Pregnancy, Breastfeeding, and Vitamin D
Abstract
1. Introduction
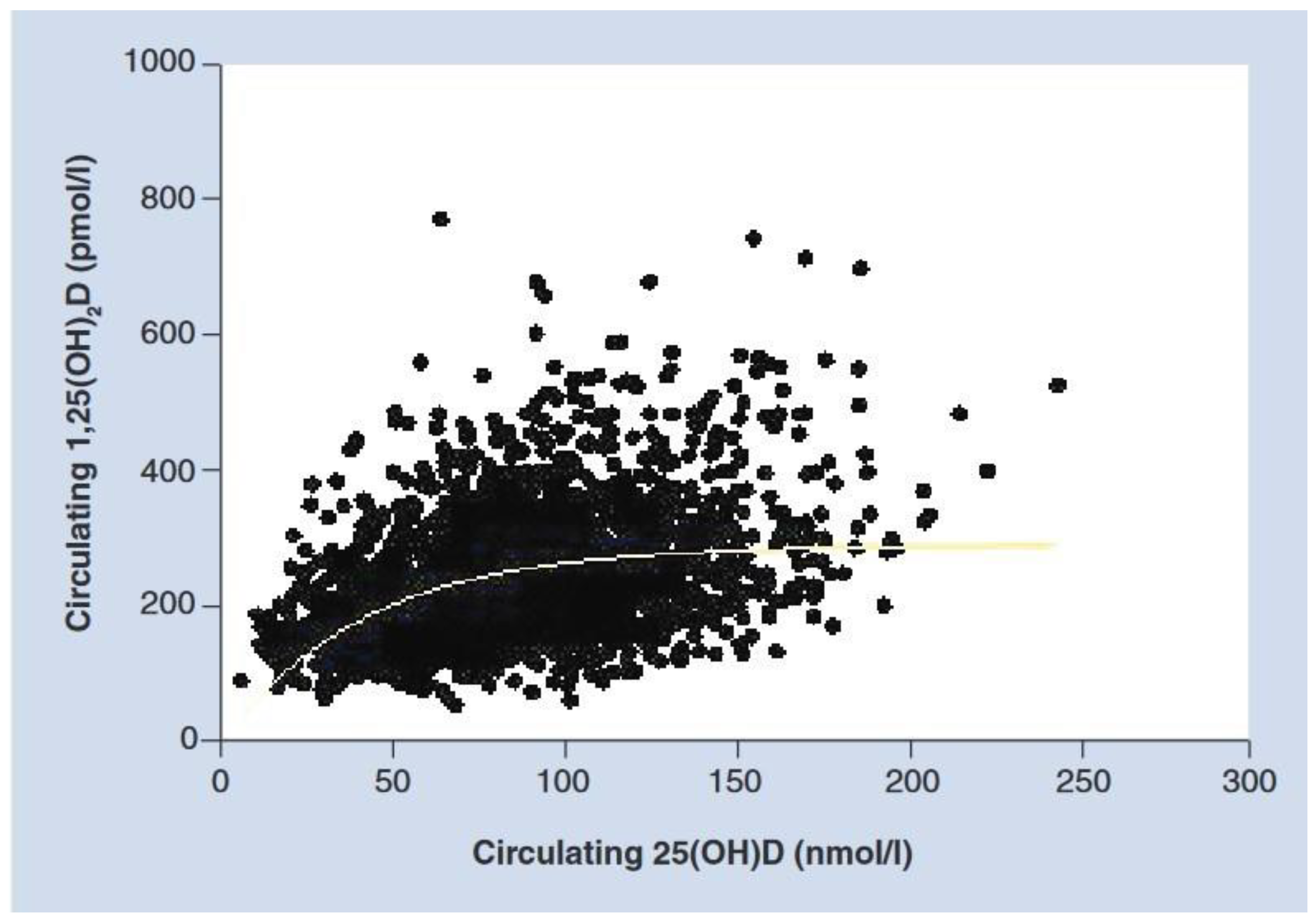
2. Vitamin D Metabolism
3. Definition of Hypovitaminosis D
- -
- Vitamin D deficiency: calcidiol levels are lower than 20 ng/mL (<50 nmol/L);
- -
- Vitamin D insufficiency: calcidiol levels range between 20 and 29 ng/mL (51–74 nmol/L);
- -
- Vitamin D sufficiency: calcidiol levels are equal to or higher than 30 ng/mL (>75 nmol/L).
4. Vitamin D Metabolism during Pregnancy
5. Vitamin D Deficiency in Pregnant and Nursing Mothers
6. Consequences of Vitamin D Deficiency during Pregnancy
7. Maternal Vitamin D Supplementation during Pregnancy
8. Vitamin D Deficiency in the Neonatal Period
9. Vitamin D Content in Breast Milk
10. Pharmacological Vitamin D Supplementation in the Infant
| Exclusive Supplementation in the Infant | |
|---|---|
| Huynh et al., 2017 [61] | 50,000 UI in the newborn (single dose) |
| Mother exclusive supplementation | |
| Hollis et al., 2015 [34] | 6500 UI daily |
| Chandy et al., 2016 [64] | 120,000 UI monthly |
| Naik et al., 2017 [65] | 60,000 UI daily for the first 10 days after delivery (total of 600,000 UI) |
| Trivedi et al., 2020 [24] | 60,000 UI in immediate postpartum and after 6, 10 and 14 weeks (total of 240,000 UI) |
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PTH | Parathyroid hormone |
| 25(OH)D | 25-hydroxicholecalciferol |
| 1,25(OH)2D | 1,25-hydroxicholecalciferol |
| VDBP | vitamin D binding protein |
References
- WHO. Global Strategy on Infant and Young Child Feeding, Document WHA55 A55/15. 2002. Available online: https://apps.who.int/gb/archive/pdf_files/WHA55/ea5515.pdf (accessed on 8 December 2022).
- Hossein-Nezhad, A.; Holick, M.F. Vitamin D for health: A global perspective. Mayo Clin. Proc. 2013, 88, 720–755. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.L.; Hollis, B.W. The extraordinary metabolism of vitamin D. eLife 2022, 11, e77539. [Google Scholar] [CrossRef] [PubMed]
- Karras, S.N.; Wagner, C.L.; Castracane, V.D. Understanding vitamin D metabolism in pregnancy: From physiology to pathophysiology and clinical outcomes. Metabolism 2018, 86, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Grayson, R.; Hewison, M. Vitamin D and human pregnancy. Fetal Matern. Med. Rev. 2011, 22, 67–90. [Google Scholar] [CrossRef]
- Sotunde, O.F.; Laliberte, A.; Weiler, H.A. Maternal risk factors and newborn infant vitamin D status: A scoping literature review. Nutr. Res. 2019, 63, 1–20. [Google Scholar] [CrossRef]
- Saraf, R.; Morton, S.M.B.; Camargo, C.A.; Grant, C.C. Global summary of maternal and newborn vitamin D status—A systematic review. Matern. Child Nutr. 2016, 12, 647–668. [Google Scholar] [CrossRef]
- van der Pligt, P.; Willcox, J.; Szymlek-Gay, E.A.; Murray, E.; Worsley, A.; Daly, R.M. Associations of maternal vitamin D deficiency with pregnancy and neonatal complications in developing countries: A systematic review. Nutrients 2018, 10, 640. [Google Scholar] [CrossRef]
- Aghajafari, F.; Nagulesapillai, T.; Ronksley, P.E.; Tough, S.C.; O’Beirne, M.; Rabi, D.M. Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: Systematic review and meta-analysis of observational studies. BMJ 2013, 346, f1169. [Google Scholar] [CrossRef]
- Palacios, C.; Kostiuk, L.K.; Peña-Rosas, J.P. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst. Rev. 2019, 7, CD008873. [Google Scholar] [CrossRef] [PubMed]
- Morales, E.; Guxens, M.; Llop, S.; Rodríguez-Bernal, C.L.; Tardón, A.; Riaño, I.; Ibarluzea, J.; Lertxundi, N.; Espada, M.; Rodriguez, A.; et al. Circulating 25-Hydroxyvitamin D3 in Pregnancy and Infant Neuropsychological Development. Pediatrics 2012, 130, e913–e920. [Google Scholar] [CrossRef]
- Pacheco-González, R.M.; García-Marcos, L.; Morales, E. Prenatal vitamin D status and respiratory and allergic outcomes in childhood: A meta-analysis of observational studies. Pediatr. Allergy Immunol. 2018, 29, 243–253. [Google Scholar] [CrossRef]
- Palermo, N.E.; Holick, M.F. Vitamin D, bone health, and other health benefits in pediatric patients. J. Pediatr. Rehabil. Med. 2014, 7, 179–192. [Google Scholar] [CrossRef]
- Misra, M.; Pacaud, D.; Petryk, A.; Collett-Solberg, P.F.; Kappy, M. Vitamin D deficiency in children and its management: Review of current knowledge and recommendations. Pediatrics 2008, 122, 398–417. [Google Scholar] [CrossRef]
- Bouillon, R. Vitamin D metabolism revised: Fall of dogmas. J. Bone Miner Res. 2019, 34, 1985–1992. [Google Scholar] [CrossRef]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. Med. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Vieth, R. Why the minimum desirable serum 25-hydroxyvitamin D level should be 75 nmol/L (30 ng/mL). Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 681–691. [Google Scholar] [CrossRef]
- Hollis, B.W.; Johnson, D.; Hulsey, T.C.; Ebeling, M.; Wagner, C.L. Vitamin D supplementation during pregnancy: Double-blind, randomized clinical trial of safety and effectiveness. J. Bone Miner. Res. 2011, 26, 2341–2357. [Google Scholar] [CrossRef]
- Olmos-Ortiz, A.; Avila, E.; Duland-Carbajal, M.; Díaz, L. Regulation of calcitriol byosinthesis and activity: Focus on gestational vitamin D deficiency and adverse pregnancy outcomes. Nutrients 2015, 7, 443–480. [Google Scholar] [CrossRef]
- Hollis, B.W.; Wagner, C.L. New insights into the vitamin D requirements during pregnancy. Bone Res. 2017, 5, 17030. [Google Scholar] [CrossRef]
- Kovacs, C.S. Maternal mineral and bone metabolism during pregnancy, lactation, and post-weaning recovery. Physiol. Rev. 2016, 96, 449–547. [Google Scholar] [CrossRef]
- Wagner, C.L.; Hollis, B.W. Early-life effects of vitamin D: A focus on pregnancy and lactation. Ann. Nutr. Metab. 2020, 76 (Suppl. S2), 16–28. [Google Scholar] [CrossRef]
- Trivedi, M.; Faridi, M.M.A.; Aggarwal, A.; Madhu, S.V.; Malhotra, R.K. Oral vitamin D supplementation to mothers during lactation-effect of 25(OH) D concentration on exclusively breastfed infants at 6 months of age: A randomized double-blind placebo-controlled trial. Breastfeed. Med. 2020, 15, 237–245. [Google Scholar] [CrossRef]
- Adams, J.S.; Hewison, M. Extrarenal expression of the 25-hydroxyvitamin D-1-hydroxylase. Arch. Biochem. Biophys. 2012, 523, 95–102. [Google Scholar] [CrossRef]
- Bikle, D.D.; Schwartz, J. Vitamin D binding protein, total and free vitamin D levels in different physiological and pathophysiological conditions. Front. Endocrinol. 2019, 10, 317. [Google Scholar] [CrossRef]
- Mahadevan, S.; Kumaravel, V.; Bharath, R. Calcium and bone disorders in pregnancy. Indian J. Endocrinol. Metab. 2012, 16, 358–363. [Google Scholar] [CrossRef]
- Wagner, C.L.; Hollis, B.W.; Kotsa, K.; Fakhoury, H.; Karras, S.N. Vitamin D administration during pregnancy as prevention for pregnancy, neonatal and postnatal complications. Rev. Endocr. Metab. Disord. 2017, 18, 307–322. [Google Scholar] [CrossRef]
- Tamblyn, J.A.; Hewison, M.; Wagner, C.L.; Bulmer, J.N.; Kilby, M.D. Immunological role of vitamin D at the maternal-fetal interface. J. Endocrinol. 2015, 224, R107–R121. [Google Scholar] [CrossRef]
- Hart, P.H.; Lucas, R.M.; Walsh, J.P.; Zosky, G.R.; Whitehouse, A.J.; Zhu, K.; Allen, K.L.; Kusel, M.M.; Anderson, D.; Mountain, J.A. Vitamin D in fetal development: Findings from a birth cohort study. Pediatrics 2015, 135, e167–e173. [Google Scholar] [CrossRef]
- Hou, W.; Yan, X.T.; Bai, C.M.; Zhang, X.V.; Hui, L.Y.; Yu, X.W. Decreased serum vitamin D levels in early spontaneous pregnancy loss. Eur. J. Clin. Nutr. 2016, 70, 1004–1008. [Google Scholar] [CrossRef]
- Eckhardt, C.L.; Gernand, A.D.; Roth, D.E.; Bodnar, L.M. Maternal vitamin D status and infant anthropometry in a US multicentre cohort study. Ann. Hum. Biol. 2015, 42, 215–222. [Google Scholar] [CrossRef]
- Agarwal, S.; Kovilam, O.; Agrawal, D.K. Vitamin D and its impact on maternal-fetal outcomes in pregnancy: A critical review. Crit. Rev. Food Sci. Nutr. 2018, 58, 755–769. [Google Scholar] [CrossRef]
- Hollis, B.W.; Wagner, C.L.; Howard, C.R.; Ebeling, M.; Shary, J.R.; Smith, P.G.; Taylor, S.N.; Morella, K.; Lawrence, R.A.; Hulsey, T.C. Maternal versus infant vitamin D supplementation during lactation: A randomized controlled trial. Pediatrics 2015, 136, 625–634. [Google Scholar] [CrossRef]
- Streym, S.; Højskov, C.S.; Møller, U.K.; Heickendorff, L.; Vestergaard, P.; Mosekilde, L.; Rejnmark, L. Vitamin D content in human breast milk: A 9-mo follow-up study. Am. J. Clin. Nutr. 2016, 103, 107–114. [Google Scholar] [CrossRef]
- Holick, M.F. A call to action: Pregnant women indeed require vitamin D supplementation for better health outcomes. J. Clin. Endocrinol. Metab. 2019, 104, 13–15. [Google Scholar] [CrossRef]
- Hollis, B.W.; Wagner, C.L. Substantial vitamin D supplementation is required during the prenatal period to improve birth outcomes. Nutrients 2022, 14, 899. [Google Scholar] [CrossRef]
- Rostami, M.; Tehrani, F.R.; Simbar, M.; Bidhendi Yarandi, R.; Minooee, S.; Hollis, B.W.; Hosseinpanah, F. Effectiveness of prenatal vitamin D deficiency screening and treatment program: A stratified randomized field trial. J. Clin. Endocrinol. Metab. 2018, 103, 2936–2948. [Google Scholar] [CrossRef]
- Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Dawodu, A.; Saadi, H.F.; Bekdache, G.; Javed, Y.; Altaye, M.; Hollis, B.W. Randomized controlled trial (RCT) of vitamin D supplementation in pregnancy in a population with endemic vitamin D deficiency. J. Clin. Endocrinol. Metab. 2013, 98, 2337–2346. [Google Scholar] [CrossRef]
- Roth, D.E.; Morris, S.K.; Zlotkin, S.; Gernand, A.D.; Ahmed, T.; Shanta, S.S.; Papp, E.; Korsiak, J.; Shi, J.; Islam, M.M.; et al. Vitamin D supplementation in pregnancy and lactation and infant growth. N. Engl. J. Med. 2018, 379, 535–546. [Google Scholar] [CrossRef]
- Litonjua, A.A.; Carey, V.J.; Laranjo, N.; Stubbs, B.J.; Mirzakhani, H.; O’Connor, G.T.; Sandel, M.; Beigelman, A.; Bacharier, L.B.; Zeiger, R.S.; et al. Six-year follow-up of a trial of antenatal vitamin D for asthma reduction. N. Engl. J. Med. 2020, 382, 525–533. [Google Scholar] [CrossRef]
- Mumford, S.L.; Garbose, R.A.; Kim, K.; Kissell, K.; Kuhr, D.L.; Omosigho, U.R.; Perkins, N.J.; Galai, N.; Silver, R.M.; Sjaarda, L.A.; et al. Association of preconception serum 25-hydroxyvitamin D concentrations with livebirth and pregnancy loss: A prospective cohort study. Lancet Diabetes Endocrinol. 2018, 6, 725–732. [Google Scholar] [CrossRef]
- Bayramoğlu, E.; Akkoç, G.; Ağbaş, A.; Akgün, Ö.; Yurdakul, K.; Selçuk Duru, H.N.; Elevli, M. The association between vitamin D levels and the clinical severity and inflammation markers in pediatric COVID-19 patients: Single-center experience from a pandemic hospital. Eur. J. Pediatr. 2021, 180, 2699–2705. [Google Scholar] [CrossRef]
- Mansur, J.L.; Oliveri, B.; Giacoia, E.; Fusaro, D.; Costanzo, P.R. Vitamin D: Before, during and after pregnancy: Effect on neonates and children. Nutrients 2022, 14, 1900. [Google Scholar] [CrossRef]
- Domenici, R.; Vierucci, F. Exclusive breastfeeding and vitamin D supplementation: ¿a positive synergistic effect on prevention of childhood infections? Int. J. Environ. Res. Public Health 2022, 19, 2973. [Google Scholar] [CrossRef]
- Stoutjesdijk, E.; Schaafsma, A.; Nhien, N.V.; Khor, G.L.; Kema, I.P.; Hollis, B.W.; Dijck-Brouwer, D.J.; Muskiet, F.A. Milk vitamin D in relation to the ‘adequate intake’ for 0–6-month-old infants: A study in lactating women with different cultural backgrounds, living at different latitudes. Br. J. Nutr. 2017, 118, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Tsugawa, N.; Nishino, M.; Kuwabara, A.; Ogasawara, H.; Kamao, M.; Kobayashi, S.; Yamamura, J.; Higurashi, S. Comparison of vitamin D and 25-hydroxyvitamin D concentrations in human breast milk between 1989 and 2016–2017. Nutrients 2021, 13, 573. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.S.; Ahn, Y.M.; Kim, A.E.; Shin, S.M. Breastfeeding and vitamin D. Clin. Exp. Pediatr. 2022, 65, 418–429. [Google Scholar] [CrossRef]
- Bae, Y.J.; Kratzsch, J. Vitamin D and calcium in the human breast milk. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 39–45. [Google Scholar] [CrossRef]
- Munns, C.F.; Shaw, N.; Kiely, M.; Specker, B.L.; Thacher, T.D.; Ozono, K.; Michigami, T.; Tiosano, D.; Mughal, M.Z.; Mäkitie, O.; et al. Global consensus recommendations on prevention and management of nutritional rickets. J. Clin. Endocrinol. Metab. 2016, 101, 394–415. [Google Scholar] [CrossRef]
- Dawodu, A.; Tsang, R.C. Maternal vitamin D status: Effect on milk vitamin D content and vitamin D status of breastfeeding infants. Adv. Nutr. 2012, 3, 353–361. [Google Scholar] [CrossRef]
- Garnacho, G.M.; Salido, R.; Moreno, J.C. Effects of solar radiation and an update on photoprotection. An. Pediatr. 2020, 92, 377.e1–377.e9. [Google Scholar] [CrossRef]
- Wagner, C.L.; Howard, C.; Hulsey, T.C.; Lawrence, R.A.; Taylor, S.N.; Will, H.; Ebeling, M.; Hutson, J.; Hollis, B.W. Circulating 25-hydroxyvitamin D levels in fully breastfed infants on oral vitamin D supplementation. Int. J. Endocrinol. 2010, 2010, 235035. [Google Scholar] [CrossRef]
- Golden, N.H.; Abrams, S.A. Optimizing bone health in children and adolescents. Pediatrics 2014, 134, e1229–e1243. [Google Scholar] [CrossRef] [PubMed]
- Braegger, C.; Campoy, C.; Colomb, V.; Decsi, T.; Domellof, M.; Fewtrell, M.; Hojsak, I.; Mihatsch, W.; Molgaard, C.; Shamir, R.; et al. Vitamin D in the healthy European paediatric population. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 692–701. [Google Scholar] [CrossRef]
- Martínez-Suárez, V.; Moreno-Villares, J.M.; Dalmau-Serra, J. Recommended intake of calcium and vitamin D. Positioning of the Nutrition Committee of the AEP. An. Pediatr. 2012, 77, 57.e1–57.e8. [Google Scholar]
- Saggese, G.; Vierucci, F.; Prodam, F.; Cardinale, F.; Cetin, I.; Chiappini, E.; de’Angelis, G.L.; Massari, M.; Miraglia Del Giudice, E.; Miraglia Del Giudice, M.; et al. Vitamin D in pediatric age: Consensus of the Italian Pediatric Society and the Italian Society of Preventive and Social Pediatrics, jointly with the Italian Federation of Pediatricians. Ital. J. Pediatr. 2018, 44, 51. [Google Scholar] [CrossRef]
- Tan, M.L.; Abrams, S.A.; Osborn, D.A. Vitamin D supplementation for term breastfed infants to prevent vitamin D deficiency and improve bone health. Cochrane Database Syst. Rev. 2020, 12, Cd013046. [Google Scholar] [CrossRef]
- Moodley, A.; Spector, S.A. Single high dose vitamin D at birth corrects vitamin D deficiency in infants in Mexico. Int. J. Food Sci. Nutr. 2015, 66, 336–341. [Google Scholar] [CrossRef][Green Version]
- Huynh, J.; Lu, T.; Liew, D.; Doery, J.C.; Tudball, R.; Jona, M.; Bhamjee, R.; Rodda, C.P. Vitamin D in newborns. A randomised controlled trial comparing daily and single oral bolus vitamin D in infants. J. Paediatr. Child Health 2017, 53, 163–169. [Google Scholar] [CrossRef]
- Dawodu, A.; Salameh, K.M.; Al-Janahi, N.S.; Bener, A.; Elkum, N. The effect of high-dose postpartum maternal vitamin D supplementation alone compared with maternal plus infant vitamin D supplementation in breastfeeding infants in a high-risk population. a randomized controlled trial. Nutrients 2019, 11, 1632. [Google Scholar] [CrossRef]
- Corsello, A.; Milani, G.P.; Giannì, M.L.; Dipasquale, V.; Romano, C.; Agostoni, C. Different vitamin D supplementation strategies in the first years of life: A systematic review. Healthcare 2022, 10, 1023. [Google Scholar] [CrossRef] [PubMed]
- Chandy, D.D.; Kare, J.; Singh, S.N.; Agarwal, A.; Das, V.; Singh, U.; Ramesh, V.; Bhatia, V. Effect of vitamin D supplementation, directly or via breast milk for term infants, on serum 25 hydroxyvitamin D and related biochemistry, and propensity to infection: A randomised placebo-controlled trial. Br. J. Nutr. 2016, 116, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Naik, P.; Faridi, M.M.A.; Batra, P.; Madhu, S.V. Oral supplementation of parturient mothers with vitamin D and its effect on 25OHD status of exclusively breastfed infants at 6 months of age: A double-blind randomized placebo controlled trial. Breastfeed Med. 2017, 12, 621–628. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, K.M.; Taghivand, M.; Zuchniak, A.; Onoyovwi, A.; Korsiak, J.; Leung, M.; Roth, D.E. Vitamin D in breastfed infants: Systematic review of alternatives to daily supplementation. Adv. Nutr. 2020, 11, 144–159. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durá-Travé, T.; Gallinas-Victoriano, F. Pregnancy, Breastfeeding, and Vitamin D. Int. J. Mol. Sci. 2023, 24, 11881. https://doi.org/10.3390/ijms241511881
Durá-Travé T, Gallinas-Victoriano F. Pregnancy, Breastfeeding, and Vitamin D. International Journal of Molecular Sciences. 2023; 24(15):11881. https://doi.org/10.3390/ijms241511881
Chicago/Turabian StyleDurá-Travé, Teodoro, and Fidel Gallinas-Victoriano. 2023. "Pregnancy, Breastfeeding, and Vitamin D" International Journal of Molecular Sciences 24, no. 15: 11881. https://doi.org/10.3390/ijms241511881
APA StyleDurá-Travé, T., & Gallinas-Victoriano, F. (2023). Pregnancy, Breastfeeding, and Vitamin D. International Journal of Molecular Sciences, 24(15), 11881. https://doi.org/10.3390/ijms241511881






