JAK3 Y841 Autophosphorylation Is Critical for STAT5B Activation, Kinase Domain Stability and Dimer Formation
Abstract
1. Introduction
2. Results
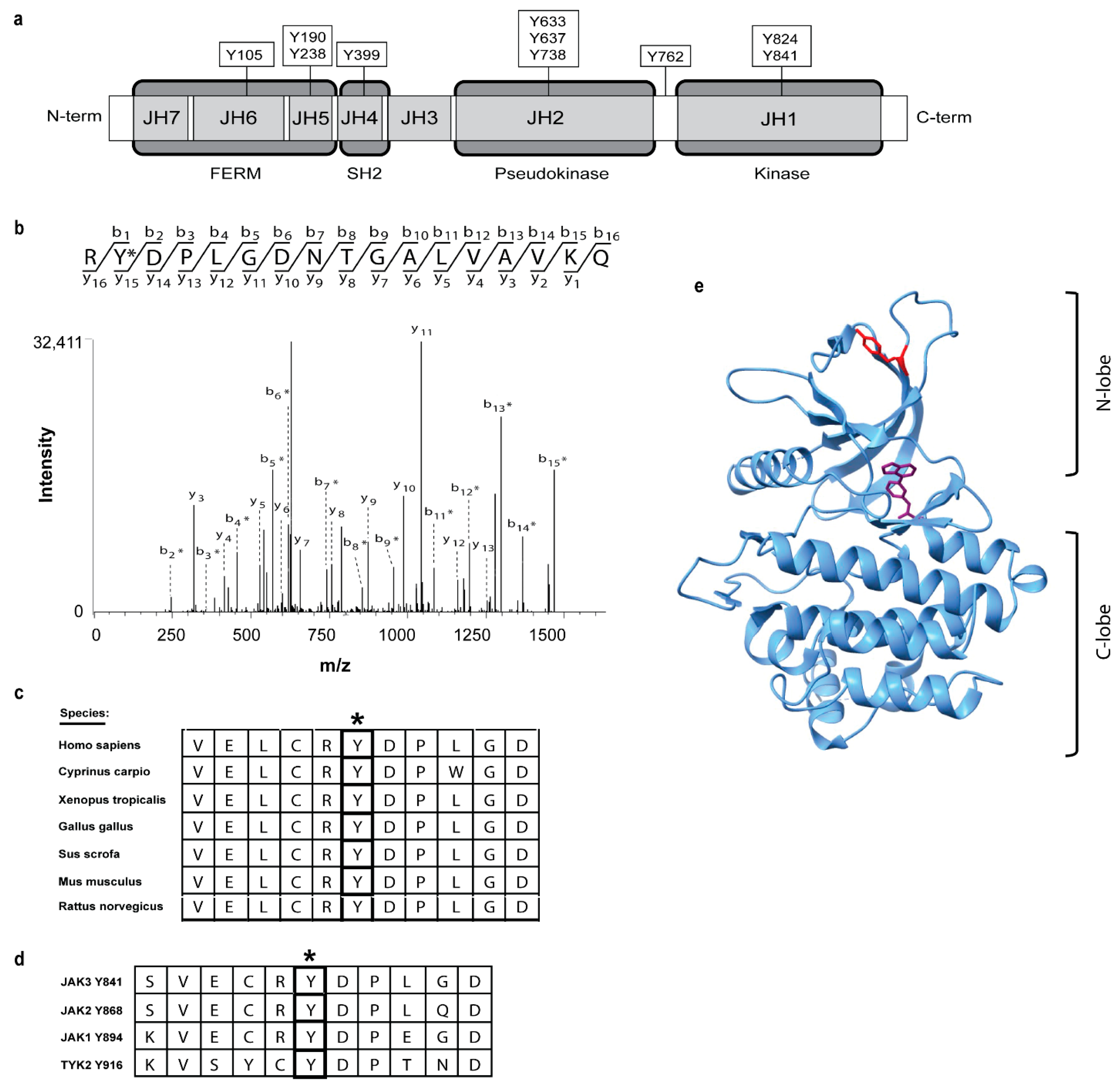
2.1. Identification of Ten Novel JAK3 Tyrosine Phosphorylation Sites
2.2. JAK3 Kinase Domain Residues Y841 and Y929 Are Critical for Activation of Downstream Effector STAT5B
2.3. JAK3 Y841 Represents a Constitutively Autophosphorylated Residue
2.4. JAK3 pY841 Is Constitutively Phosphorylated in Leukemic JAK3 A573V-Positive Cells and Various T-Cell Lines
2.5. Phosphorylation of Y841 Decreases JAK3 JH1 Kinase Domain pH Sensitivity
2.6. JH1 Kinase Domain Electrostatic Potential Is Altered by Y841 Phosphorylation to Favor Dimerization and Structural Stability
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Treatments
4.2. Transfections
4.3. Identification of Ten Novel JAK3 Tyrosine Phosphorylation Sites via Mass Spectrometry
4.4. Generation of Monoclonal Anti-pY841 Antibodies
4.5. Dot Blot Analysis
4.6. Solubilization of Proteins, Immunoprecipitation and Western Blot
4.7. Autokinase Assay
4.8. Relative Folding Energy Calculation
4.9. Structural Modeling of JAK3
4.10. Molecular Dynamic Simulation
4.11. Electrostatic Calculation
4.12. JAK3 JH1 Kinase Domain Dimer Docking
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leonard, W.J.; Lin, J.X.; O’Shea, J.J. The gamma(c) Family of Cytokines: Basic Biology to Therapeutic Ramifications. Immunity 2019, 50, 832–850. [Google Scholar] [CrossRef]
- Lin, J.X.; Leonard, W.J. The Common Cytokine Receptor gamma Chain Family of Cytokines. Cold Spring Harb. Perspect. Biol. 2018, 10, a028449. [Google Scholar] [CrossRef]
- Morris, R.; Kershaw, N.J.; Babon, J.J. The molecular details of cytokine signaling via the JAK/STAT pathway. Protein Sci. 2018, 27, 1984–2009. [Google Scholar] [CrossRef]
- Buitenhuis, M.; Coffer, P.J.; Koenderman, L. Signal transducer and activator of transcription 5 (STAT5). Int. J. Biochem. Cell Biol. 2004, 36, 2120–2124. [Google Scholar] [CrossRef]
- Delespine-Carmagnat, M.; Bouvier, G.; Allee, G.; Fagard, R.; Bertoglio, J. Biochemical analysis of interleukin-2 receptor beta chain phosphorylation by p56(lck). FEBS Lett. 1999, 447, 241–246. [Google Scholar] [CrossRef]
- Leonard, W.J.; O’Shea, J.J. Jaks and STATs: Biological implications. Annu. Rev. Immunol. 1998, 16, 293–322. [Google Scholar] [CrossRef]
- Pesu, M.; Laurence, A.; Kishore, N.; Zwillich, S.H.; Chan, G.; O’Shea, J.J. Therapeutic targeting of Janus kinases. Immunol. Rev. 2008, 223, 132–142. [Google Scholar] [CrossRef]
- Ferrao, R.; Wallweber, H.J.; Ho, H.; Tam, C.; Franke, Y.; Quinn, J.; Lupardus, P.J. The Structural Basis for Class II Cytokine Receptor Recognition by JAK1. Structure 2016, 24, 897–905. [Google Scholar] [CrossRef]
- Ferrao, R.; Lupardus, P.J. The Janus Kinase (JAK) FERM and SH2 Domains: Bringing Specificity to JAK-Receptor Interactions. Front. Endocrinol. 2017, 8, 71. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wlodawer, A.; Lubkowski, J. Crystal Structure of a Complex of the Intracellular Domain of Interferon lambda Receptor 1 (IFNLR1) and the FERM/SH2 Domains of Human JAK1. J. Mol. Biol. 2016, 428, 4651–4668. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.M.; Zhang, Q.; Young, S.N.; Reese, M.L.; Bailey, F.P.; Eyers, P.A.; Ungureanu, D.; Hammaren, H.; Silvennoinen, O.; Varghese, L.N.; et al. A robust methodology to subclassify pseudokinases based on their nucleotide-binding properties. Biochem. J. 2014, 457, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Ungureanu, D.; Wu, J.; Pekkala, T.; Niranjan, Y.; Young, C.; Jensen, O.N.; Xu, C.F.; Neubert, T.A.; Skoda, R.C.; Hubbard, S.R.; et al. The pseudokinase domain of JAK2 is a dual-specificity protein kinase that negatively regulates cytokine signaling. Nat. Struct. Mol. Biol. 2011, 18, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Saharinen, P.; Takaluoma, K.; Silvennoinen, O. Regulation of the Jak2 tyrosine kinase by its pseudokinase domain. Mol. Cell. Biol. 2000, 20, 3387–3395. [Google Scholar] [CrossRef] [PubMed]
- Lupardus, P.J.; Ultsch, M.; Wallweber, H.; Bir Kohli, P.; Johnson, A.R.; Eigenbrot, C. Structure of the pseudokinase-kinase domains from protein kinase TYK2 reveals a mechanism for Janus kinase (JAK) autoinhibition. Proc. Natl. Acad. Sci. USA 2014, 111, 8025–8030. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Rose, P.; Barber, D.; Hanratty, W.P.; Lee, S.; Roberts, T.M.; D’Andrea, A.D.; Dearolf, C.R. Mutation in the Jak kinase JH2 domain hyperactivates Drosophila and mammalian Jak-Stat pathways. Mol. Cell. Biol. 1997, 17, 1562–1571. [Google Scholar] [CrossRef] [PubMed]
- Saharinen, P.; Silvennoinen, O. The pseudokinase domain is required for suppression of basal activity of Jak2 and Jak3 tyrosine kinases and for cytokine-inducible activation of signal transduction. J. Biol. Chem. 2002, 277, 47954–47963. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Witthuhn, B.A.; Matsuda, T.; Kohlhuber, F.; Kerr, I.M.; Ihle, J.N. Activation of Jak2 catalytic activity requires phosphorylation of Y1007 in the kinase activation loop. Mol. Cell. Biol. 1997, 17, 2497–2501. [Google Scholar] [CrossRef]
- Kurzer, J.H.; Argetsinger, L.S.; Zhou, Y.J.; Kouadio, J.L.; O’Shea, J.J.; Carter-Su, C. Tyrosine 813 is a site of JAK2 autophosphorylation critical for activation of JAK2 by SH2-B beta. Mol. Cell. Biol. 2004, 24, 4557–4570. [Google Scholar] [CrossRef]
- Cheng, H.; Ross, J.A.; Frost, J.A.; Kirken, R.A. Phosphorylation of human Jak3 at tyrosines 904 and 939 positively regulates its activity. Mol. Cell. Biol. 2008, 28, 2271–2282. [Google Scholar] [CrossRef]
- Bai, Y.; Li, J.; Fang, B.; Edwards, A.; Zhang, G.; Bui, M.; Eschrich, S.; Altiok, S.; Koomen, J.; Haura, E.B. Phosphoproteomics identifies driver tyrosine kinases in sarcoma cell lines and tumors. Cancer Res. 2012, 72, 2501–2511. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Hanson, E.P.; Chen, Y.Q.; Magnuson, K.; Chen, M.; Swann, P.G.; Wange, R.L.; Changelian, P.S.; O’Shea, J.J. Distinct tyrosine phosphorylation sites in JAK3 kinase domain positively and negatively regulate its enzymatic activity. Proc. Natl. Acad. Sci. USA 1997, 94, 13850–13855. [Google Scholar] [CrossRef]
- Nolen, B.; Taylor, S.; Ghosh, G. Regulation of protein kinases; controlling activity through activation segment conformation. Mol. Cell 2004, 15, 661–675. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.N.; Noble, M.E.; Owen, D.J. Active and inactive protein kinases: Structural basis for regulation. Cell 1996, 85, 149–158. [Google Scholar] [CrossRef]
- Martinez, G.S.; Ross, J.A.; Kirken, R.A. Transforming Mutations of Jak3 (A573V and M511I) Show Differential Sensitivity to Selective Jak3 Inhibitors. Clin. Cancer Drugs 2016, 3, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.A.; Rodriguez, G.; Kirken, R.A. Analysis of Janus tyrosine kinase phosphorylation and activation. Methods Mol. Biol. 2013, 967, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Grant, A.H.; Rodriguez, A.C.; Rodriguez Moncivais, O.J.; Sun, S.; Li, L.; Mohl, J.E.; Leung, M.Y.; Kirken, R.A.; Rodriguez, G. JAK1 Pseudokinase V666G Mutant Dominantly Impairs JAK3 Phosphorylation and IL-2 Signaling. Int. J. Mol. Sci. 2023, 24, 6805. [Google Scholar] [CrossRef] [PubMed]
- Rochman, Y.; Spolski, R.; Leonard, W.J. New insights into the regulation of T cells by gamma(c) family cytokines. Nat. Rev. Immunol. 2009, 9, 480–490. [Google Scholar] [CrossRef]
- Jeong, E.H.; Koo, D.H.; Lee, S.H.; Bang, K.B.; Park, E.H.; Seol, J.S.; Lee, J.Y.; Pyo, J.S.; Kim, D.H.; Lee, H.J.; et al. Aggressive classical Kaposi’s sarcoma mimicking malignant lymphoma. Pathol. Oncol. Res. 2012, 18, 1067–1069. [Google Scholar] [CrossRef]
- Bouchekioua, A.; Scourzic, L.; de Wever, O.; Zhang, Y.; Cervera, P.; Aline-Fardin, A.; Mercher, T.; Gaulard, P.; Nyga, R.; Jeziorowska, D.; et al. JAK3 deregulation by activating mutations confers invasive growth advantage in extranodal nasal-type natural killer cell lymphoma. Leukemia 2014, 28, 338–348. [Google Scholar] [CrossRef]
- Bergmann, A.K.; Schneppenheim, S.; Seifert, M.; Betts, M.J.; Haake, A.; Lopez, C.; Maria Murga Penas, E.; Vater, I.; Jayne, S.; Dyer, M.J.; et al. Recurrent mutation of JAK3 in T-cell prolymphocytic leukemia. Genes Chromosomes Cancer 2014, 53, 309–316. [Google Scholar] [CrossRef]
- Yamashita, Y.; Yuan, J.; Suetake, I.; Suzuki, H.; Ishikawa, Y.; Choi, Y.L.; Ueno, T.; Soda, M.; Hamada, T.; Haruta, H.; et al. Array-based genomic resequencing of human leukemia. Oncogene 2010, 29, 3723–3731. [Google Scholar] [CrossRef] [PubMed]
- Bunn, P.A., Jr.; Foss, F.M. T-cell lymphoma cell lines (HUT102 and HUT78) established at the National Cancer Institute: History and importance to understanding the biology, clinical features, and therapy of cutaneous T-cell lymphomas (CTCL) and adult T-cell leukemia-lymphomas (ATLL). J. Cell. Biochem. Suppl. 1996, 24, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.A.; Nagy, Z.S.; Cheng, H.; Stepkowski, S.M.; Kirken, R.A. Regulation of T cell homeostasis by JAKs and STATs. Arch. Immunol. Ther. Exp. 2007, 55, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Osinalde, N.; Moss, H.; Arrizabalaga, O.; Omaetxebarria, M.J.; Blagoev, B.; Zubiaga, A.M.; Fullaondo, A.; Arizmendi, J.M.; Kratchmarova, I. Interleukin-2 signaling pathway analysis by quantitative phosphoproteomics. J. Proteom. 2011, 75, 177–191. [Google Scholar] [CrossRef]
- Sun, S.; Rodriguez, G.; Xie, Y.; Guo, W.; Hernandez, A.E.L.; Sanchez, J.E.; Kirken, R.A.; Li, L. Phosphorylation of Tyrosine 841 Plays a Significant Role in JAK3 Activation. Life 2023, 13, 981. [Google Scholar] [CrossRef]
- Glassman, C.R.; Tsutsumi, N.; Saxton, R.A.; Lupardus, P.J.; Jude, K.M.; Garcia, K.C. Structure of a Janus kinase cytokine receptor complex reveals the basis for dimeric activation. Science 2022, 376, 163–169. [Google Scholar] [CrossRef]
- Asnafi, V. HiJAKing T-ALL. Blood 2014, 124, 3038–3040. [Google Scholar] [CrossRef][Green Version]
- Li, G.; Waite, E.; Wolfson, J. T-cell prolymphocytic leukemia in an adolescent with ataxia-telangiectasia: Novel approach with a JAK3 inhibitor (tofacitinib). Blood Adv. 2017, 1, 2724–2728. [Google Scholar] [CrossRef]
- Springuel, L.; Hornakova, T.; Losdyck, E.; Lambert, F.; Leroy, E.; Constantinescu, S.N.; Flex, E.; Tartaglia, M.; Knoops, L.; Renauld, J.C. Cooperating JAK1 and JAK3 mutants increase resistance to JAK inhibitors. Blood 2014, 124, 3924–3931. [Google Scholar] [CrossRef][Green Version]
- Haan, C.; Behrmann, I.; Haan, S. Perspectives for the use of structural information and chemical genetics to develop inhibitors of Janus kinases. J. Cell. Mol. Med. 2010, 14, 504–527. [Google Scholar] [CrossRef]
- Hammaren, H.M.; Virtanen, A.T.; Raivola, J.; Silvennoinen, O. The regulation of JAKs in cytokine signaling and its breakdown in disease. Cytokine 2019, 118, 48–63. [Google Scholar] [CrossRef]
- Raivola, J.; Haikarainen, T.; Abraham, B.G.; Silvennoinen, O. Janus Kinases in Leukemia. Cancers 2021, 13, 800. [Google Scholar] [CrossRef]
- Ott, N.; Faletti, L.; Heeg, M.; Andreani, V.; Grimbacher, B. JAKs and STATs from a Clinical Perspective: Loss-of-Function Mutations, Gain-of-Function Mutations, and Their Multidimensional Consequences. J. Clin. Immunol. 2023, 43, 1326–1359. [Google Scholar] [CrossRef]
- Knighton, D.R.; Zheng, J.H.; Ten Eyck, L.F.; Ashford, V.A.; Xuong, N.H.; Taylor, S.S.; Sowadski, J.M. Crystal structure of the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase. Science 1991, 253, 407–414. [Google Scholar] [CrossRef]
- Carrera, A.C.; Alexandrov, K.; Roberts, T.M. The conserved lysine of the catalytic domain of protein kinases is actively involved in the phosphotransfer reaction and not required for anchoring ATP. Proc. Natl. Acad. Sci. USA 1993, 90, 442–446. [Google Scholar] [CrossRef]
- Buechler, J.A.; Vedvick, T.A.; Taylor, S.S. Differential labeling of the catalytic subunit of cAMP-dependent protein kinase with acetic anhydride: Substrate-induced conformational changes. Biochemistry 1989, 28, 3018–3024. [Google Scholar] [CrossRef]
- Yodoi, J.; Teshigawara, K.; Nikaido, T.; Fukui, K.; Noma, T.; Honjo, T.; Takigawa, M.; Sasaki, M.; Minato, N.; Tsudo, M.; et al. TCGF (IL 2)-receptor inducing factor(s). I. Regulation of IL 2 receptor on a natural killer-like cell line (YT cells). J. Immunol. 1985, 134, 1623–1630. [Google Scholar] [CrossRef]
- Miyoshi, I.; Kubonishi, I.; Yoshimoto, S.; Shiraishi, Y. A T-cell line derived from normal human cord leukocytes by co-culturing with human leukemic T-cells. Gan 1981, 72, 978–981. [Google Scholar] [PubMed]
- Gazdar, A.F.; Carney, D.N.; Bunn, P.A.; Russell, E.K.; Jaffe, E.S.; Schechter, G.P.; Guccion, J.G. Mitogen requirements for the in vitro propagation of cutaneous T-cell lymphomas. Blood 1980, 55, 409–417. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, J.J.; McVicar, D.W.; Bailey, T.L.; Burns, C.; Smyth, M.J. Activation of human peripheral blood T lymphocytes by pharmacological induction of protein-tyrosine phosphorylation. Proc. Natl. Acad. Sci. USA 1992, 89, 10306–10310. [Google Scholar] [CrossRef]
- Shevchenko, A.; Wilm, M.; Vorm, O.; Mann, M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 1996, 68, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Gygi, S.P. Proteomics: The move to mixtures. J. Mass. Spectrom. 2001, 36, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Eng, J.K.; McCormack, A.L.; Yates, J.R. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J. Am. Soc. Mass. Spectrom. 1994, 5, 976–989. [Google Scholar] [CrossRef] [PubMed]
- Beausoleil, S.A.; Villen, J.; Gerber, S.A.; Rush, J.; Gygi, S.P. A probability-based approach for high-throughput protein phosphorylation analysis and site localization. Nat. Biotechnol. 2006, 24, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Medina, B.E.; Ross, J.A.; Kirken, R.A. Interleukin-2 Receptor beta Thr-450 Phosphorylation Is a Positive Regulator for Receptor Complex Stability and Activation of Signaling Molecules. J. Biol. Chem. 2015, 290, 20972–20983. [Google Scholar] [CrossRef]
- Malabarba, M.G.; Rui, H.; Deutsch, H.H.; Chung, J.; Kalthoff, F.S.; Farrar, W.L.; Kirken, R.A. Interleukin-13 is a potent activator of JAK3 and STAT6 in cells expressing interleukin-2 receptor-gamma and interleukin-4 receptor-alpha. Biochem. J. 1996, 319 Pt 3, 865–872. [Google Scholar] [CrossRef]
- Sondergaard, C.R.; Olsson, M.H.; Rostkowski, M.; Jensen, J.H. Improved Treatment of Ligands and Coupling Effects in Empirical Calculation and Rationalization of pKa Values. J. Chem. Theory Comput. 2011, 7, 2284–2295. [Google Scholar] [CrossRef]
- Olsson, M.H.; Sondergaard, C.R.; Rostkowski, M.; Jensen, J.H. PROPKA3: Consistent Treatment of Internal and Surface Residues in Empirical pKa Predictions. J. Chem. Theory Comput. 2011, 7, 525–537. [Google Scholar] [CrossRef]
- Zhou, H.X. A Gaussian-chain model for treating residual charge-charge interactions in the unfolded state of proteins. Proc. Natl. Acad. Sci. USA 2002, 99, 3569–3574. [Google Scholar] [CrossRef]
- Tanford, C. Protein denaturation. C. Theoretical models for the mechanism of denaturation. Adv. Protein Chem. 1970, 24, 1–95. [Google Scholar]
- Zheng, W.; Zhang, C.; Li, Y.; Pearce, R.; Bell, E.W.; Zhang, Y. Folding non-homologous proteins by coupling deep-learning contact maps with I-TASSER assembly simulations. Cell Rep. Methods 2021, 1, 100014. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. CHARMM-GUI: A web-based graphical user interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; MacKerell, A.D., Jr. CHARMM36 all-atom additive protein force field: Validation based on comparison to NMR data. J. Comput. Chem. 2013, 34, 2135–2145. [Google Scholar] [CrossRef]
- Phillips, J.C.; Braun, R.; Wang, W.; Gumbart, J.; Tajkhorshid, E.; Villa, E.; Chipot, C.; Skeel, R.D.; Kale, L.; Schulten, K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005, 26, 1781–1802. [Google Scholar] [CrossRef]
- Price, D.J.; Brooks, C.L., 3rd. A modified TIP3P water potential for simulation with Ewald summation. J. Chem. Phys. 2004, 121, 10096–10103. [Google Scholar] [CrossRef]
- Li, L.; Li, C.; Sarkar, S.; Zhang, J.; Witham, S.; Zhang, Z.; Wang, L.; Smith, N.; Petukh, M.; Alexov, E. DelPhi: A comprehensive suite for DelPhi software and associated resources. BMC Biophys. 2012, 5, 9. [Google Scholar] [CrossRef]
- Dolinsky, T.J.; Nielsen, J.E.; McCammon, J.A.; Baker, N.A. PDB2PQR: An automated pipeline for the setup of Poisson-Boltzmann electrostatics calculations. Nucleic Acids Res. 2004, 32, W665–W667. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Pierce, B.G.; Wiehe, K.; Hwang, H.; Kim, B.H.; Vreven, T.; Weng, Z. ZDOCK server: Interactive docking prediction of protein-protein complexes and symmetric multimers. Bioinformatics 2014, 30, 1771–1773. [Google Scholar] [CrossRef]
- Xian, Y.; Xie, Y.; Silva, S.M.; Karki, C.B.; Qiu, W.; Li, L. StructureMan: A Structure Manipulation Tool to Study Large Scale Biomolecular Interactions. Front. Mol. Biosci. 2020, 7, 627087. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez, G.; Martinez, G.S.; Negrete, O.D.; Sun, S.; Guo, W.; Xie, Y.; Li, L.; Xiao, C.; Ross, J.A.; Kirken, R.A. JAK3 Y841 Autophosphorylation Is Critical for STAT5B Activation, Kinase Domain Stability and Dimer Formation. Int. J. Mol. Sci. 2023, 24, 11928. https://doi.org/10.3390/ijms241511928
Rodriguez G, Martinez GS, Negrete OD, Sun S, Guo W, Xie Y, Li L, Xiao C, Ross JA, Kirken RA. JAK3 Y841 Autophosphorylation Is Critical for STAT5B Activation, Kinase Domain Stability and Dimer Formation. International Journal of Molecular Sciences. 2023; 24(15):11928. https://doi.org/10.3390/ijms241511928
Chicago/Turabian StyleRodriguez, Georgialina, George Steven Martinez, Omar Daniel Negrete, Shengjie Sun, Wenhan Guo, Yixin Xie, Lin Li, Chuan Xiao, Jeremy Aaron Ross, and Robert Arthur Kirken. 2023. "JAK3 Y841 Autophosphorylation Is Critical for STAT5B Activation, Kinase Domain Stability and Dimer Formation" International Journal of Molecular Sciences 24, no. 15: 11928. https://doi.org/10.3390/ijms241511928
APA StyleRodriguez, G., Martinez, G. S., Negrete, O. D., Sun, S., Guo, W., Xie, Y., Li, L., Xiao, C., Ross, J. A., & Kirken, R. A. (2023). JAK3 Y841 Autophosphorylation Is Critical for STAT5B Activation, Kinase Domain Stability and Dimer Formation. International Journal of Molecular Sciences, 24(15), 11928. https://doi.org/10.3390/ijms241511928







