Aging Model for Analyzing Drug-Induced Proarrhythmia Risks Using Cardiomyocytes Differentiated from Progeria-Patient-Derived Induced Pluripotent Stem Cells
Abstract
1. Introduction
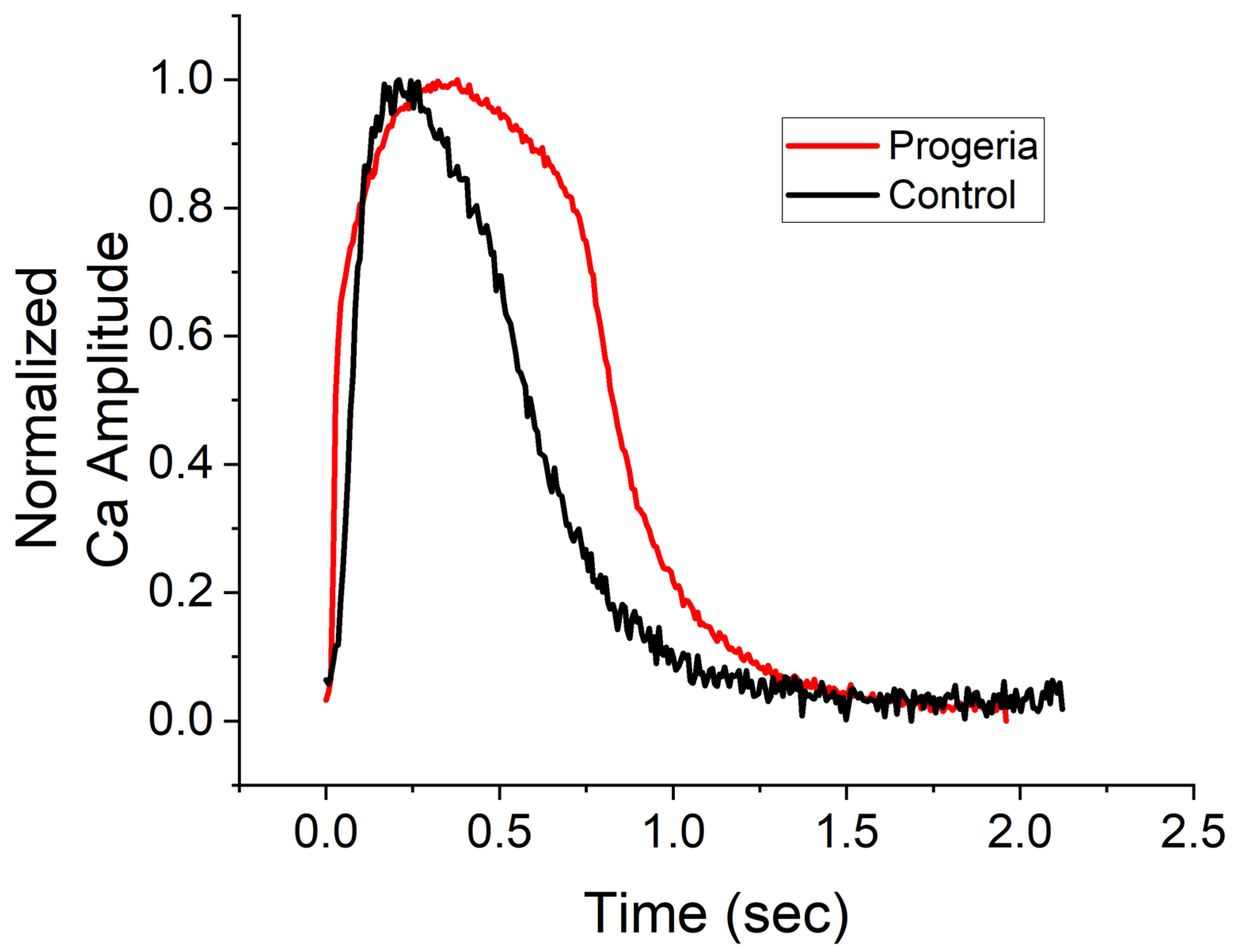
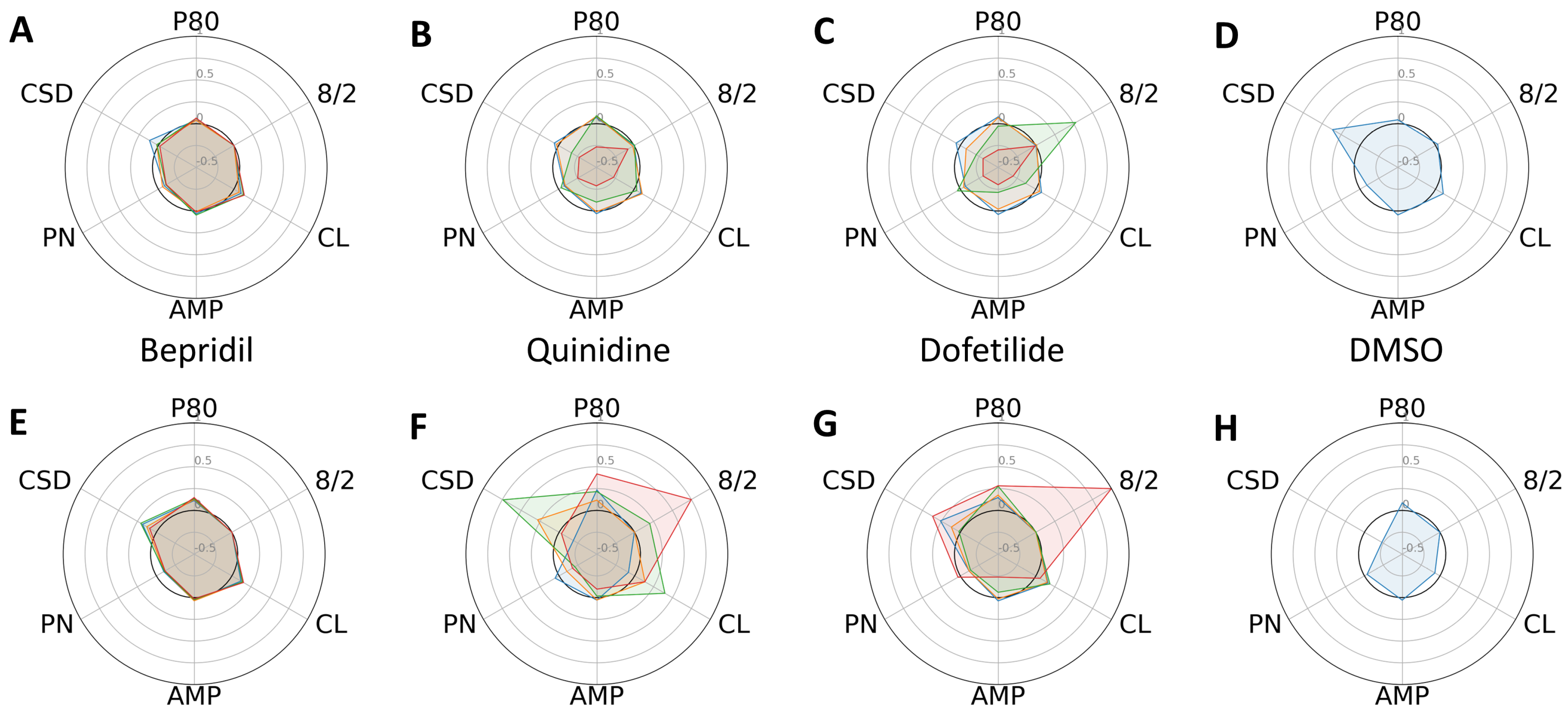
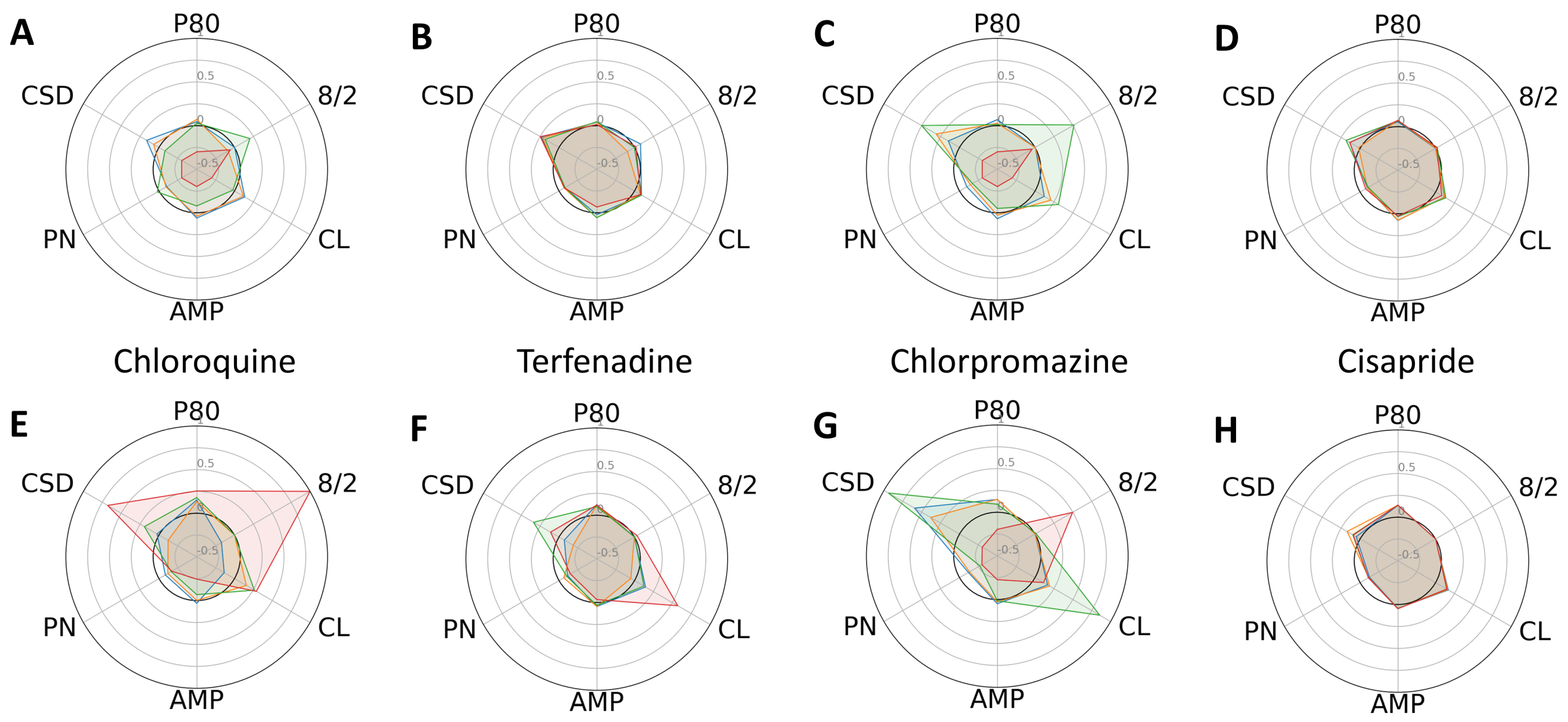
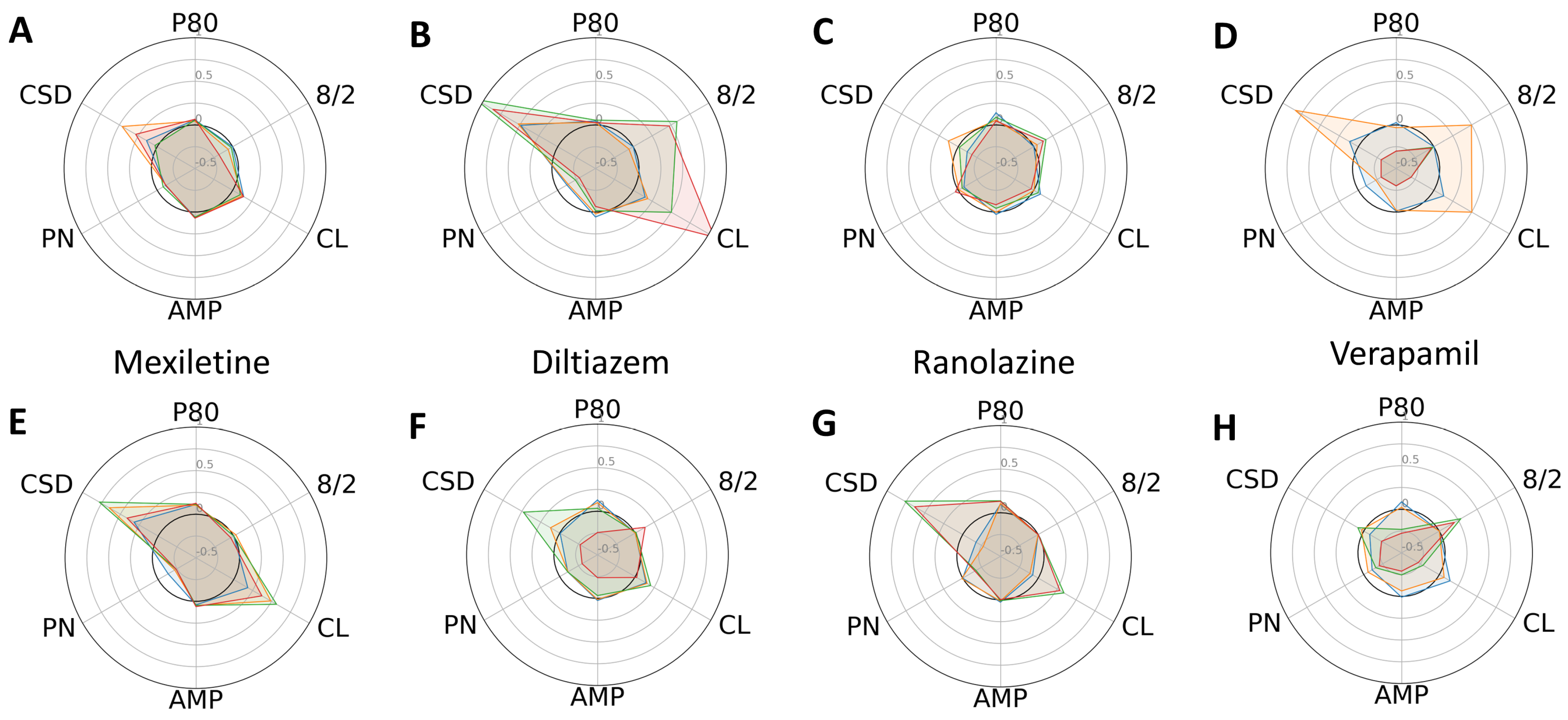
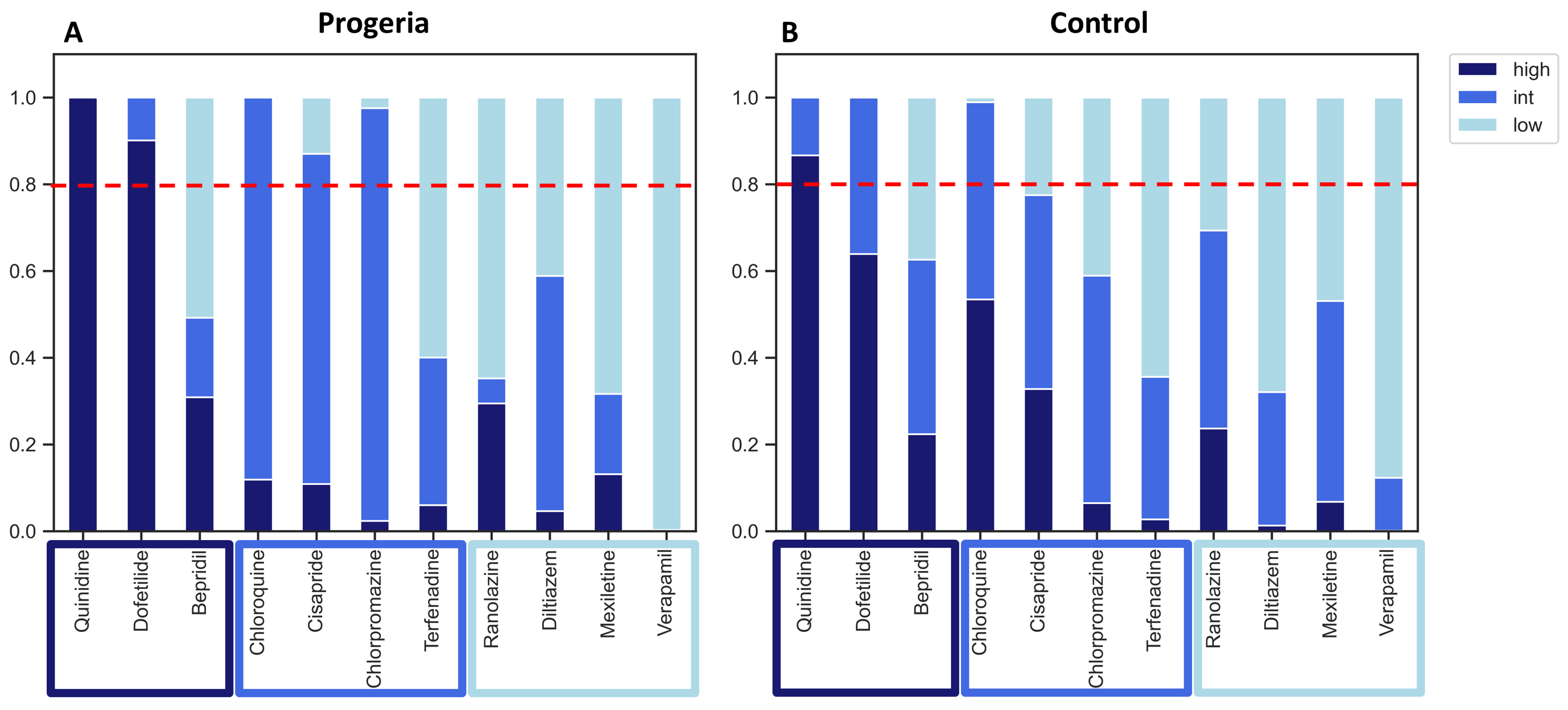
2. Results
3. Discussion
4. Methods
4.1. iPSC Culture
4.2. iPSC Differentiation and Cardiomyocyte Purification
4.3. Compounds
| Drug | Category | Cmax [nM] | Dose1 [nM] | Dose2 [nM] | Dose3 [nM] | Dose4 [nM] |
|---|---|---|---|---|---|---|
| Dofetilide | High | 2.14 | 0.3 | 1 | 3 | 10 |
| Bepridil | High | 31.5 | 35 | 105 | 210 | 315 |
| Quinidine | High | 843 | 100 | 300 | 900 | 2700 |
| Terfenadine | Mid | 0.286 | 0.08 | 0.8 | 8 | 80 |
| Chloroquine | Mid | 250 | 250 | 750 | 2500 | 25,000 |
| Chlorpromazine | Mid | 34.5 | 35 | 350 | 1050 | 3500 |
| Cisapride | Mid | 2.58 | 2.5 | 7.5 | 25 | 125 |
| Diltiazem | Low | 128 | 3 | 10 | 30 | 90 |
| Mexiletine | Low | 2500 | 625 | 1250 | 2500 | 3750 |
| Ranolazine | Low | 1948 | 1000 | 2300 | 6900 | 15,000 |
| Verapamil | Low | 45 | 1 | 10 | 50 | 150 |
4.4. Assay
4.5. Radial Plots
4.6. Logistic Regression
- model = LogisticRegression(multi_class = ‘multinomial’, solver = ‘lbfgs’, random_state = 0, max_iter = 1000)
- result = model.fit(x_train,y_train)
- probabilities = model.predict_proba(drug)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kantor, E.D.; Rehm, C.D.; Haas, J.S.; Chan, A.T.; Giovannucci, E.L. Trends in Prescription Drug Use Among Adults in the United States from 1999–2012. JAMA 2015, 314, 1818–1831. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Albert, N.M.; Allen, L.A.; Bluemke, D.A.; Butler, J.; Fonarow, G.C.; Ikonomidis, J.S.; Khavjou, O.; Konstam, M.A.; Maddox, T.M.; et al. Forecasting the impact of heart failure in the United States: A policy statement from the American Heart Association. Circ. Heart Fail. 2013, 6, 606–619. [Google Scholar] [CrossRef] [PubMed]
- Page, R.L., II; O’Bryant, C.L.; Cheng, D.; Dow, T.J.; Ky, B.; Stein, C.M.; Spencer, A.P.; Trupp, R.J.; Lindenfeld, J.; American Heart Association Clinical Pharmacology; et al. Drugs That May Cause or Exacerbate Heart Failure. Circulation 2016, 134, e32–e69. [Google Scholar] [CrossRef] [PubMed]
- Cobretti, M.R.; Page, R.L., II; Linnebur, S.A.; Deininger, K.M.; Ambardekar, A.V.; Lindenfeld, J.; Aquilante, C.L. Medication regimen complexity in ambulatory older adults with heart failure. Clin. Interv. Aging 2017, 12, 679–686. [Google Scholar] [CrossRef]
- Reddy, P.; Shenoy, C.; Blaes, A.H. Cardio-oncology in the older adult. J. Geriatr. Oncol. 2017, 8, 308–314. [Google Scholar] [CrossRef]
- Stoltzfus, K.C.; Zhang, Y.; Sturgeon, K.; Sinoway, L.I.; Trifiletti, D.M.; Chinchilli, V.M.; Zaorsky, N.G. Fatal heart disease among cancer patients. Nat. Commun. 2020, 11, 2011. [Google Scholar] [CrossRef]
- Ewer, M.S.; Ewer, S.M. Cardiotoxicity of anticancer treatments. Nat. Rev. Cardiol. 2015, 12, 547–558. [Google Scholar] [CrossRef]
- Stevens, J.L.; Baker, T.K. The future of drug safety testing: Expanding the view and narrowing the focus. Drug Discov. Today 2009, 14, 162–167. [Google Scholar] [CrossRef]
- Beamish, J.A.; He, P.; Kottke-Marchant, K.; Marchant, R.E. Molecular regulation of contractile smooth muscle cell phenotype: Implications for vascular tissue engineering. Tissue Eng. Part B Rev. 2010, 16, 467–491. [Google Scholar] [CrossRef]
- Himmel, H.M. Drug-induced functional cardiotoxicity screening in stem cell-derived human and mouse cardiomyocytes: Effects of reference compounds. J. Pharmacol. Toxicol. Methods 2013, 68, 97–111. [Google Scholar] [CrossRef]
- He, J.Q.; Ma, Y.; Lee, Y.; Thomson, J.A.; Kamp, T.J. Human Embryonic Stem Cells Develop into Multiple Types of Cardiac Myocytes: Action Potential Characterization. Circ. Res. 2003, 93, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Lieu, D.K.; Fu, J.-D.; Chiamvimonvat, N.; Tung, K.C.; McNerney, G.P.; Huser, T.; Keller, G.; Kong, C.-W.; Li, R.A.; L, P.; et al. Mechanism-Based Facilitated Maturation of Human Pluripotent Stem Cell–Derived Cardiomyocytes. Circ. Arrhythmia Electrophysiol. 2013, 6, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Colatsky, T.; Fermini, B.; Gintant, G.; Pierson, J.B.; Sager, P.; Sekino, Y.; Strauss, D.G.; Stockbridge, N. The Comprehensive In Vitro Proarrhythmia Assay (CiPA) initiative—Update on progress. J. Pharmacol. Toxicol. Methods 2016, 81, 15–20. [Google Scholar] [CrossRef]
- Folch, J.; Busquets, O.; Ettcheto, M.; López, E.S.; Pallàs, M.; Beas-Zarate, C.; Marin, M.; Casadesus, G.; Olloquequi, J.; Auladell, C.; et al. Experimental Models for Aging and their Potential for Novel Drug Discovery. Curr. Neuropharmacol. 2018, 16, 1466–1483. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.R.; Venable, S.; Roberts, T.W.; Metter, E.J.; Monticone, R.; Schneider, E.L. Relationship between in vivo age and in vitro aging: Assessment of 669 cell cultures derived from members of the Baltimore Longitudinal Study of Aging. J. Gerontol. Ser. A 2002, 57, B239–B246. [Google Scholar] [CrossRef]
- Hamczyk, M.R.; del Campo, L.; Andrés, V. Aging in the Cardiovascular System: Lessons from Hutchinson-Gilford Progeria Syndrome. Annu. Rev. Physiol. 2018, 80, 27–48. [Google Scholar] [CrossRef] [PubMed]
- Prakash, A.; Gordon, L.B.; E Kleinman, M.; D’Agostino, R.; Massaro, J.; Kieran, M.W.; Gerhard-Herman, M.; Smoot, L.B. Abstract 11518: Diastolic Left Ventricular Dysfunction is a Common and Early Cardiac Abnormality in Hutchinson-Gilford Progeria Syndrome. Circulation 2015, 132 (Suppl. S3), A11518. [Google Scholar] [CrossRef]
- Eriksson, M.; Brown, W.T.; Gordon, L.B.; Glynn, M.W.; Singer, J.; Scott, L.; Erdos, M.R.; Robbins, C.M.; Moses, T.Y.; Berglund, P.; et al. Recurrent de novo point mutations in lamin A cause Hutchinson–Gilford progeria syndrome. Nature 2003, 423, 293. [Google Scholar] [CrossRef]
- Cao, K.; Blair, C.D.; Faddah, D.A.; Kieckhaefer, J.E.; Olive, M.; Erdos, M.R.; Nabel, E.G.; Collins, F.S. Progerin and telomere dysfunction collaborate to trigger cellular senescence in normal human fibroblasts. J. Clin. Investig. 2011, 121, 2833–2844. [Google Scholar] [CrossRef]
- Scaffidi, P.; Misteli, T. Lamin A-Dependent Nuclear Defects in Human Aging. Science 2006, 312, 1059–1063. [Google Scholar] [CrossRef]
- McClintock, D.; Ratner, D.; Lokuge, M.; Owens, D.M.; Gordon, L.B.; Collins, F.S.; Djabali, K. The Mutant Form of Lamin A that Causes Hutchinson-Gilford Progeria Is a Biomarker of Cellular Aging in Human Skin. PLoS ONE 2007, 2, e1269. [Google Scholar] [CrossRef]
- Olive, M.; Harten, I.; Mitchell, R.; Beers, J.K.; Djabali, K.; Cao, K.; Erdos, M.R.; Blair, C.; Funke, B.; Smoot, L.; et al. Cardiovascular Pathology in Hutchinson-Gilford Progeria: Correlation with the Vascular Pathology of Aging. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2301–2309. [Google Scholar] [CrossRef]
- Janczewski, A.; Spurgeon, H.A.; Lakatta, E.G. Action Potential Prolongation in Cardiac Myocytes of Old Rats is an Adaptation to Sustain Youthful Intracellular Ca2+ Regulation. J. Mol. Cell. Cardiol. 2002, 34, 641–648. [Google Scholar] [CrossRef]
- Morita, N.; Sovari, A.A.; Xie, Y.; Fishbein, M.C.; Mandel, W.J.; Garfinkel, A.; Lin, S.F.; Chen, P.S.; Xie, L.H.; Chen, F.; et al. Increased susceptibility of aged hearts to ventricular fibrillation during oxidative stress. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1594–H1605. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, J.; Zheng, K.; Thai, S.; Simpson, R.J.; Kinlaw, A.C.; Xu, Y.; Wei, J.; Cui, X.; Buse, J.B.; et al. Serious Cardiovascular Adverse Events Associated with Hydroxychloroquine/Chloroquine Alone or with Azithromycin in Patients with COVID-19: A Pharmacovigilance Analysis of the FDA Adverse Event Reporting System (FAERS). Drugs-Real World Outcomes 2022, 9, 231–241. [Google Scholar] [CrossRef]
- Wang, J.C.; Kiyosue, T.; Kiriyama, K.; Arita, M. Bepridil differentially inhibits two delayed rectifier K(+) currents, I(Kr) and I(Ks), in guinea-pig ventricular myocytes. Br. J. Pharmacol. 1999, 128, 1733–1738. [Google Scholar] [CrossRef]
- Rabkin, S.W.; Cheng, X.-B.J.; Thompson, D.J. Detailed analysis of the impact of age on the QT interval. J. Geriatr. Cardiol. 2016, 13, 740–748. [Google Scholar]
- Schwartz, P.J.; Woosley, R.L. Predicting the Unpredictable: Drug-Induced QT Prolongation and Torsades de Pointes. J. Am. Coll. Cardiol. 2016, 67, 1639–1650. [Google Scholar] [CrossRef] [PubMed]
- Prakash, A.; Gordon, L.B.; Kleinman, M.E.; Gurary, E.B.; Massaro, J.; D’agostino, R.; Kieran, M.W.; Gerhard-Herman, M.; Smoot, L. Cardiac Abnormalities in Patients with Hutchinson-Gilford Progeria Syndrome. JAMA Cardiol. 2018, 3, 326–334. [Google Scholar] [CrossRef]
- Filgueiras-Rama, D.; Torres, J.R.; Andrés, V. Electrocardiographic Abnormalities in Patients with Hutchinson-Gilford Progeria Syndrome. JAMA Cardiol. 2018, 3, 1024–1025. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Torres, J.; Calvo, C.J.; Llach, A.; Guzmán-Martínez, G.; Caballero, R.; González-Gómez, C.; Jiménez-Borreguero, L.J.; Guadix, J.A.; Osorio, F.G.; López-Otín, C.; et al. Cardiac electrical defects in progeroid mice and Hutchinson-Gilford progeria syndrome patients with nuclear lamina alterations. Proc. Natl. Acad. Sci. USA 2016, 113, E7250–E7259. [Google Scholar] [CrossRef] [PubMed]
- Litviňuková, M.; Talavera-López, C.; Maatz, H.; Reichart, D.; Worth, C.L.; Lindberg, E.L.; Kanda, M.; Polanski, K.; Heinig, M.; Lee, M.; et al. Cells of the adult human heart. Nature 2020, 588, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, M.J.; Daily, N.J.; Wang, A.; Conway, M.K.; Wakatsuki, T. Genetic and Tissue Engineering Approaches to Modeling the Mechanics of Human Heart Failure for Drug Discovery. Front. Cardiovasc. Med. 2018, 5, 120. [Google Scholar] [CrossRef] [PubMed]
- Olivetti, G.; Melissari, M.; Capasso, J.M.; Anversa, P. Cardiomyopathy of the aging human heart. Myocyte loss and reactive cellular hypertrophy. Circ. Res. 1991, 68, 1560–1568. [Google Scholar] [CrossRef] [PubMed]
- Seidman, J.G.; Seidman, C. The Genetic Basis for Cardiomyopathy: From Mutation Identification to Mechanistic Paradigms. Cell 2001, 104, 557–567. [Google Scholar] [CrossRef]
- Ahmad, T.; Pencina, M.J.; Schulte, P.J.; O’brien, E.; Whellan, D.J.; Piña, I.L.; Kitzman, D.W.; Lee, K.L.; O’connor, C.M.; Felker, G.M. Clinical Implications of Chronic Heart Failure Phenotypes Defined by Cluster Analysis. J. Am. Coll. Cardiol. 2014, 64, 1765–1774. [Google Scholar] [CrossRef]
- Patel, D.; Stohlman, J.; Dang, Q.; Strauss, D.G.; Blinova, K. Assessment of Proarrhythmic Potential of Drugs in Optogenetically Paced Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Toxicol. Sci. 2019, 170, 167–179. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daily, N.; Elson, J.; Wakatsuki, T. Aging Model for Analyzing Drug-Induced Proarrhythmia Risks Using Cardiomyocytes Differentiated from Progeria-Patient-Derived Induced Pluripotent Stem Cells. Int. J. Mol. Sci. 2023, 24, 11959. https://doi.org/10.3390/ijms241511959
Daily N, Elson J, Wakatsuki T. Aging Model for Analyzing Drug-Induced Proarrhythmia Risks Using Cardiomyocytes Differentiated from Progeria-Patient-Derived Induced Pluripotent Stem Cells. International Journal of Molecular Sciences. 2023; 24(15):11959. https://doi.org/10.3390/ijms241511959
Chicago/Turabian StyleDaily, Neil, Julian Elson, and Tetsuro Wakatsuki. 2023. "Aging Model for Analyzing Drug-Induced Proarrhythmia Risks Using Cardiomyocytes Differentiated from Progeria-Patient-Derived Induced Pluripotent Stem Cells" International Journal of Molecular Sciences 24, no. 15: 11959. https://doi.org/10.3390/ijms241511959
APA StyleDaily, N., Elson, J., & Wakatsuki, T. (2023). Aging Model for Analyzing Drug-Induced Proarrhythmia Risks Using Cardiomyocytes Differentiated from Progeria-Patient-Derived Induced Pluripotent Stem Cells. International Journal of Molecular Sciences, 24(15), 11959. https://doi.org/10.3390/ijms241511959






