Vitamin D and Bone fragility in Individuals with Osteogenesis Imperfecta: A Scoping Review
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design
2.1.1. Definition of the Research Question
- -
- P: individuals affected by OI;
- -
- I: vitamin D status, measurement, and supplementation;
- -
- C: healthy subjects;
- -
- O: vitamin D status and supplementation.
2.1.2. Search and Selection of the Studies
2.2. Data Extraction
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| SECTION | ITEM | PRISMA-ScR CHECKLIST ITEM | REPORTED ON PAGE # |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a scoping review. | 1 |
| ABSTRACT | |||
| Structured summary | 2 | Provide a structured summary that includes (as applicable): background, objectives, eligibility criteria, sources of evidence, charting methods, results, and conclusions that relate to the review questions and objectives. | 1 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known. Explain why the review questions/objectives lend themselves to a scoping review approach. | 1 |
| Objectives | 4 | Provide an explicit statement of the questions and objectives being addressed with reference to their key elements (e.g., population or participants, concepts, and context) or other relevant key elements used to conceptualize the review questions and/or objectives. | 1–4 |
| METHODS | |||
| Protocol and registration | 5 | Indicate whether a review protocol exists; state if and where it can be accessed (e.g., a Web address); and if available, provide registration information, including the registration number. | 4 |
| Eligibility criteria | 6 | Specify characteristics of the sources of evidence used as eligibility criteria (e.g., years considered, language, and publication status), and provide a rationale. | 4–5 |
| Information sources * | 7 | Describe all information sources in the search (e.g., databases with dates of coverage and contact with authors to identify additional sources), as well as the date the most recent search was executed. | 4–5 |
| Search | 8 | Present the full electronic search strategy for at least 1 database, including any limits used, such that it could be repeated. | 5 |
| Selection of sources of evidence † | 9 | State the process for selecting sources of evidence (i.e., screening and eligibility) included in the scoping review. | 5 |
| Data charting process ‡ | 10 | Describe the methods of charting data from the included sources of evidence (e.g., calibrated forms or forms that have been tested by the team before their use, and whether data charting was done independently or in duplicate) and any processes for obtaining and confirming data from investigators. | 6 |
| Data items | 11 | List and define all variables for which data were sought and any assumptions and simplifications made. | 6 |
| Critical appraisal of individual sources of evidence § | 12 | If done, provide a rationale for conducting a critical appraisal of included sources of evidence; describe the methods used and how this information was used in any data synthesis (if appropriate). | - |
| Synthesis of results | 13 | Describe the methods of handling and summarizing the data that were charted. | 5–12 |
| RESULTS | |||
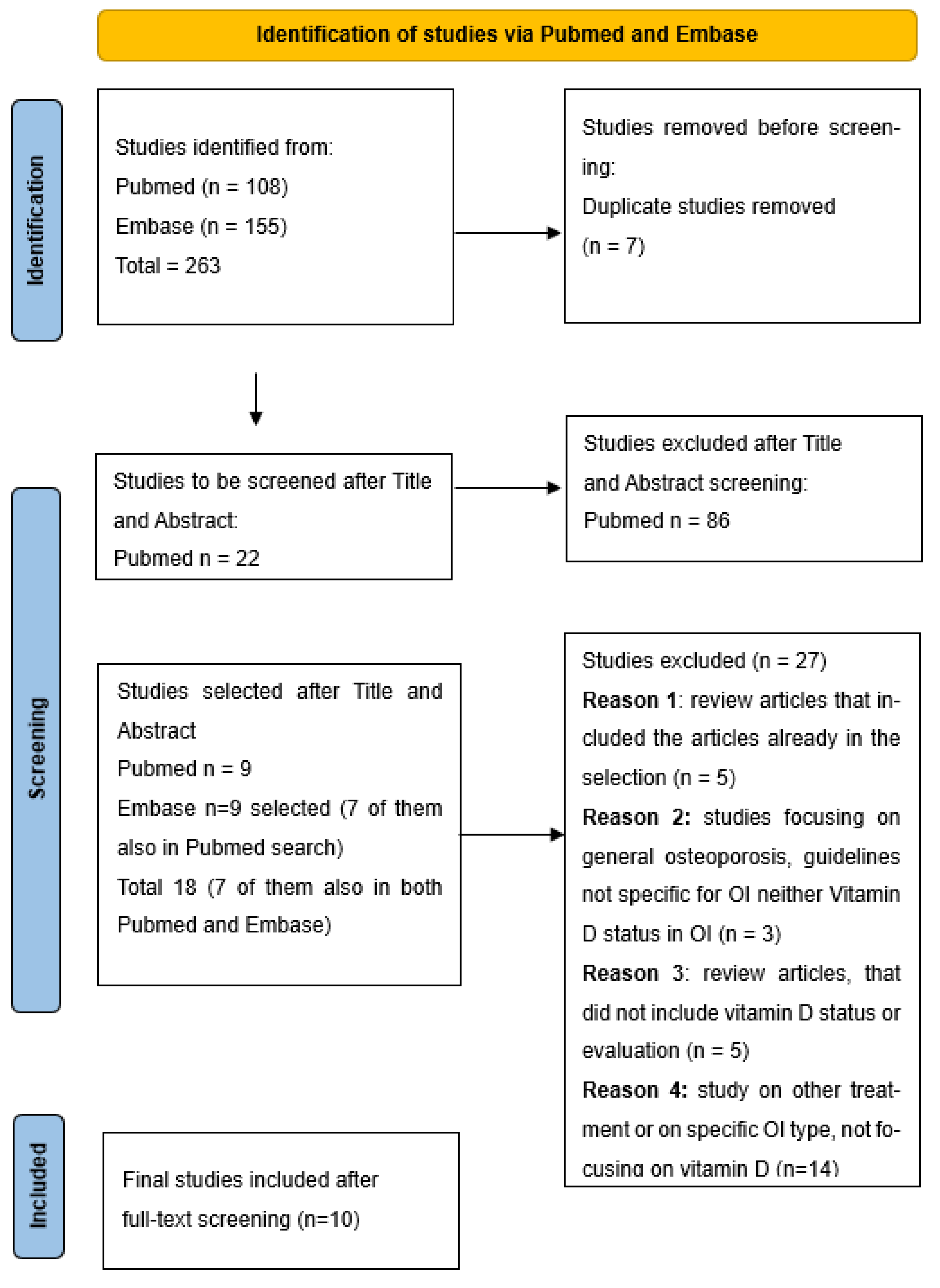
| Selection of sources of evidence | 14 | Give numbers of sources of evidence screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally using a flow diagram. | 5, 6 |
| Characteristics of sources of evidence | 15 | For each source of evidence, present characteristics for which data were charted and provide the citations. | 6 |
| Critical appraisal within sources of evidence | 16 | If done, present data on critical appraisal of included sources of evidence (see item 12). | - |
| Results of individual sources of evidence | 17 | For each included source of evidence, present the relevant data that were charted that relate to the review questions and objectives. | 7–12 |
| Synthesis of results | 18 | Summarize and/or present the charting results as they relate to the review questions and objectives. | 7–13 |
| DISCUSSION | |||
| Summary of evidence | 19 | Summarize the main results (including an overview of concepts, themes, and types of evidence available), link to the review questions and objectives, and consider the relevance to key groups. | 13,14 |
| Limitations | 20 | Discuss the limitations of the scoping review process. | 15 |
| Conclusions | 21 | Provide a general interpretation of the results with respect to the review questions and objectives, as well as potential implications and/or next steps. | 15 |
| FUNDING | |||
| Funding | 22 | Describe sources of funding for the included sources of evidence, as well as sources of funding for the scoping review. Describe the role of the funders of the scoping review. | |
References
- Kulie, T.; Groff, A.; Redmer, J.; Hounshell, J.; Schrager, S. Vitamin D: An Evidence-Based Review. J. Am. Board. Fam. Med. 2009, 22, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Saponaro, F.; Saba, A.; Zucchi, R. An Update on Vitamin D Metabolism. Int. J. Mol. Sci. 2020, 21, 6573. [Google Scholar] [CrossRef]
- Bikle, D.; Christakos, S. New aspects of vitamin D metabolism and action—Addressing the skin as source and target. Nat. Rev. Endocrinol. 2020, 16, 234–252. [Google Scholar] [CrossRef]
- Bikle, D.D. Extraskeletal actions of vitamin D. Ann. N. Y. Acad. Sci. 2016, 1376, 29. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Marcocci, C.; Carmeliet, G.; Bikle, D.; White, J.H.; Dawson-Hughes, B.; Lips, P.; Munns, C.F.; Lazaretti-Castro, M.; Giustina, A.; et al. Skeletal and Extraskeletal Actions of Vitamin D: Current Evidence and Outstanding Questions. Endocr. Rev. 2019, 40, 1109–1151. [Google Scholar] [CrossRef]
- Sîrbe, C.; Rednic, S.; Grama, A.; Pop, T.L. An Update on the Effects of Vitamin D on the Immune System and Autoimmune Diseases. Int. J. Mol. Sci. 2022, 23, 9784. [Google Scholar] [CrossRef]
- Cui, X.; Eyles, D.W. Vitamin D and the Central Nervous System: Causative and Preventative Mechanisms in Brain Disorders. Nutrients 2022, 14, 4353. [Google Scholar] [CrossRef]
- Triantos, C.; Aggeletopoulou, I.; Mantzaris, G.J.; Mouzaki, A. Molecular basis of vitamin D action in inflammatory bowel disease. Autoimmun. Rev. 2022, 21, 103136. [Google Scholar] [CrossRef] [PubMed]
- Argano, C.; Mallaci Bocchio, R.; Lo Monaco, M.; Scibetta, S.; Natoli, G.; Cavezzi, A.; Troiani, E.; Corrao, S. An Overview of Systematic Reviews of the Role of Vitamin D on Inflammation in Patients with Diabetes and the Potentiality of Its Application on Diabetic Patients with COVID-19. Int. J. Mol. Sci. 2022, 23, 2873. [Google Scholar] [CrossRef]
- Prono, F.; Bernardi, K.; Ferri, R.; Bruni, O. The Role of Vitamin D in Sleep Disorders of Children and Adolescents: A SystematicReview. Int. J. Mol. Sci. 2022, 23, 1430. [Google Scholar] [CrossRef]
- Charoenngam, N. Vitamin D and Rheumatic Diseases: A Review of Clinical Evidence. Int. J. Mol. Sci. 2021, 22, 10659. [Google Scholar] [CrossRef]
- Albergamo, A.; Apprato, G.; Silvagno, F. The Role of Vitamin D in Supporting Health in the COVID-19 Era. Int. J. Mol. Sci. 2022, 23, 3621. [Google Scholar] [CrossRef] [PubMed]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Tmava Berisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0, an update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef]
- Cashman, K.D.; Dowling, K.G.; Škrabáková, Z.; Gonzalez-Gross, M.; Valtueña, J.; De Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Mølgaard, C.; et al. Vitamin D deficiency in Europe: Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef]
- Cashman, K.D. Vitamin D deficiency: Defining, prevalence, causes, and strategies of addressing. Calcif. Tissue Int. 2019, 106, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Schleicher, R.L.; Sternberg, M.R.; Looker, A.C.; Yetley, E.A.; Lacher, D.A.; Sempos, C.T.; Taylor, C.L.; Durazo-Arvizu, R.A.; Maw, K.L.; Chaudhary-Webb, M.; et al. National estimates of serum total 25-Hydroxyvitamin D and metabolite concentrations measured by liquid chromatography–Tandem mass spectrometry in the US population during 2007–2010. J. Nutr. 2016, 146, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Sarafin, K.; Durazo-Arvizu, R.; Tian, L.; Phinney, K.W.; Tai, S.; Camara, J.E.; Merkel, J.; Green, E.; Sempos, C.T.; Brooks, S.P. Standardizing 25-hydroxyvitamin D values from the Canadian Health Measures Survey. Am. J. Clin. Nutr. 2015, 102, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Mithal, A.; Wahl, D.A.; Bonjour, J.P.; Burckhardt, P.; Dawson-Hughes, B.; Eisman, J.A.; El-Hajj Fuleihan, G.; Josse, R.G.; Lips, P.; Morales-Torres, J. IOF Committee of Scientific Advisors (CSA) Nutrition Working Group, Global vitamin D status and determinants of hypovitaminosis D. Osteoporos. Int. 2009, 20, 1807–1820. [Google Scholar] [CrossRef]
- Van Schoor, N.M.; Lips, P. Worldwide vitamin D status. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 671–680. [Google Scholar] [CrossRef]
- Wahl, D.A.; Cooper, C.; Ebeling, P.R.; Eggersdorfer, M.; Hilger, J.; Hoffmann, K.; Josse, R.; Kanis, J.A.; Mithal, A.; Pierroz, D.D.; et al. A global representation of vitamin D status in healthy populations. Arch. Osteoporos. 2012, 7, 155–172. [Google Scholar] [CrossRef]
- Palacios, C.; Gonzalez, L. Is vitamin D deficiency a major global public health problem? J. Steroid Biochem. Mol. Biol. 2014, 144, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Vierucci, F.; Del Pistoia, M.; Fanos, M.; Gori, M.; Carlone, G.; Erba, P.; Massimetti, G.; Federico, G.; Saggese, G. Vitamin D status and predictors of hypovitaminosis D in Italian children and adolescents: A cross-sectional study. Eur. J. Pediatr. 2013, 172, 1607–1617. [Google Scholar] [CrossRef]
- Horton-French, K.; Dunlop, E.; Lucas, R.M.; Pereira, G.; Black, L.J. Prevalence and predictors of vitamin D deficiency in a nationally representative sample of Australian adolescents and young adults. Eur. J. Clin. Nutr. 2021, 75, 1627–1636. [Google Scholar] [CrossRef]
- Rabufetti, A.; Milani, G.P.; Lava, S.A.G.; Edefonti, V.; Bianchetti, M.G.; Stettbacher, A.; Muggli, F.; Simonetti, G. Vitamin D Status Among Male Late Adolescents Living in Southern Switzerland: Role of Body Composition and Lifestyle. Nutrients 2019, 11, 2727. [Google Scholar] [CrossRef]
- Islam, M.Z.; Bhuiyan, N.H.; Akhtaruzzaman, M.; Allardt, C.L.; Fogelholm, M. Vitamin D deficiency in Bangladesh: A review of prevalence, causes and recommendations for mitigation. Asia. Pac. J. Clin. Nutr. 2022, 31, 167–180. [Google Scholar] [PubMed]
- Mogire, R.M.; Mutua, A.; Kimita, W.; Kamau, A.; Bejon, P.; Pettifor, J.M.; Adeyemo, A.; Williams, T.N.; Atkinson, S.H. Prevalence of vitamin D deficiency in Africa: A systematic review and meta-analysis. Lancet Glob. Health 2020, 8, e134–e142. [Google Scholar] [CrossRef] [PubMed]
- Akkermans, M.D.; van der Horst-Graat, J.M.; Eussen, S.R.; van Goudoever, J.B.; Brus, F.J. Iron and Vitamin D Deficiency in Healthy Young Children in Western Europe Despite Current Nutritional Recommendations. Pediatr. Gastroenterol. Nutr. 2016, 62, 635–642. [Google Scholar] [CrossRef]
- Yu, S.; Fang, H.; Han, J.; Cheng, X.; Xia, L.; Li, S.; Liu, M.; Tao, Z.; Wang, L.; Hou, L.; et al. The high prevalence of hypovitaminosis D in China: A multicenter vitamin D status survey. Medicine 2015, 94, e585. [Google Scholar] [CrossRef]
- Holick, M.F.; Chen, T.C. Vitamin D deficiency: A worldwide problem with health consequences. Am. J. Clin. Nutr. 2008, 87, 1080S–1086S. [Google Scholar] [CrossRef] [PubMed]
- Hollis, B.W.; Wagner, C.L. Normal serum vitamin D levels. N. Engl. J. Med. 2005, 352, 515–516. [Google Scholar]
- Hussain, S.; Yates, C.; Campbell, M.J. Vitamin D and Systems Biology. Nutrients 2022, 14, 5197. [Google Scholar] [CrossRef] [PubMed]
- Lips, P. Vitamin D physiology. Prog. Biophys. Mol. Biol. 2006, 92, 4–8. [Google Scholar] [CrossRef]
- Bickle, D.D. Vitamin D and Bone. Curr. Osteoporos. Rep. 2012, 10, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, T.O.; Shaw, N.J.; Portale, A.A.; Ward, L.M.; Abrams, S.A.; Pettifor, J.M. Rickets. Nat. Rev. Dis. Primers 2017, 3, 17101. [Google Scholar] [CrossRef] [PubMed]
- Haffner, D.; Leifheit-Nestler, M.; Grund, A.; Schnabel, D. Rickets guidance: Part I-diagnostic workup. Pediatr. Nephrol. 2022, 37, 2013–2036. [Google Scholar] [CrossRef]
- OMIM. Available online: https://www.ncbi.nlm.nih.gov/omim (accessed on 1 January 2023).
- Jovanovic, M.; Guterman-Ram, G.; Marini, J.C. Osteogenesis Imperfecta: Mechanisms and Signaling Pathways Connecting Classical and Rare OI Types. Endocr. Rev. 2022, 43, 61–90. [Google Scholar] [CrossRef]
- Kang, H.; Aryal AC, S.; Marini, J.C. Osteogenesis imperfecta: New genes reveal novel mechanisms in bone dysplasia. Transl. Res. 2017, 181, 27–48. [Google Scholar] [CrossRef]
- Sillence, D.O.; Senn, A.; Danks, D.M. Genetic heterogeneity in osteogenesis imperfecta. J. Med. Genet. 1979, 16, 101–116. [Google Scholar] [CrossRef]
- Forlino, A.; Marini, J.C. Osteogenesis imperfecta. Lancet 2016, 387, 1657–1671. [Google Scholar] [CrossRef]
- Unger, S.; Ferreira, C.R.; Mortier, G.R.; Ali, H.; Bertola, D.R.; Calder, A.; Cohn, D.H.; Cormier-Daire, V.; Girisha, K.M.; Hall, C.; et al. Nosology of genetic skeletal disorders: 2023 revision. Am. J. Med. Genet. Part A 2023, 191, 1164–1209. [Google Scholar] [CrossRef]
- Claeys, L.; Storoni, S.; Eekhoff, M.; Elting, M.; Wisse, L.; Pals, G.; Bravenboer, N.; Maugeri, A.; Micha, D. Collagen transport and related pathways in Osteogenesis Imperfecta. Hum. Genet. 2021, 140, 1121–1141. [Google Scholar] [CrossRef]
- Nijhuis, W.H.; Eastwood, D.M.; Allgrove, J.; Hvid, I.; Weinans, H.H.; Bank, R.A.; Sakkers, R.J. Current concepts in osteogenesis imperfecta: Bone structure, biomechanics and medical management. J. Child. Orthop. 2019, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.J.; Mönkkönen, J.; Munoz, M.A. Molecular mechanisms of action of bisphosphonates and new insights into their effects outside the skeleton. Bone 2020, 139, 115493. [Google Scholar] [CrossRef] [PubMed]
- Bishop, N.; Adami, S.; Ahmed, S.F.; Antón, J.; Arundel, P.; Burren, C.P.; Devogelaer, J.-P.; Hangartner, T.; Hosszú, E.; Lane, J.M.; et al. Risedronate in children with osteogenesis imperfecta: A randomised, double-blind, placebo-controlled trial. Lancet 2013, 382, 1424–1432. [Google Scholar] [CrossRef] [PubMed]
- Dwan, K.; Phillipi, C.A.; Steiner, R.D.; Basel, D. Bisphosphonate therapy for osteogenesis imperfecta. Cochrane Database Syst. Rev. 2014, 7, CD005088. [Google Scholar]
- Hald, J.D.; Evangelou, E.; Langdahl, B.L.; Ralston, S.H. Bisphosphonates for the prevention of fractures in osteogenesis imperfecta: Meta-analysis of placebo-controlled trials. J. Bone Miner. Res. 2015, 30, 929–933. [Google Scholar] [CrossRef]
- Marini, J.C.; Dang Do, A.N. Osteogenesis Imperfecta. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Ralston, S.H.; Gaston, M.S. Management of Osteogenesis Imperfecta. Front. Endocrinol. 2020, 10, 924. [Google Scholar] [CrossRef]
- Rossi, V.; Lee, B.; Marom, R. Osteogenesis imperfecta: Advancements in genetics and treatment. Curr. Opin. Pediatr. 2019, 31, 708–715. [Google Scholar] [CrossRef]
- Botor, M.; Fus-Kujawa, A.; Uroczynska, M.; Stepien, K.L.; Galicka, A.; Gawron, K.; Sieron, A.L. Osteogenesis Imperfecta: Current and Prospective Therapies. Biomolecules 2021, 11, 1493. [Google Scholar] [CrossRef] [PubMed]
- Marom, R.; Rabenhorst, B.M.; Morello, R. Osteogenesis imperfecta: An update on clinical features and therapies. Eur. J. Endocrinol. 2020, 183, R95–R106. [Google Scholar] [CrossRef]
- Hoyer-Kuhn, H.; Rehberg, M.; Netzer, C.; Schoenau, E.; Semler, O. Individualized treatment with denosumab in children with osteogenesis imperfecta - follow up of a trial cohort. Orphanet. J. Rare Dis. 2019, 14, 219. [Google Scholar] [CrossRef] [PubMed]
- Glorieux, F.H.; Devogelaer, J.P.; Durigova, M.; Goemaere, S.; Hemsley, S.; Jakob, F.; Junker, U.; Ruckle, J.; Seefried, L.; Winkle, P.J.J. BPS804 Anti-Sclerostin Antibody in Adults with Moderate Osteogenesis Imperfecta: Results of a Randomized Phase 2a Trial. Bone Miner. Res. 2017, 32, 1496–1504. [Google Scholar] [CrossRef]
- Song, I.W.; Nagamani, S.C.; Nguyen, D.; Grafe, I.; Sutton, V.R.; Gannon, F.H.; Munivez, E.; Jiang, M.M.; Tran, A.; Wallace, M.; et al. Targeting TGF-β for treatment of osteogenesis imperfecta. J. Clin. Invest. 2022, 132, e152571. [Google Scholar] [CrossRef]
- Schindeler, A.; Lee, L.R.; O’Donohue, A.K.; Ginn, S.L.; Munns, C.F. Curative Cell and Gene Therapy for Osteogenesis Imperfecta. J. Bone Miner. Res. 2022, 37, 826–836. [Google Scholar] [CrossRef]
- Torok, G. The vitamin D treatment in massive doses in osteogenesis imperfecta. Ann. Paediatr. 1948, 170, 304–308. [Google Scholar] [PubMed]
- Sbyrakis, S.; Mengreli, C.; Côté, G.B.; Morakis, A. Vitamin D and related research in osteogenesis imperfecta. Prog. Clin. Biol. Res. 1982, 104, 367–376. [Google Scholar]
- Karimian, P.; Ebrahimi, H.K.; Jafarnejad, S.; Delavar, M.A. Effects of vitamin D on bone density in healthy children: A systematic review. J. Fam. Med. Prim. Care 2022, 11, 870–878. [Google Scholar] [CrossRef]
- Beck, J.J.; Mahan, S.T.; Nowicki, P.; Schreiber, V.M.; Minkowitz, B. What Is New in Pediatric Bone Health. J. Pediatr. Orthop. 2021, 41, e594–e599. [Google Scholar] [CrossRef]
- Winzenberg, T.M.; Powell, S.; Shaw, K.A.; Jones, G. Vitamin D supplementation for improving bone mineral density in children. Cochrane Database Syst. Rev. 2010, 10, CD006944. [Google Scholar] [CrossRef]
- Reichrath, J.; März, W.; DEGruijl, F.R.; Vieth, R.; Grant, W.B.; Slominski, A.T.; Holick, M.F.; Vogt, T.; Pilz, S. An Appraisal to Address Health Consequences of Vitamin D Deficiency with Food Fortification and Supplements: Time to Act! Anticancer Res. 2022, 42, 5009–5015. [Google Scholar] [CrossRef] [PubMed]
- Sakka, S.D.; Cheung, M.S. Management of primary and secondary osteoporosis in children. Ther. Adv. Musculoskelet. Dis. 2020, 12, 1759720X20969262. [Google Scholar] [CrossRef]
- Marr, C.; Seasman, A.; Bishop, N. Managing the patient with osteogenesis imperfecta: A multidisciplinary approach. J. Multidiscip. Healthc. 2017, 10, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Keating, S.; Mikhael, M.; Lim, J. Osteogenesis Imperfecta: Multidisciplinary and Goal-Centered Care. AJP Rep. 2022, 12, e144–e147. [Google Scholar] [CrossRef] [PubMed]
- Mäkitie, O.; Zillikens, M.C. Early-Onset Osteoporosis. Calcif. Tissue Int. 2022, 110, 546–561. [Google Scholar] [CrossRef]
- Saraff, V.; Högler, W. ENDOCRINOLOGY AND ADOLESCENCE: Osteoporosis in children: Diagnosis and management. Eur. J. Endocrinol. 2015, 173, R185–R197. [Google Scholar] [CrossRef]
- Akaike, A.; Suzuki, D.; Okuyama, S.; Kudo, Y.; Shimizu, H.; Takanashi, S.; Makino, K.; Yokoyama, J.; Nakaji, S. Associations between physical physique/fitness in children and bone development during puberty: A 4-year longitudinal study. Sci. Rep. 2022, 12, 13427. [Google Scholar] [CrossRef]
- Tortolani, P.J.; McCarthy, E.F.; Sponseller, P.D. Bone mineral density deficiency in children. J. Am. Acad. Orthop. Surg. 2002, 10, 57–66. [Google Scholar] [CrossRef]
- Charoenngam, N.; Cevik, M.B.; Holick, M.F. Diagnosis and management of pediatric metabolic bone diseases associated with skeletal fragility. Curr. Opin. Pediatr. 2020, 32, 560–573. [Google Scholar] [CrossRef]
- Galindo-Zavala, R.; Bou-Torrent, R.; Magallares-López, B.; Mir-Perelló, C.; Palmou-Fontana, N.; Sevilla-Pérez, B.; Medrano-San Ildefonso, M.; González-Fernández, M.I.; Román-Pascual, A.; Alcañiz-Rodríguez, P.; et al. Expert panel consensus recommendations for diagnosis and treatment of secondary osteoporosis in children. Pediatr. Rheumatol. Online J. 2020, 18, 20. [Google Scholar] [CrossRef] [PubMed]
- Mohsenzade, P.; Amirhakimi, A.; Honar, N.; Saki, F.; Omrani, G.H.R.; Dabbaghmanesh, M. Bone density, fractures and the associated factors in iranian children and adolescent with Osteogenesis Imperfecta. BMC Pediatr. 2021, 21, 37. [Google Scholar] [CrossRef] [PubMed]
- Nazim, W.S.; Temtamy, S.A.; Sayed, O.; Otaify, G.A.; Ibrahim, M.M.; Aglan, M.S.; Gouda, A.S. Bone turnover markers in osteogenesis imperfecta and effect of bisphosphonate treatment: First Egyptian study. Int. J. Pharm. Clin. Res. 2019, 11, 68–73. [Google Scholar]
- Zambrano, M.B.; Brizola, E.; Pinheiro, B.; Vanz, A.P.; Mello, E.D.; Félix, T.M.J. Study of the Determinants of Vitamin D Status in Pediatric Patients With Osteogenesis Imperfecta. Am. Coll. Nutr. 2016, 35, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Plante, L.; Veilleux, L.N.; Glorieux, F.H.; Weiler, H.; Rauch, F. Effect of high-dose vitamin D supplementation on bone density in youth with osteogenesis imperfecta: A randomized controlled trial. Bone 2016, 86, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Wilsford, L.D.; Sullivan, E.; Mazur, L.J. Risk factors for vitamin D deficiency in children with osteogenesis imperfecta. J. Pediatr. Orthop. 2013, 33, 5759. [Google Scholar] [CrossRef]
- Chagas, C.E.A.; Roque, J.P.; Peters, B.S.E.; Lazaretti-Castro, M.; Martini, L.A. Do patients with osteogenesis imperfecta need individualized nutritional support? Nutrition 2012, 28, 138–142. [Google Scholar] [CrossRef]
- Wekre, L.L.; Eriksen, E.F.; Falch, J.A. Bone mass, bone markers and prevalence of fractures in adults with osteogenesis imperfecta. Arch. Osteoporos. 2011, 6, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Edouard, T.; Glorieux, F.H.; Rauch, F. Relationship between vitamin D status and bone mineralization, mass, and metabolism in children with osteogenesis imperfecta: Histomorphometric study. J. Bone Min. Res. 2011, 26, 2245–2251. [Google Scholar] [CrossRef] [PubMed]
- Edouard, T.; Glorieux, F.H.; Rauch, F. Predictors and correlates of vitamin D status in children and adolescents with osteogenesis imperfecta. J. Clin. Endocrinol. Metab. 2011, 96, 3193–3198. [Google Scholar] [CrossRef]
- Bowden, S.A.; Robinson, R.F.; Carr, R.; Mahan, J.D. 2008 Prevalence of vitamin D deficiency and insufficiency in children with osteopenia or osteoporosis referred to a pediatric metabolic bone clinic. Pediatrics 2018, 121, 1585–1590. [Google Scholar] [CrossRef]
- Miller, W.L.; Imel, E.A. Rickets, Vitamin D, and Ca/P Metabolism. Horm. Res. Paediatr. 2022, 95, 579–592. [Google Scholar] [CrossRef]
- Foo, L.H.; Zhang, Q.; Zhu, K.; Ma, G.; Hu, X.; Greenfield, H.; Fraser, D.R. Low vitamin D status has an adverse influence on bone mass, bone turnover, and muscle strength in Chinese adolescent girls. J. Nutr. 2009, 139, 1002–1007. [Google Scholar] [CrossRef]
- Emadzadeh, M.; Mehdizadeh, A.; Sharifan, P.; Khoshakhlagh, M.; Sahebi, R.; Sadeghi, R.; AFerns, G.; Ghayour-Mobarhan, M. The Effects of Vitamin D Fortified Products on Bone Biomarkers: A Systematic Review and Meta-Analysis. Iran. J. Public Health 2022, 51, 278–291. [Google Scholar] [CrossRef] [PubMed]
- Jesudason, D.; Need, A.G.; Horowitz, M.; O’Loughlin, P.D.; Morris, H.A.; Nordin, B.E. Relationship between serum 25-hydroxyvitamin D and bone resorption markers in vitamin D insufficiency. Bone 2002, 31, 626–663. [Google Scholar] [CrossRef]
- Gordon, C.M.; DePeter, K.C.; Feldman, H.A.; Grace, E.; Emans, S.J. Prevalence of vitamin D deficiency among healthy adolescents. Arch. Pediatr. Adolesc. Med. 2004, 158, 531–537. [Google Scholar] [CrossRef]
- Hill, T.R.; Cotter, A.A.; Mitchell, S.; Boreham, C.A.; Dubitzky, W.; Murray, L.; Strain, J.J.; Flynn, A.; Robson, P.J.; Wallace, J.M.W.; et al. Vitamin D status and parathyroid hormone relationship in adolescents and its association with bone health parameters: Analysis of the Northern Ireland Young Heart’s Project. Osteoporos. Int. 2010, 21, 695–700. [Google Scholar] [CrossRef]
- Outila, T.A.; Kärkkäinen, M.U.; Lamberg-Allardt, C.J. Vitamin D status affects serum parathyroid hormone concentrations during winter in female adolescents: Associations with forearm bone mineral density. Am. J. Clin. Nutr. 2001, 74, 206–210. [Google Scholar] [CrossRef]
- Guillemant, J.; Cabrol, S.; Allemandou, A.; Peres, G.; Guillemant, S. Vitamin D-dependent seasonal variation of PTH in growing male adolescents. Bone 1995, 17, 513–516. [Google Scholar] [CrossRef]
- Dong, Y.; Pollock, N.; Stallmann-Jorgensen, I.S.; Gutin, B.; Lan, L.; Chen, T.C.; Keeton, D.; Petty, K.; Holick, M.F.; Zhu, H. Low 25-hydroxyvitamin D levels in adolescents: Race, season, adiposity, physical activity, and fitness. Pediatrics 2010, 125, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Stallmann-Jorgensen, I.S.; Pollock, N.K.; Harris, R.A.; Keeton, D.; Huang, Y.; Li, K.; Bassali, R.; Guo, D.-H.; Thomas, J. A 16-week randomized clinical trial of 2000 international units daily vitamin D3 supplementation in black youth: 25-hydroxyvitamin D, adiposity, and arterial stiffness. J. Clin. Endocrinol. Metab. 2010, 95, 4584–4591. [Google Scholar] [CrossRef]
- Dimitri, P.; Wales, J.K.; Bishop, N. Fat and bone in children: Differential effects of obesity on bone size and mass according to fracture history. J. Bone Miner. Res. 2010, 25, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Reid, I.R. Fat and bone. Arch. Biochem. Biophys. 2010, 503, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Zheng, X.; Gao, W.L.; Tao, F.; Chen, Y. Association between serum vitamin D levels and visceral adipose tissue among adolescents: A cross-sectional observational study in NHANES 2011–2015. BMC Pediatr. 2022, 22, 634. [Google Scholar] [CrossRef]
- Gammone, M.A.; Danese, A.; D’Orazio, N. Prevalence of 25(OH)D insufficiency and overweight/obesity in an adult population from the Central Italy. Clin. Ther. 2022, 173, 334–341. [Google Scholar]
- Alemzadeh, R.; Kichler, J.; Babar, G.; Calhoun, M. Hypovitaminosis D in obese children and adolescents: Relationship with adiposity, insulin sensitivity, ethnicity, and season. Metabolism 2008, 57, 183–191. [Google Scholar] [CrossRef]
- White, Z.; White, S.; Dalvie, T.; Kruger, M.C.; Van Zyl, A.; Becker, P. Bone Health, body composition, and vitamin D status of black preadolescent children in South Africa. Nutrients 2019, 11, 1243. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, F.S.; Castell, E.C.; Marco, F.C.; Ruiz, M.J.; Rico, J.A.Q.; Roca, A.P.N. Influence of weight status on bone mineral content measured by DXA in children. BMC Pediatr. 2021, 21, 185. [Google Scholar] [CrossRef]
- Crepaldi, G.; Romanato, G.; Tonin, P.; Maggi, S. Osteoporosis and body composition. J. Endocrinol. Invest. 2007, 30, 42–47. [Google Scholar]
- Chevrel, G.; Meunier, P.J. Osteogenesis imperfecta: Lifelong management is imperative and feasible. Jt. Bone Spine 2001, 68, 125–129. [Google Scholar] [CrossRef]
- Marom, R.; Lee, Y.C.; Grafe, I.; Lee, B. Pharmacological and biological therapeutic strategies for osteogenesis imperfecta. Am. J. Med. Genet. C Semin. Med. Genet. 2016, 172, 367–383. [Google Scholar] [CrossRef] [PubMed]
- Cho, T.J.; Cho, T.J.; Ko, J.M.; Kim, H.; Shin, H.I.; Yoo, W.J.; Shin, C.H. Management of Osteogenesis Imperfecta: A Multidisciplinary Comprehensive Approach. Clin. Orthop. Surg. 2020, 12, 417–429. [Google Scholar] [CrossRef]
- Freitas, R.; Sousa, S.; Godinho, F. The relevance of a multidisciplinary care in the management of patients with Osteogenesis Imperfecta. Acta Reumatol. Port. 2021, 46, 372–373. [Google Scholar] [PubMed]
- Thomas, I.H.; DiMeglio, L.A. Advances in the Classification and Treatment of Osteogenesis Imperfecta. Curr. Osteoporos. Rep. 2016, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L.; et al. PRISMA Exten-sion for Scoping Reviews (PRISMAScR): Checklist and Explanation. Ann Intern Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
| OMIM | OI Type in OMIM | Sillence OI Type | Inheritance | Defective Gene | Mechanism |
|---|---|---|---|---|---|
| 166200 | I | 1 | AD | COL1A1, COL1A2 | Defects in collagen structure and processing |
| 166210, 259420, 166220 | II–IV | 2–4 | AD | COL1A1, COL1A2 | Defects in collagen structure and processing |
| 610967 | V | 3, 4 OI with calcification of interosseous membranes and/or hypertrophic callus (OI type 5), | AD | IFITM5 | Bone mineralization defect |
| 613982 | VI | 3 | AR | SERPINF1 | Bone mineralization defect |
| 610682 | VII | 2, 3, 4 | AR | CRTAP | Defect in collagen modification |
| 610915 | VIII | 2, 3 | AR | LEPRE1, (P3H1) | Defect in collagen modification |
| 259440 | IX | 2, 3, 4 | AR | PPIB | Defect in collagen modification |
| 613848 | X | 3 | AR | SERPINH1 | Defect in collagen folding and cross-linking |
| 610968 | XI | 3, 4 | AR | FKBP10 | Defect in collagen folding and cross-linking |
| 613849 | XII | 4 | AR | SP7 | Osteoblast function and differentiation |
| 614856 | XIII | 3 | AR | BMP1 | Procollagen processing |
| 615066 | XIV | 3 | AR | TMEM38B | Defects in collagen modification |
| 615220 | XV | 3, 4 | AD (Osteoporosis, WNT1-related)/AR | WNT1 | Osteoblast function and differentiation |
| 616229 | XVI | 3 | AR | CREB3L1 | Osteoblast function and differentiation |
| 616507 | XVII | 3 | AR | SPARC | Osteoblast function and differentiation |
| 617952 | XVIII | 3 | AR | TENT5A | Defect in BMP/TGFβ signaling pathway |
| 301014 | XIX | 3 | XR | MBTPS2 | Osteoblast function and differentiation |
| 618644 | XX | 3 | AR | MESD | Defect in Wnt signaling |
| 619131 | XXI | 3 | AR | KDELR2 | Defect in collagen folding and cross-linking |
| 619795 | XXII | 3 | AR | CCD134 | Dysregulation of the RAS/MAPK signaling pathway |
| Author Year Country Study Design | Population Characteristics | OI Type/Reported Severity/Genetic Testing | Vitamin D | Study Aim (s) and Main Results | Summary | Strength/ Limitation of the Study | |
|---|---|---|---|---|---|---|---|
| 1 | Mohsenzade et al., 2021 [71] Iran Case control study | 23 children affected by OI, 23 age–gender-matched controls; 9 males, 14 females | 6 cases OI I 17 cases OI IV No molecular analysis data | Vitamin D deficiency was found in 43% of OI patients vs. 56% of controls Vitamin D levels were higher in OI patients (p = 0.033) | Aim: to assess the BMD and vitamin D level in children with OI in Iran Results: 43.4% of OI children had vitamin D deficiency No association between vitamin D levels and BMD parameters | Vitamin D deficiency is prevalent in OI patients. | Strength: -Case-control study assessing vitamin D status, BMD, and volumetric BMD in children Limitations: -Small sample -Self-reported data from a standard questionnaire -All the patients had received vitamin D supplements since the time of diagnosis |
| 2 | Nazim et al., 2019 [72] Egypt Case control study | 26 children affected by OI, 26 controls; 13 males, 13 females in OI group | 9 cases OI I 11 cases OI III 6 cases OI IV No molecular analysis data | 25(OH) vitamin D lower than the reference range in 4 patients and > 100 µg/L in 5 cases. Note: In the study all patients were on vitamin D oral supplement | Aim: evaluate bone turnover markers in the Egyptian bone patients and the effect of bisphosphonate treatment in these markers Results: Serum calcium measurement, osteocalcin, P1NP are valuable for monitoring the effect of bisphosphonate treatment | 4/26 patients showed low levels of vitamin D | Strengths: -Case-control study -Biochemical measurements, markers of bone formation, and markers of type I collagen degradation evaluation -Measurements at baseline, 6 months of treatment, and 12 months of treatment Limitations: -Small sample -Bisphosphonate treatment -Important variables not evaluated (number and location of fractures, Tanner stage, dietary vitamin D intake, and body composition) |
| 3 | Zambrano et al., 2016 [73] Brazil Cross-sectional study | 52 patients affected by OI Age 1–19 y 29 females, 23 males | 24 cases OI I 5 cases OI III 23 cases OI IV No molecular analysis data | Vitamin D deficiency was found in 35.5% and vitamin D insufficiency was found in 51.9% of OI patients; in 88.4% of cases vitamin D levels were insufficient or deficient | Aim: to assess the relationship between determinants of vitamin D status in pediatric patients with OI. Results: Vitamin D levels were insufficient or deficient in 88% of cases. Vitamin D levels were associated to LS- BMD z-score and were positive correlated to height. No significant difference in OI type No correlation with season of assessment No correlation with PTH or circulating bone markers was found | High prevalence of vitamin D low levels Correlation between vitamin D levels and LS BMD Z-score and height | Strengths: -Different outcomes assessed as vitamin D status, BMD, information about sun exposition, mobility, and bisphosphonate therapy Limitations: -Small sample -Blood samples collected in autumn/winter. -There are no longitudinal data -There are no data about vitamin D supplementation effects. -Important variables not reported (the number/location of fractures, Tanner stage, dietary vitamin D intake, and body composition) |
| 4 | Plante et al., 2016 [74] Canada Clinical randomized controlled trial. | 60 individuals affected by OI Age 6 to 18.9 y; 35 females and 25 males Population was stratified for baseline bisphosphonate treatment and pubertal stage | 23 cases OI 25 cases OI IV 12 cases OI III, V, or VI | Baseline vitamin D concentration, 80% > 50 nmol/L | Aim: to evaluate the efficacy of high-dose vitamin D supplementation on LS-aBMD in children with OI. Results: No significant differences in LS-aBMD z-score changes were detected between treatment groups Increase in vitamin D OH level after supplementation significantly higher in group receiving 2000 IU vitamin D | No significant differences in LS-aBMD z-score changes | Strengths: -Randomized controlled trial -Evaluation of vitamin D supplementation -Patients under bisphosphonate treatment in the previous 2 years were excluded Limitations: -No collected data reflecting endogenous vitamin D synthesis, such as skin pigmentation or sun exposure. -Simultaneous treatment with intravenous bisphosphonates in high proportion of participants |
| 5 | Wilsford et al., 2013 [75] USA Retrospective chart review | 80 children with OI; charts of 44 children (26 female) had documentation of the variables of interest. | 15 cases OI I 12 cases OI III 17 cases OI IV No molecular analysis data | Almost 80% of children with OI had insufficient or deficient levels of vitamin D | Aim: to evaluate the prevalence of vitamin D deficiency and possible risk factors influencing the vitamin D serum levels in patients with (OI). Results: Significant correlations with low vitamin D levels were found for older age (p < 0.001), African American descent (p = 0.01), BMI (p < 0.001), BMI percentile (p = 0.30), consumption of soda (p = 0.009), and pamidronate therapy (p = 0.004). | High prevalence of vitamin D deficiency or insufficient levels. Significant correlations with low vitamin D levels and BMI | Strengths: -Evaluation of several relevant parameters (season of year, level of ambulation, BMI, type of OI, time spent outdoors, and use of sunscreen before playing outdoors) Limitations: -Retrospective study -Missing number and location of fractures as main outcome. -Thirty-four (79.5%) patients had a history of pamidronate therapy |
| 6 | Chagas et al., 2012 [76] Brazil Cross-sectional study | 26 patients affected by OI 13 type I OI and 13 type III OI 8 healthy controls Note: all patients were in treatment with pamidronate | 13 cases OI I 13 cases OI III No molecular genetic testing information reported | 69% type I patients 77% type III patients showed insufficient vitamin D levels 8% type III OI presented Vitamin D deficiency | Aim: Evaluate nutritional status, bone mineral density and biochemical parameters in OI subjects Results: in patients with OI number of fractures was positively related to body mass index and the percentage of body fat and negative correlated to lean body mass. Even taking dietary supplements, 12% of subjects did not achieve vitamin D recommendations | High prevalence of insufficient vitamin D levels in both type I and type III OI | Strengths: -Equal number of OI type 1 and type 3 patients. -First study in which a nutritional evaluation was performed in subjects with OI and body composition information collected. Limitations: -Small sample -Missing number and location of fractures as main outcome -No information about season -All patients were in treatment with pamidronate |
| 7 | Wekre et al., 2011 [77] Norway Case series | 97 adult OI patients 41 males and 56 females Type I OI 74 Type III OI 9 Type IV OI 11 Unclassified 2 | 75 cases OI I 9 cases OI III 11 cases OI IV 2 unclassified cases No molecular analysis information | All patients showed normal levels of PTH, calcium and Vitamin D. OI type III displayed significantly lower values for 25 vitamin (OH) D (p = 0.05) than persons with type I and IV | Aim: Assess bone mass, bone turnover and prevalence of fractures in adult OI patients Results osteoporotic T scores in only 10% of patients Bone turnover markers were normal in the vast majority of patients. In adults with OI type III, bone turnover tended to be increased and osteoporosis more prevalent Seventeen persons (16 females and 1 male) were underbisphosphonates and/or hormone replacement therapy. There were no significant differences in anti-osteoporosis treatment between OI subtypes | Adults with OI type III, bone turnover tended to be increased, and osteoporosis more prevalent, and lower vitamin D levels than other OI types | Strengths: -Study in adult population -Prevalence and localization of fractures were evaluated Limitations: -Relatively small sample -Patient self-reported total number of fractures -No molecular analysis information -No information about bisphosphonate use in childhood -Other parameters (season, dietary vitamin D intake, sun exposure) not evaluated |
| 8 | Edouard et al. [78] 2011a Canada Retrospective study | 71 patients affected with OI type I, III, or IV Age 1.4–17.5; 36 females, 35 males | 29 cases OI I 12 cases OI III 30 cases OI IV In 63 patients a COL1A or COL2A3 mutation was identified (sequence analysis was performed in 65 patients) | Vitamin D deficiency in 52% of cases (Vitamin D concentration ≤ 50 nmol/L) Vitamin D concentration ≤ 80 nmol/L were found in 94% patients | Aim: to evaluated the relationship between vitamin D status and parameters of skeletal mineralization, mass, and metabolism in a group of pediatric osteogenesis imperfecta (OI) patients. Results: vitamin D was negative correlated with age and serum PTH levels No correlation with alkaline phosphatase levels. No seasonal variability Vitamin D levels were not related with bone formation rate, osteoid thickness, mineralization lag time. No evidence that vitamin D levels from 13 to 103 nmol/L were associated with measurement of bone mineralization, metabolism or mass in children with OI. | Deficient or low levels of vitamin D were found in more than 50% of patients Negative correlation between PTH levels and vitamin D levels was observed No seasonal variability | Strengths: -Histomorphometric parameters evaluated Limitations: -Small sample -Missing number and location of fractures as main outcome -No information about season or detailed information about treatment history |
| 9 | Edouard et al. [79] 2011b Canada Retrospective cross-sectional study | 315 patients affected with OI type I, III, or IV Age 1.1–17.9 y; 161 females and 154 males | 165 cases OI I 56 cases OI III 94 cases OI IV Collagen type I molecular testing available in 254 patients. Disease causing mutation in 222 patients | Vitamin D deficiency in 27% of cases Lowers levels in teenagers Levels decreased less markedly in winter than in other studies | Aim: evaluated vitamin D status determinants in children and adolescent OI patients Results: vitamin D levels were associated to LS-aBMD z-score in children and adolescents with OI, type I, III, IV. Vitamin D levels were inversely associated to PTH levels. | Vitamin D deficiency is prevalent in OI Lower levels of vitamin D were associated to LS-aBMD score and teenage. | Strengths: -Large sample size. -No previous treatment with bisphosphonate. Limitations: -No additional variables evaluated |
| 10 | Bowden et al., 2008 [80] USA Retrospective study | 84 children with osteopenia or osteoporosis 24 OI patients (28% of the total) | There was no information about OI type or severity Some cases underwent collagen fibroblast analysis. No information about molecular genetics | Vitamin D deficiency was observed in 26% of OI cases Insufficient levels in 7% of OI patients | Aim: To determine the prevalence of vitamin D deficiency and insufficiency in children with osteopenia or osteoporosis and to evaluate the relationship between serum vitamin D levels and bone parameters, including bone mineral density. Results: A high prevalence of vitamin D insufficiency was found in this series of children with osteopenia or osteoporosis, regardless of the etiology of bone disorder. Negative correlation between vitamin D levels and PTH levels. No effect of seasonality on vitamin D. | High prevalence of insufficient or deficient vitamin D levels. Negative correlation between Vitamin D levels and PTH levels No effect of seasonality on vitamin D | Strengths: -Data about fracture rate concurrent with drug therapy -Demographic data, and -detailed medical history and biochemical laboratory studies Limitations: -Relatively small sample -Other disease with bone fragility included. -Other important variables not reported (i.e., type of OI or OI severity, location of fractures, season) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gnoli, M.; Brizola, E.; Tremosini, M.; Di Cecco, A.; Sangiorgi, L. Vitamin D and Bone fragility in Individuals with Osteogenesis Imperfecta: A Scoping Review. Int. J. Mol. Sci. 2023, 24, 9416. https://doi.org/10.3390/ijms24119416
Gnoli M, Brizola E, Tremosini M, Di Cecco A, Sangiorgi L. Vitamin D and Bone fragility in Individuals with Osteogenesis Imperfecta: A Scoping Review. International Journal of Molecular Sciences. 2023; 24(11):9416. https://doi.org/10.3390/ijms24119416
Chicago/Turabian StyleGnoli, Maria, Evelise Brizola, Morena Tremosini, Alessia Di Cecco, and Luca Sangiorgi. 2023. "Vitamin D and Bone fragility in Individuals with Osteogenesis Imperfecta: A Scoping Review" International Journal of Molecular Sciences 24, no. 11: 9416. https://doi.org/10.3390/ijms24119416
APA StyleGnoli, M., Brizola, E., Tremosini, M., Di Cecco, A., & Sangiorgi, L. (2023). Vitamin D and Bone fragility in Individuals with Osteogenesis Imperfecta: A Scoping Review. International Journal of Molecular Sciences, 24(11), 9416. https://doi.org/10.3390/ijms24119416






