A New Role for Yeast Cells in Health and Nutrition: Antioxidant Power Assessment
Abstract
1. Introduction
2. Results
2.1. AOPY: A Transposition of the AOP1 Live Cell Assay to Yeast
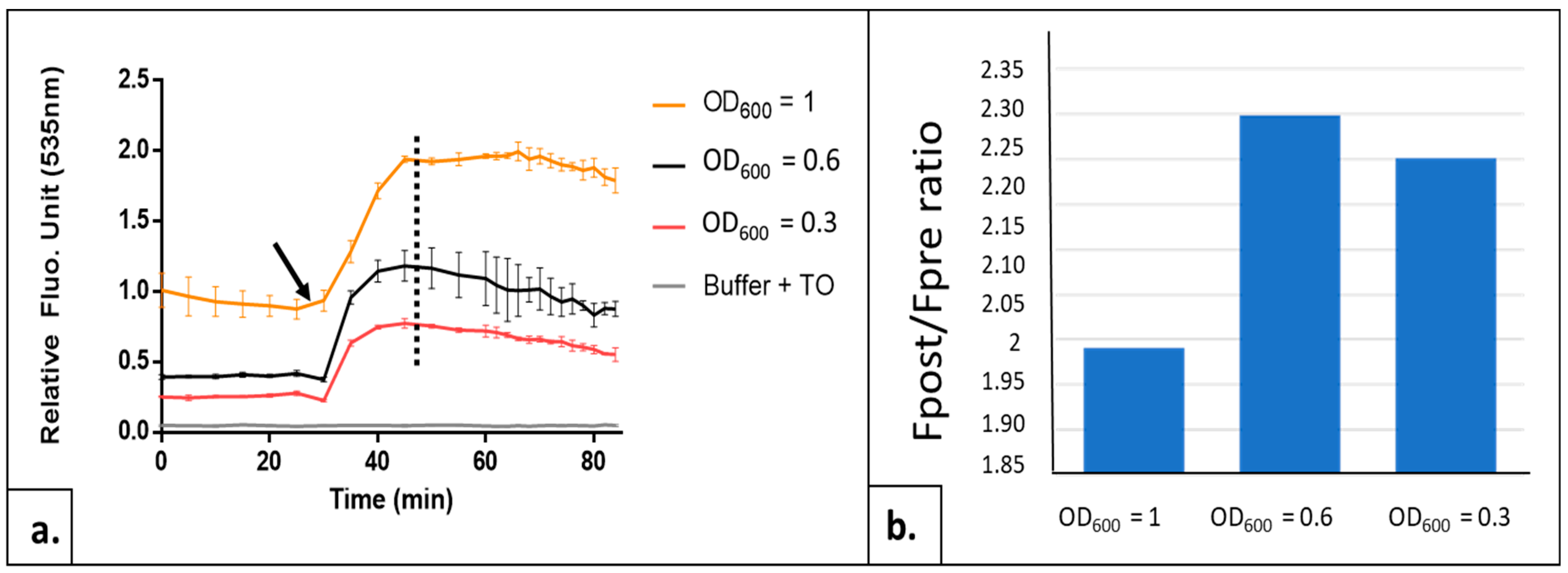
2.1.1. Adjusting the Yeast Cell Density Parameter
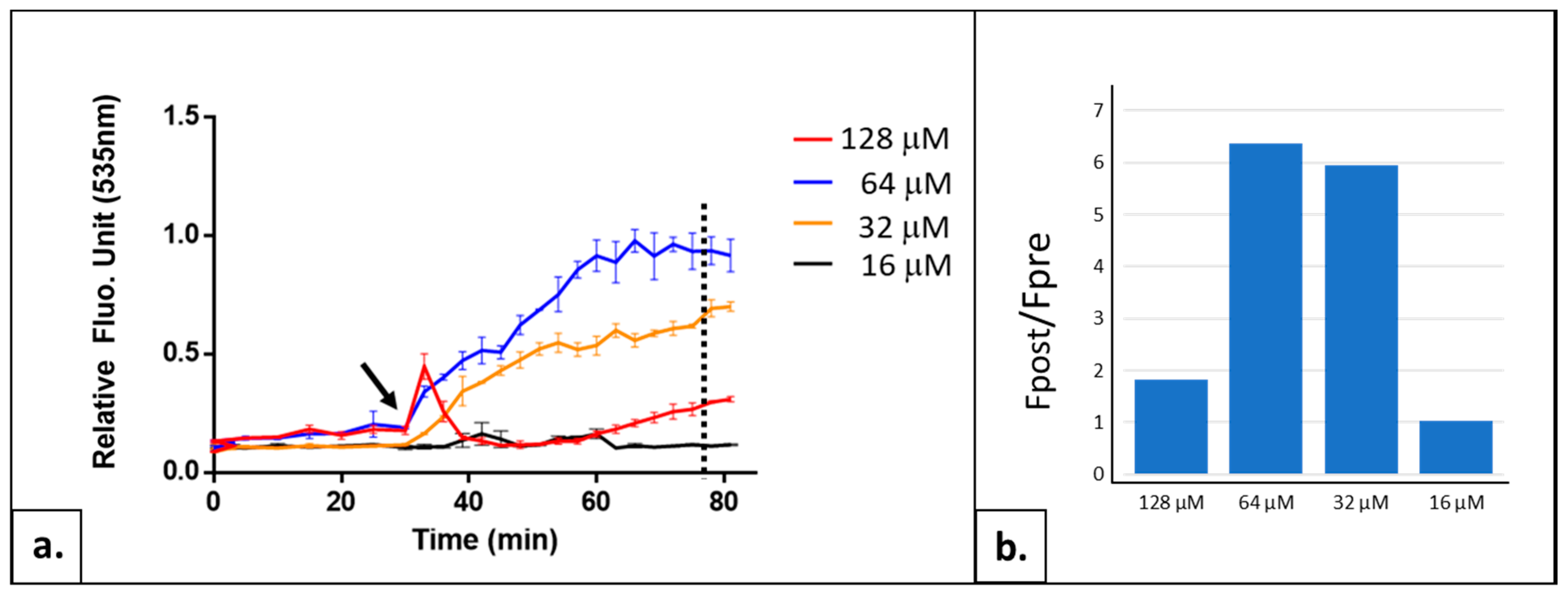
2.1.2. Adjusting the Thiazole Orange Concentration
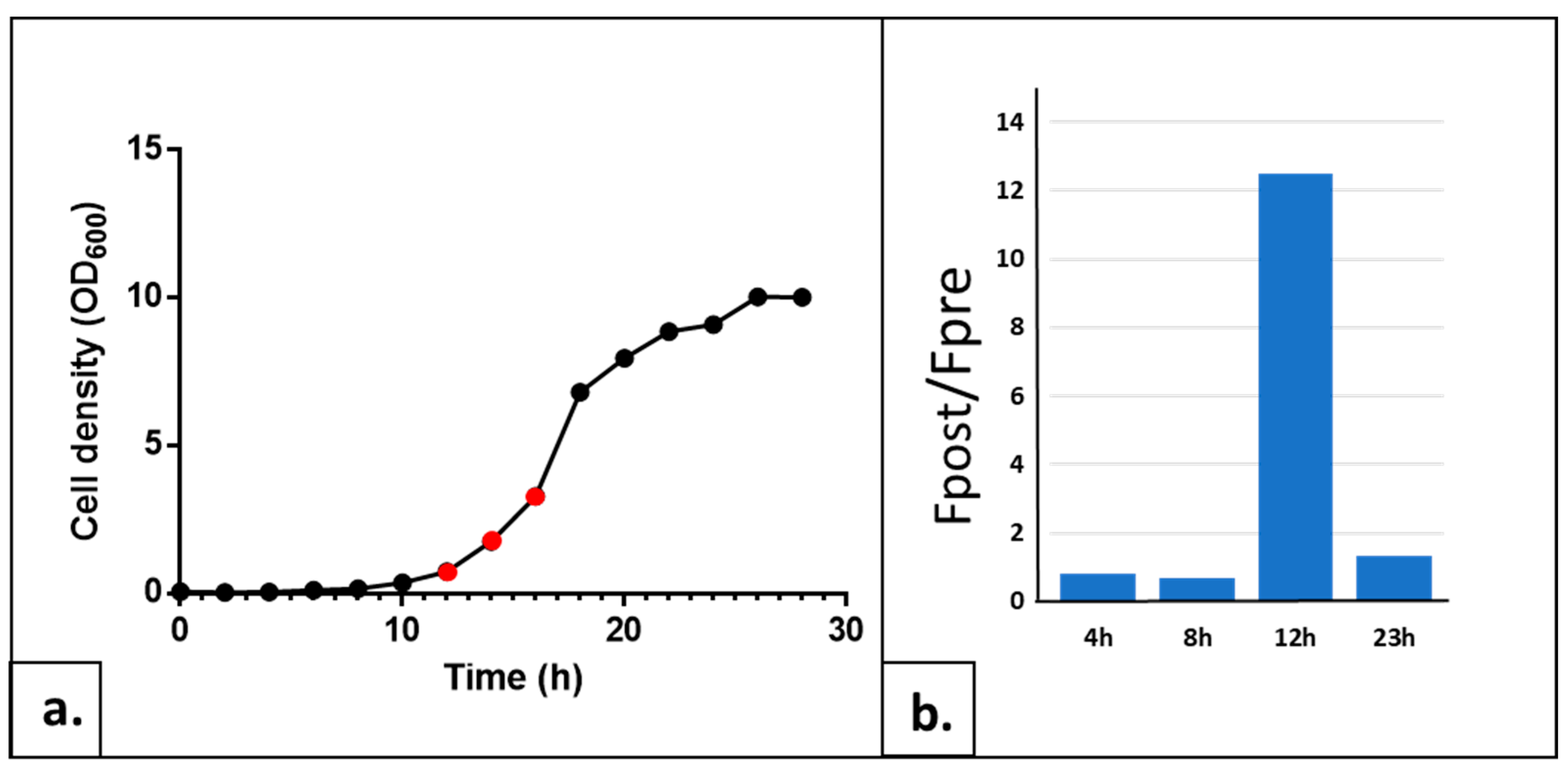
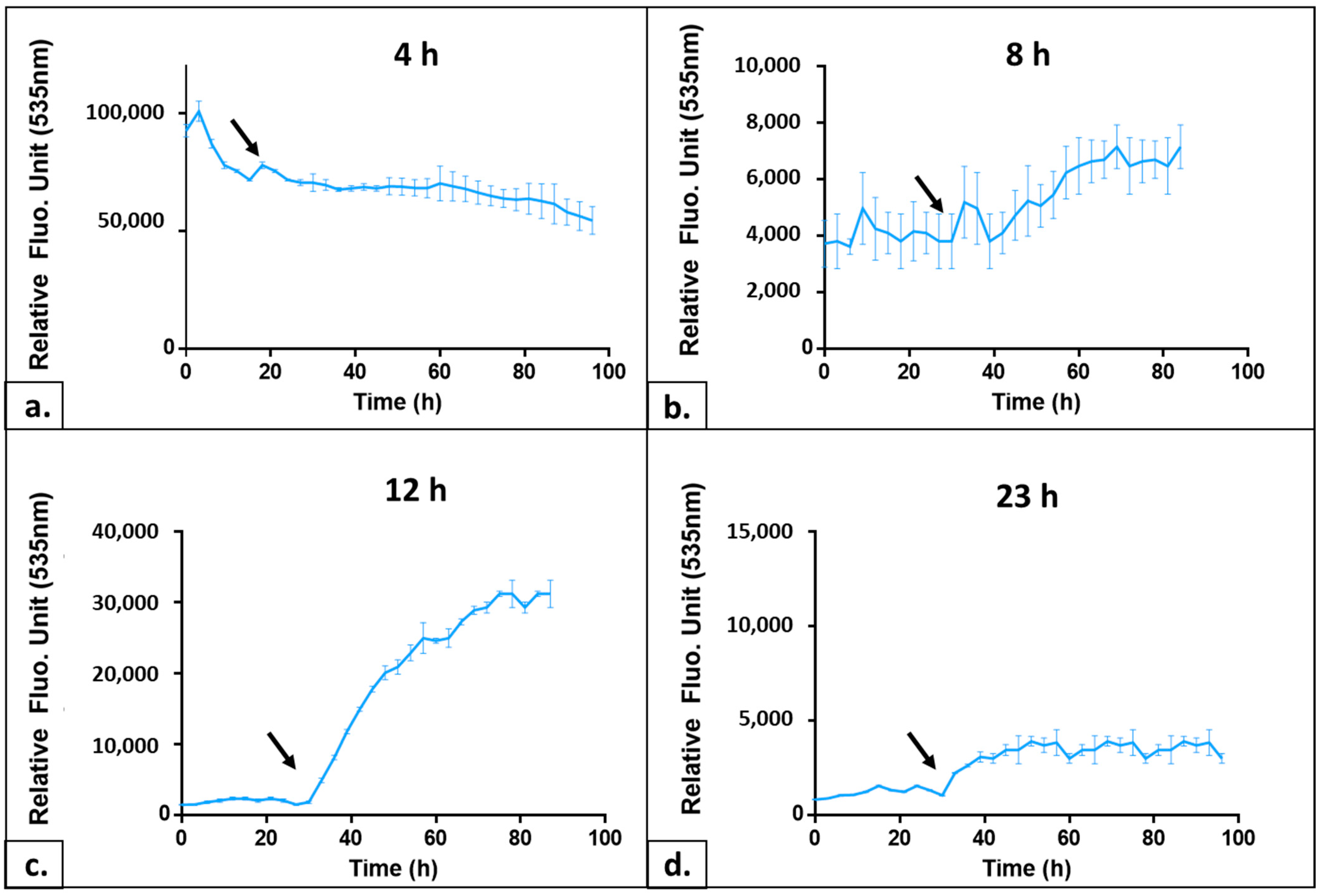
2.1.3. Determining the Optimal Growth Phase of the Yeast Culture
2.2. Testing the AOPY Response to Externally Added or Internally Produced Antioxidants
2.2.1. Testing the Response to Soluble Molecules Added to the Medium
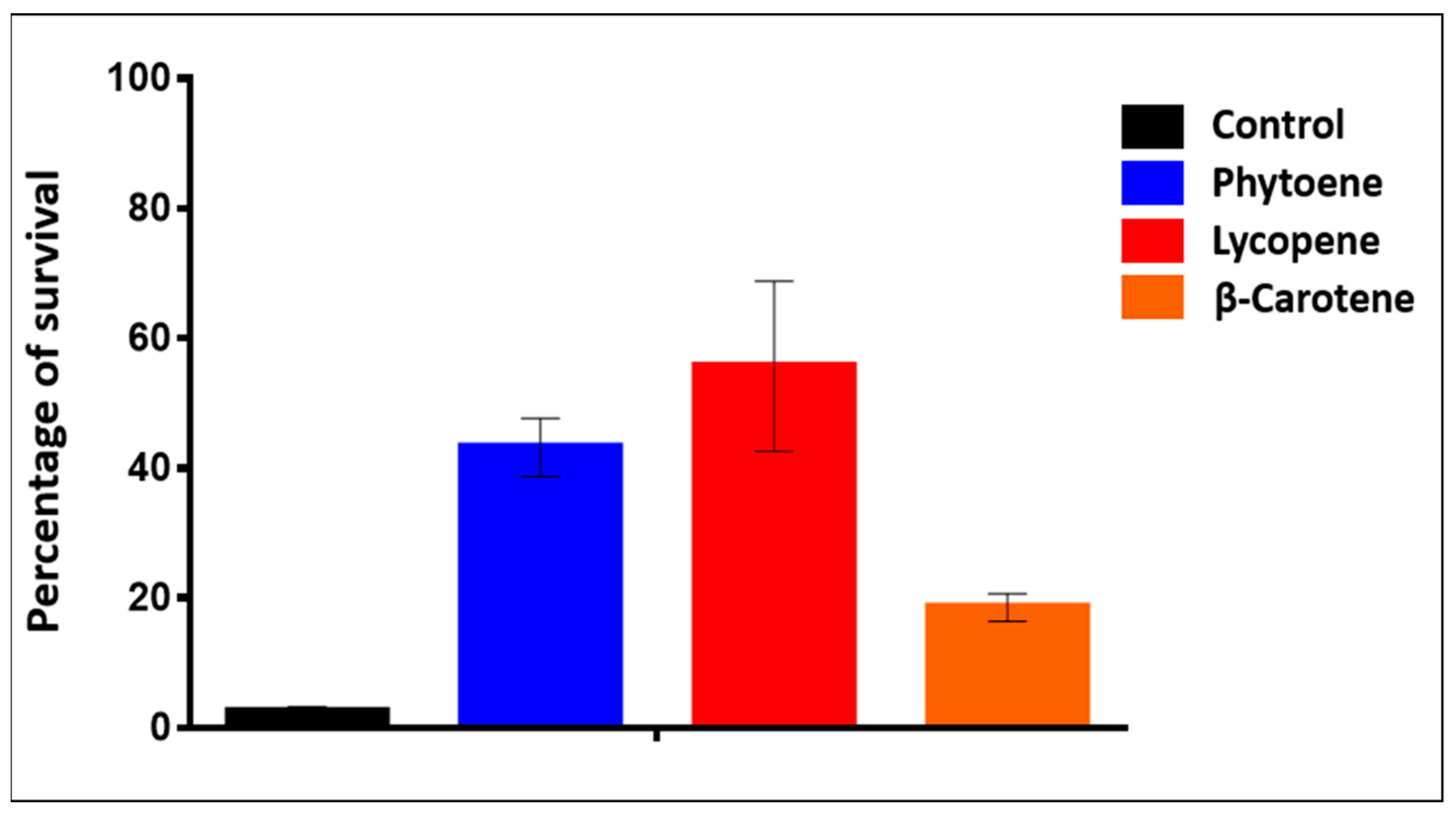
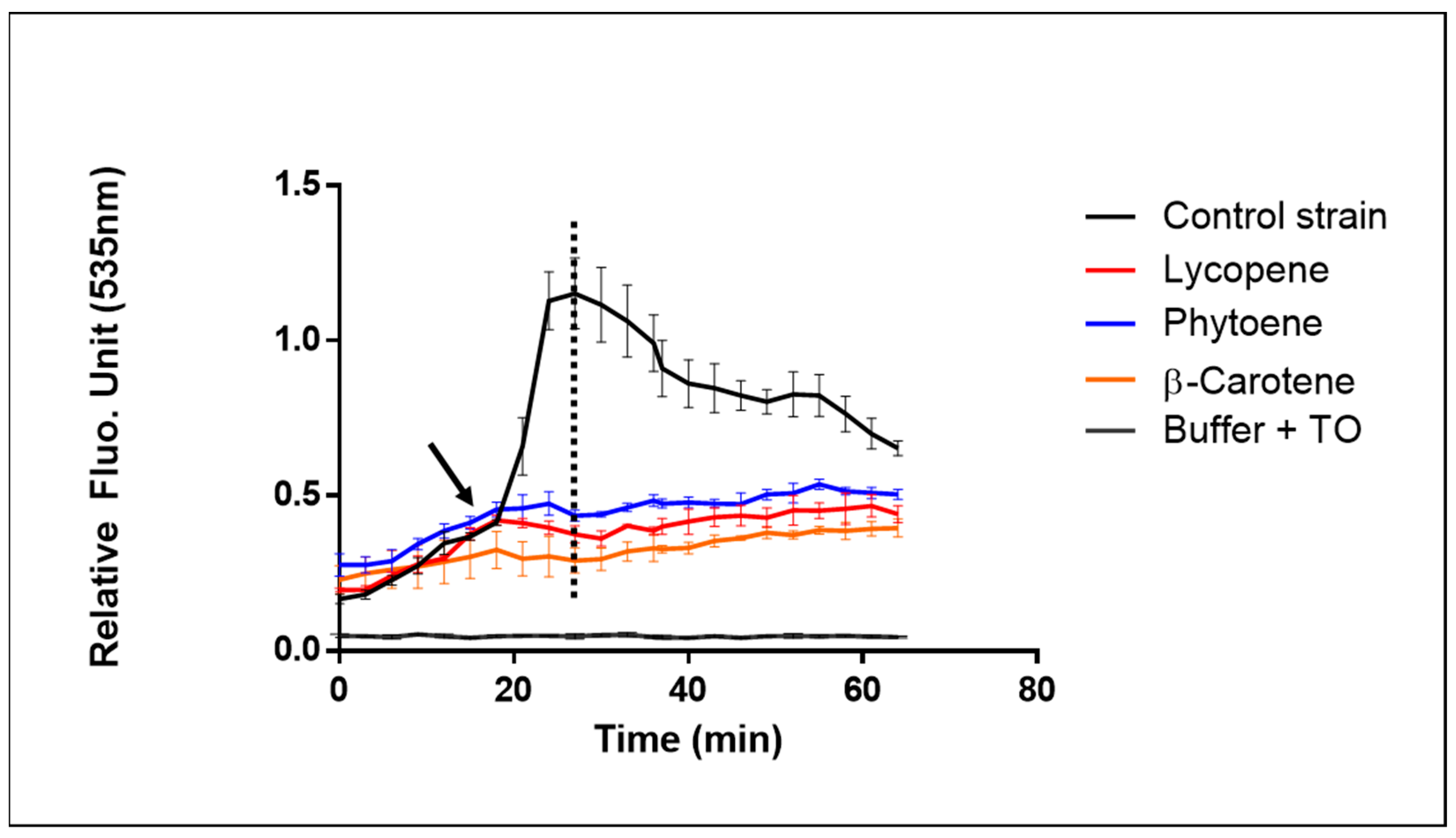
2.2.2. Testing the Response to Internally Produced Antioxidant Molecules
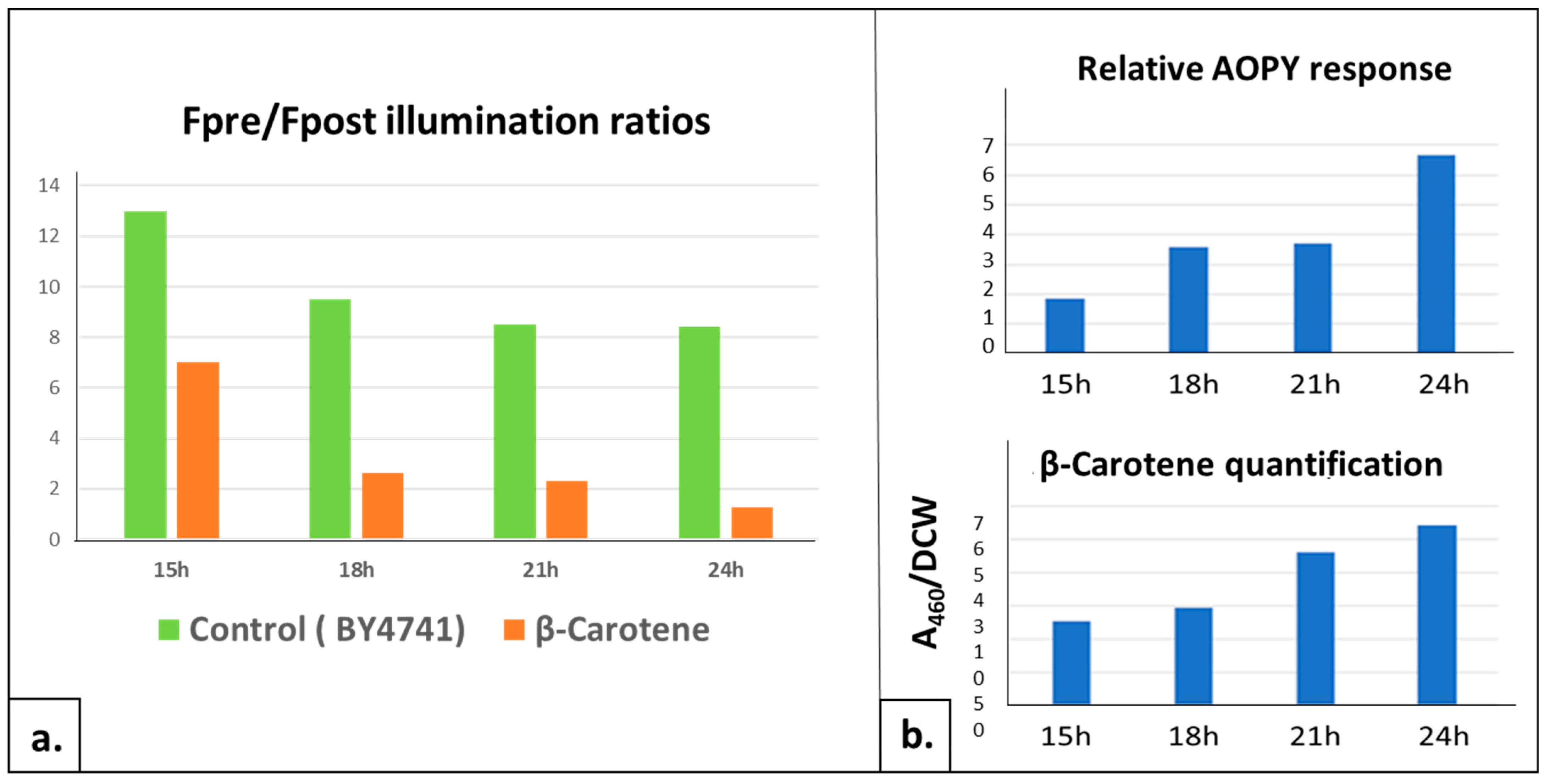
2.2.3. Quantitative Detection of Intracellularly Produced Carotenoids
3. Discussion
4. Materials and Methods
4.1. Yeast Strains Used in This Study
4.2. Yeast AOP Assay Experimental Protocol
4.2.1. AOPY Protocol Optimization
4.2.2. Test of Antioxidant Molecules with AOPY Protocol
4.3. UV Detection of Carotenoids
4.4. Viability Tests
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Derick, S.; Gironde, C.; Perio, P.; Reybier, K.; Nepveu, F.; Jauneau, A.; Furger, C. LUCS (Light-Up Cell System), a universal high throughput assay for homeostasis evaluation in live cells. Sci. Rep. 2017, 7, 18069. [Google Scholar] [CrossRef] [PubMed]
- Gironde, C.; Rigal, M.; Dufour, C.; Furger, C. AOP1, a new live cell assay for the direct and quantitative measure of intracellular antioxidant effects. Antioxidants 2020, 9, 471. [Google Scholar] [CrossRef] [PubMed]
- Furger, C.; Gironde, C.; Rigal, M.; Dufour, C.; Guillemet, D. Cell-based antioxidant properties and synergistic effects of natural plant and algal extracts pre and post intestinal barrier transport. Antioxidants 2022, 11, 565. [Google Scholar] [CrossRef] [PubMed]
- Dufour, C.; Villa-Rodriguez, J.A.; Furger, C.; Lessard-Lord, J.; Gironde, C.; Rigal, M.; Badr, A.; Desjardins, Y.; Guyonnet, D. Cellular antioxidant effect of an aronia extract and its polyphenolic fractions enriched in proanthocyanidins, phenolic acids, and anthocyanins. Antioxidants 2022, 11, 1561. [Google Scholar] [CrossRef] [PubMed]
- Ostergaard, S.; Olsson, L.; Nielsen, J. Metabolic engineering of Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2000, 64, 34–50. [Google Scholar] [CrossRef]
- Lian, J.; Mishra, S.; Zhao, H. Recent advances in metabolic engineering of Saccharomyces cerevisiae: New tools and their applications. Metab. Eng. 2018, 50, 85–108. [Google Scholar] [CrossRef]
- Jensen, M.K.; Keasling, J.D. Recent applications of synthetic biology tools for yeast metabolic engineering. FEMS Yeast Res. 2014, 15, 1–10. [Google Scholar] [CrossRef]
- Baptista, S.L.; Costa, C.E.; Cunha, J.T.; Soares, P.O.; Domingues, L. Metabolic engineering of Saccharomyces cerevisiae for the production of top value chemicals from biorefinery carbohydrates. Biotechnol. Adv. 2021, 47, 107697. [Google Scholar] [CrossRef]
- Suss, O.; Motiei, L.; Margulies, D. Broad applications of thiazole orange in fluorescent sensing of biomolecules and ions. Molecules 2021, 26, 2828. [Google Scholar] [CrossRef]
- Palková, Z.; Wilkinson, D.; Váchová, L. Aging and differentiation in yeast populations: Elders with different properties and functions. FEMS Yeast Res. 2014, 14, 96–108. [Google Scholar] [CrossRef]
- Hohmann, S.; Mager, W.H. Yeast Stress Responses; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Elliott, B.; Futcher, B. Stress resistance of yeast cells is largely independent of cell cycle phase. Yeast 1993, 9, 33–42. [Google Scholar] [CrossRef]
- Cabrera, E.; Welch, L.C.; Robinson, M.R.; Sturgeon, C.M.; Crow, M.M.; Segarra, V.A. Cryopreservation and the freeze–thaw stress response in yeast. Genes 2020, 11, 835. [Google Scholar] [CrossRef]
- Galiniak, S.; Aebisher, D.; Bartusik-Aebisher, D. Health benefits of resveratrol administration. Acta Biochim. Pol. 2019, 66, 13–21. [Google Scholar] [CrossRef]
- Alam, M.; Ali, S.; Ashraf, G.M.; Bilgrami, A.L.; Yadav, D.K.; Hassan, M.I. Epigallocatechin 3-gallate: From green tea to cancer therapeutics. Food Chem. 2022, 379, 132135. [Google Scholar] [CrossRef]
- D’Andrea, G. Quercetin: A Flavonol with multifaceted therapeutic applications? Fitoterapia 2015, 106, 256–271. [Google Scholar] [CrossRef]
- Davinelli, S.; Nielsen, M.E.; Scapagnini, G. Astaxanthin in skin health, repair, and disease: A comprehensive review. Nutrients 2018, 10, 522. [Google Scholar] [CrossRef]
- Carotenoids: Physical, Chemical, and Biological Functions and Properties; Landrum, J.T., Ed.; CRC Press: Boca Raton, FL, USA, 2009; ISBN 978-0-429-15048-7. [Google Scholar]
- Pérez-Gálvez, A.; Viera, I.; Roca, M. Carotenoids and chlorophylls as antioxidants. Antioxidants 2020, 9, 505. [Google Scholar] [CrossRef]
- Francenia Santos-Sánchez, N.; Salas-Coronado, R.; Villanueva-Cañongo, C.; Hernández-Carlos, B. Antioxidant compounds and their antioxidant mechanism. In Antioxidants; Shalaby, E., Ed.; IntechOpen: London, UK, 2019; ISBN 978-1-78923-919-5. [Google Scholar]
- Yamada, R.; Ando, Y.; Mitsui, R.; Mizobata, A.; Yoshihara, S.; Tokumoto, H.; Matsumoto, T.; Ogino, H. Improving carotenoid production in recombinant yeast, Saccharomyces cerevisiae, using ultrasound-irradiated two-phase extractive fermentation. Eng. Life Sci. 2022, 22, 4–12. [Google Scholar] [CrossRef]
- Reyes, L.H.; Gomez, J.M.; Kao, K.C. Improving carotenoids production in yeast via adaptive laboratory evolution. Metab. Eng. 2014, 21, 26–33. [Google Scholar] [CrossRef]
- Jin, J.; Wang, Y.; Yao, M.; Gu, X.; Li, B.; Liu, H.; Ding, M.; Xiao, W.; Yuan, Y. Astaxanthin overproduction in yeast by strain engineering and new gene target uncovering. Biotechnol. Biofuels 2018, 11, 230. [Google Scholar] [CrossRef]
- Verwaal, R.; Jiang, Y.; Wang, J.; Daran, J.-M.; Sandmann, G.; van den Berg, J.A.; van Ooyen, A.J.J. Heterologous carotenoid production in Saccharomyces cerevisiae induces the pleiotropic drug resistance stress response. Yeast 2010, 27, 983–998. [Google Scholar] [CrossRef] [PubMed]
- Rabeharindranto, H.; Castaño-Cerezo, S.; Lautier, T.; Garcia-Alles, L.F.; Treitz, C.; Tholey, A.; Truan, G. Enzyme-fusion strategies for redirecting and improving carotenoid synthesis in S. cerevisiae. Metab. Eng. Commun. 2019, 8, e00086. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Song, D.; Zhu, H. Metabolic engineering of Saccharomyces cerevisiae for enhanced carotenoid production from xylose-glucose mixtures. Front. Bioeng. Biotechnol. 2020, 8, 435. [Google Scholar] [CrossRef] [PubMed]
- Miazek, K.; Beton, K.; Śliwińska, A.; Brożek-Płuska, B. The effect of β-carotene, tocopherols and ascorbic acid as anti-oxidant molecules on human and animal in vitro/in vivo studies: A review of research design and analytical techniques used. Biomolecules 2022, 12, 1087. [Google Scholar] [CrossRef] [PubMed]
- Perrone, G.G.; Tan, S.-X.; Dawes, I.W. Reactive oxygen species and yeast apoptosis. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2008, 1783, 1354–1368. [Google Scholar] [CrossRef] [PubMed]
- Van der Laan, K.J.; Morita, A.; Perona-Martinez, F.P.; Schirhagl, R. Evaluation of the oxidative stress response of aging yeast cells in response to internalization of fluorescent nanodiamond biosensors. Nanomaterials 2020, 10, 372. [Google Scholar] [CrossRef]
- Pawelczak, P.; Fedoruk-Wyszomirska, A.; Wyszko, E. antiaging effect of 4-n-furfurylcytosine in yeast model manifests through enhancement of mitochondrial activity and ROS reduction. Antioxidants 2022, 11, 850. [Google Scholar] [CrossRef]
- Koziol, S.; Zagulski, M.; Bilinski, T.; Bartosz, G. Antioxidants protect the yeast Saccharomyces cerevisiae against hypertonic stress. Free Radic. Res. 2005, 39, 365–371. [Google Scholar] [CrossRef]
- James, J.; Fiji, N.; Roy, D.; Andrew MG, D.; Shihabudeen, M.S.; Chattopadhyay, D.; Thirumurugan, K. A Rapid method to assess reactive oxygen species in yeast using H2 DCF-DA. Anal. Methods 2015, 7, 8572–8575. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Liu, R.H. Cellular Antioxidant Activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J. Agric. Food Chem. 2007, 55, 8896–8907. [Google Scholar] [CrossRef]
- Ouyang, X.; Tran, Q.T.; Goodwin, S.; Wible, R.S.; Sutter, C.H.; Sutter, T.R. Yap1 activation by H2O2 or thiol-reactive chemicals elicits distinct adaptive gene responses. Free Radic. Biol. Med. 2011, 50, 1–13. [Google Scholar] [CrossRef]
- Zahoor, H.; Watchaputi, K.; Hata, J.; Pabuprapap, W.; Suksamrarn, A.; Chua, L.S.; Soontorngun, N. Model yeast as a versatile tool to examine the antioxidant and anti-ageing potential of flavonoids, extracted from medicinal plants. Front. Pharmacol. 2022, 13, 980066. [Google Scholar] [CrossRef]
- Wu, M.J.; O’Doherty, P.J.; Fernandez, H.R.; Lyons, V.; Rogers, P.J.; Dawes, I.W.; Higgins, V.J. An antioxidant screening assay based on oxidant-induced growth arrest in Saccharomyces cerevisiae: Antioxidant assay based on oxidant-induced growth arrest. FEMS Yeast Res. 2011, 11, 379–387. [Google Scholar] [CrossRef]
- Li, T.; Liu, G.-S.; Zhou, W.; Jiang, M.; Ren, Y.-H.; Tao, X.-Y.; Liu, M.; Zhao, M.; Wang, F.-Q.; Gao, B.; et al. Metabolic engineering of Saccharomyces cerevisiae to overproduce squalene. J. Agric. Food Chem. 2020, 68, 2132–2138. [Google Scholar] [CrossRef]
- Cataldo, V.F.; Arenas, N.; Salgado, V.; Camilo, C.; Ibáñez, F.; Agosin, E. Heterologous production of the epoxycarotenoid violaxanthin in Saccharomyces cerevisiae. Metab. Eng. 2020, 59, 53–63. [Google Scholar] [CrossRef]
- Lyu, X.; Zhao, G.; Ng, K.R.; Mark, R.; Chen, W.N. Metabolic engineering of Saccharomyces cerevisiae for de novo production of kaempferol. J. Agric. Food Chem. 2019, 67, 5596–5606. [Google Scholar] [CrossRef]
- Promdonkoy, P.; Sornlek, W.; Preechakul, T.; Tanapongpipat, S.; Runguphan, W. Metabolic engineering of Saccharomyces cerevisiae for production of fragrant terpenoids from agarwood and sandalwood. Fermentation 2022, 8, 429. [Google Scholar] [CrossRef]
- Šovljanski, O.; Saveljić, A.; Tomić, A.; Šeregelj, V.; Lončar, B.; Cvetković, D.; Ranitović, A.; Pezo, L.; Ćetković, G.; Markov, S.; et al. Carotenoid-producing yeasts: Selection of the best-performing strain and the total carotenoid extraction procedure. Processes 2022, 10, 1699. [Google Scholar] [CrossRef]
- Scott, K.J. Detection and measurement of carotenoids by UV/VIS spectrophotometry. Curr. Protoc. Food Anal. Chem. 2001, F2.2.1–F2.2.10. [Google Scholar] [CrossRef]
- Popescu, M.; Iancu, P.; Pleșu, V.; Bîldea, C.S.; Todasca, C.M. Different spectrophotometric methods for simultaneous quantification of lycopene and β-carotene from a binary mixture. LWT 2022, 160, 113238. [Google Scholar] [CrossRef]
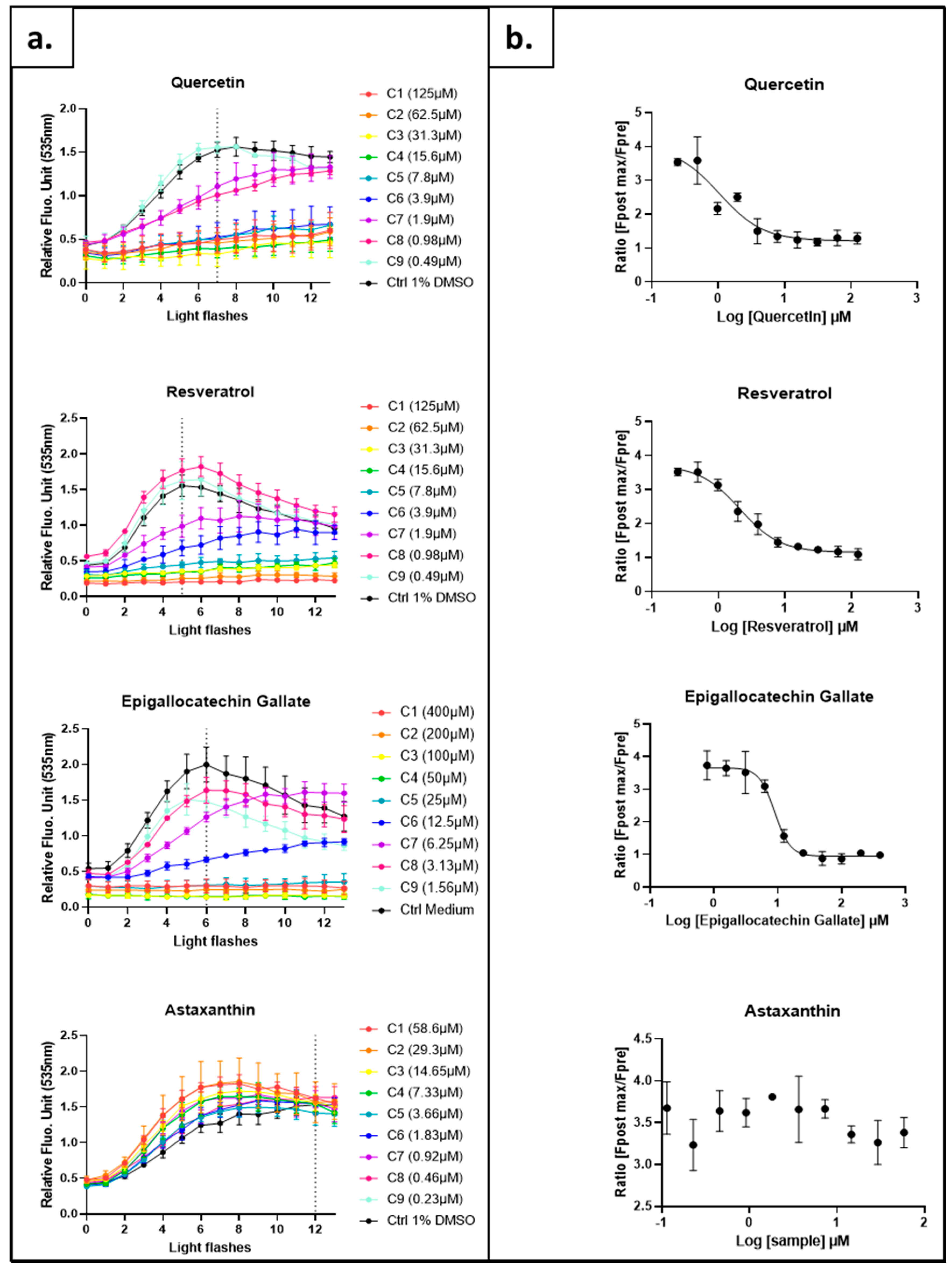
| Compound | EC50 (µM) | CI 95% (µM) | R2 |
|---|---|---|---|
| Quercetin | 1.041 | 0.02520 to 1.961 | 0.86 |
| Resveratrol | 2.086 | 1.529 to 2.680 | 0.97 |
| Epigallocatechin Gallate | 8.962 | 7.711 to 10.40 | 0.96 |
| Astaxanthin | ND | ND | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gosselin-Monplaisir, T.; Dagkesamanskaya, A.; Rigal, M.; Floch, A.; Furger, C.; Martin-Yken, H. A New Role for Yeast Cells in Health and Nutrition: Antioxidant Power Assessment. Int. J. Mol. Sci. 2023, 24, 11800. https://doi.org/10.3390/ijms241411800
Gosselin-Monplaisir T, Dagkesamanskaya A, Rigal M, Floch A, Furger C, Martin-Yken H. A New Role for Yeast Cells in Health and Nutrition: Antioxidant Power Assessment. International Journal of Molecular Sciences. 2023; 24(14):11800. https://doi.org/10.3390/ijms241411800
Chicago/Turabian StyleGosselin-Monplaisir, Thomas, Adilya Dagkesamanskaya, Mylène Rigal, Aurélie Floch, Christophe Furger, and Hélène Martin-Yken. 2023. "A New Role for Yeast Cells in Health and Nutrition: Antioxidant Power Assessment" International Journal of Molecular Sciences 24, no. 14: 11800. https://doi.org/10.3390/ijms241411800
APA StyleGosselin-Monplaisir, T., Dagkesamanskaya, A., Rigal, M., Floch, A., Furger, C., & Martin-Yken, H. (2023). A New Role for Yeast Cells in Health and Nutrition: Antioxidant Power Assessment. International Journal of Molecular Sciences, 24(14), 11800. https://doi.org/10.3390/ijms241411800






