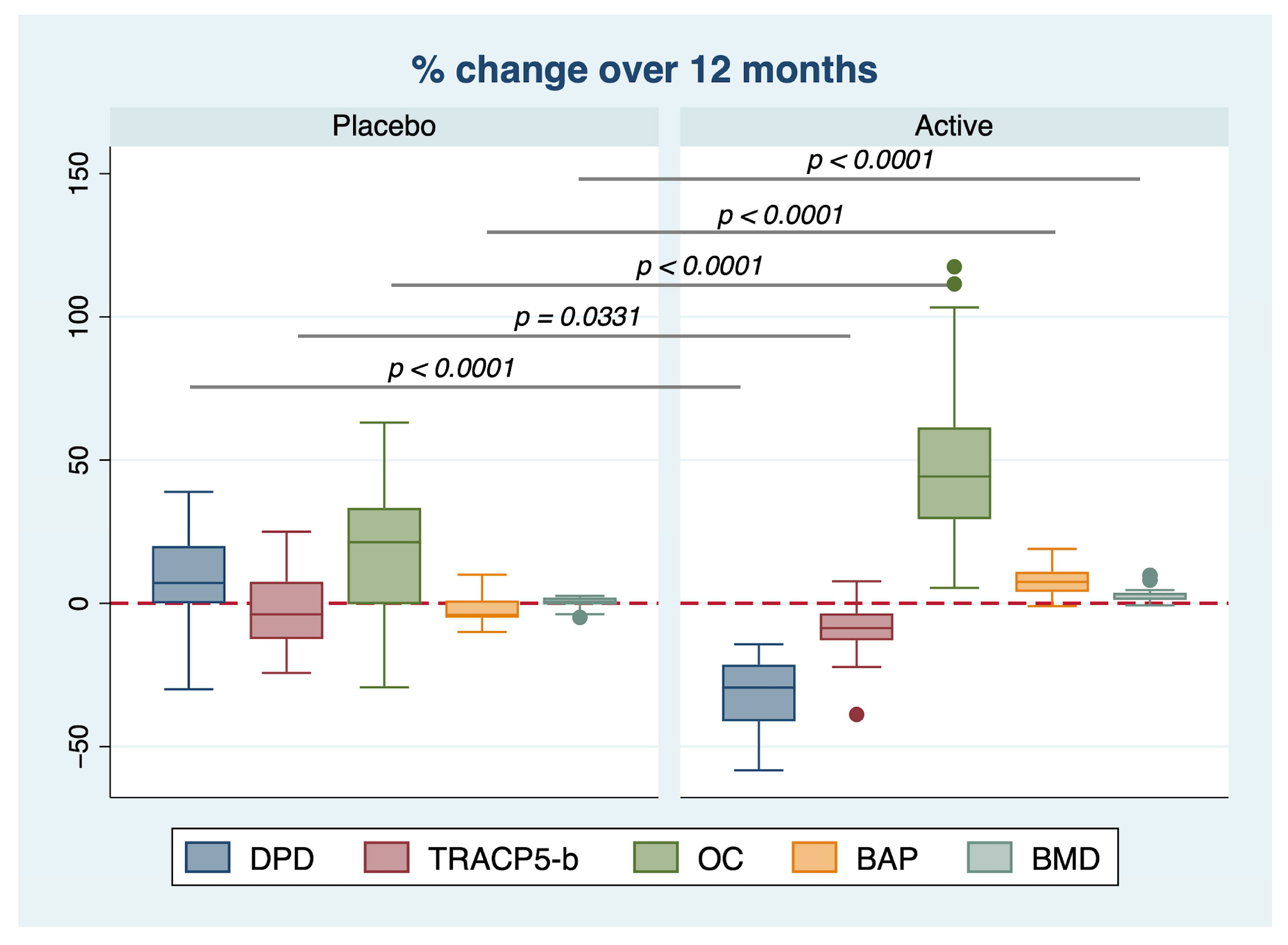
| Characteristic | Placebo | Active | p |
|---|---|---|---|
| Age, years | 52.69 ± 2.10 | 52.09 ± 1.71 | 0.931 |
| SBP, mmHg | 134.17 ± 12.87 | 134.26 ± 8.89 | 0.972 |
| DBP, mmHg | 85.17 ± 9.69 | 88.45 ± 8.46 | 0.171 |
| BMI, Kg/m2 | 23.84 ± 2.19 | 23.42 ± 1.81 | 0.355 |
| Total Cholesterol, mg/dL | 171.38 ± 29.23 | 178.03 ± 30.33 | 0.322 |
| HDL Cholesterol, mg/dL | 49.60 ± 21.94 | 50.64 ± 20.71 | 0.818 |
| LDL Cholesterol, mg/dL | 91.24 ± 34.78 | 98.08 ± 34.31 | 0.379 |
| Triglycerides, mg/dL | 108.43 ± 62.97 | 110.11 ± 66.97 | 0.904 |
| ALT, U/L | 22.95 ± 5.93 | 24.13 ± 9.49 | 0.217 |
| AST, U/L | 23.57 ± 4.41 | 24.47 ± 5.25 | 0.406 |
| Glycemia, mg/dL | 93.60 ± 28.98 | 91.08 ± 16.74 | 0.635 |
| DPD, pmol/μmol | 13.73 ± 2.98 | 14.40 ± 3.05 | 0.395 |
| TRACP-5b, U/L | 4.29 ± 0.81 | 4.11 ± 0.91 | 0.422 |
| Osteocalcin, ng/mL | 23.53 ± 5.67 | 22.95 ± 5.20 | 0.679 |
| BAP, μ/mL | 45.77 ± 2.30 | 45.13 ± 3.00 | 0.363 |
| BMD, SD | 0.827 ± 0.097 | 0.867 ± 0.119 | 0.211 |
| Var_BMD | Beta | [95% Conf. Interval] | p | |
|---|---|---|---|---|
| Lowest | Highest | |||
| Var_BAP | 0.216 | 0.08 | 0.350 | 0.002 |
| Var_OC | −0.014 | −0.040 | 0.012 | 0.285 |
| Var_TRACP-5b | 0.008 | −0.046 | 0.062 | 0.771 |
| Var_DPD | 0.052 | 0.008 | 0.095 | 0.020 |
| Group | ||||
| Active | 3.394 | 1.245 | 5.544 | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corbi, G.; Nobile, V.; Conti, V.; Cannavo, A.; Sorrenti, V.; Medoro, A.; Scapagnini, G.; Davinelli, S. Equol and Resveratrol Improve Bone Turnover Biomarkers in Postmenopausal Women: A Clinical Trial. Int. J. Mol. Sci. 2023, 24, 12063. https://doi.org/10.3390/ijms241512063
Corbi G, Nobile V, Conti V, Cannavo A, Sorrenti V, Medoro A, Scapagnini G, Davinelli S. Equol and Resveratrol Improve Bone Turnover Biomarkers in Postmenopausal Women: A Clinical Trial. International Journal of Molecular Sciences. 2023; 24(15):12063. https://doi.org/10.3390/ijms241512063
Chicago/Turabian StyleCorbi, Graziamaria, Vincenzo Nobile, Valeria Conti, Alessandro Cannavo, Vincenzo Sorrenti, Alessandro Medoro, Giovanni Scapagnini, and Sergio Davinelli. 2023. "Equol and Resveratrol Improve Bone Turnover Biomarkers in Postmenopausal Women: A Clinical Trial" International Journal of Molecular Sciences 24, no. 15: 12063. https://doi.org/10.3390/ijms241512063
APA StyleCorbi, G., Nobile, V., Conti, V., Cannavo, A., Sorrenti, V., Medoro, A., Scapagnini, G., & Davinelli, S. (2023). Equol and Resveratrol Improve Bone Turnover Biomarkers in Postmenopausal Women: A Clinical Trial. International Journal of Molecular Sciences, 24(15), 12063. https://doi.org/10.3390/ijms241512063










