Hyperacusis in the Adult Fmr1-KO Mouse Model of Fragile X Syndrome: The Therapeutic Relevance of Cochlear Alterations and BKCa Channels
Abstract
1. Introduction
2. Results
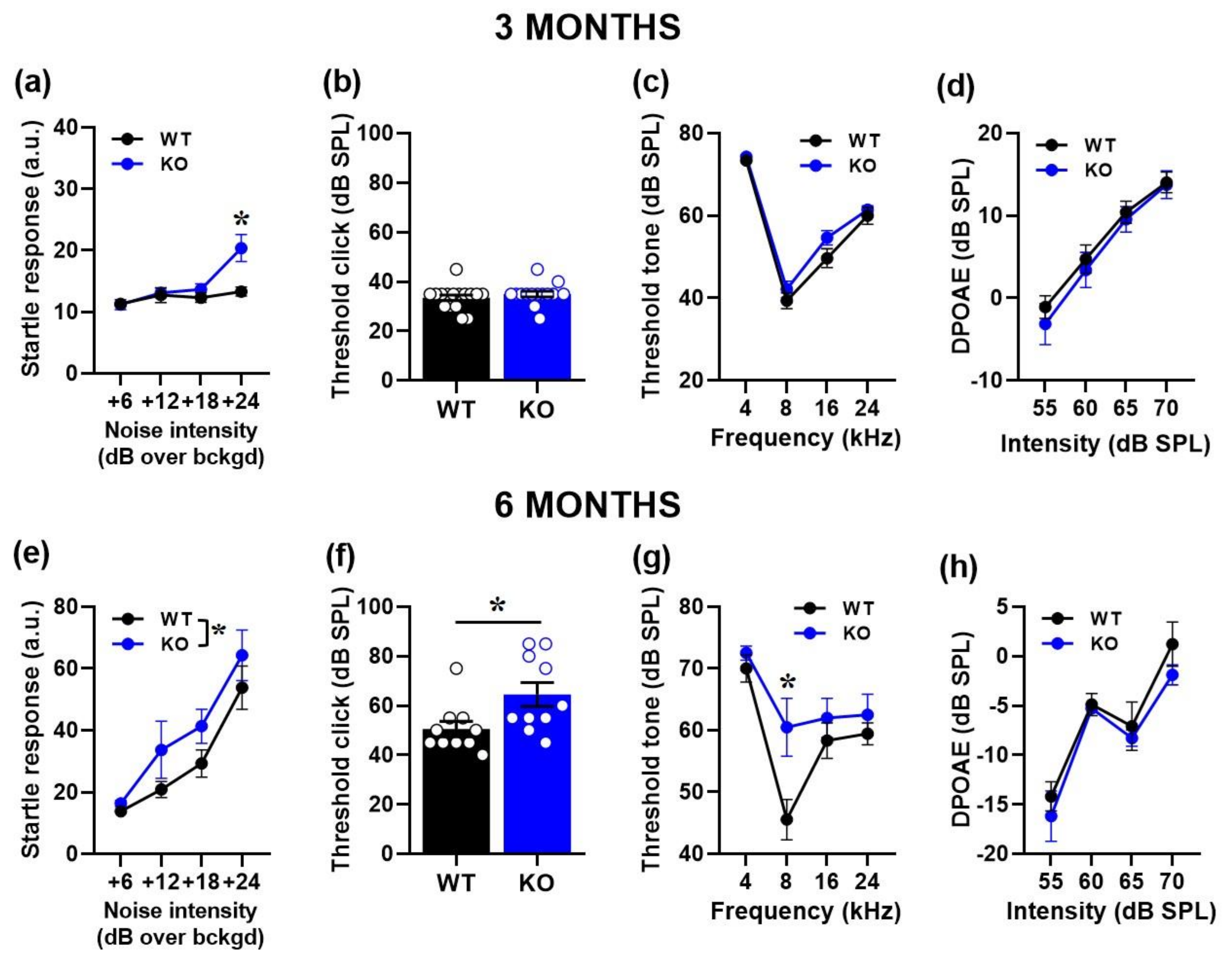
2.1. Hyperacusis-like Phenotypes in Fmr1-KO Mice at 3 and 6 Months of Age: Behavioral and Electrophysiological Measurements
2.1.1. Acoustic Startle Response (ASR)
2.1.2. Auditory Brainstem Responses (ABRs) and Distortion Product Otoacoustic Emissions (DPOAEs)
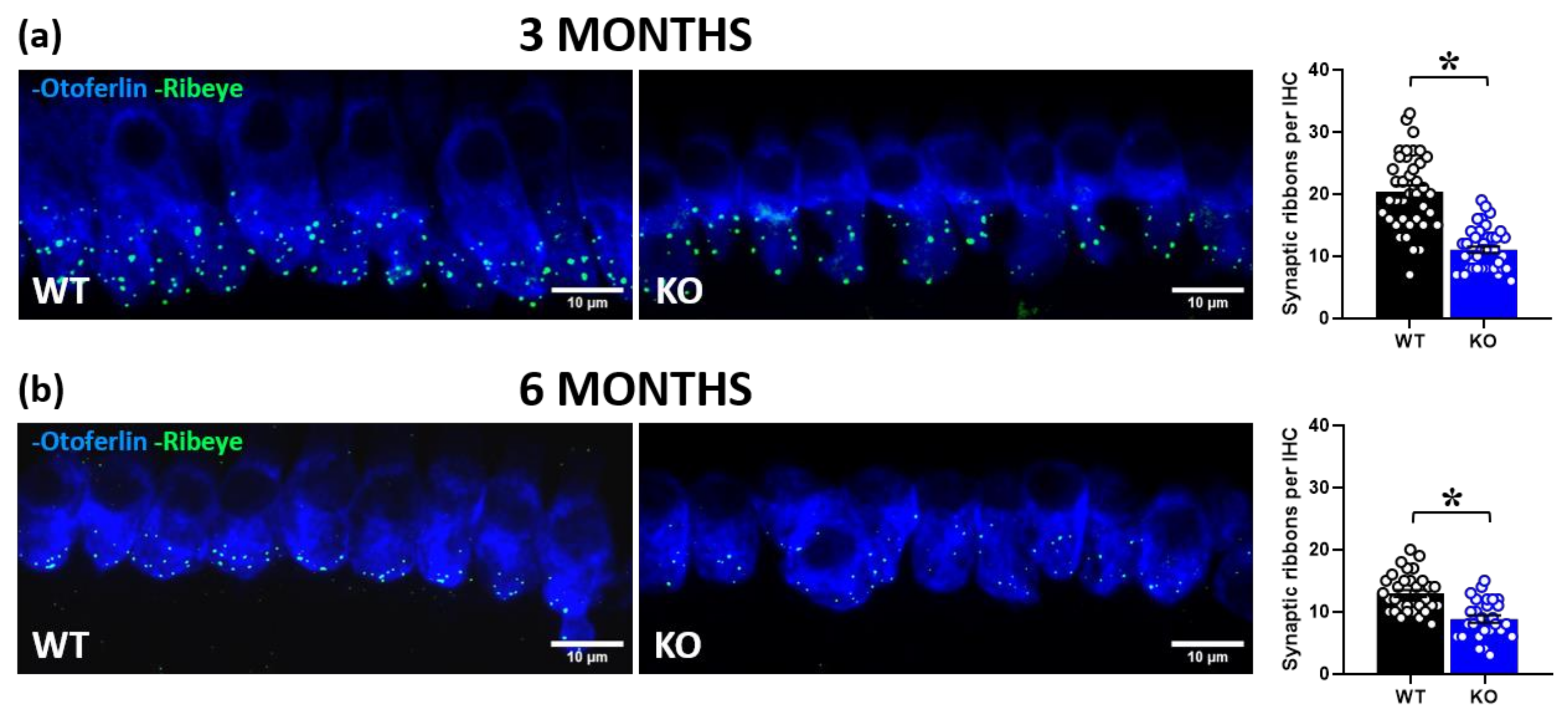
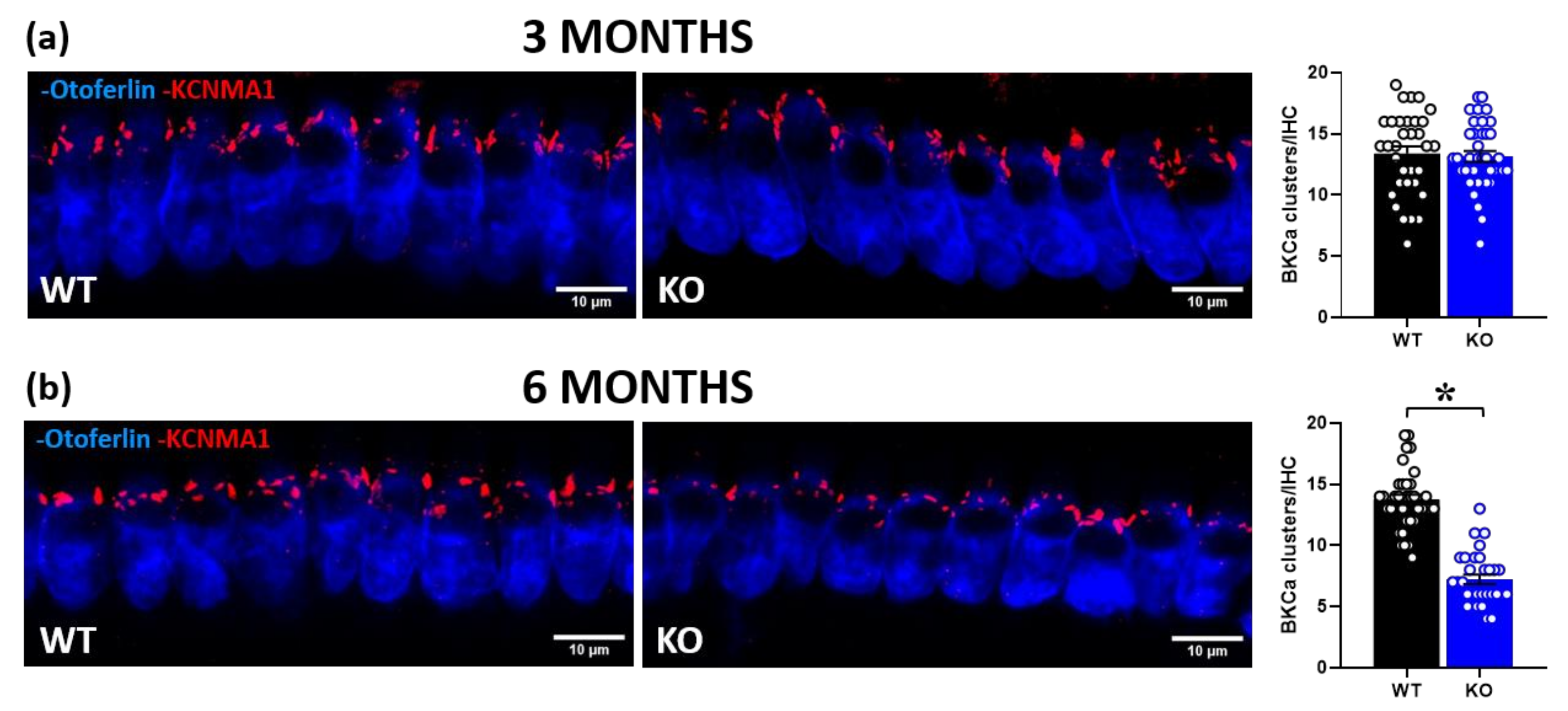
2.2. Immunohistochemical Analysis in Inner Hair Cells (IHCs)
2.2.1. RIBEYE Expression in IHCs
2.2.2. KCNMA1 Expression in IHCs
2.3. Therapeutic Effects of Chlorzoxazone (CHLOR) in Fmr1-KO Mice
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Acoustic Startle Response
4.3. Auditory Brainstem Response (ABRs) and Distortion Product Otoacoustic Emissions (DPOAEs)
4.4. Tissue Preparation and Immunocytochemistry
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hagerman, R.J.; Berry-Kravis, E.; Hazlett, H.C.; Bailey, D.B.; Moine, H.; Kooy, R.F.; Tassone, F.; Gantois, I.; Sonenberg, N.; Mandel, J.L.; et al. Fragile X syndrome. Nat. Rev. Dis. Primers 2017, 3, 17065. [Google Scholar] [CrossRef]
- Greenough, W.T.; Klintsova, A.Y.; Irwin, S.A.; Galvez, R.; Bates, K.E.; Weiler, I.J. Synaptic regulation of protein synthesis and the fragile X protein. Proc. Natl. Acad. Sci. USA 2001, 98, 7101–7106. [Google Scholar] [CrossRef]
- Berry-Kravis, E.; Raspa, M.; Loggin-Hester, L.; Bishop, E.; Holiday, D.; Bailey, D.B., Jr. Seizures in fragile X syndrome: Characteristics and comorbid diagnoses. Am. J. Intellect. Dev. Disabil. 2010, 115, 461–472. [Google Scholar] [CrossRef]
- Cordeiro, L.; Ballinger, E.; Hagerman, R.; Hessl, D. Clinical assessment of DSM-IV anxiety disorders in fragile X syndrome: Prevalence and characterization. J. Neurodev. Disord. 2011, 3, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Hogan, A.L.; Caravella, K.E.; Ezell, J.; Rague, L.; Hills, K.; Roberts, J.E. Autism Spectrum Disorder Symptoms in Infants with Fragile X Syndrome: A Prospective Case Series. J. Autism Dev. Disord. 2017, 47, 1628–1644. [Google Scholar] [CrossRef]
- Miller, L.J.; McIntosh, D.N.; McGrath, J.; Shyu, V.; Lampe, M.; Taylor, A.K.; Tassone, F.; Neitzel, K.; Stackhouse, T.; Hagerman, R.J. Electrodermal responses to sensory stimuli in individuals with fragile X syndrome: A preliminary report. Am. J. Med. Genet. 1999, 83, 268–279. [Google Scholar] [CrossRef]
- Rotschafer, S.E.; Razak, K.A. Auditory processing in fragile X syndrome. Front. Cell. Neurosci. 2014, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.B.; Comeau, M.; Clarke, K.D. Hyperacusis in Williams syndrome. J. Otolaryngol. 2001, 30, 90–92. [Google Scholar] [CrossRef]
- Ralli, M.; Romani, M.; Zodda, A.; Russo, F.Y.; Altissimi, G.; Orlando, M.P.; Cammeresi, M.G.; Penge, R.; Turchetta, R. Hyperacusis in Children with Attention Deficit Hyperactivity Disorder: A Preliminary Study. Int. J. Environ. Res. Public Health 2020, 17, 3045. [Google Scholar] [CrossRef] [PubMed]
- Thabet, E.M.; Zaghloul, H.S. Auditory profile and high resolution CT scan in autism spectrum disorders children with auditory hypersensitivity. Eur. Arch. Otorhinolaryngol. 2013, 270, 2353–2358. [Google Scholar] [CrossRef]
- Pyott, S.; Duncan, R. BK Channels in the Vertebrate Inner Ear. Int. Rev. Neurobiol. 2016, 128, 369–399. [Google Scholar] [PubMed]
- Skinner, L.J.; Enée, V.; Beurg, M.; Jung, H.H.; Ryan, A.F.; Hafidi, A.; Aran, J.M.; Dulon, D. Contribution of BK Ca2+-activated K+ channels to auditory neurotransmission in the Guinea pig cochlea. J. Neurophysiol. 2003, 90, 320–332. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Peineau, T.; Belleudy, S.; Pietropaolo, S.; Bouleau, Y.; Dulon, D. Synaptic Release Potentiation at Aging Auditory Ribbon Synapses. Front. Aging Neurosci. 2021, 13, 756449. [Google Scholar] [CrossRef] [PubMed]
- Khimich, D.; Nouvian, R.; Pujol, R.; Dieck, S.T.; Egner, A.; Gundelfinger, E.D.; Moser, T. Hair cell synaptic ribbons are essential for synchronous auditory signalling. Nature 2005, 434, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, C.; Moser, T. Relating structure and function of inner hair cell ribbon synapses. Cell Tissue Res. 2015, 361, 95–114. [Google Scholar] [CrossRef]
- Sah, P.; Faber, E.L. Channels underlying neuronal calcium-activated potassium currents. Prog. Neurobiol. 2002, 66, 345–353. [Google Scholar] [CrossRef]
- Hébert, B.; Pietropaolo, S.; Même, S.; Laudier, B.; Laugeray, A.; Doisne, N.; Quartier, A.; Lefeuvre, S.; Got, L.; Cahard, D.; et al. Rescue of fragile X syndrome phenotypes in Fmr1 KO mice by a BKCa channel opener molecule. Orphanet J. Rare Dis. 2014, 9, 124. [Google Scholar] [CrossRef]
- Laumonnier, F.; Roger, S.; Guérin, P.; Molinari, F.; M’rad, R.; Cahard, D.; Belhadj, A.; Halayem, M.; Persico, A.; Elia, M.; et al. Association of a functional deficit of the BKCa channel, a synaptic regulator of neuronal excitability, with autism and mental retardation. Am. J. Psychiatry 2006, 163, 1622–1629. [Google Scholar] [CrossRef]
- Liang, L.; Li, X.; Moutton, S.; Vergano, S.A.S.; Cogné, B.; Saint-Martin, A.; Hurst, A.C.E.; Hu, Y.; Bodamer, O.; Thevenon, J.; et al. De novo loss-of-function KCNMA1 variants are associated with a new multiple malformation syndrome and a broad spectrum of developmental and neurological phenotypes. Hum. Mol. Genet. 2019, 28, 2937–2951. [Google Scholar] [CrossRef]
- Khattak, S.; Brimble, E.; Zhang, W.; Zaslavsky, K.; Strong, E.; Ross, P.J.; Hendry, J.; Mital, S.; Salter, M.W.; Osborne, L.R.; et al. Human induced pluripotent stem cell derived neurons as a model for Williams-Beuren syndrome. Mol. Brain 2015, 8, 77. [Google Scholar] [CrossRef]
- Lemaire-Mayo, V.; Piquemal, M.; Crusio, W.E.; Louette, E.; Pietropaolo, S. Therapeutic effects of Chlorzoxazone, a BKCa channel agonist, in a mouse model of Fragile X syndrome. bioRxiv 2020. preprint. [Google Scholar] [CrossRef]
- Zhang, Y.; Bonnan, A.; Bony, G.; Ferezou, I.; Pietropaolo, S.; Ginger, M.; Sans, N.; Rossier, J.; Oostra, B.; LeMasson, G.; et al. Dendritic channelopathies contribute to neocortical and sensory hyperexcitability in Fmr1−/y mice. Nat. Neurosci. 2014, 17, 1701–1709. [Google Scholar] [CrossRef] [PubMed]
- Consorthium, The Dutch-Belgian Fragile X; Bakker, C.E.; Verheij, C.; Willemsen, R.; van der Helm, R.; Oerlemans, F.; Vermey, M.; Bygrave, A.; Hoogeveen, A.; Oostra, B.A. Fmr1 knockout mice: A model to study fragile X mental retardation. Cell 1994, 78, 23–33. [Google Scholar]
- Kat, R.; Arroyo-Araujo, M.; de Vries, R.B.; Koopmans, M.A.; de Boer, S.F.; Kas, M.J. Translational validity and methodological underreporting in animal research: A systematic review and meta-analysis of the Fragile X syndrome (Fmr1 KO) rodent model. Neurosci. Biobehav. Rev. 2022, 139, 104722. [Google Scholar] [CrossRef] [PubMed]
- Gaudissard, J.; Ginger, M.; Premoli, M.; Memo, M.; Frick, A.; Pietropaolo, S. Behavioral abnormalities in the Fmr1-KO2 mouse model of fragile X syndrome: The relevance of early life phases. Autism Res. 2017, 10, 1584–1596. [Google Scholar] [CrossRef]
- Gauducheau, M.; Lemaire-Mayo, V.; D’Amato, F.R.; Oddi, D.; Crusio, W.E.; Pietropaolo, S. Age-specific autistic-like behaviors in heterozygous Fmr1-KO female mice. Autism Res. 2017, 10, 1067–1078. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Sethna, F.; Wang, H. Behavioral analysis of male and female Fmr1 knockout mice on C57BL/6 background. Behav. Brain Res. 2014, 271, 72–78. [Google Scholar] [CrossRef]
- Frankland, P.W.; Wang, Y.; Rosner, B.; Shimizu, T.; Balleine, B.W.; Dykens, E.M.; Ornitz, E.M.; Silva, A.J. Sensorimotor gating abnormalities in young males with fragile X syndrome and Fmr1-knockout mice. Mol. Psychiatry 2004, 9, 417–425. [Google Scholar] [CrossRef]
- Olmos-Serrano, J.L.; Corbin, J.G.; Burns, M.P. The GABA(A) receptor agonist THIP ameliorates specific behavioral deficits in the mouse model of fragile X syndrome. Dev. Neurosci. 2011, 33, 395–403. [Google Scholar] [CrossRef]
- Michalon, A.; Sidorov, M.; Ballard, T.M.; Ozmen, L.; Spooren, W.; Wettstein, J.G.; Jaeschke, G.; Bear, M.F.; Lindemann, L. Chronic pharmacological mGlu5 inhibition corrects fragile X in adult mice. Neuron 2012, 74, 49–56. [Google Scholar] [CrossRef]
- Nielsen, D.M.; Derber, W.J.; McClellan, D.A.; Crnic, L.S. Alterations in the auditory startle response in Fmr1 targeted mutant mouse models of fragile X syndrome. Brain Res. 2002, 927, 8–17. [Google Scholar] [CrossRef]
- El-Hassar, L.; Song, L.; Tan, W.J.; Large, C.H.; Alvaro, G.; Santos-Sacchi, J.; Kaczmarek, L.K. Modulators of Kv3 Potassium Channels Rescue the Auditory Function of Fragile X Mice. J. Neurosci. 2019, 39, 4797–4813. [Google Scholar] [CrossRef] [PubMed]
- Rotschafer, S.E.; Marshak, S.; Cramer, K.S. Deletion of Fmr1 alters function and synaptic inputs in the auditory brainstem. PLoS ONE 2015, 10, e0117266. [Google Scholar] [CrossRef] [PubMed]
- Pietropaolo, S.; Guilleminot, A.; Martin, B.; D’Amato, F.R.; Crusio, W.E. Genetic-background modulation of core and variable autistic-like symptoms in Fmr1 knock-out mice. PLoS ONE 2011, 6, e17073. [Google Scholar] [CrossRef] [PubMed]
- Spencer, C.M.; Alekseyenko, O.; Hamilton, S.M.; Thomas, A.M.; Serysheva, E.; Yuva-Paylor, L.A.; Paylor, R. Modifying behavioral phenotypes in Fmr1KO mice: Genetic background differences reveal autistic-like responses. Autism Res. 2011, 4, 40–56. [Google Scholar] [CrossRef]
- Johnson, K.R.; Erway, L.C.; Cook, S.A.; Willott, J.F.; Zheng, Q.Y. A major gene affecting age-related hearing loss in C57BL/6J mice. Hear. Res. 1997, 114, 83–92. [Google Scholar] [CrossRef]
- Noben-Trauth, K.; Zheng, Q.Y.; Johnson, K.R. Association of cadherin 23 with polygenic inheritance and genetic modification of sensorineural hearing loss. Nat. Genet. 2003, 35, 21–23. [Google Scholar] [CrossRef]
- Chen, L.; Toth, M. Fragile X mice develop sensory hyperreactivity to auditory stimuli. Neuroscience 2001, 103, 1043–1050. [Google Scholar] [CrossRef]
- Garcia-Pino, E.; Gessele, N.; Koch, U. Enhanced Excitatory Connectivity and Disturbed Sound Processing in the Auditory Brainstem of Fragile X Mice. J. Neurosci. 2017, 37, 7403–7419. [Google Scholar] [CrossRef]
- Kim, H.; Gibboni, R.; Kirkhart, C.; Bao, S. Impaired critical period plasticity in primary auditory cortex of fragile X model mice. J. Neurosci. 2013, 33, 15686–15692. [Google Scholar] [CrossRef]
- Razak, K.A.; Binder, D.K.; Ethell, I.M. Neural Correlates of Auditory Hypersensitivity in Fragile X Syndrome. Front. Psychiatry 2021, 12, 720752. [Google Scholar] [CrossRef] [PubMed]
- Rotschafer, S.; Razak, K. Altered auditory processing in a mouse model of fragile X syndrome. Brain Res. 2013, 1506, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Rotschafer, S.E.; Cramer, K.S. Developmental Emergence of Phenotypes in the Auditory Brainstem Nuclei of Fmr1 Knockout Mice. eNeuro 2017, 4, ENEURO.0264-17.2017. [Google Scholar] [CrossRef]
- Lee, U.S.; Cui, J. BK channel activation: Structural and functional insights. Trends Neurosci. 2010, 33, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, F.; Konigstorfer, A.; Sudhof, T.C. RIBEYE, a component of synaptic ribbons: A protein’s journey through evolution provides insight into synaptic ribbon function. Neuron 2000, 28, 857–872. [Google Scholar] [CrossRef]
- Musumeci, S.A.; Bosco, P.; Calabrese, G.; Bakker, C.; Sarro, G.B.; Elia, M.; Ferri, R.; Oostra, B.A. Audiogenic seizures susceptibility in transgenic mice with fragile X syndrome. Epilepsia 2000, 41, 19–23. [Google Scholar] [CrossRef]
- Petroni, V.; Subashi, E.; Premoli, M.; Memo, M.; Lemaire, V.; Pietropaolo, S. Long-term behavioral effects of prenatal stress in the Fmr1-knock-out mouse model for fragile X syndrome. Front. Cell. Neurosci. 2022, 16, 917183. [Google Scholar] [CrossRef]
- Sergeyenko, Y.; Lall, K.; Liberman, M.C.; Kujawa, S.G. Age-related cochlear synaptopathy: An early-onset contributor to auditory functional decline. J. Neurosci. 2013, 33, 13686–13694. [Google Scholar] [CrossRef]
- Deng, P.Y.; Klyachko, V.A. Genetic upregulation of BK channel activity normalizes multiple synaptic and circuit defects in a mouse model of fragile X syndrome. J. Physiol. 2016, 594, 83–97. [Google Scholar] [CrossRef]
- Deng, P.-Y.; Rotman, Z.; Blundon, J.A.; Cho, Y.; Cui, J.; Cavalli, V.; Zakharenko, S.S.; Klyachko, V.A. FMRP regulates neurotransmitter release and synaptic information transmission by modulating action potential duration via BK channels. Neuron 2013, 77, 696–711. [Google Scholar] [CrossRef]
- Kshatri, A.; Cerrada, A.; Gimeno, R.; Bartolomé-Martín, D.; Rojas, P.; Giraldez, T. Differential regulation of BK channels by fragile X mental retardation protein. J. Gen. Physiol. 2020, 152, e201912502. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Coghill, L.S.; MacDonald, S.H.F.; Armstrong, D.L.; Shipston, M.J. Leucine zipper domain targets cAMP-dependent protein kinase to mammalian BK channels. J. Biol. Chem. 2003, 278, 8669–8677. [Google Scholar] [CrossRef] [PubMed]
- Van Welie, I.; du Lac, S. Bidirectional control of BK channel open probability by CAMKII and PKC in medial vestibular nucleus neurons. J. Neurophysiol. 2011, 105, 1651–1659. [Google Scholar] [CrossRef]
- Wang, Z.W. Regulation of synaptic transmission by presynaptic CaMKII and BK channels. Mol. Neurobiol. 2008, 38, 153–166. [Google Scholar] [CrossRef]
- Darnell, J.C.; Van Driesche, S.J.; Zhang, C.; Hung, K.Y.S.; Mele, A.; Fraser, C.E.; Stone, E.F.; Chen, C.; Fak, J.J.; Chi, S.W.; et al. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell 2011, 146, 247–261. [Google Scholar] [CrossRef]
- Brown, M.R.; Kaczmarek, L.K. Potassium channel modulation and auditory processing. Hear. Res. 2011, 279, 32–42. [Google Scholar] [CrossRef]
- Eatock, R.A. Auditory physiology: Listening with K+ channels. Curr. Biol. 2003, 13, R767–R769. [Google Scholar] [CrossRef]
- Adusei, D.C.; Pacey, L.K.; Chen, D.; Hampson, D.R. Early developmental alterations in GABAergic protein expression in fragile X knockout mice. Neuropharmacology 2010, 59, 167–171. [Google Scholar] [CrossRef]
- Oliver, D.; Taberner, A.M.; Thurm, H.; Sausbier, M.; Arntz, C.; Ruth, P.; Fakler, B.; Liberman, M.C. The role of BKCa channels in electrical signal encoding in the mammalian auditory periphery. J. Neurosci. 2006, 26, 6181–6189. [Google Scholar] [CrossRef] [PubMed]
- Oddi, D.; Subashi, E.; Middei, S.; Bellocchio, L.; Lemaire-Mayo, V.; Guzmán, M.; Crusio, W.E.; D’Amato, F.R.; Pietropaolo, S. Early social enrichment rescues adult behavioral and brain abnormalities in a mouse model of fragile X syndrome. Neuropsychopharmacology 2015, 40, 1113–1122. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferraguto, C.; Bouleau, Y.; Peineau, T.; Dulon, D.; Pietropaolo, S. Hyperacusis in the Adult Fmr1-KO Mouse Model of Fragile X Syndrome: The Therapeutic Relevance of Cochlear Alterations and BKCa Channels. Int. J. Mol. Sci. 2023, 24, 11863. https://doi.org/10.3390/ijms241411863
Ferraguto C, Bouleau Y, Peineau T, Dulon D, Pietropaolo S. Hyperacusis in the Adult Fmr1-KO Mouse Model of Fragile X Syndrome: The Therapeutic Relevance of Cochlear Alterations and BKCa Channels. International Journal of Molecular Sciences. 2023; 24(14):11863. https://doi.org/10.3390/ijms241411863
Chicago/Turabian StyleFerraguto, Celeste, Yohan Bouleau, Thibault Peineau, Didier Dulon, and Susanna Pietropaolo. 2023. "Hyperacusis in the Adult Fmr1-KO Mouse Model of Fragile X Syndrome: The Therapeutic Relevance of Cochlear Alterations and BKCa Channels" International Journal of Molecular Sciences 24, no. 14: 11863. https://doi.org/10.3390/ijms241411863
APA StyleFerraguto, C., Bouleau, Y., Peineau, T., Dulon, D., & Pietropaolo, S. (2023). Hyperacusis in the Adult Fmr1-KO Mouse Model of Fragile X Syndrome: The Therapeutic Relevance of Cochlear Alterations and BKCa Channels. International Journal of Molecular Sciences, 24(14), 11863. https://doi.org/10.3390/ijms241411863





