PPARG: A Novel Target for Yellow Tea in Kidney Stone Prevention
Abstract
1. Introduction
2. Results
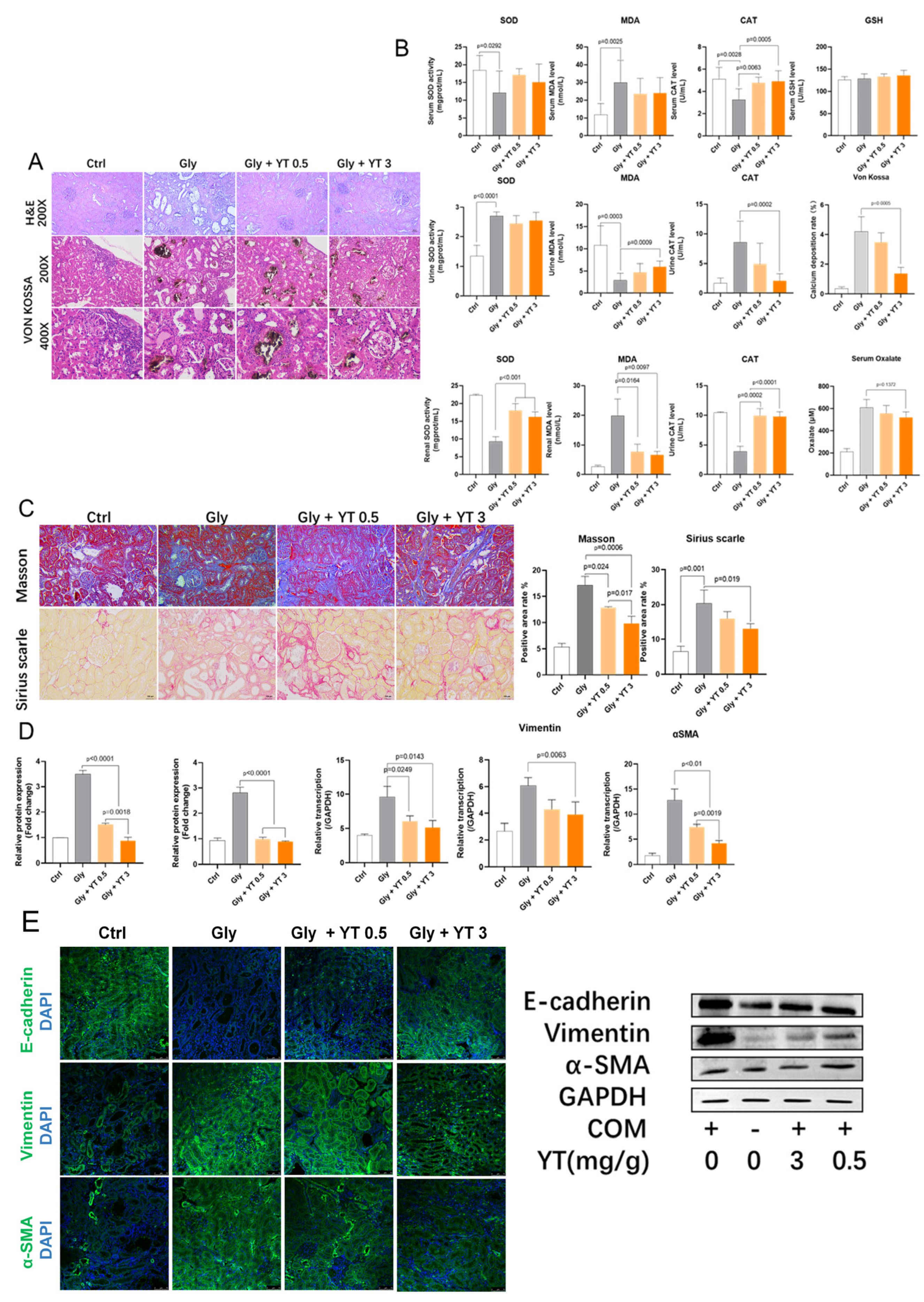
2.1. The Effects of Yellow Tea on Kidney Stone Formation in Rats
2.1.1. Yellow Tea Inhibits Renal Crystal Deposition and Oxidative Stress in Rats
2.1.2. Yellow Tea Alleviates Renal Fibrosis in Rats
2.1.3. Yellow Tea Inhibits Renal Epithelial–Mesenchymal Transition in Rats
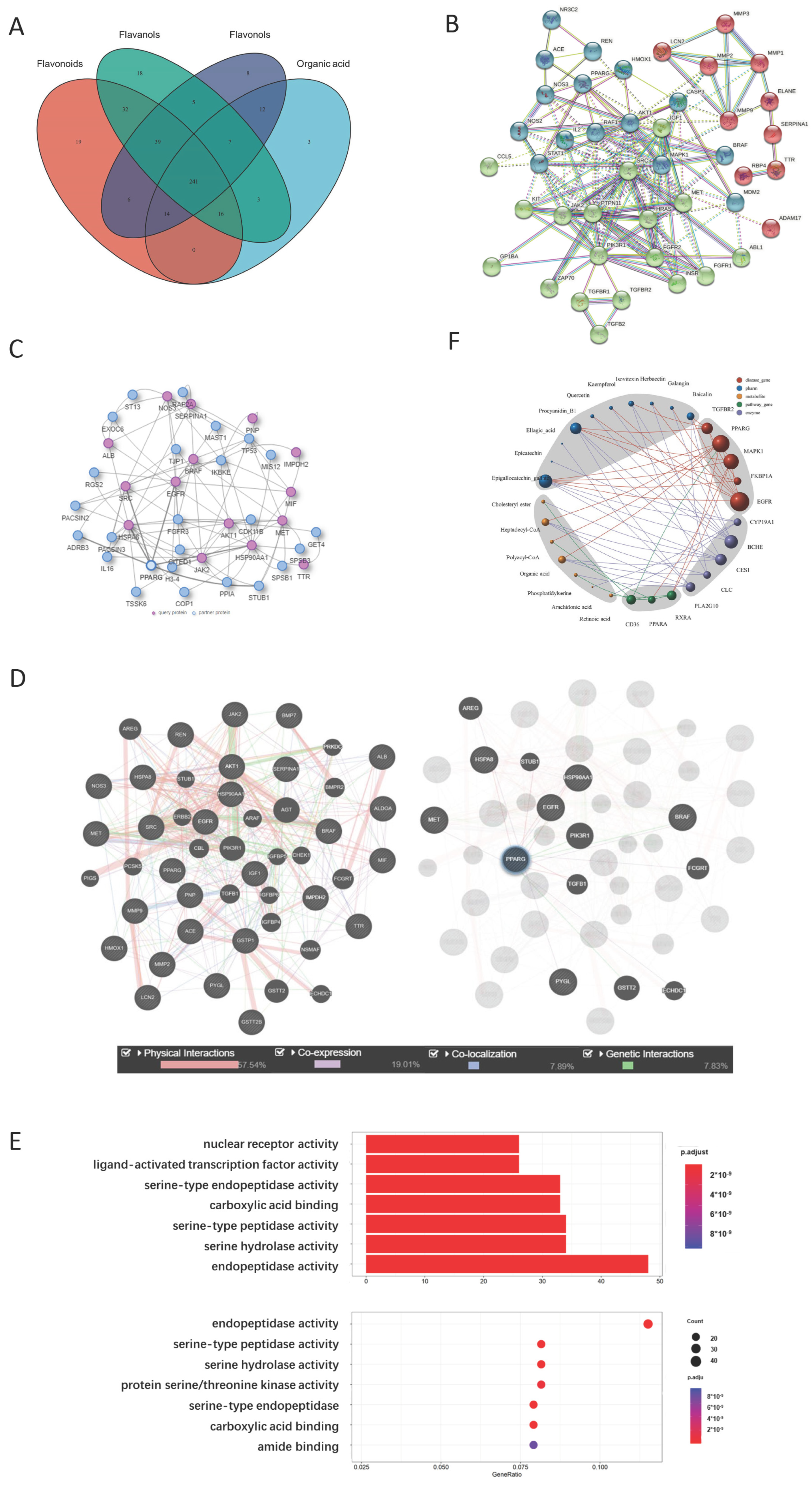
2.2. Yellow Tea Compound Target Screening Results
2.2.1. Representative Molecules of Yellow Tea and Their Target Network
2.2.2. PPARG Is the Center of the Yellow Tea and Kidney Disease Target Network
2.2.3. Metabolite Target Network Construction Based on PPARG Protein
2.2.4. GO Analysis Shows That Yellow Tea Affects Kidney Stones through Ligand-Activated Transcription Factor Activity
2.2.5. Network Analysis Shows That Isovitexin and Other Flavonoids Synergistically Act on PPARG Protein
2.3. QSAR Evaluation Shows That Catechins Have High PPARG Agonist Scores
2.4. Flavonoids Regulate PPARG Transcription in HK-2 Cells
2.4.1. Flavonoid Pretreatment Increased Cell Viability after H2O2 Stimulation
2.4.2. Flavonoid Pretreatment Upregulated PPARG and Downregulated TGF-Β1 Gene Transcription
2.4.3. Fatty Acid Treatment Upregulated PPARG Gene Transcription in HK-2 Cells
2.4.4. Flavonoid Pretreatment Upregulated CLC Gene Transcription in Co-Cultured Eosinophils
2.4.5. Flavonoid Pretreatment Upregulated Free Fatty Acid Concentration in Co-Cultured HK-2 Cells
2.4.6. Co-Cultured HK-2 Cells Had a Higher PPARG Transcription Level
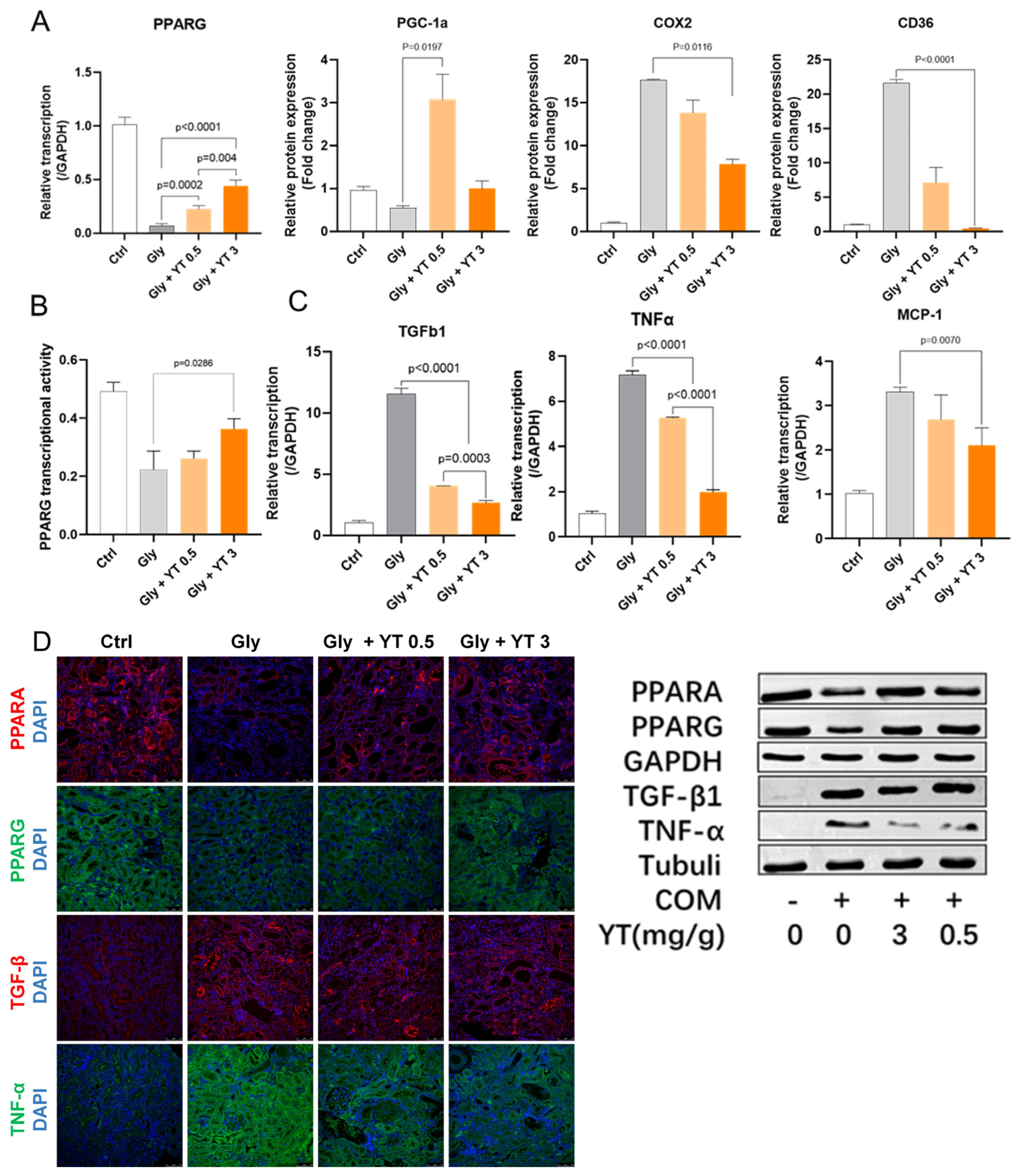
2.5. Yellow Tea Regulates PPARG Transcription Factor Activity
2.5.1. Yellow Tea Regulated Kidney PPARG Pathway Protein Transcription in Nephrolithiasis Rats
2.5.2. Yellow Tea Inhibited Downregulation of PPARG Transcription Factor Activity in Nephrolithiasis Rat Kidney
2.5.3. Yellow Tea Inhibited Inflammation- and Fibrosis-Related Cytokine Transcription in Nephrolithiasis Rat Kidney
2.5.4. Yellow Tea Affected Protein Expression in Nephrolithiasis Rats
3. Discussion
4. Materials and Methods
4.1. Animal Model of Renal Oxalate Stones and Yellow Tea Administration
4.2. Histological Methods to Analyze Renal Tissue Pathology
4.3. Biochemical and Molecular Marker Measurements in Serum, Urine, and Cell Samples
4.4. qRT–PCR
4.5. Western Blotting
4.6. Immunofluorescence Staining
4.7. Compound Structure Acquisition and Pharmacophore Prediction
4.8. Target Screening, Network Generation, and GO Analysis
4.9. Screening Metabolite-Related Targets and Construction of Synergistic Network
4.10. QSAR Model Validation of PPARG Agonist Activity of Flavonoids
4.11. Cell Culture and Treatments
4.12. Cell Viability Assay
4.13. Biochemical Marker Detection
4.14. PPARG Transcription Factor Activity Assay
4.15. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, S.R.; Pearle, M.S.; Robertson, W.G.; Gambaro, G.; Canales, B.K.; Doizi, S.; Traxer, O.; Tiselius, H.-G. Kidney stones. Nat. Rev. Dis. Prim. 2016, 2, 16008. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y. Epidemiology of Stone Disease Over a 40-Year Period in Japan. In Urolithiasis: Basic Science and Clinical Practice; Talati, J.J., Tiselius, H.-G., Albala, D.M., Ye, Z., Talati, J.J., Tiselius, H.-G., Albala, D.M., Ye, Z., Eds.; Springer: London, UK, 2012; pp. 89–96. [Google Scholar] [CrossRef]
- Finlayson, B. Physicochemical aspects of urolithiasis. Kidney Int. 1978, 13, 344–360. [Google Scholar] [CrossRef] [PubMed]
- Afshar, K.; Jafari, S.; Marks, A.J.; Eftekhari, A.; E MacNeily, A. Nonsteroidal anti-inflammatory drugs (NSAIDs) and non-opioids for acute renal colic. Cochrane Database Syst. Rev. 2015, CD006027. [Google Scholar] [CrossRef] [PubMed]
- Kasote, D.M.; Jagtap, S.; Thapa, D.; Khyade, M.; Russell, W. Herbal remedies for urinary stones used in India and China: A review. J. Ethnopharmacol. 2017, 203, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, M.; Zhao, J.; Wang, Y.-H.; Tang, Q.; Khan, I.A. Yellow tea (Camellia sinensis L.), a promising Chinese tea: Processing, chemical constituents and health benefits. Food Res. Int. 2018, 107, 567–577. [Google Scholar] [CrossRef]
- Wang, C.; Liu, J.; Sang, S.; Ao, X.; Su, M.; Hu, B.; Li, H. Effects of Tea Treatments against High-Fat Diet-Induced Disorder by Regulating Lipid Metabolism and the Gut Microbiota. Comput. Math. Methods Med. 2022, 2022, 9336080. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, W.; Yang, L.; Chen, L.; Stimpson, S.A.; Diehl, A.M. PPARγ agonists prevent TGFβ1/Smad3-signaling in human hepatic stellate cells. Biochem. Biophys. Res. Commun. 2006, 350, 385–391. [Google Scholar] [CrossRef]
- Casas, A.I.; Hassan, A.A.; Larsen, S.J.; Gomez-Rangel, V.; Elbatreek, M.; Kleikers, P.W.M.; Guney, E.; Egea, J.; López, M.G.; Baumbach, J.; et al. From single drug targets to synergistic network pharmacology in ischemic stroke. Proc. Natl. Acad. Sci. USA 2019, 116, 7129–7136. [Google Scholar] [CrossRef]
- Danishuddin; Khan, A. U. Descriptors and their selection methods in QSAR analysis: Paradigm for drug design. Drug Discov. Today 2016, 21, 1291–1302. [Google Scholar] [CrossRef]
- Sorokin, I.; Mamoulakis, C.; Miyazawa, K.; Rodgers, A.; Talati, J.; Lotan, Y. Epidemiology of stone disease across the world. World J. Urol. 2017, 35, 1301–1320. [Google Scholar] [CrossRef]
- López, M.; Hoppe, B. History, epidemiology and regional diversities of urolithiasis. Pediatr. Nephrol. 2010, 25, 49–59. [Google Scholar] [CrossRef]
- Chaussy, C.; Schmiedt, E.; Jocham, D.; Brendel, W.; Forssmann, B.; Walther, V. First Clinical Experience with Extracorporeally Induced Destruction of Kidney Stones by Shock Waves. J. Urol. 2017, 197, S160–S163. [Google Scholar] [CrossRef]
- Alelign, T.; Petros, B. Kidney Stone Disease: An Update on Current Concepts. Adv. Urol. 2018, 2018, 3068365. [Google Scholar] [CrossRef]
- Anders, H.-J.; Ryu, M. Renal microenvironments and macrophage phenotypes determine progression or resolution of renal inflammation and fibrosis. Kidney Int. 2011, 80, 915–925. [Google Scholar] [CrossRef]
- Ding, T.; Zhao, T.; Li, Y.; Liu, Z.; Ding, J.; Ji, B.; Wang, Y.; Guo, Z. Vitexin exerts protective effects against calcium oxalate crystal-induced kidney pyroptosis in vivo and in vitro. Phytomedicine 2021, 86, 153562. [Google Scholar] [CrossRef]
- Vargas, F.; Romecín, P.; Guillen, A.I.G.; Wangesteen, R.; Vargas-Tendero, P.; Paredes, M.D.; Atucha, N.M.; García-Estañ, J. Flavonoids in Kidney Health and Disease. Front. Physiol. 2018, 9, 394. [Google Scholar] [CrossRef]
- Saito, A. EMT and EndMT: Regulated in similar ways? J. Biochem. 2013, 153, 493–495. [Google Scholar] [CrossRef]
- Sang, S.; Wang, L.; Liang, T.; Su, M.; Li, H. Potential role of tea drinking in preventing hyperuricaemia in rats: Biochemical and molecular evidence. Chin. Med. 2022, 17, 108. [Google Scholar] [CrossRef]
- Toffoli, B.; Gilardi, F.; Winkler, C.; Soderberg, M.; Kowalczuk, L.; Arsenijevic, Y.; Bamberg, K.; Bonny, O.; Desvergne, B. Nephropathy in Pparg-null mice highlights PPARγ systemic activities in metabolism and in the immune system. PLoS ONE 2017, 12, e0171474. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef] [PubMed]
- Gomes, I.; Mathur, S.K.; Espenshade, B.M.; Mori, Y.; Varga, J.; Ackerman, S.J. Eosinophil-fibroblast interactions induce fibroblast IL-6 secretion and extracellular matrix gene expression: Implications in fibrogenesis. J. Allergy Clin. Immunol. 2005, 116, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Li, L.; Zhang, Y.; Zheng, L.; Xu, M.; Rong, R.; Zhu, T. Baicalin Ameliorates H2O2 Induced Cytotoxicity in HK-2 Cells through the Inhibition of ER Stress and the Activation of Nrf2 Signaling. Int. J. Mol. Sci. 2014, 15, 12507–12522. [Google Scholar] [CrossRef] [PubMed]
- Small, D.M.; Morais, C.; Coombes, J.S.; Bennett, N.C.; Johnson, D.W.; Gobe, G.C. Oxidative stress-induced alterations in PPAR-γ and associated mitochondrial destabilization contribute to kidney cell apoptosis. Am. J. Physiol. Physiol. 2014, 307, F814–F822. [Google Scholar] [CrossRef]

| Formula | Molecule | Molecular Weight | Class | OB (%) | DL |
|---|---|---|---|---|---|
| C22H18O11 | (-)-Epigallocatechin gallate | 458.08396 | Flavan-3-ol | 55.09 | 0.77 |
| C21H18O11 | Baicalein 7-O-Glucuronide | 446.08371 | Flavone | 40.12 | 0.75 |
| C21H20O10 | Isovitexin | 432.10466 | Flavone | 31.29 | 0.72 |
| C30H26O12 | Procyanidin B1 | 578.14173 | Flavan-3-ol | 67.87 | 0.66 |
| C14H6O8 | Ellagic acid | 302.00551 | Organic Acid | 43.06 | 0.43 |
| C15H10O7 | Quercetin | 302.04179 | Flavonol | 46.43 | 0.28 |
| C15H10O7 | Herbacetin | 302.04178 | Flavonol | 36.07 | 0.27 |
| C15H14O6 | Epicatechin | 290.07823 | Flavan-3-ol | 48.96 | 0.24 |
| C15H10O6 | Kaempferol | 286.04692 | Flavonol | 41.88 | 0.24 |
| C15H10O5 | Galangin | 270.05195 | Flavonol | 45.55 | 0.21 |
| HMDB ID | Molecular | Formula | Molecular Weight |
|---|---|---|---|
| HMDB0000039 | Butyric acid | C4H8O2 | 88 |
| HMDB0000220 | Palmitic acid | C16H32O2 | 256 |
| HMDB0000827 | Stearic acid | C18H36O2 | 284 |
| HMDB0001338 | Palmityl-CoA | C37H66N7O17P3S | 1006 |
| HMDB0002212 | Arachidic acid | C20H40O2 | 313 |
| HMDB0002259 | Heptadecanoic acid | C17H34O2 | 270 |
| HMDB0002845 | Hexanoyl-CoA | C27H46N7O17P3S | 866 |
| Molecule | CHEMBL ID | pEC50 (PPARG) |
|---|---|---|
| Rosiglitazone | CHEMBL121 | 7.12 |
| Chiglitazar | CHEMBL4650349 | 6.68 |
| Pioglitazone | CHEMBL595 | 6.60 |
| Isovitexin | CHEMBL465360 | 6.29 |
| Epigallocatechin gallate | CHEMBL297453 | 5.60 |
| Baicalin | CHEMBL485818 | 5.57 |
| Ellagic acid | CHEMBL6246 | 5.39 |
| Procyanidin B1 | CHEMBL504937 | 5.24 |
| Herbacetin | CHEMBL611029 | 5.21 |
| Galangin | CHEMBL309490 | 5.06 |
| Kaempferol | CHEMBL150 | 5.06 |
| Quercetin | CHEMBL50 | 5.05 |
| Epicatechin | CHEMBL129482 | 4.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, M.; Sang, S.; Liang, T.; Li, H. PPARG: A Novel Target for Yellow Tea in Kidney Stone Prevention. Int. J. Mol. Sci. 2023, 24, 11955. https://doi.org/10.3390/ijms241511955
Su M, Sang S, Liang T, Li H. PPARG: A Novel Target for Yellow Tea in Kidney Stone Prevention. International Journal of Molecular Sciences. 2023; 24(15):11955. https://doi.org/10.3390/ijms241511955
Chicago/Turabian StyleSu, Mingjie, Siyao Sang, Taotao Liang, and Hui Li. 2023. "PPARG: A Novel Target for Yellow Tea in Kidney Stone Prevention" International Journal of Molecular Sciences 24, no. 15: 11955. https://doi.org/10.3390/ijms241511955
APA StyleSu, M., Sang, S., Liang, T., & Li, H. (2023). PPARG: A Novel Target for Yellow Tea in Kidney Stone Prevention. International Journal of Molecular Sciences, 24(15), 11955. https://doi.org/10.3390/ijms241511955






