Abstract
Symptoms of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) are common in rheumatic diseases, but no studies report the frequency of these in early systemic sclerosis. There are no known biomarkers that can distinguish between patients with ME/CFS, although mitochondrial abnormalities are often demonstrated. We sought to assess the prevalence of ME/CFS in limited cutaneous SSc (lcSSc) patients early in their disease (<5 years from the onset of non-Raynaud’s symptoms) and to determine if alterations in mitochondrial electron transport chain (ETC) transcripts and mitochondrial DNA (mtDNA) integrity could be used to distinguish between fatigued and non-fatigued patients. All SSc patients met ACR/EULAR classification criteria. ME/CFS-related symptoms were assessed through validated questionnaires, and the expression of ETC transcripts and mtDNA integrity were quantified via qPCR. SSc patients with ME/CFS could be distinguished from non-fatigued patients through ETC gene analysis; specifically, reduced expression of ND4 and CyB and increased expression of Cox7C. ND4 and CyB expression correlated with indicators of disease severity. Further prospective and functional studies are needed to determine if this altered signature can be further utilized to better identify ME/CFS in SSc patients, and whether ME/CFS in early SSc disease could predict more severe disease outcomes.
1. Introduction
Systemic sclerosis (SSc) is a heterogeneous disease characterized by immune dysregulation, vasculopathy and fibrosis of the skin and visceral organs [1]. These changes can be present early in the disease (<5 years), with patients having Raynaud’s phenomenon (RP) SSc-specific autoantibodies (e.g., anticentromere antibodies (ACA), anti-Scl70 autoantibodies) and a SSc-pattern that is detected using nailfold video capillaroscopy (NVC). The most common clinical manifestations present in early SSc include puffy fingers, digital pits/ulcers and/or interstitial lung disease [1]. Early in SSc, skin fibrosis may not be clinically apparent in many patients [1]. Many SSc patients may suffer from nonspecific symptoms such as fatigue or muscle pain, which can be mistaken for fibromyalgia (FM). These symptoms may have profound effects on the quality of life in patients with SSc, and potentially result in loss of income [2].
Fatigue in SSc is common in patients with long-standing SSc with rates ranging between 48–79% [2,3]. In many studies, it has been suggested that lung fibrosis is an important driver of fatigue, but this is not well established [2]. In contrast to symptoms of just being tired, ME/CFS is characterized by severe fatigue associated with unrefreshing sleep, post-exertional malaise, cognitive dysfunction and dysautonomia symptoms such as orthostatic intolerance. Many patients may also develop widespread pain, resulting in comorbid FM [4].
Both fatigue and FM are frequently associated with other rheumatic diseases, though studies assessing the frequency of ME/CFS and FM in established SSc are few [5,6,7]. Perrot et al. found that 27.8% of SSc patients (n = 122) presented with FM, and that these comorbid patients had higher BMIs, greater pain intensity and more diffuse pain [6]. In patients with long standing SSc, fatigue is associated with increased skin fibrosis, which is known to be associated with a poor outcome [2]. Importantly, no studies have quantified the frequency of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and FM in SSc patients particularly early on in their course of the disease [3]
Though the etiology of ME/CFS and FM remains poorly defined, mitochondrial dysregulation may play a prominent role in their pathophysiology [8,9]. Importantly, many patients with idiopathic FM demonstrate dysregulated expression of proteins in the mitochondrial electron transport chain (ETC) (e.g., ND4 (complex I), Cytochrome B (CyB, complex III) and COX7C (complex IV)) [8]. Mitochondria are responsible for regulating metabolic activity and the generation of important intermediates, and play integral roles in critical cellular decisions such as cell death and proliferation. Notably, oxidative phosphorylation, utilizing the ETC, produces important metabolic intermediates and ATP [10].
Though the majority of mitochondrial proteins are nuclear-encoded, a subset of core ETC proteins are encoded by the mitochondrial genome (mtDNA). Hence, mitochondrial biogenesis requires coordinated expression from both genomes. ND4 and CyB are mitochondrially encoded and Cox7C is encoded by the nucleus [10]. Loss of mitochondrial functions as a result of altered ETC gene expression has been linked to aging and diseases associated with accelerated aging in the immune system, such as atherosclerosis, Alzheimer’s and multiple sclerosis [11,12,13,14,15]. Notably, oxidative stress and the disruption of oxygen homeostasis has been linked to the development of vascular remodeling and fibrosis [16,17].
Based on clinical observations in our SSc patient cohort, we hypothesized that many patients with early SSc (disease duration <5 years) may fulfill the diagnostic criteria for ME/CFS and/or FM. We postulate that markers of mitochondrial dysfunction may be preferentially dysregulated in patients with SSc suffering from ME/CFS, compared to those with SSc without ME/CFS.
2. Results
2.1. Demographic and Clinical Characteristics of Study Population
Consent for inclusion into this study was obtained from 12 fatigued and 12 non-fatigued, sequentially recruited lcSSc patients with non-Raynaud’s related symptoms <5 years that were age- and sex-matched to healthy controls. The 12 fatigued patients fulfilled the classification criteria for ME/CFS (SSc-CFS); the 12 non-fatigued patients did not (SSc-NCFS). All SSc patients had Raynaud’s phenomenon with a SSc profile on nailfold video-capillaroscopy (NVC) [18]. Median modified Rodnan skin scores (mRSS) were zero. Median forced expiratory volume in one second (FEV1)/forced vital capacity (FVC) ratios—77.5 (72.8;82.3); single-breath diffusing capacity of the lung (DLCO SB)—18.61 (15.8;22.3); FEV1—2.55 (2.1;3.0); and FVC—3.29 (2.6;3.8) values were within normal reference ranges. A total of 87.5% (21/24) of SSc patients had puffy fingers; few (25% (6/24)) had digital ulcers; 37.5% (9/24) reported arthralgia’s; and none suffered joint contractures. Gastrointestinal symptoms were reported by 29.1% (7/24) of patients, and interstitial lung disease (ILD) (confirmed by chest computer tomography) was documented in 16.6% (4/24).
Comparing SSc patients with ME/CFS (SSc-CFS), SSc without ME/CFS (SSc-NCFS) and healthy controls (HC), we found no statistical differences between age, gender, body mass index (BMI) and C-reactive protein (CRP) (Table 1). Thyroid-stimulating hormone levels were significantly higher in the SSc-CFS group (2.63) when compared to both SSc-NCFS (1.54, p = 0.007) and HC (1.29, p = 0.009), though all individuals had levels within the normal reference range (0.2–4.0 mU/L). SSc disease-related factors, including mRSS score, digital ulcers, autoantibodies, puffy fingers, lung function (DLCOSB, FEV, FVC) and ILD, were similar between SSc-CFS and SSc-NCFS patients (Table 1). Arthralgia was, however, more often present in SSc-CFS patients (X2 = 4.44, p = 0.035). Immunomodulatory and vasodilator medication use was similar between SSc-CFS and SSc-NCFS patients (X2 = 1.53, p = 0.675), though immunomodulatory therapy was associated with higher rates of arthralgia (X2 = 15.1, p = 0.002), and vasodilator use (without immunomodulators) associated with digital ulcers (X2 = 9.53, p = 0.025) (Supplementary Table S2).

Table 1.
Patient demographics and clinical findings. SSc-CFS patients are compared to SSc-NCFS patients and healthy controls.
2.2. ME/CFS and FM in SSc
As we predicted, severe fatigue in SSc-CFS patients (as defined by the MFI (67.5) and FACIT (24)) was significantly higher than in the SSc-NCFS group (39, p < 0.001, 48, p < 0.001) (Table 1, Supplementary Table S1). A total of 66.6% (8/12) of SSc-CFS patients also fulfilled the diagnostic criteria for FM. Pain, as measured by the widespread pain index (WPI), was significantly higher in SSc-CFS (6.5) patients than both SSc-NCFS patients (1, p < 0.001) and HCs (0.5, p < 0.001). The symptom severity score (SSS), which quantifies the severity of fatigue, tiredness and cognitive impairment in the preceding week, was higher in SSc-CFS patients (7) than both SSc-NCFS (3, p < 0.001) and HCs (1, p < 0.001) (Supplementary Table S1). Quality of life, as measured by the physical (PCS) and mental (MCS) components of the SF-36, was significantly lower in SSc-CFS patients when compared with both SSc-NCFS and HCs (p < 0.001) (Table 1).
Rates of depression were similar in all patient and control groups (Table 1), though HCs (0.5, p < 0.001) and SSc-NCFS (1.5, p = 0.002) patients had significantly lower HADS_depression scores than SSc-CFS patients (5) (Supplementary Table S1). Rates of anxiety, as well as HADS_anxiety scores, were significantly higher in SSc-CFS, compared to both SSc-NCFS and HCs (p < 0.050) (Table 1, Supplementary Table S1). Similar rates of sleep disturbance were seen for SSc-CFS patients and SSc-NCFS patients (X2 = 2.25, p = 0.133) (Table 1), but SSc-CFS patients had significantly higher Pittsburgh Sleep Quality Index (PSQI) scores (13) than SSc-NCFS patients (3.5, p < 0.001) (Supplementary Table S1). Cognitive failure was similar between all groups (Table 1, Supplementary Table S1). Immunomodulatory and vasodilator medication use was not associated with symptoms related to ME/CFS in our SSc patients (Supplementary Table S2).
2.3. ETC Differential Expression and Cell Free Mitochondrial DNA Integrity
We investigated expression levels of genes within the ETC that have previously been associated with altered mitochondrial functions in ME/CFS and/or FM, using whole-blood RNA purified from PAX gene extractions. Compared to SSc-NCFS patients (0.47), SSc-CFS patients showed a trend of reduced expression of ND4 (0.34, p = 0.051) (Figure 1A). CyB expression was significantly lower for SSc-CFS (0.21) when compared to both HCs (0.42, p = 0.030) and SSc-NCFS patients (0.38, p = 0.044) (Figure 1A). Intriguingly, we also saw increased expression of Cox7C in SSc-CFS (−0.86) when compared to SSc-NCFS (−1.11, p = 0.031) (Figure 1A). This was not associated with differences in nuclear-encoded CoxIV protein levels, between SSc-CFS and SSc-NCFS patients (Supplementary Figure S1). Dloop expression as a measure of mtDNA transcription, was not different between the groups. Additionally, protein levels of TFAM were similar between SSc-CFS and SSc-NCFS patients (Supplementary Figure S1). TFAM is a core mitochondrial transcription factor, known to directly control mtDNA copy number [19]. We did not appreciate any significant differences in mtDNA integrity between SSc-CFS (1.03), SSc-NCFS (1.04, p = 0.137) and HCs (1.05, p = 0.251), though SSc-CFS patients appeared to have lower levels (Figure 1B).

Figure 1.
Mitochondrial electron transport chain gene expression and mitochondrial cell-free DNA. Mitochondrially encoded ND4 and CyB gene expression was reduced in SSc-CFS patients, compared to SSc-NCFS patients. Nuclear-encoded Cox7C expression was significantly higher in SSc-CFS patients. Mitochondrial transcription, as indicated by Dloop expression, was not significantly different between disease groups (A). Cell-free mitochondrial DNA integrity was not significantly different between each group (B). mtDNA integrity refers to the ratio of mtDNA230 to mtDNA79 fragments. Median and interquartile range are indicated. (p-values: + = 0.050, * <0.050).
We individually assessed the correlates of ETC gene expression and mtDNA integrity in our SSc patients. We found that both CyB and Cox7C correlated with fatigue as measured by the FACIT (0.40, p = 0.057; −0.46, p = 0.030), whilst mtDNA integrity correlated with fatigue as measured by the MFI (−0.59, p = 0.003) (Supplemental Table S2 and Figure S2). Both CyB expression and mtDNA integrity correlated with reduced quality of life as measured by SF36-Vitality (0.40, p = 0.052; 0.49, p = 0.018) (Supplemental Table S3 and Figure S2). A noteworthy finding was the correlation of both ND4 and CyB expression with disease-related factors, specifically increased skin involvement (mRSS) (−0.41, p = 0.045; −0.45, p = 0.024) and reduced lung diffusion capacity (DLCO SB) (0.48, p = 0.018; 0.49, p = 0.015). ND4 expression was also significantly correlated with FVC and FEV1 values (0.46, p = 0.024; 0.46, p = 0.022) (Supplemental Table S3 and Figure S3).
3. Discussion
We show for the first time that many patients with early SSc (disease duration <5 years) fulfill the classification criteria for ME/CFS and FM. Remarkably, the rate of both ME/CFS and FM in these patients is consistent with values described in long-standing SSc [2]. As previously documented in fatigued patients with long-standing SSc, we found that early SSc disease patients had significantly higher scores with regards to fibromyalgia symptoms (WPI and SSS), sleep disturbances, anxiety and depression [2]. SSc-CFS patients could not be distinguished from SSc-NCFS patients with regards to disease-related factors such as skin thickening, lung function, ILD, CRP, digital ulcers and gastrointestinal disturbances.
Though in the normal range, TSH levels were shown to be higher in SSc-CFS patients; this is in keeping with previous studies, which have shown a significant increase in TSH levels in SSc patients compared to healthy controls, with mean values staying within normal ranges [19]. Increased TSH levels have, however, been shown to correlate with SSc disease severity, as indicated by decreased FEV1 and FVC values [20]. Rates of arthralgia were higher in SSc-CFS patients, which is consistent with symptoms of fibromyalgia, and possibly the presence of an interferon signature [21]. Of interest is the finding that a trend was evident for the reduced presence of anticentromere antibodies (ACA) in SSc-CFS patients. These antibodies are useful prognostic markers in SSc disease progression, where their presence is associated with a less fibrotic disease profile [22] and a reduced risk of other features such as malignancy [23]. Additionally, ACAs are described as being protective against scleroderma renal crisis and interstitial lung disease, even in patients with diffuse SSc [24]. Another important finding was that the use of immunomodulator and vasodilator medications did not impact the development of fatigue in our patients. Though these therapies were respectively associated with arthralgia and digital ulcers, this is likely due to treatment bias.
Looking at potential markers of mitochondrial dysregulation, we found that mitochondrially encoded genes ND4 and CyB had reduced expression in SSc-CFS patients, whilst nuclear gene Cox7C had elevated expression. Increased levels of Cox7C (without changes in CoxIV) is intriguing, since this suggests that Cox7C may be providing a compensatory role in patients with ME/CFS. In addition to stabilizing Complex IV, Cox7C has also recently been implicated in promoting more efficient energy utilization via respirasome supercomplexes [25]. Supercomplexes may play a role in stabilizing individual ETC complexes, reducing reactive oxygen species (ROS) production and increasing ETC efficiency [25].
Mitochondrial suppression is recognized as an early (and required) feature of immune cell activation [26]. It is also established that loss of mitochondrial ETC transcripts (particularly in ETC Complex I) may promote various inflammatory diseases [8]. Fibrosis in SSc is associated with metabolic dysregulation [27]. In particular, Zhou et al. showed that impaired TFAM expression led to mitochondrial damage, reduced OXPHOS capacity and transcriptional changes that drive tissue fibrosis in SSc patients [28]. Similarly, preclinical animal models of SSc with altered mitochondrial functions develop more severe fibrotic complications [29]. Furthermore, patients with diffuse SSc have alterations in molecules (such as CD38), which may result in more suppressed mitochondrial functions [30].
An intriguing finding with regards to our ETC gene expression was that increased age, BMI and CRP did not seem to play a factor in this altered gene signature. In particular, obesity has been linked to decreased mitochondrial gene expression in adipose tissues [25], and the majority of our SSc patients were overweight. We instead found that reduced ND4 and CyB gene expression was correlated with increased skin involvement and reduced DLCO SB, and that ND4 expression was correlated with reduced FVC and FEV1 values. DLCO SB, FVC and FEV1 are considered surrogate markers for SSc disease progression and the development of ILD, which is the leading cause of mortality in SSc patients [26].
We postulate that the presence of ME/CFS with associated mitochondrial dysfunction in lcSSc patients with less than 5 years of disease may be associated with increased risk of developing more severe disease-specific complications in the future. The altered ETC gene signature in these patients may also hint at the mechanisms underlying disease progression. Future large prospective studies assessing these patients and disease-associated complications over time are required to verify this hypothesis.
The heterogeneity of ME/CFS has made diagnosis and treatment of these patients challenging. Conducting clinical trials for these patients has been most challenging, with inconsistent conclusions likely stemming from patient heterogeneity. Hence, biomarkers that can strategically identify patients more harmoniously may inform future clinical trials. It is well established that fatigue in rheumatic diseases such as SSc can be explained, at least in part, by their disease. In our SSc cohort of patients early in their disease, clinical variables associated with SSc could not distinguish SSc-CFS patients from SSc-NCFS patients. Both fatigue and mitochondrial dysfunction have, however, been linked to more severe SSc outcomes [27,28]. In our study, a simple assay measuring differences in ETC gene expression was able to distinguish these two groups (Figure 2). Additionally, these changes in ETC gene expression also showed a connection with increased skin and lung function measures.
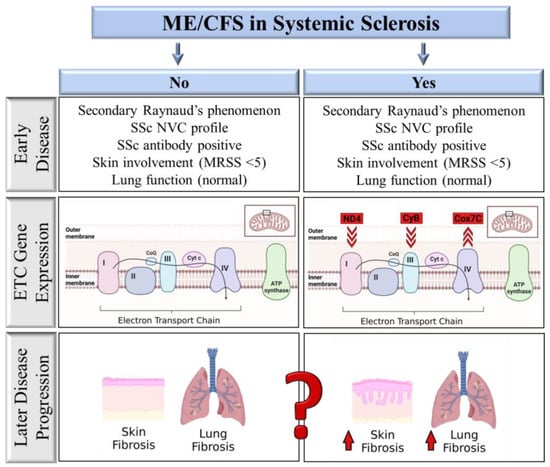
Figure 2.
Comparison of factors associated with ME/CFS in SSc patients. SSc-CFS and SSc-NCFS patients are not distinguishable from each other based on early SSc disease-related factors. Electron transport chain gene (ETC) expression distinguishes SSc-CFS patients from SSc-NCFS patients, with reduced ND4 and CyB expression and increased Cox7C gene expression. ETC gene expression correlates with measures of disease severity, including skin thickening and lung function.
There are several limitations to our study. These include the small sample size and single-center design. Our exploratory results will need to be replicated and confirmed in larger, more ethnically diverse cohorts. Though we did not directly measure changes of electron transport chain activity in cells, other groups assessing patients with ME/CFS have [9,29]. The observed gene expression changes will thus require follow-up in functional assays to further elucidate the possible mechanisms affecting mitochondrial function in SSc patients who meet the criteria for ME/CFS.
4. Materials and Methods
4.1. Participants
Participants included in our study were referred to the Rheumatology Clinics at the University of Alberta. The study was approved by the University of Alberta Research Ethics Board (Pro00085583, Pro00090050), and all research was performed in accordance with relevant guidelines. All methods were performed in accordance with the Declaration of Helsinki. Patients were recruited to the study during their scheduled rheumatology appointments. Written informed consent was obtained from all the study participants. Patients were not involved in setting the research question or outcome measures.
All of our SSc patients meeting the 2013 European Alliance of Associations for Rheumatology/American College of Rheumatology (EULAR/ACR) classification criteria for SSc were invited to take part [30]. All patients were tested for antinuclear antibodies and SSc-specific autoantibodies (Supplementary Table S4) [31] in a reference clinical laboratory (MitogenDx laboratories, Calgary, Alberta). Patients underwent simultaneous clinical and nailfold capillaroscopy (NVC) assessments at the time of enrollment [32]. All patients with SSc had an SSc-spectrum capillaroscopy pattern [1,18]. Consecutive SSc patients who had non-Raynaud’s related symptoms (Autoantibodies, positive NVC) for less that <5 years, who gave informed consent, participated in this study. Patients were screened for the use of both immunomodulatory (Hydroxychloroquine, Methotrexate, Folate, Mycophenolate, Prednisone) and vasodilator medications (Amlodipine, Nifedipine, Sildenafil, Tadalafil). None of the patients included were treated with rituximab or tocilizumab.
FM was diagnosed using the modified 2010 and 2016 American College of Rheumatology classification criteria for fibromyalgia [33,34]. Diagnosis of ME/CFS was based on both the International and Canadian Consensus Criteria [35,36,37]. Clinical parameters such as BMI, and laboratory parameters such C-reactive protein (CRP) and thyroid-stimulating hormone (TSH) levels were also collected. Healthy controls (HC)s were volunteers that did not suffer from a rheumatic disease, hypothyroidism or diabetes requiring insulin. Similarly, patients with idiopathic FM who visited our rheumatology clinic and who met the FM 2010 and 2016 classification criteria [33,34] and did not suffer from an underlying inflammatory condition, hypothyroidism and/or diabetes were included in our study.
4.2. Questionnaires
Enrolled patients were assessed for the presence of ME/CFS and/or FM using validated questionnaires (e.g., DePaul symptom questionnaire (DSQ-2), wide-scale pain index (WPI) and symptom severity scale (SSS), and the 36-Item Short Form Survey (SF-36)) (Supplementary Table S6). Comorbidities (e.g., anxiety, sleep disturbances and depression) were also assessed using validated questionnaires (Supplementary Table S4).
4.3. Mitochondrial Electron Transport Chain Expression
We selected 12 sequential patients from each of our SSc groups (SSc with ME/CFS (SSc-CFS) and SSc without ME/CFS (SSc-NCFS)) and 9 patients for the healthy and idiopathic FM controls for mitochondrial gene expression analyses. Blood-derived mRNA was extracted from PAXgene tubes using the PAXgene Blood RNA Kit (PreAnalytiX). Subsequently, real-time PCR was carried out using Taqman Fast Virus 1 step Master Mix (Applied Biosystems) and D-loop (MT-7S), ND4, MT-CYB, COX7C, β-Actin and GAPDH expression kits (Hs02596861_s1, Hs02596876_g1, Hs02596867_s1, Hs01595220_g1, Hs99999903_m1, Hs99999905_m1). Gene expression was normalized to GAPDH. Expression was calculated as the log(10) of ddCT. PCRs were conducted in duplicate, with three replicates and the three means averaged. The median log(ddCT) values were determined for each patient group and visualized as a violin plot.
4.4. Cell-Free Mitochondrial DNA Analysis
Cell-free DNA was extracted from 500 μL serum using the QIAamp UltraSens Virus Kit (QIAGEN), according to the manufacturer’s instructions. Quantitative analysis of mtDNA in serum was performed by SYBR Green quantitative real-time polymerase chain reaction (PCR). Two previously published primer sets were used to analyze the levels of a 79 bp fragment of the mitochondrial 16S-RNA (corresponding to DNA released by apoptotic cells) and 230 bp fragment (corresponding to mtDNA released by necrosis), then normalized to cell-free GAPDH DNA from the same sample, as previously described [38,39] (Supplementary Table S5). Mitochondrial fragmentation was defined as mtDNA integrity, which was calculated as the relative amount of mtDNA230 to mtDNA79. Each 10 μL reaction consisted of 5 μL Fast SYBR Green master mix (Applied Biosystems), 1 μL DNA and 0.5 μL of forward and reverse primer (8000 nM). PCR conditions were as follows: 95 °C for 15 min, followed by 40 cycles of 95 °C for 15 s, 60 °C for 30 s, 72 °C for 30 s. Melt curve analysis was applied to confirm specificity, at 95 °C for 15 s, 60 °C for 15 s and 95 °C for 15 s. PCRs were conducted in triplicate, with the mean of two replicate runs used for analysis.
4.5. Statistical Analysis
All statistical analyses were completed using STATA 17 (StataCorp). For continuous variables, median and interquartile range are indicated with the exact p-value, as determined by the Wilcoxon rank-sum test. For categorical variables, chi-square analysis was carried out with Fisher’s exact test. Variables are indicated as the total number of positive responses and the percentage of positive responses within the population. Spearman’s rank-order was used for correlation coefficient analysis.
5. Conclusions
ME/CFS plays a pivotal role in early morbidity in a subset of SSc patients. We identified a mitochondrial signature in SSc patients with ME/CFS, which distinguishes them from SSc patients without this comorbidity. Gene expression changes in mitochondrially encoded ND4 and CyB also correlated with measures of disease severity. Further investigations into the functional consequences of the observed mitochondrial changes may lead to the facilitation of therapeutic intervention studies and possible treatment options, a current unmet need in SSc patients with ME/CFS. Together, our data suggest that the presence of ME/CFS in SSc reflects an independent manifestation of SSc, which we hypothesize may be linked to poor disease-related outcomes. Future studies assessing this possibility in SSc and possibly in other rheumatic diseases should be tested.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms241512057/s1. References [40,41,42,43,44,45,46] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, C.v.E., A.L.M., J.W.C.T. and M.S.O.; data curation, D.R. and N.M.; formal analysis, C.v.E.; funding acquisition, M.J.L., J.W.C.T. and M.S.O.; writing—original draft, C.v.E. and M.S.O.; writing—review and editing, C.v.E., M.J.L., A.L.M., J.W.C.T. and M.S.O. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by an unrestricted research grant from Boehringer Ingelheim, Canada (IIS 1199-0431) through an unrestricted investigator-initiated study and Scleroderma Canada (MO and ML), the Dutch Kidney Foundation (17PhD01); and Arthritis Society (19-0558). Additionally, MO is funded by an Arthritis Society/IMHA STAR career development award (award 00049).
Institutional Review Board Statement
The study was approved by the University of Alberta Research Ethics Board (Pro00085583, Pro00090050), and all research was performed in accordance with relevant guidelines. All methods were performed in accordance with the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets generated during the current study are not publicly available due to privacy/ethical issues but are available from the corresponding author on reasonable request.
Acknowledgments
We would like to thank A.S. Russell for his patient referrals, which contributed to this study. We are grateful to the patients of our Rheumatology Clinic and the healthy volunteers who took part in this study. Figure 2 was created with BioRender.com (accessed 14 July 2023).
Conflicts of Interest
J.W.C.T. received speaker honoraria from Pfizer, Medexus and Otsuka, and advisory board honoraria from Mallinckrodt and Otsuka. M.S.O. received speaker honoraria from Boehringer Ingelheim. M.J.L. received advisory board honoraria from Boehringer Ingelheim, Janssen, Mallinckrodt and Pfizer. All other authors declare that they do not have any competing interest.
References
- Minier, T.; Guiducci, S.; Bellando-Randone, S.; Bruni, C.; Lepri, G.; Czirják, L.; Distler, O.; A Walker, U.; Fransen, J.; Allanore, Y.; et al. Preliminary analysis of the Very Early Diagnosis of Systemic Sclerosis (VEDOSS) EUSTAR multicentre study: Evidence for puffy fingers as a pivotal sign for suspicion of systemic sclerosis. Ann. Rheum. Dis. 2014, 73, 2087–2093. [Google Scholar] [CrossRef]
- Basta, F.; Afeltra, A.; Margiotta, D.P.E. Fatigue in systemic sclerosis: A systematic review. Ann. Rheum. Dis. 2018, 36, 150–160. [Google Scholar]
- Van Eeden, C.; Osman, M.S.; Tervaert, J.W.C. Fatigue in ANCA-associated vasculitis (AAV) and systemic sclerosis (SSc): Similarities with Myalgic encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). A critical review of the literature. Expert Rev. Clin. Immunol. 2022, 18, 1049–1070. [Google Scholar] [CrossRef] [PubMed]
- Afari, N.; Buchwald, D. Chronic Fatigue Syndrome: A Review. Am. J. Psychiatry 2003, 160, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Ostuni, P.; Botsios, C.; Sfriso, P.; Bertagnin, A.; Cozzi, F.; Doria, A.; Todesco, S. Prevalence and clinical features of fibromyalgia in systemic lupus erythematosus, systemic sclerosis and Sjögren’s syndrome. Minerva Medica 2002, 93, 203–209. [Google Scholar]
- Perrot, S.; Peixoto, M.; Dieude, P.; Hachulla, E.; Avouac, J.; Ottaviani, S.; Allanore, Y. Patient phenotypes in fibromyalgia comorbid with systemic sclerosis or rheumatoid arthritis: Influence of diagnostic and screening tests. Screening with the FiRST questionnaire, diagnosis with the ACR 1990 and revised ACR 2010 criteria. Ann. Rheum. Dis. 2017, 35, 35–42. [Google Scholar]
- El-Rabbat, M.S.; Mahmoud, N.K.; Gheita, T.A. Clinical significance of fibromyalgia syndrome in different rheumatic diseases: Relation to disease activity and quality of life. Reumatol. Clin. 2018, 14, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Castro-Marrero, J.; Cordero, M.D.; Sáez-Francas, N.; Jimenez-Gutierrez, C.; Aguilar-Montilla, F.J.; Aliste, L.; Alegre-Martin, J. Could Mitochondrial Dysfunction Be a Differentiating Marker Between Chronic Fatigue Syndrome and Fibromyalgia. Antioxid. Redox Signal. 2013, 19, 1855–1860. [Google Scholar] [CrossRef] [PubMed]
- Missailidis, D.; Annesley, S.J.; Allan, C.Y.; Sanislav, O.; Lidbury, B.A.; Lewis, D.P.; Fisher, P.R. An Isolated Complex V Inefficiency and Dysregulated Mitochondrial Function in Immortalized Lymphocytes from ME/CFS Patients. Int. J. Mol. Sci. 2020, 21, 1074. [Google Scholar] [CrossRef]
- Cogliati, S.; Lorenzi, I.; Rigoni, G.; Caicci, F.; Soriano, M.E. Regulation of Mitochondrial Electron Transport Chain Assembly. J. Mol. Biol. 2018, 430, 4849–4873. [Google Scholar] [CrossRef]
- Lane, R.K.; Hilsabeck, T.; Rea, S.L. The role of mitochondrial dysfunction in age-related diseases. Biochim. Biophys. Acta 2015, 1847, 1387–1400. [Google Scholar] [CrossRef] [PubMed]
- Pandit, A.; Vadnal, J.; Houston, S.; Freeman, E.; McDonough, J. Impaired regulation of electron transport chain subunit genes by nuclear respiratory factor 2 in multiple sclerosis. J. Neurol. Sci. 2009, 279, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.P.; Reinhold, J.; Yu, H.; Starks, L.; Uryga, A.K.; Foote, K.; Finigan, A.; Figg, N.; Pung, Y.-F.; Logan, A.; et al. Mitochondrial Respiration Is Reduced in Atherosclerosis, Promoting Necrotic Core Formation and Reducing Relative Fibrous Cap Thickness. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 2322–2332. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Sobenin, I.A.; Revin, V.V.; Orekhov, A.N.; Bobryshev, Y.V. Mitochondrial Aging and Age-Related Dysfunction of Mitochondria. BioMed. Res. Int. 2014, 2014, 238463. [Google Scholar] [CrossRef] [PubMed]
- Zahn, J.M.; Sonu, R.; Vogel, H.; Crane, E.; Mazan-Mamczarz, K.; Rabkin, R.; Davis, R.W.; Becker, K.G.; Owen, A.B.; Kim, S.K. Transcriptional Profiling of Aging in Human Muscle Reveals a Common Aging Signature. PLOS Genet. 2006, 2, e115. [Google Scholar] [CrossRef]
- Dromparis, P.; Michelakis, E.D. Mitochondria in Vascular Health and Disease. Annu. Rev. Physiol. 2013, 75, 95–126. [Google Scholar] [CrossRef]
- Li, X.; Zhang, W.; Cao, Q.; Wang, Z.; Zhao, M.; Xu, L.; Zhuang, Q. Mitochondrial dysfunction in fibrotic diseases. Cell Death Discov. 2020, 6, 80. [Google Scholar] [CrossRef]
- Ma, Z.; Mulder, D.J.; Gniadecki, R.; Tervaert, J.W.C.; Osman, M. Methods of Assessing Nailfold Capillaroscopy Compared to Video Capillaroscopy in Patients with Systemic Sclerosis—A Critical Review of the Literature. Diagnostics 2023, 13, 2204. [Google Scholar] [CrossRef]
- Fallahi, P.; Ruffilli, I.; Giuggioli, D.; Colaci, M.; Ferrari, S.M.; Antonelli, A.; Ferri, C. Associations between Systemic Sclerosis and Thyroid Diseases. Front. Endocrinol. 2017, 8, 266. [Google Scholar] [CrossRef]
- Singh, P.K.; Sharma, S.K.; Sinha, A.; Dutta, P. Can thyroid volume predict thyroid dysfunction in patients with systemic sclerosis? A prospective cross-sectional study from a tertiary care center in North West India. Clin. Rheumatol. 2016, 35, 765–769. [Google Scholar] [CrossRef]
- Fineschi, S.; Klar, J.; Gustafsson, K.A.; Jonsson, K.; Karlsson, B.; Dahl, N. Inflammation and Interferon Signatures in Peripheral B-Lymphocytes and Sera of Individuals with Fibromyalgia. Front. Immunol. 2022, 13, 874490. [Google Scholar] [CrossRef] [PubMed]
- Powell, F.C.; Winkelmann, R.K.; Venencie-Lemarchand, F.; Spurbeck, J.L.; Schroeter, A.L. The Anticentromere Antibody: Disease Specificity and Clinical Significance. Mayo Clin. Proc. 1984, 59, 700–706. [Google Scholar] [CrossRef]
- Igusa, T.; Hummers, L.K.; Visvanathan, K.; Richardson, C.; Wigley, F.M.; Casciola-Rosen, L.; Rosen, A.; A Shah, A. Autoantibodies and scleroderma phenotype define subgroups at high-risk and low-risk for cancer. Ann. Rheum. Dis. 2018, 77, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Caetano, J.; I Nihtyanova, S.; Harvey, J.; Denton, C.P.; Ong, V.H. Distinctive clinical phenotype of anti-centromere antibody-positive diffuse systemic sclerosis. Rheumatol. Adv. Pract. 2018, 2, rky002. [Google Scholar] [CrossRef]
- Miao, Z.; Alvarez, M.; Ko, A.; Bhagat, Y.; Rahmani, E.; Jew, B.; Heinonen, S.; Muñoz-Hernandez, L.L.; Herrera-Hernandez, M.; Aguilar-Salinas, C.; et al. The causal effect of obesity on prediabetes and insulin resistance reveals the important role of adipose tissue in insulin resistance. PLOS Genet. 2020, 16, e1009018. [Google Scholar] [CrossRef] [PubMed]
- Distler, O.; Assassi, S.; Cottin, V.; Cutolo, M.; Danoff, S.K.; Denton, C.P.; Distler, J.H.; Hoffmann-Vold, A.-M.; Johnson, S.R.; Ladner, U.M.; et al. Predictors of progression in systemic sclerosis patients with interstitial lung disease. Eur. Respir. J. 2020, 55, 1902026. [Google Scholar] [CrossRef]
- Bueno, M.; Papazoglou, A.; Valenzi, E.; Rojas, M.; Lafyatis, R.; Mora, A.L. Mitochondria, Aging, and Cellular Senescence: Implications for Scleroderma. Curr. Rheumatol. Rep. 2020, 22, 37. [Google Scholar] [CrossRef]
- Sandusky, S.B.; McGuire, L.; Smith, M.T.; Wigley, F.M.; Haythornthwaite, J.A. Fatigue: An overlooked determinant of physical function in scleroderma. Rheumatology 2009, 48, 165–169. [Google Scholar] [CrossRef]
- Tomas, C.; Brown, A.; Strassheim, V.; Elson, J.L.; Newton, J.; Manning, P. Cellular bioenergetics is impaired in patients with chronic fatigue syndrome. PLoS ONE 2017, 12, e0186802. [Google Scholar] [CrossRef]
- Van den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.P.; Medsger, T.A., Jr.; Carreira, P.E.; et al. 2013 classification criteria for systemic sclerosis: An American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 2013, 65, 2737–2747. [Google Scholar] [CrossRef]
- Koenig, M.; Joyal, F.; Fritzler, M.J.; Roussin, A.; Abrahamowicz, M.; Boire, G.; Goulet, J.-R.; Rich, E.; Grodzicky, T.; Raymond, Y.; et al. Autoantibodies and microvascular damage are independent predictive factors for the progression of Raynaud’s phenomenon to systemic sclerosis: A twenty-year prospective study of 586 patients, with validation of proposed criteria for early systemic sclerosis. Arthritis Rheum. 2008, 58, 3902–3912. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.; van Eeden, C.; Moazab, N.; Redmond, D.; Phan, C.; Keeling, S.; Gniadecki, R.; Tervaert, J.W.C.; Osman, M. Nailfold Capillaroscopy Abnormalities Correlate with Disease Activity in Adult Dermatomyositis. Front. Med. 2021, 8, 708432. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Häuser, W.; Katz, R.L.; Mease, P.J.; Russel, A.S.; Russell, I.J.; Walitt, R. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin. Arthritis Rheum. 2016, 46, 319–329. [Google Scholar] [CrossRef]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.-A.; Goldenberg, D.L.; Katz, R.S.; Mease, P.; Russell, A.S.; Russell, I.J.; Winfield, J.B.; Yunus, M.B. The American College of Rheumatology Preliminary Diagnostic Criteria for Fibromyalgia and Measurement of Symptom Severity. Arthritis Care Res. 2010, 62, 600–610. [Google Scholar] [CrossRef]
- Clayton, E.W. Beyond myalgic encephalomyelitis/chronic fatigue syndrome: An IOM report on redefining an illness. JAMA 2015, 313, 1101–1102. [Google Scholar] [CrossRef] [PubMed]
- Carruthers, B.M.; Van De Sande, M.I.; De Meirleir, K.L.; Klimas, N.G.; Broderick, G.; Mitchell, T.; Staines, D.; Powles, A.C.; Speight, N.; Vallings, R.; et al. Myalgic encephalomyelitis: International Consensus Criteria. J. Intern. Med. 2011, 270, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Van Eeden, C.; Mohazab, N.; Redmond, D.; Yacyshyn, E.; Clifford, A.; Russell, A.S.; Osman, M.S.; Tervaert, J.W.C. Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and fibromyalgia: PR3-versus MPO-ANCA-associated vasculitis, an exploratory cross-sectional study. Lancet Reg. Health Am. 2023, 20, 100460. [Google Scholar]
- Ellinger, J.; Müller, S.C.; Wernert, N.; von Ruecker, A.; Bastian, P.J. Mitochondrial DNA in serum of patients with prostate cancer: A predictor of biochemical recurrence after prostatectomy. BJU Int. 2008, 102, 628–632. [Google Scholar] [CrossRef]
- Meng, X.; Schwarzenbach, H.; Yang, Y.; Müller, V.; Li, N.; Tian, D.; Shen, Y.; Gong, Z. Circulating Mitochondrial DNA is Linked to Progression and Prognosis of Epithelial Ovarian Cancer. Transl. Oncol. 2019, 12, 1213–1220. [Google Scholar] [CrossRef]
- Bedree, H.; Sunnquist, M.; Jason, L.A. The DePaul Symptom Questionnaire-2: A validation study. Fatigue Biomed. Heal. Behav. 2019, 7, 166–179. [Google Scholar] [CrossRef]
- Smets, E.M.A.; Garssen, B.; Bonke, B.; De Haes, J.C.J.M. The multidimensional fatigue inventory (MFI) psychometric qualities of an instrument to assess fatigue. J. Psychosom. Res. 1995, 39, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Yellen, S.B.; Cella, D.F.; Webster, K.; Blendowski, C.; Kaplan, E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J. Pain Symptom Manag. 1997, 13, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Ware, J.E., Jr.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, C. International experiences with the Hospital Anxiety and Depression Scale-a review of validation data and clinical results. J. Psychosom. Res. 1997, 42, 17–41. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., III; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Broadbent, D.E.; Cooper, P.F.; FitzGerald, P.; Parkes, K.R. The Cognitive Failures Questionnaire (CFQ) and its correlates. Br. J. Clin. Psychol. 1982, 21, 1–16. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).