KY19382 Accelerates Cutaneous Wound Healing via Activation of the Wnt/β-Catenin Signaling Pathway
Abstract
1. Introduction
2. Results
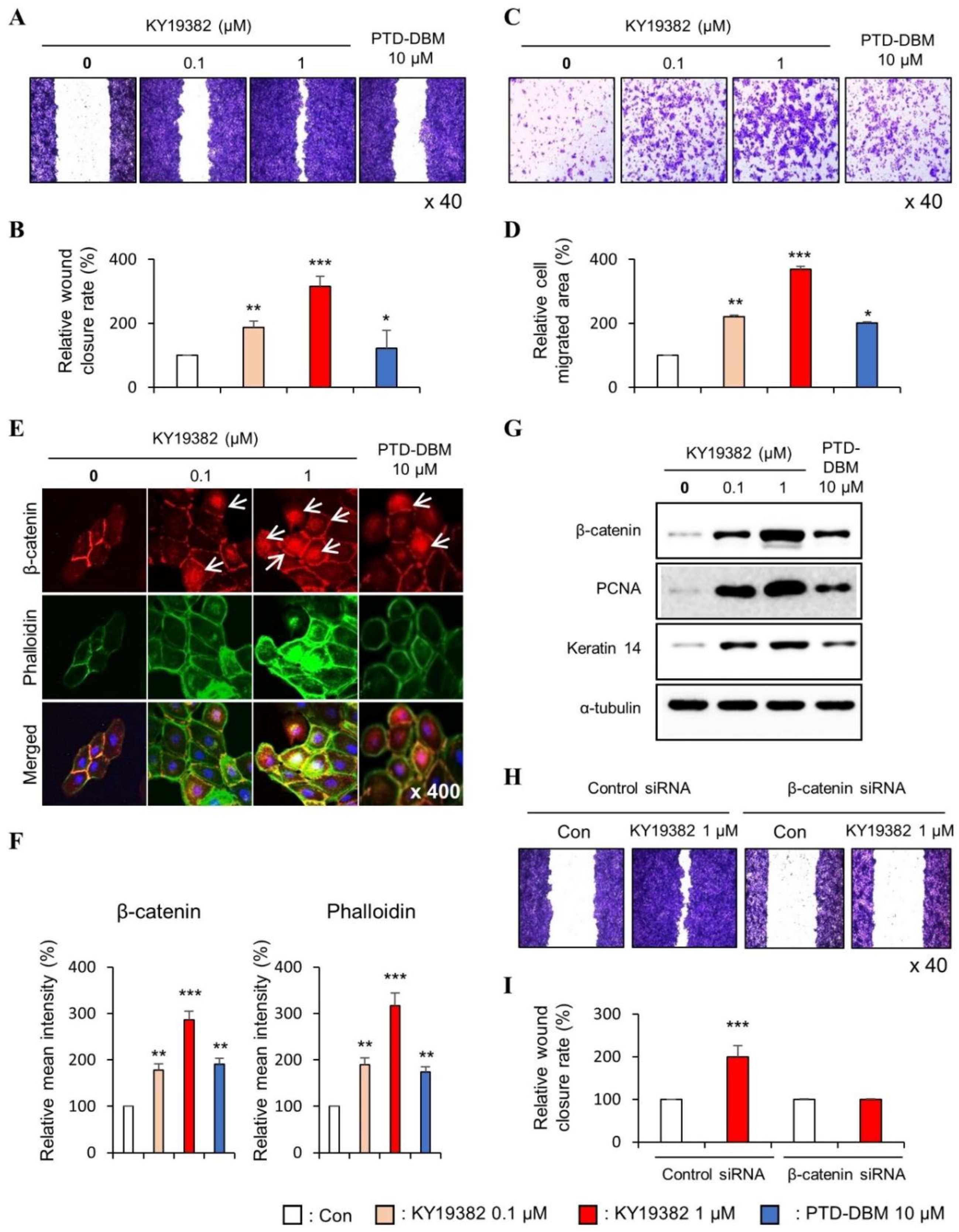
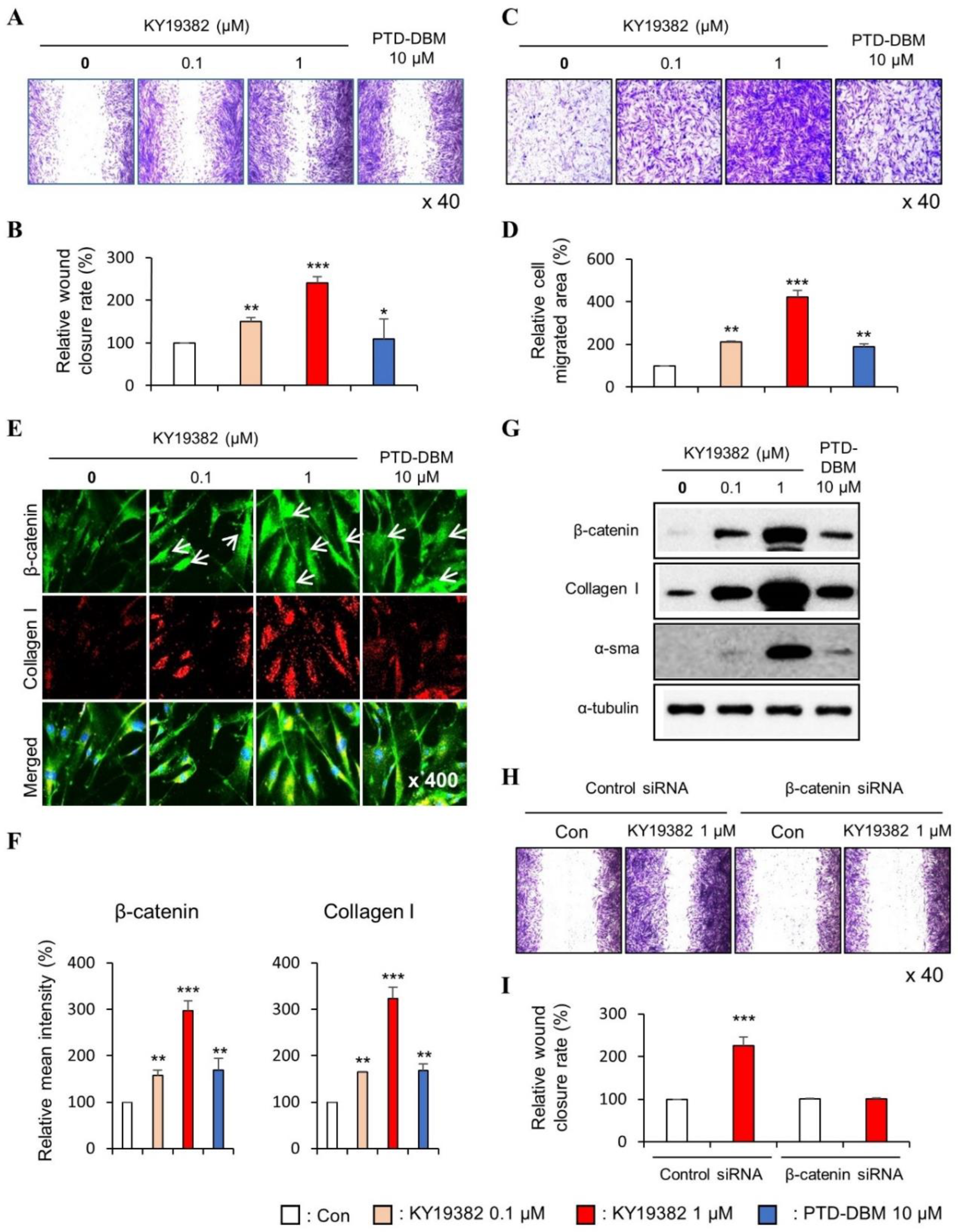
2.1. KY19382 Enhances the Migration of Human Keratinocytes and Dermal Fibroblasts via Activation of the Wnt/β-Catenin Signaling Pathway
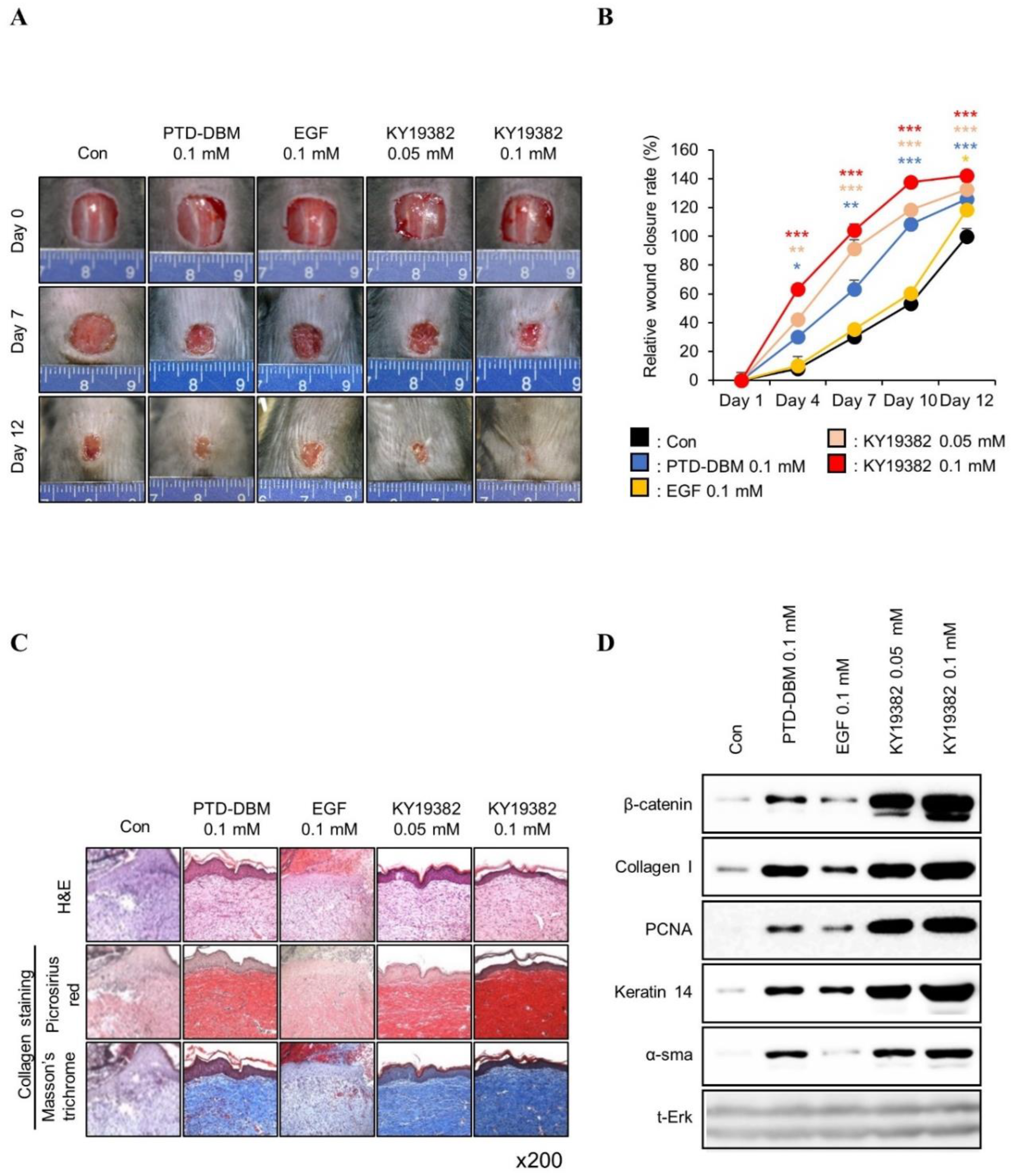
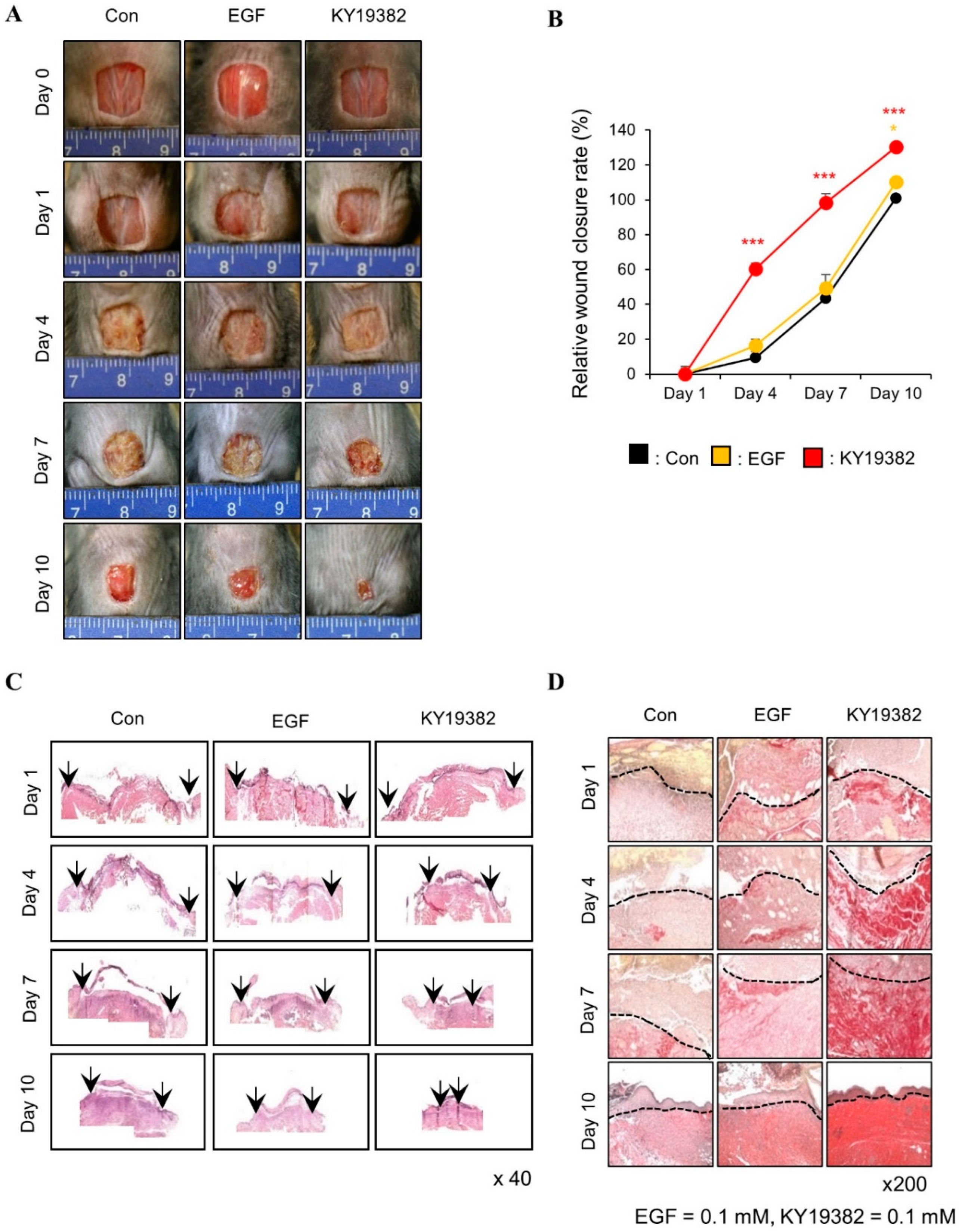
2.2. KY19382 Accelerates Cutaneous Wound Healing In Vivo with Activation of the Wnt/β-Catenin Pathway
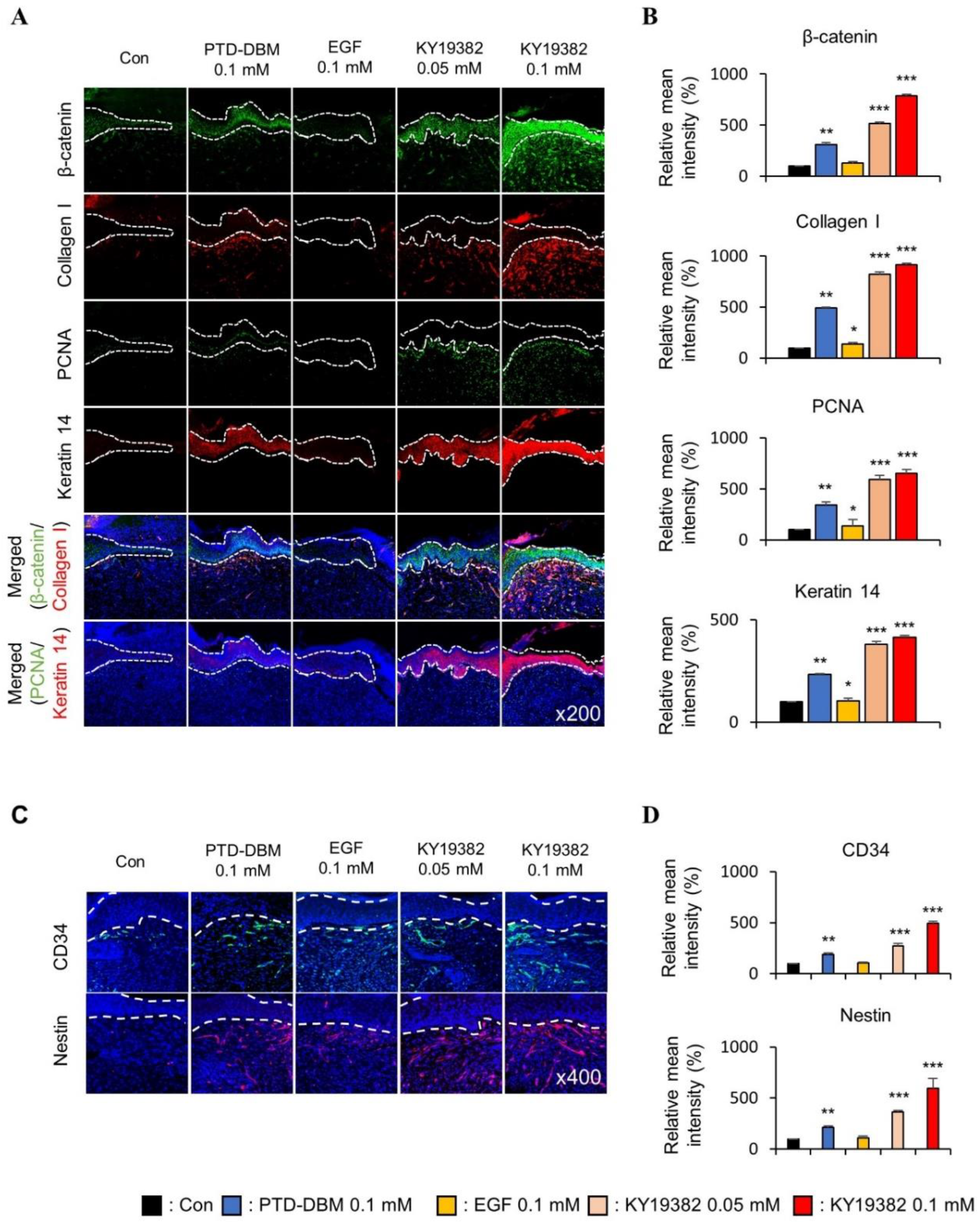
2.3. KY19382 Enhances Re-Epithelialization and Collagen Deposition at an Early Stage of Cutaneous Wound Healing
2.4. KY19382 Activates Stem Cells and Accelerates Wound Healing at an Early Stage of Cutaneous Wound Healing with the Induction of the Wnt/β-Catenin Pathway
3. Discussion
4. Materials and Methods
4.1. Cell Culture and In Vitro Scratch Assay
4.2. Transwell Migration Assay
4.3. Immunoblot Analysis
4.4. Immunocytochemistry
4.5. β-Catenin Knockdown by Small Interfering RNA Transfection
4.6. Animals and In Vivo Wound Healing Assay
4.7. Immunohistochemical Analysis
4.8. TOPFLASH Reporter Luciferase Assay
4.9. CellTiter-Glo Luminescent Cell Viability Assay
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Werner, S.; Grose, R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Velnar, T.; Bailey, T.; Smrkolj, V. The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.C. Antibiotic toxicity, interactions and resistance development. Periodontology 2000 2002, 28, 280–297. [Google Scholar] [CrossRef]
- Shah, J.B. The history of wound care. J. Am. Col. Certif. Wound Spec. 2011, 3, 65–66. [Google Scholar] [CrossRef]
- Choi, S.; Yoon, M.; Choi, K.Y. Approaches for Regenerative Healing of Cutaneous Wound with an Emphasis on Strategies Activating the Wnt/beta-Catenin Pathway. Adv. Wound Care 2022, 11, 70–86. [Google Scholar] [CrossRef]
- Lau, K.; Paus, R.; Tiede, S.; Day, P.; Bayat, A. Exploring the role of stem cells in cutaneous wound healing. Exp. Dermatol. 2009, 18, 921–933. [Google Scholar] [CrossRef] [PubMed]
- Volk, S.W.; Theoret, C. Translating stem cell therapies: The role of companion animals in regenerative medicine. Wound Repair Regen. 2013, 21, 382–394. [Google Scholar] [CrossRef]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Clevers, H.; Nusse, R. Wnt/beta-catenin signaling and disease. Cell 2012, 149, 1192–1205. [Google Scholar] [CrossRef]
- Burgy, O.; Konigshoff, M. The WNT signaling pathways in wound healing and fibrosis. Matrix Biol. 2018, 68–69, 67–80. [Google Scholar] [CrossRef]
- Lim, X.; Nusse, R. Wnt signaling in skin development, homeostasis, and disease. Cold Spring Harb. Perspect. Biol. 2013, 5, a008029. [Google Scholar] [CrossRef]
- Miki, T.; Yasuda, S.Y.; Kahn, M. Wnt/beta-catenin signaling in embryonic stem cell self-renewal and somatic cell reprogramming. Stem. Cell. Rev. Rep. 2011, 7, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Bastakoty, D.; Young, P.P. Wnt/beta-catenin pathway in tissue injury: Roles in pathology and therapeutic opportunities for regeneration. FASEB J. 2016, 30, 3271–3284. [Google Scholar] [CrossRef]
- Steinhart, Z.; Angers, S. Wnt signaling in development and tissue homeostasis. Development 2018, 145, dev146589. [Google Scholar] [CrossRef]
- Cheon, S.S.; Nadesan, P.; Poon, R.; Alman, B.A. Growth factors regulate beta-catenin-mediated TCF-dependent transcriptional activation in fibroblasts during the proliferative phase of wound healing. Exp. Cell Res. 2004, 293, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Wang, C.; Shi, C.; Sun, F.; Xu, X.; Qian, W.; Nie, S.; Han, X. Activated Wnt signaling induces myofibroblast differentiation of mesenchymal stem cells, contributing to pulmonary fibrosis. Int. J. Mol. Med. 2014, 33, 1097–1109. [Google Scholar] [CrossRef]
- Houschyar, K.S.; Momeni, A.; Pyles, M.N.; Maan, Z.N.; Whittam, A.J.; Siemers, F. Wnt signaling induces epithelial differentiation during cutaneous wound healing. Organogenesis 2015, 11, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Akhmetshina, A.; Palumbo, K.; Dees, C.; Bergmann, C.; Venalis, P.; Zerr, P.; Horn, A.; Kireva, T.; Beyer, C.; Zwerina, J.; et al. Activation of canonical Wnt signalling is required for TGF-beta-mediated fibrosis. Nat. Commun. 2012, 3, 735. [Google Scholar] [CrossRef]
- Shi, Y.; Shu, B.; Yang, R.; Xu, Y.; Xing, B.; Liu, J.; Chen, L.; Qi, S.; Liu, X.; Wang, P.; et al. Wnt and Notch signaling pathway involved in wound healing by targeting c-Myc and Hes1 separately. Stem. Cell Res. Ther. 2015, 6, 120. [Google Scholar] [CrossRef]
- Lee, S.H.; Zahoor, M.; Hwang, J.K.; Min, D.S.; Choi, K.Y. Valproic acid induces cutaneous wound healing in vivo and enhances keratinocyte motility. PLoS ONE 2012, 7, e48791. [Google Scholar] [CrossRef]
- Seo, S.H.; Lee, S.H.; Cha, P.H.; Kim, M.Y.; Min, D.S.; Choi, K.Y. Polygonum aviculare L. and its active compounds, quercitrin hydrate, caffeic acid, and rutin, activate the Wnt/beta-catenin pathway and induce cutaneous wound healing. Phytother. Res. 2016, 30, 848–854. [Google Scholar] [CrossRef]
- Yoon, M.; Seo, S.H.; Choi, S.; Han, G.; Choi, K.Y. Euodia daniellii Hemsl. Extract and Its Active Component Hesperidin Accelerate Cutaneous Wound Healing via Activation of Wnt/beta-Catenin Signaling Pathway. Molecules 2022, 27, 7134. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, M.Y.; Kim, H.Y.; Lee, Y.M.; Kim, H.; Nam, K.A.; Roh, M.R.; Min, D.S.; Chung, K.Y.; Choi, K.Y. The Dishevelled-binding protein CXXC5 negatively regulates cutaneous wound healing. J. Exp. Med. 2015, 212, 1061–1080. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Seo, S.H.; Lee, D.H.; Pi, L.Q.; Lee, W.S.; Choi, K.Y. Targeting of CXXC5 by a Competing Peptide Stimulates Hair Regrowth and Wound-Induced Hair Neogenesis. J. Investig. Dermatol. 2017, 137, 2260–2269. [Google Scholar] [CrossRef]
- Choi, S.; Kim, H.Y.; Cha, P.H.; Seo, S.H.; Lee, C.; Choi, Y.; Shin, W.; Heo, Y.; Han, G.; Lee, W.; et al. CXXC5 mediates growth plate senescence and is a target for enhancement of longitudinal bone growth. Life Sci. Alliance 2019, 2. [Google Scholar] [CrossRef] [PubMed]
- Ryu, Y.C.; Lee, D.H.; Shim, J.; Park, J.; Kim, Y.R.; Choi, S.; Bak, S.S.; Sung, Y.K.; Lee, S.H.; Choi, K.Y. KY19382, a novel activator of Wnt/beta-catenin signalling, promotes hair regrowth and hair follicle neogenesis. Br. J. Pharmacol. 2021, 178, 2533–2546. [Google Scholar] [CrossRef]
- Wojtowicz, A.M.; Oliveira, S.; Carlson, M.W.; Zawadzka, A.; Rousseau, C.F.; Baksh, D. The importance of both fibroblasts and keratinocytes in a bilayered living cellular construct used in wound healing. Wound Repair Regen. 2014, 22, 246–255. [Google Scholar] [CrossRef]
- Chipev, C.C.; Simon, M. Phenotypic differences between dermal fibroblasts from different body sites determine their responses to tension and TGFbeta1. BMC Dermatol. 2002, 2, 13. [Google Scholar] [CrossRef] [PubMed]
- Hardwicke, J.; Schmaljohann, D.; Boyce, D.; Thomas, D. Epidermal growth factor therapy and wound healing—Past, present and future perspectives. Surgeon 2008, 6, 172–177. [Google Scholar] [CrossRef]
- Ojeh, N.; Pastar, I.; Tomic-Canic, M.; Stojadinovic, O. Stem Cells in Skin Regeneration, Wound Healing, and Their Clinical Applications. Int. J. Mol. Sci. 2015, 16, 25476–25501. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.C.; Costa, T.F.; Andrade, Z.A.; Medrado, A.R. Wound healing—A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K. Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv. Wound Care 2019, 8, 39–48. [Google Scholar] [CrossRef]
- Duscher, D.; Barrera, J.; Wong, V.W.; Maan, Z.N.; Whittam, A.J.; Januszyk, M.; Gurtner, G.C. Stem cells in wound healing: The future of regenerative medicine? A mini-review. Gerontology 2016, 62, 216–225. [Google Scholar] [CrossRef]
- Metcalfe, A.D.; Ferguson, M.W. Tissue engineering of replacement skin: The crossroads of biomaterials, wound healing, embryonic development, stem cells and regeneration. J. R. Soc. Interface 2007, 4, 413–437. [Google Scholar] [CrossRef]
- Cheon, S.; Poon, R.; Yu, C.; Khoury, M.; Shenker, R.; Fish, J.; Alman, B.A. Prolonged beta-catenin stabilization and tcf-dependent transcriptional activation in hyperplastic cutaneous wounds. Lab. Investig. 2005, 85, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Pedroso, D.C.; Tellechea, A.; Moura, L.; Fidalgo-Carvalho, I.; Duarte, J.; Carvalho, E.; Ferreira, L. Improved survival, vascular differentiation and wound healing potential of stem cells co-cultured with endothelial cells. PLoS ONE 2011, 6, e16114. [Google Scholar] [CrossRef]
- Aki, R.; Amoh, Y.; Li, L.; Katsuoka, K.; Hoffman, R.M. Nestin-expressing interfollicular blood vessel network contributes to skin transplant survival and wound healing. J. Cell Biochem. 2010, 110, 80–86. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, M.; Kim, E.; Seo, S.H.; Kim, G.-U.; Choi, K.-Y. KY19382 Accelerates Cutaneous Wound Healing via Activation of the Wnt/β-Catenin Signaling Pathway. Int. J. Mol. Sci. 2023, 24, 11742. https://doi.org/10.3390/ijms241411742
Yoon M, Kim E, Seo SH, Kim G-U, Choi K-Y. KY19382 Accelerates Cutaneous Wound Healing via Activation of the Wnt/β-Catenin Signaling Pathway. International Journal of Molecular Sciences. 2023; 24(14):11742. https://doi.org/10.3390/ijms241411742
Chicago/Turabian StyleYoon, Minguen, Eunhwan Kim, Seol Hwa Seo, Geon-Uk Kim, and Kang-Yell Choi. 2023. "KY19382 Accelerates Cutaneous Wound Healing via Activation of the Wnt/β-Catenin Signaling Pathway" International Journal of Molecular Sciences 24, no. 14: 11742. https://doi.org/10.3390/ijms241411742
APA StyleYoon, M., Kim, E., Seo, S. H., Kim, G.-U., & Choi, K.-Y. (2023). KY19382 Accelerates Cutaneous Wound Healing via Activation of the Wnt/β-Catenin Signaling Pathway. International Journal of Molecular Sciences, 24(14), 11742. https://doi.org/10.3390/ijms241411742





