Amyloid Beta Peptides Lead to Mast Cell Activation in a Novel 3D Hydrogel Model
Abstract
1. Introduction
2. Results
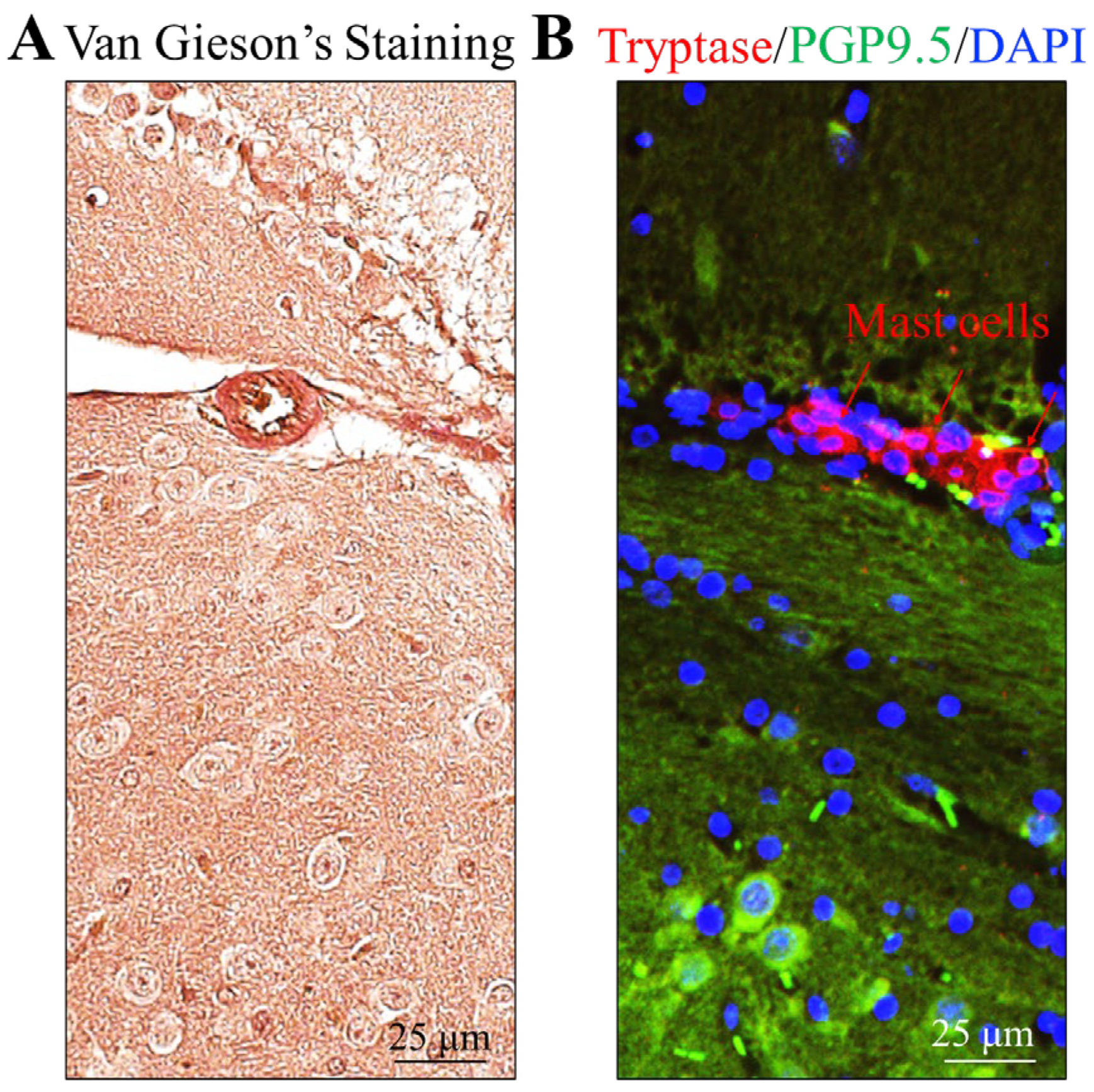
2.1. MCs Are Present in Mouse Brain Surrounded by Collagen-Rich Extracellular Matrix
2.2. MCs Grown in 3D Collagen Gels Proliferate Similar to MCs Cultured in Suspension
2.3. MCs Cultured in 3D Collagen Gels Respond to IL-33 and IgE/anti-IgE to Release Inflammatory Mediators
2.4. Aβ Peptides Stimulate the Release of Cytokines from Human MCs in the 3D Collagen Gel
3. Discussion
4. Materials and Methods
4.1. Tissue Preparation
4.2. Human Mast Cell Culture
4.3. 3D Collagen Gel Culture Preparation
4.4. Mast Cell Treatments
4.5. ELISA and Cytokine Array Analysis
4.6. EdU Cell Proliferation Assay
4.7. Live/Dead Assay
4.8. Histological and Immunofluorescence (IF) Staining
4.9. Microscopy
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| Aβ | Amyloid-beta |
| MCs | Mast cells |
| IgE/anti-IgE | Immunoglobulin E/anti-Immunoglobulin E |
| IL-33 | Interleukin-33 |
| 3D cell culture | Three-dimensional cell culture |
| ECM | Extracellular matrix |
References
- Alzheimer’s Association Report. 2023 Alzheimer’s disease facts and figures. Alzheimers Dement. 2023, 19, 1598–1695. [Google Scholar] [CrossRef]
- Bloom, G.S. Amyloid-beta and tau: The trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014, 71, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Kummer, M.P.; Latz, E. Innate immune activation in neurodegenerative disease. Nat. Rev. Immunol. 2014, 14, 463–477. [Google Scholar] [CrossRef]
- McGeer, P.L.; Schwab, C.; Parent, A.; Doudet, D. Presence of reactive microglia in monkey substantia nigra years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine administration. Ann. Neurol. 2003, 54, 599–604. [Google Scholar] [CrossRef]
- Maslinska, D.; Laure-Kamionowska, M.; Maslinski, K.T.; Gujski, M.; Maslinski, S. Distribution of tryptase-containing mast cells and metallothionein reactive astrocytes in human brains with amyloid deposits. Inflamm. Res. 2007, 56 (Suppl. S1), S17–S18. [Google Scholar] [CrossRef]
- Town, T.; Tan, J.; Flavell, R.A.; Mullan, M. T-cells in Alzheimer’s disease. Neuromol. Med. 2005, 7, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Valent, P.; Akin, C. Mast Cells, Mastocytosis, and Related Disorders. N. Engl. J. Med. 2015, 373, 1885–1886. [Google Scholar] [CrossRef]
- Krystel-Whittemore, M.; Dileepan, K.N.; Wood, J.G. Mast Cell: A Multi-Functional Master Cell. Front. Immunol. 2015, 6, 620. [Google Scholar] [CrossRef]
- Nautiyal, K.M.; Liu, C.; Dong, X.; Silver, R. Blood-borne donor mast cell precursors migrate to mast cell-rich brain regions in the adult mouse. J. Neuroimmunol. 2011, 240–241, 142–146. [Google Scholar] [CrossRef]
- Alanazi, S.; Grujic, M.; Lampinen, M.; Rollman, O.; Sommerhoff, C.P.; Pejler, G.; Melo, F.R. Mast Cell beta-Tryptase Is Enzymatically Stabilized by DNA. Int. J. Mol. Sci. 2020, 21, 5065. [Google Scholar] [CrossRef]
- Varricchi, G.; Marone, G. Mast Cells: Fascinating but Still Elusive after 140 Years from Their Discovery. Int. J. Mol. Sci. 2020, 21, 464. [Google Scholar] [CrossRef] [PubMed]
- da Silva, E.Z.; Jamur, M.C.; Oliver, C. Mast cell function: A new vision of an old cell. J. Histochem. Cytochem. 2014, 62, 698–738. [Google Scholar] [CrossRef] [PubMed]
- Harcha, P.A.; Vargas, A.; Yi, C.; Koulakoff, A.A.; Giaume, C.; Saez, J.C. Hemichannels Are Required for Amyloid beta-Peptide-Induced Degranulation and Are Activated in Brain Mast Cells of APPswe/PS1dE9 Mice. J. Neurosci. 2015, 35, 9526–9538. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Niederhoffer, N.; Levy, R.; Sick, E.; Andre, P.; Coupin, G.; Lombard, Y.; Gies, J.P. Amyloid beta peptides trigger CD47-dependent mast cell secretory and phagocytic responses. Int. J. Immunopathol. Pharmacol. 2009, 22, 473–483. [Google Scholar] [CrossRef]
- Venegas, C.; Heneka, M.T. Danger-associated molecular patterns in Alzheimer’s disease. J. Leukoc. Biol. 2017, 101, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Sedeyn, J.C.; Wu, H.; Hobbs, R.D.; Levin, E.C.; Nagele, R.G.; Venkataraman, V. Histamine Induces Alzheimer’s Disease-Like Blood Brain Barrier Breach and Local Cellular Responses in Mouse Brain Organotypic Cultures. Biomed. Res. Int. 2015, 2015, 937148. [Google Scholar] [CrossRef]
- Nelson, R.B.; Siman, R.; Iqbal, M.A.; Potter, H. Identification of a chymotrypsin-like mast cell protease in rat brain capable of generating the N-terminus of the Alzheimer amyloid beta-protein. J. Neurochem. 1993, 61, 567–577. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Dong, H.; Xu, Y.; Zhang, S. Induction of Microglial Activation by Mediators Released from Mast Cells. Cell. Physiol. Biochem. 2016, 38, 1520–1531. [Google Scholar] [CrossRef]
- Jones, M.K.; Nair, A.; Gupta, M. Mast Cells in Neurodegenerative Disease. Front. Cell. Neurosci. 2019, 13, 171. [Google Scholar] [CrossRef]
- Dong, H.; Wang, Y.; Zhang, X.; Zhang, X.; Qian, Y.; Ding, H.; Zhang, S. Stabilization of Brain Mast Cells Alleviates LPS-Induced Neuroinflammation by Inhibiting Microglia Activation. Front. Cell. Neurosci. 2019, 13, 191. [Google Scholar] [CrossRef] [PubMed]
- Villain, N. Therapeutic news in Alzheimer’s disease: Soon a disease-modifying therapy? Rev. Neurol. 2022, 178, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Radinger, M.; Jensen, B.M.; Kuehn, H.S.; Kirshenbaum, A.; Gilfillan, A.M. Generation, isolation, and maintenance of human mast cells and mast cell lines derived from peripheral blood or cord blood. Curr. Protoc. Immunol. 2010, 90, 7–37. [Google Scholar] [CrossRef]
- Ogawa, M.; Nakahata, T.; Leary, A.G.; Sterk, A.R.; Ishizaka, K.; Ishizaka, T. Suspension culture of human mast cells/basophils from umbilical cord blood mononuclear cells. Proc. Natl. Acad. Sci. USA 1983, 80, 4494–4498. [Google Scholar] [CrossRef]
- Park, D.W.; Choi, D.S.; Ryu, H.S.; Kwon, H.C.; Joo, H.; Min, C.K. A well-defined in vitro three-dimensional culture of human endometrium and its applicability to endometrial cancer invasion. Cancer Lett. 2003, 195, 185–192. [Google Scholar] [CrossRef]
- Chen, S.S.; Revoltella, R.P.; Papini, S.; Michelini, M.; Fitzgerald, W.; Zimmerberg, J.; Margolis, L. Multilineage differentiation of rhesus monkey embryonic stem cells in three-dimensional culture systems. Stem. Cells 2003, 21, 281–295. [Google Scholar] [CrossRef]
- O’Shaughnessy, T.J.; Lin, H.J.; Ma, W. Functional synapse formation among rat cortical neurons grown on three-dimensional collagen gels. Neurosci. Lett. 2003, 340, 169–172. [Google Scholar] [CrossRef]
- Skold, C.M.; Ohkuni, Y.; Liu, X.D.; Numerof, R.; Rennard, S.I. Co-cultured human mast cells stimulate fibroblast-mediated contraction of collagen gels. Inflammation 2001, 25, 47–51. [Google Scholar] [CrossRef]
- Margulis, A.; Nocka, K.H.; Brennan, A.M.; Deng, B.; Fleming, M.; Goldman, S.J.; Kasaian, M.T. Mast cell-dependent contraction of human airway smooth muscle cell-containing collagen gels: Influence of cytokines, matrix metalloproteases, and serine proteases. J. Immunol. 2009, 183, 1739–1750. [Google Scholar] [CrossRef]
- Stevens, R.L.; Somerville, L.L.; Sewell, D.; Swafford, J.R.; Caulfield, J.P.; Levi-Schaffer, F.; Hubbard, J.R.; Dayton, E.T. Serosal mast cells maintain their viability and promote the metabolism of cartilage proteoglycans when cocultured with chondrocytes. Arthritis Rheum. 1992, 35, 325–335. [Google Scholar] [CrossRef]
- Mair, T.S.; Sherlock, C.E.; Fews, D.; Harley, R.; Pearson, G.R. Idiopathic Fibrosis of the Tunica Muscularis of the Large Intestine in Five Horses with Colic. J. Comp. Pathol. 2016, 154, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Yarden, Y.; Kuang, W.J.; Yang-Feng, T.; Coussens, L.; Munemitsu, S.; Dull, T.J.; Chen, E.; Schlessinger, J.; Francke, U.; Ullrich, A. Human proto-oncogene c-kit: A new cell surface receptor tyrosine kinase for an unidentified ligand. EMBO J. 1987, 6, 3341–3351. [Google Scholar] [CrossRef] [PubMed]
- Kirshenbaum, A.S.; Yin, Y.; Sundstrom, J.B.; Bandara, G.; Metcalfe, D.D. Description and Characterization of a Novel Human Mast Cell Line for Scientific Study. Int. J. Mol. Sci. 2019, 20, 5520. [Google Scholar] [CrossRef]
- Novoa, C.; Salazar, P.; Cisternas, P.; Gherardelli, C.; Vera-Salazar, R.; Zolezzi, J.M.; Inestrosa, N.C. Inflammation context in Alzheimer’s disease, a relationship intricate to define. Biol. Res. 2022, 55, 39. [Google Scholar] [CrossRef] [PubMed]
- Reverchon, F.; de Concini, V.; Larrigaldie, V.; Benmerzoug, S.; Briault, S.; Togbe, D.; Ryffel, B.; Quesniaux, V.F.J.; Menuet, A. Hippocampal interleukin-33 mediates neuroinflammation-induced cognitive impairments. J. Neuroinflamm. 2020, 17, 268. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.; Hua, F.; Zhang, L.; Lin, Y.; Fang, P.; Chen, S.; Ying, J.; Wang, X. Dual roles of interleukin-33 in cognitive function by regulating central nervous system inflammation. J. Transl. Med. 2022, 20, 369. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, J.; Kucharska, E.; Garcez, M.L.; Rodrigues, M.S.; Quevedo, J.; Moreno-Gonzalez, I.; Budni, J. Inflammatory Cascade in Alzheimer’s Disease Pathogenesis: A Review of Experimental Findings. Cells 2021, 10, 2581. [Google Scholar] [CrossRef]
- Bourgognon, J.M.; Cavanagh, J. The role of cytokines in modulating learning and memory and brain plasticity. Brain Neurosci. Adv. 2020, 4, 2398212820979802. [Google Scholar] [CrossRef]
- Morgan, A.R.; Touchard, S.; Leckey, C.; O’Hagan, C.; Nevado-Holgado, A.J.; Consortium, N.; Barkhof, F.; Bertram, L.; Blin, O.; Bos, I.; et al. Inflammatory biomarkers in Alzheimer’s disease plasma. Alzheimers Dement. 2019, 15, 776–787. [Google Scholar] [CrossRef]
- Cojocaru, I.M.; Cojocaru, M.; Miu, G.; Sapira, V. Study of interleukin-6 production in Alzheimer’s disease. Rom. J. Intern. Med. 2011, 49, 55–58. [Google Scholar]
- Tripathy, D.; Thirumangalakudi, L.; Grammas, P. RANTES upregulation in the Alzheimer’s disease brain: A possible neuroprotective role. Neurobiol. Aging 2010, 31, 8–16. [Google Scholar] [CrossRef]
- Tarkowski, E.; Wallin, A.; Regland, B.; Blennow, K.; Tarkowski, A. Local and systemic GM-CSF increase in Alzheimer’s disease and vascular dementia. Acta Neurol. Scand. 2001, 103, 166–174. [Google Scholar] [CrossRef]
- Brombacher, T.M.; Nono, J.K.; De Gouveia, K.S.; Makena, N.; Darby, M.; Womersley, J.; Tamgue, O.; Brombacher, F. IL-13-Mediated Regulation of Learning and Memory. J. Immunol. 2017, 198, 2681–2688. [Google Scholar] [CrossRef]
- Ng, A.; Tam, W.W.; Zhang, M.W.; Ho, C.S.; Husain, S.F.; McIntyre, R.S.; Ho, R.C. IL-1beta, IL-6, TNF- alpha and CRP in Elderly Patients with Depression or Alzheimer’s disease: Systematic Review and Meta-Analysis. Sci. Rep. 2018, 8, 12050. [Google Scholar] [CrossRef] [PubMed]
- Griffin, W.S.; Stanley, L.C.; Ling, C.; White, L.; MacLeod, V.; Perrot, L.J.; White, C.L., 3rd; Araoz, C. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc. Natl. Acad. Sci. USA 1989, 86, 7611–7615. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chai, Y.L.; Hilal, S.; Ikram, M.K.; Venketasubramanian, N.; Wong, B.S.; Chen, C.P.; Lai, M.K. Serum IL-8 is a marker of white-matter hyperintensities in patients with Alzheimer’s disease. Alzheimers Dement. 2017, 7, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Tsilioni, I.; Theoharides, T.C. Recombinant SARS-CoV-2 Spike Protein Stimulates Secretion of Chymase, Tryptase, and IL-1beta from Human Mast Cells, Augmented by IL-33. Int. J. Mol. Sci. 2023, 24, 9487. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Leeman, S.E. Effect of IL-33 on de novo synthesized mediators from human mast cells. J. Allergy Clin. Immunol. 2019, 143, 451. [Google Scholar] [CrossRef]
- He, Z.; Song, J.; Hua, J.; Yang, M.; Ma, Y.; Yu, T.; Feng, J.; Liu, B.; Wang, X.; Li, Y.; et al. Mast cells are essential intermediaries in regulating IL-33/ST2 signaling for an immune network favorable to mucosal healing in experimentally inflamed colons. Cell Death. Dis. 2018, 9, 1173. [Google Scholar] [CrossRef]
- Ronnberg, E.; Ghaib, A.; Ceriol, C.; Enoksson, M.; Arock, M.; Safholm, J.; Ekoff, M.; Nilsson, G. Divergent Effects of Acute and Prolonged Interleukin 33 Exposure on Mast Cell IgE-Mediated Functions. Front. Immunol. 2019, 10, 1361. [Google Scholar] [CrossRef]
- Weng, Z.; Patel, A.B.; Panagiotidou, S.; Theoharides, T.C. The novel flavone tetramethoxyluteolin is a potent inhibitor of human mast cells. J. Allergy Clin. Immunol. 2015, 135, 1044–1052. [Google Scholar] [CrossRef]
- Asadi, S.; Theoharides, T.C. Corticotropin-releasing hormone and extracellular mitochondria augment IgE-stimulated human mast-cell vascular endothelial growth factor release, which is inhibited by luteolin. J. Neuroinflamm. 2012, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.; Zhang, B.; Asadi, S.; Sismanopoulos, N.; Butcher, A.; Fu, X.; Katsarou-Katsari, A.; Antoniou, C.; Theoharides, T.C. Quercetin is more effective than cromolyn in blocking human mast cell cytokine release and inhibits contact dermatitis and photosensitivity in humans. PLoS ONE 2012, 7, e33805. [Google Scholar] [CrossRef]
- Sarlus, H.; Hoglund, C.O.; Karshikoff, B.; Wang, X.; Lekander, M.; Schultzberg, M.; Oprica, M. Allergy influences the inflammatory status of the brain and enhances tau-phosphorylation. J. Cell. Mol. Med. 2012, 16, 2401–2412. [Google Scholar] [CrossRef] [PubMed]
- Joh, H.K.; Kwon, H.; Son, K.Y.; Yun, J.M.; Cho, S.H.; Han, K.; Park, J.H.; Cho, B. Allergic Diseases and Risk of Incident Dementia and Alzheimer’s Disease. Ann. Neurol. 2023, 93, 384–397. [Google Scholar] [CrossRef]
- Sarlus, H.; Eyjolfsdottir, H.; Eriksdotter, M.; Oprica, M.; Schultzberg, M. Influence of Allergy on Immunoglobulins and Amyloid-beta in the Cerebrospinal Fluid of Patients with Alzheimer’s Disease. J. Alzheimers Dis. 2015, 48, 495–505. [Google Scholar] [CrossRef]
- Naslund, J.; Schierhorn, A.; Hellman, U.; Lannfelt, L.; Roses, A.D.; Tjernberg, L.O.; Silberring, J.; Gandy, S.E.; Winblad, B.; Greengard, P.; et al. Relative abundance of Alzheimer A beta amyloid peptide variants in Alzheimer disease and normal aging. Proc. Natl. Acad. Sci. USA 1994, 91, 8378–8382. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Takio, K.; Ogawara, M.; Selkoe, D.J. Mass spectrometry of purified amyloid beta protein in Alzheimer’s disease. J. Biol. Chem. 1992, 267, 17082–17086. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Swomley, A.M.; Sultana, R. Amyloid beta-peptide (1-42)-induced oxidative stress in Alzheimer disease: Importance in disease pathogenesis and progression. Antioxid. Redox. Signal. 2013, 19, 823–835. [Google Scholar] [CrossRef]
- Smith, A.K.; Klimov, D.K. De novo aggregation of Alzheimer’s Abeta25-35 peptides in a lipid bilayer. Sci. Rep. 2019, 9, 7161. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Alysandratos, K.D.; Angelidou, A.; Delivanis, D.A.; Sismanopoulos, N.; Zhang, B.; Asadi, S.; Vasiadi, M.; Weng, Z.; Miniati, A.; et al. Mast cells and inflammation. Biochim. Biophys Acta 2012, 1822, 21–33. [Google Scholar] [CrossRef]
- Harcha, P.A.; Garces, P.; Arredondo, C.; Fernandez, G.; Saez, J.C.; van Zundert, B. Mast Cell and Astrocyte Hemichannels and Their Role in Alzheimer’s Disease, ALS, and Harmful Stress Conditions. Int. J. Mol. Sci. 2021, 22, 1924. [Google Scholar] [CrossRef] [PubMed]
- Shaik-Dasthagirisaheb, Y.B.; Conti, P. The Role of Mast Cells in Alzheimer’s Disease. Adv. Clin. Exp. Med. 2016, 25, 781–787. [Google Scholar] [CrossRef]
- Chaicharoenaudomrung, N.; Kunhorm, P.; Noisa, P. Three-dimensional cell culture systems as an in vitro platform for cancer and stem cell modeling. World J. Stem. Cells. 2019, 11, 1065–1083. [Google Scholar] [CrossRef] [PubMed]
- Langhans, S.A. Three-Dimensional In Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front. Pharmacol. 2018, 9, 6. [Google Scholar] [CrossRef]
- Linka, K.; Reiter, N.; Wurges, J.; Schicht, M.; Brauer, L.; Cyron, C.J.; Paulsen, F.; Budday, S. Unraveling the Local Relation Between Tissue Composition and Human Brain Mechanics Through Machine Learning. Front. Bioeng. Biotechnol. 2021, 9, 704738. [Google Scholar] [CrossRef]
- Smagin, D.A.; Galyamina, A.G.; Kovalenko, I.L.; Babenko, V.N.; Kudryavtseva, N.N. Aberrant Expression of Collagen Gene Family in the Brain Regions of Male Mice with Behavioral Psychopathologies Induced by Chronic Agonistic Interactions. Biomed. Res. Int. 2019, 2019, 7276389. [Google Scholar] [CrossRef]
- Ma, W.; Fitzgerald, W.; Liu, Q.Y.; O’Shaughnessy, T.J.; Maric, D.; Lin, H.J.; Alkon, D.L.; Barker, J.L. CNS stem and progenitor cell differentiation into functional neuronal circuits in three-dimensional collagen gels. Exp. Neurol. 2004, 190, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Brannvall, K.; Bergman, K.; Wallenquist, U.; Svahn, S.; Bowden, T.; Hilborn, J.; Forsberg-Nilsson, K. Enhanced neuronal differentiation in a three-dimensional collagen-hyaluronan matrix. J. Neurosci. Res. 2007, 85, 2138–2146. [Google Scholar] [CrossRef]
- Reilly, J.F.; Games, D.; Rydel, R.E.; Freedman, S.; Schenk, D.; Young, W.G.; Morrison, J.H.; Bloom, F.E. Amyloid deposition in the hippocampus and entorhinal cortex: Quantitative analysis of a transgenic mouse model. Proc. Natl. Acad. Sci. USA 2003, 100, 4837–4842. [Google Scholar] [CrossRef]
- Huijbers, W.; Mormino, E.C.; Schultz, A.P.; Wigman, S.; Ward, A.M.; Larvie, M.; Amariglio, R.E.; Marshall, G.A.; Rentz, D.M.; Johnson, K.A.; et al. Amyloid-beta deposition in mild cognitive impairment is associated with increased hippocampal activity, atrophy and clinical progression. Brain 2015, 138, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Vyas, Y.; Montgomery, J.M.; Cheyne, J.E. Hippocampal Deficits in Amyloid-beta-Related Rodent Models of Alzheimer’s Disease. Front. Neurosci. 2020, 14, 266. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Shi, X.; Li, X.; Zou, J.; Zhou, C.; Liu, W.; Shao, H.; Chen, H.; Shi, L. Neurotransmitter and neuropeptide regulation of mast cell function: A systematic review. J. Neuroinflamm. 2020, 17, 356. [Google Scholar] [CrossRef]
- Undem, B.J.; Riccio, M.M.; Weinreich, D.; Ellis, J.L.; Myers, A.C. Neurophysiology of mast cell-nerve interactions in the airways. Int. Arch. Allergy Immunol. 1995, 107, 199–201. [Google Scholar] [CrossRef] [PubMed]
- Spanos, C.; Pang, X.; Ligris, K.; Letourneau, R.; Alferes, L.; Alexacos, N.; Sant, G.R.; Theoharides, T.C. Stress-induced bladder mast cell activation: Implications for interstitial cystitis. J. Urol. 1997, 157, 669–672. [Google Scholar] [CrossRef]
- Kulka, M.; Sheen, C.H.; Tancowny, B.P.; Grammer, L.C.; Schleimer, R.P. Neuropeptides activate human mast cell degranulation and chemokine production. Immunology 2008, 123, 398–410. [Google Scholar] [CrossRef]
- Taracanova, A.; Alevizos, M.; Karagkouni, A.; Weng, Z.; Norwitz, E.; Conti, P.; Leeman, S.E.; Theoharides, T.C. SP and IL-33 together markedly enhance TNF synthesis and secretion from human mast cells mediated by the interaction of their receptors. Proc. Natl. Acad. Sci. USA 2017, 114, E4002–E4009. [Google Scholar] [CrossRef]
- Asadi, S.; Alysandratos, K.D.; Angelidou, A.; Miniati, A.; Sismanopoulos, N.; Vasiadi, M.; Zhang, B.; Kalogeromitros, D.; Theoharides, T.C. Substance P (SP) induces expression of functional corticotropin-releasing hormone receptor-1 (CRHR-1) in human mast cells. J. Investig. Dermatol. 2012, 132, 324–329. [Google Scholar] [CrossRef]
- Taracanova, A.; Tsilioni, I.; Conti, P.; Norwitz, E.R.; Leeman, S.E.; Theoharides, T.C. Substance P and IL-33 administered together stimulate a marked secretion of IL-1beta from human mast cells, inhibited by methoxyluteolin. Proc. Natl. Acad. Sci. USA 2018, 115, E9381–E9390. [Google Scholar] [CrossRef]
- Kabata, H.; Artis, D. Neuro-immune crosstalk and allergic inflammation. J. Clin. Investig. 2019, 129, 1475–1482. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Tsilioni, I. Humoral Innate Immunity and Acute-Phase Proteins. N. Engl. J. Med. 2023, 388, 1725. [Google Scholar] [CrossRef] [PubMed]
- Findeis, M.A. The role of amyloid beta peptide 42 in Alzheimer’s disease. Pharmacol. Ther. 2007, 116, 266–286. [Google Scholar] [CrossRef] [PubMed]
- Combs, C.K.; Karlo, J.C.; Kao, S.C.; Landreth, G.E. beta-Amyloid stimulation of microglia and monocytes results in TNFalpha-dependent expression of inducible nitric oxide synthase and neuronal apoptosis. J. Neurosci. 2001, 21, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Sanz, J.M.; Chiozzi, P.; Ferrari, D.; Colaianna, M.; Idzko, M.; Falzoni, S.; Fellin, R.; Trabace, L.; Di Virgilio, F. Activation of microglia by amyloid beta requires P2X7 receptor expression. J. Immunol. 2009, 182, 4378–4385. [Google Scholar] [CrossRef] [PubMed]
- Sultan, F.A. Dissection of Different Areas from Mouse Hippocampus. Bio. Protoc. 2013, 3, e955. [Google Scholar] [CrossRef]
- Zeng, L.; Kempf, H.; Murtaugh, L.C.; Sato, M.E.; Lassar, A.B. Shh establishes an Nkx3.2/Sox9 autoregulatory loop that is maintained by BMP signals to induce somitic chondrogenesis. Genes Dev. 2002, 16, 1990–2005. [Google Scholar] [CrossRef] [PubMed]
- Cairns, D.; Lee, P.; Uchimura, T.; Seufert, C.; Kwon, H.; Zeng, L. The role of muscle cells in regulating cartilage matrix production. J. Orthop. Res. 2010, 28, 529–536. [Google Scholar] [CrossRef]
- Hollander, J.M.; Li, L.; Rawal, M.; Wang, S.K.; Shu, Y.; Zhang, M.; Nielsen, H.C.; Rosen, C.J.; Zeng, L. A critical bioenergetic switch is regulated by IGF2 during murine cartilage development. Commun. Biol. 2022, 5, 1230. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Liu, S.; Zeng, L.; Tsilioni, I. Amyloid Beta Peptides Lead to Mast Cell Activation in a Novel 3D Hydrogel Model. Int. J. Mol. Sci. 2023, 24, 12002. https://doi.org/10.3390/ijms241512002
Liu J, Liu S, Zeng L, Tsilioni I. Amyloid Beta Peptides Lead to Mast Cell Activation in a Novel 3D Hydrogel Model. International Journal of Molecular Sciences. 2023; 24(15):12002. https://doi.org/10.3390/ijms241512002
Chicago/Turabian StyleLiu, Jingshu, Sihan Liu, Li Zeng, and Irene Tsilioni. 2023. "Amyloid Beta Peptides Lead to Mast Cell Activation in a Novel 3D Hydrogel Model" International Journal of Molecular Sciences 24, no. 15: 12002. https://doi.org/10.3390/ijms241512002
APA StyleLiu, J., Liu, S., Zeng, L., & Tsilioni, I. (2023). Amyloid Beta Peptides Lead to Mast Cell Activation in a Novel 3D Hydrogel Model. International Journal of Molecular Sciences, 24(15), 12002. https://doi.org/10.3390/ijms241512002



