Immunosuppressive Activity of Exosomes from Granulocytic Myeloid-Derived Suppressor Cells in a Murine Model of Immune Bone Marrow Failure
Abstract
:1. Introduction
2. Results
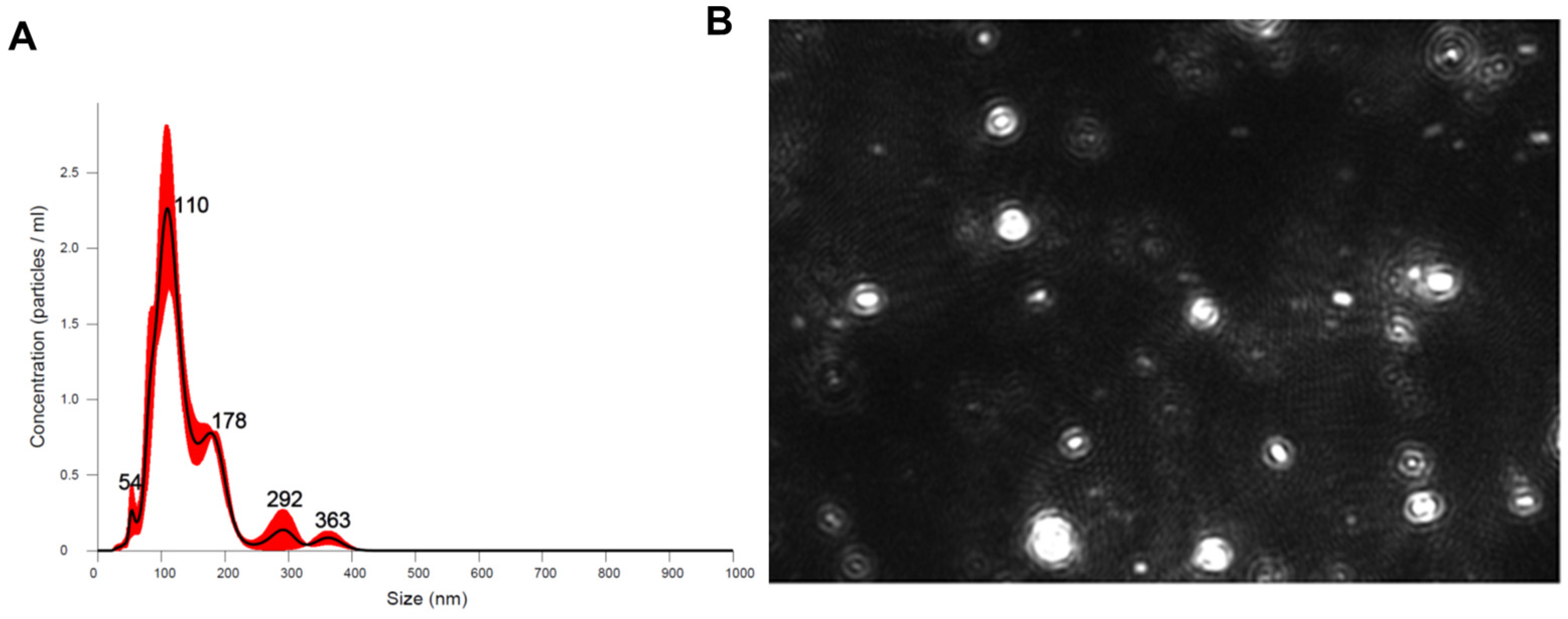
2.1. G-MDSC-exos Confirmation and Quantification
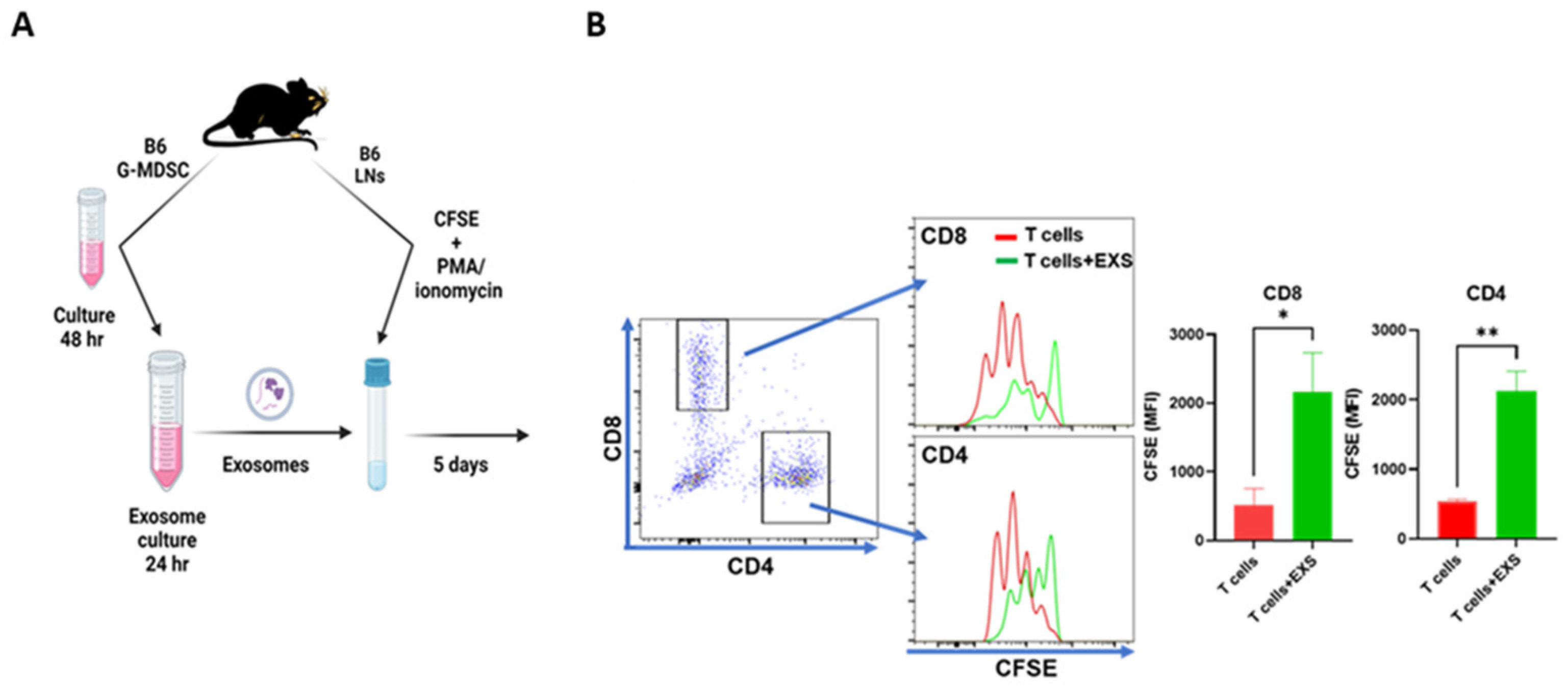
2.2. Suppression of T-Cell Proliferation In Vitro
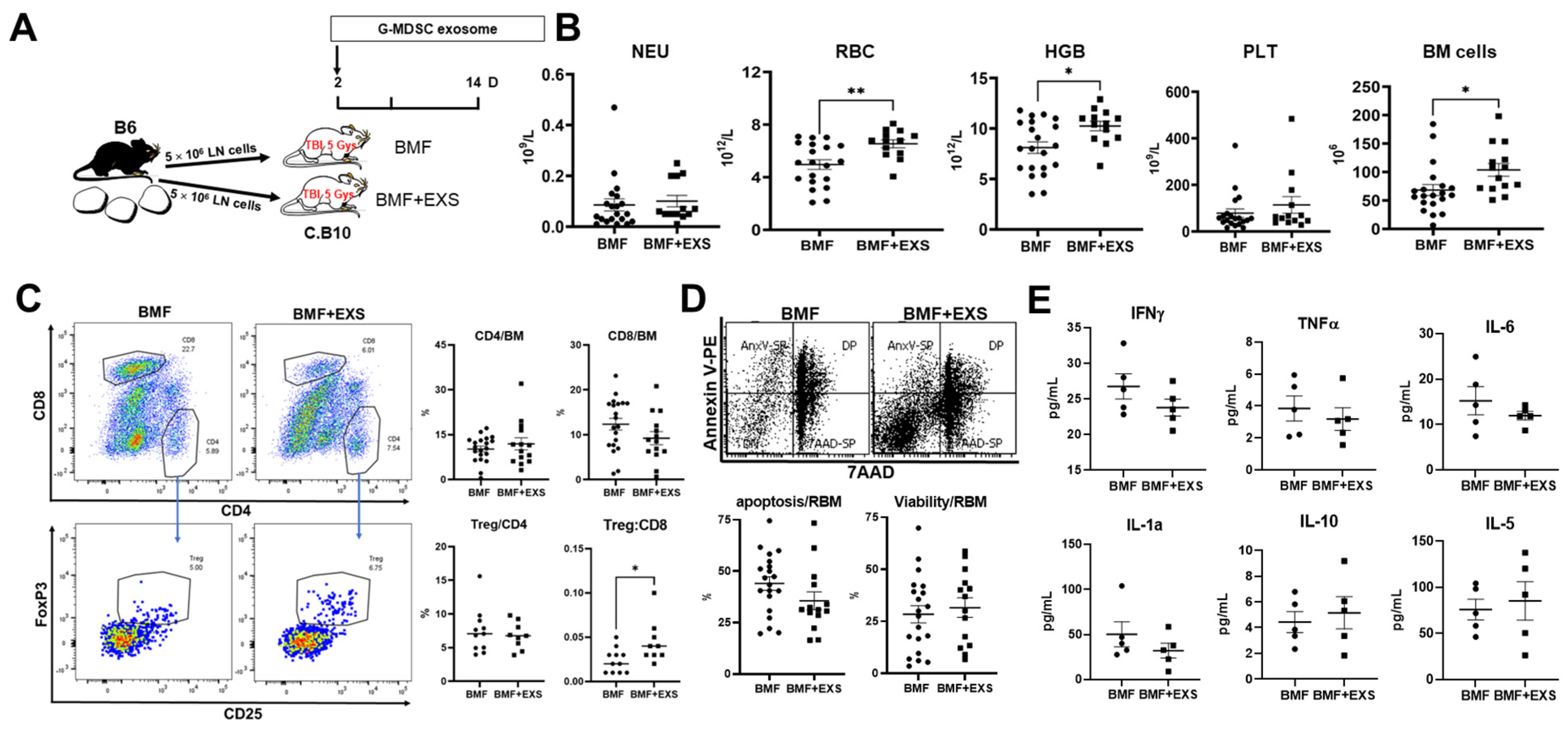
2.3. Attenuation of Immune BMF In Vivo
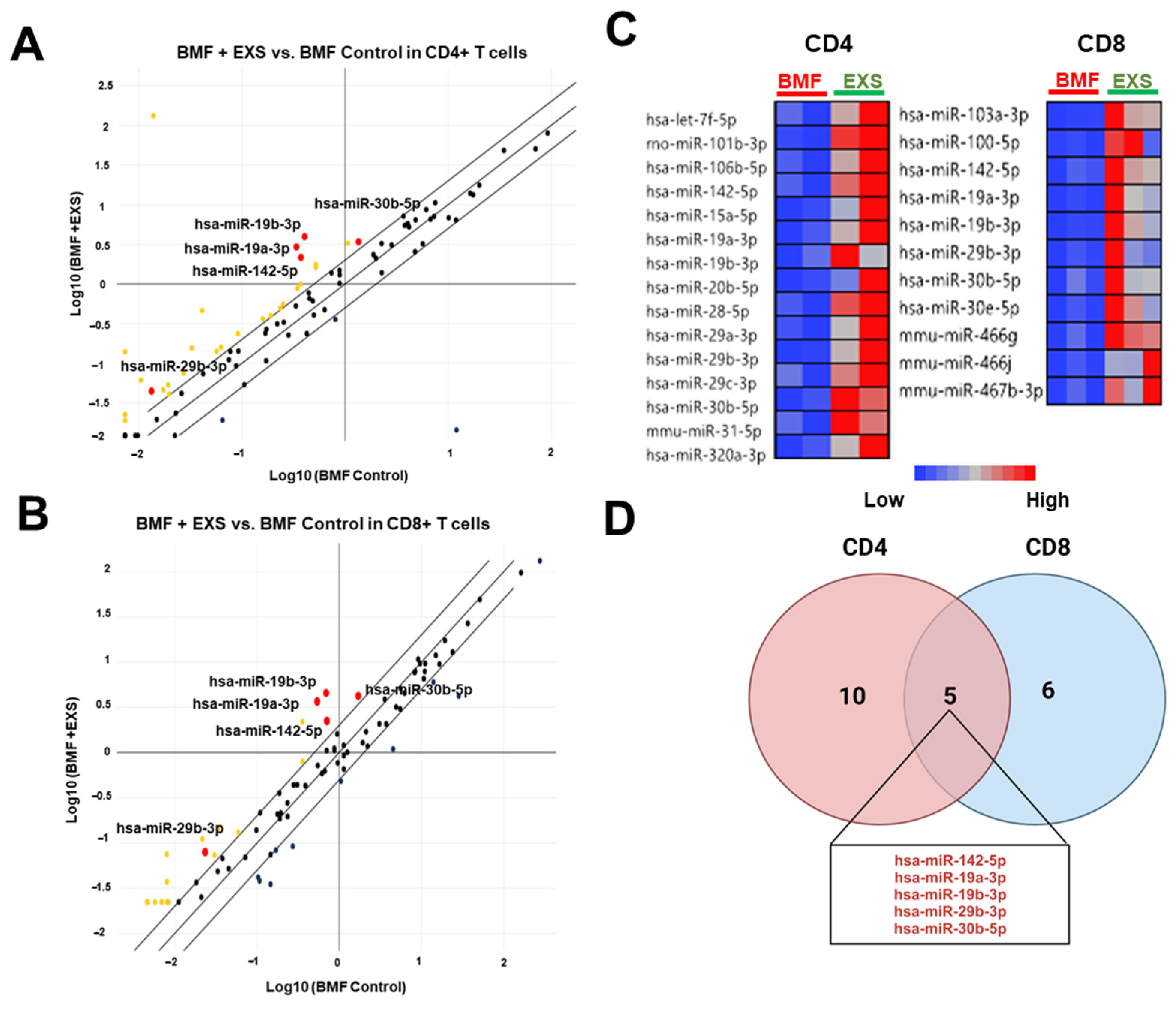
2.4. Modulation of miRNA Delivery into CD4+ and CD8+ T Cells
3. Discussion
4. Materials and Methods
4.1. Animals and Exosome Preparation
4.2. T-Cell Proliferation In Vitro
4.3. Induction of AA/BMF and G-MDSC Exosome Therapy
4.4. Cell Counts and Flow Cytometry
4.5. Cytokine Measurement
4.6. Cell Sorting and RNA Isolation
4.7. Quantitative Real-Time RT-PCR (RT-qPCR)
4.8. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Gurung, S. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal. 2021, 19, 47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bi, J.; Huang, J.; Tang, Y.; Du, S.; Li, P. Exosome: A Review of Its Classification, Isolation Techniques, Storage, Diagnostic and Targeted Therapy Applications. Int. J. Nanomed. 2020, 15, 6917–6934. [Google Scholar] [CrossRef] [PubMed]
- Basu, J.; Ludlow, J.W. Exosomes for repair, regeneration and rejuvenation. Expert. Opin. Biol. Ther. 2016, 16, 489–506. [Google Scholar] [CrossRef]
- Chen, J.; Ellison, F.M.; Eckhaus, M.A.; Smith, A.L.; Keyvanfar, K.; Calado, R.T.; Young, N.S. Minor antigen h60-mediated aplastic anemia is ameliorated by immunosuppression and the infusion of regulatory T cells. J. Immunol. 2007, 178, 4159–4168. [Google Scholar] [CrossRef]
- Young, N.S. Aplastic Anemia. N. Engl. J. Med. 2018, 379, 1643–1656. [Google Scholar] [CrossRef]
- Peslak, S.A.; Olson, T.; Babushok, D.V. Diagnosis and Treatment of Aplastic Anemia. Curr. Treat. Options Oncol. 2017, 18, 70. [Google Scholar] [CrossRef]
- Bacigalupo, A. How I treat acquired aplastic anemia. Blood 2017, 129, 1428–1436. [Google Scholar] [CrossRef]
- Bloom, M.L.; Wolk, A.G.; Simon-Stoos, K.L.; Bard, J.S.; Chen, J.; Young, N.S. A mouse model of lymphocyte infusion-induced bone marrow failure. Exp. Hematol. 2004, 32, 1163–1172. [Google Scholar] [CrossRef]
- McCabe, A.; Smith, J.N.P.; Costello, A.; Maloney, J.; Katikaneni, D.; MacNamara, K.C. Hematopoietic stem cell loss and hematopoietic failure in severe aplastic anemia is driven by macrophages and aberrant podoplanin expression. Haematologica 2018, 103, 1451–1461. [Google Scholar] [CrossRef]
- Tang, D.; Liu, S.; Sun, H.; Qin, X.; Zhou, N.; Zheng, W.; Zhang, M.; Zhou, H.; Tuersunayi, A.; Duan, C.; et al. All-trans-retinoic acid shifts Th1 towards Th2 cell differentiation by targeting NFAT1 signalling to ameliorate immune-mediated aplastic anaemia. Br. J. Haematol. 2020, 191, 906–919. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Kim, J.; Gonzalez-Matias, G.; Aggarwal, N.; Manley, A.L.; Wu, Z.; Solorzano, S.; Batchu, S.; Gao, S.; Chen, J.; et al. Granulocytic myeloid-derived suppressor cells to prevent and treat murine immune-mediated bone marrow failure. Blood Adv. 2023, 7, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Ma, P.; Cao, X.; Wang, J.; Yu, X.; Luo, X.; Lu, J.; Hou, W.; Zhang, Z.; Yan, Y.; et al. Myoblast-derived exosomes promote the repair and regeneration of injured skeletal muscle in mice. FEBS Open Biol. 2022, 12, 2213–2226. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Han, B.; Hai, Y.; Sun, D.; Yin, P. Mechanism of Action of Mesenchymal Stem Cell-Derived Exosomes in the Intervertebral Disc Degeneration Treatment and Bone Repair and Regeneration. Front. Cell Dev. Biol. 2021, 9, 833840. [Google Scholar] [CrossRef]
- Sun, S.J.; Wei, R.; Li, F.; Liao, S.Y.; Tse, H.F. Mesenchymal stromal cell-derived exosomes in cardiac regeneration and repair. Stem Cell Rep. 2021, 16, 1662–1673. [Google Scholar] [CrossRef]
- Mancuso, T.; Barone, A.; Salatino, A.; Molinaro, C.; Marino, F.; Scalise, M.; Torella, M.; De Angelis, A.; Urbanek, K.; Torella, D.; et al. Unravelling the Biology of Adult Cardiac Stem Cell-Derived Exosomes to Foster Endogenous Cardiac Regeneration and Repair. Int. J. Mol. Sci. 2020, 21, 3725. [Google Scholar] [CrossRef]
- Zhu, D. G-MDSC-Derived Exosomes Attenuate Collagen-Induced Arthritis by Impairing Th1 and Th17 Cell Responses; Elsevier: Amsterdam, The Netherlands, 2019; Volume 1865. [Google Scholar]
- Wang, Y.; Tian, J.; Tang, X.; Rui, K.; Tian, X.; Ma, J.; Ma, B.; Xu, H.; Lu, L.; Wang, S. Exosomes released by granulocytic myeloid-derived suppressor cells attenuate DSS-induced colitis in mice. Oncotarget 2016, 7, 15356–15368. [Google Scholar] [CrossRef]
- Dietz, S.; Schwarz, J.; Ruhle, J.; Schaller, M.; Fehrenbacher, B.; Marme, A.; Schmid, E.; Peter, A.; Poets, C.F.; Gille, C.; et al. Extracellular vesicles released by myeloid-derived suppressor cells from pregnant women modulate adaptive immune responses. Cell Immunol. 2021, 361, 104276. [Google Scholar] [CrossRef]
- Wu, X.; Zhu, D.; Tian, J.; Tang, X.; Guo, H.; Ma, J.; Xu, H.; Wang, S. Granulocytic Myeloid-Derived Suppressor Cell Exosomal Prostaglandin E2 Ameliorates Collagen-Induced Arthritis by Enhancing IL-10(+) B Cells. Front. Immunol. 2020, 11, 588500. [Google Scholar] [CrossRef]
- Hosokawa, K. Identification of novel microRNA signatures linked to acquired aplastic anemia. Haematologica 2015, 100, 1534–1545. [Google Scholar] [CrossRef]
- Ding, S. Decreased microRNA-142-3p/5p expression causes CD4+ T cell activation and B cell hyperstimulation in systemic lupus erythematosus. Arthritis Rheum. 2012, 64, 2953–2963. [Google Scholar] [PubMed]
- Jin, P. Bioinformatics analysis of mRNA profiles and identification of microRNA-mRNA network in CD4+ T cells in seasonal allergic rhinitis. J. Int. Med. Res. 2022, 50, 3000605221113918. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manley, A.L.; Chen, J.; Fitzgerald, W.; Feng, X.; Young, N.S. Immunosuppressive Activity of Exosomes from Granulocytic Myeloid-Derived Suppressor Cells in a Murine Model of Immune Bone Marrow Failure. Int. J. Mol. Sci. 2023, 24, 14661. https://doi.org/10.3390/ijms241914661
Manley AL, Chen J, Fitzgerald W, Feng X, Young NS. Immunosuppressive Activity of Exosomes from Granulocytic Myeloid-Derived Suppressor Cells in a Murine Model of Immune Bone Marrow Failure. International Journal of Molecular Sciences. 2023; 24(19):14661. https://doi.org/10.3390/ijms241914661
Chicago/Turabian StyleManley, Ash Lee, Jichun Chen, Wendy Fitzgerald, Xingmin Feng, and Neal S. Young. 2023. "Immunosuppressive Activity of Exosomes from Granulocytic Myeloid-Derived Suppressor Cells in a Murine Model of Immune Bone Marrow Failure" International Journal of Molecular Sciences 24, no. 19: 14661. https://doi.org/10.3390/ijms241914661
APA StyleManley, A. L., Chen, J., Fitzgerald, W., Feng, X., & Young, N. S. (2023). Immunosuppressive Activity of Exosomes from Granulocytic Myeloid-Derived Suppressor Cells in a Murine Model of Immune Bone Marrow Failure. International Journal of Molecular Sciences, 24(19), 14661. https://doi.org/10.3390/ijms241914661







