Mesenchymal Stem Cell Therapy in Traumatic Spinal Cord Injury: A Systematic Review
Abstract
1. Introduction
1.1. General Overview of Traumatic Spinal Cord Injury
1.2. Pathophysiology of Traumatic Spinal Cord Injury
- 1.
- In the acute phase, lasting between 2 and 48 h, a series of alterations occur, among which the most important are:
- Release of free radicals from lipid peroxidation of the cell membrane due to the initial trauma [12].
- Spinal cord ischemia is associated with initial vasospasm, with subsequent reperfusion and the development of edema further exacerbating the injury [13].
- Ionic alterations such as increased intracellular Ca2+ concentration, failure of Na+/K+ ATPase pumps, activation of voltage-dependent Na+ channels, and massive depolarization.
- 2.
- During the subacute phase, lasting approximately 2 days to 2 weeks, there is increased perilesional phagocytic activity in an attempt to regenerate the destroyed axons and astrocyte hyperplasia and hypertrophy around the injury, resulting in a glial scar, which acts as a physical and chemical barrier to axon regeneration [15].
- 3.
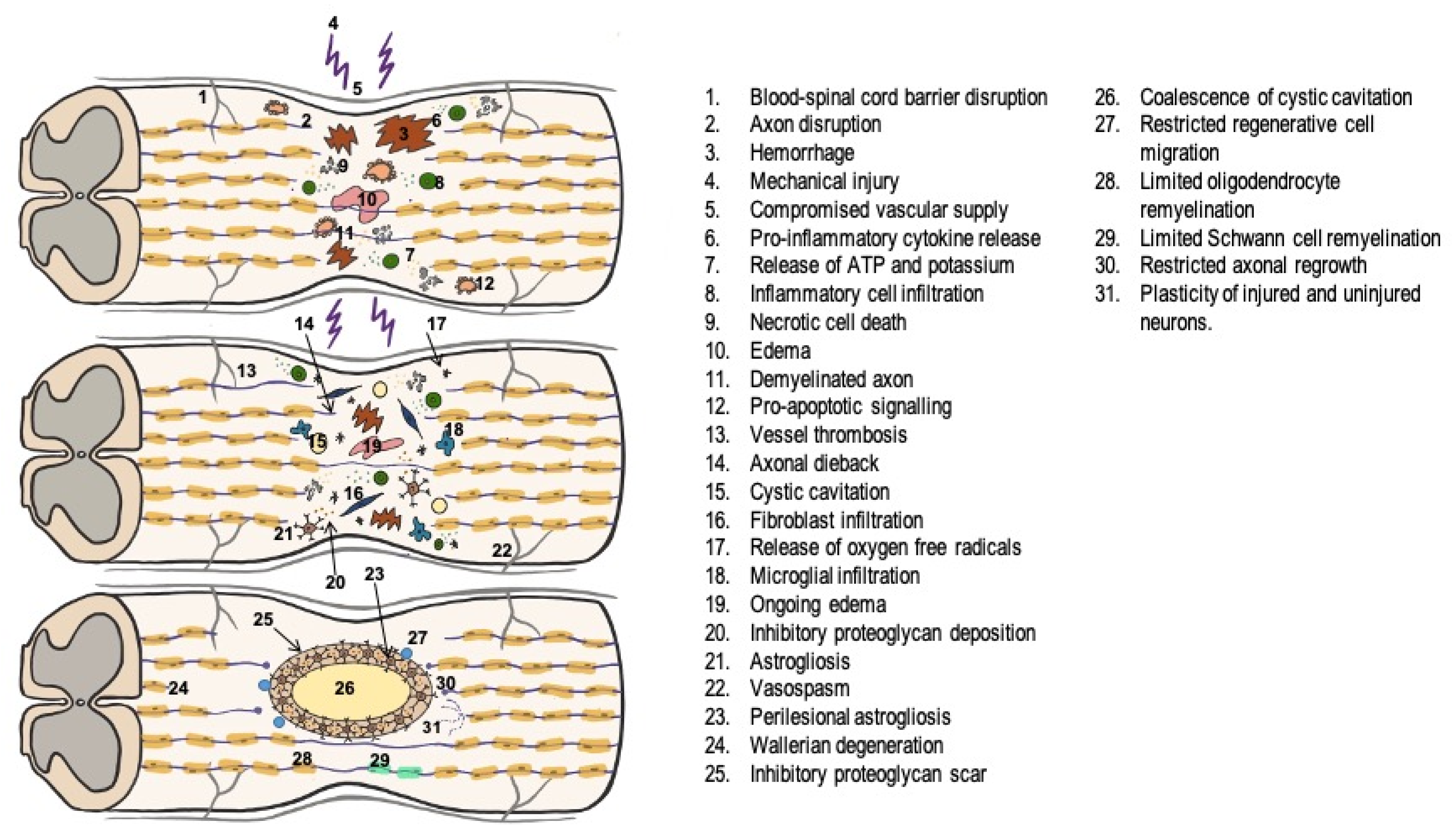
1.3. Assessment and Management of Traumatic Spinal Cord Injury
- Airway: maintaining the airway permeable and protecting the spinal cord.
- Breathing: controlling breathing and ventilation.
- Circulation: maintaining a proper hemodynamic status.
- Disability: performing a correct neurological examination.
1.4. Outcomes after Traumatic Spinal Cord Injury
1.5. Available Therapies
1.6. Cell Therapies
1.6.1. Glial Cells
- Schwann cells
- Astrocytes:
- Oligodendrocytes:
- Olfactory Ensheathing Cells:
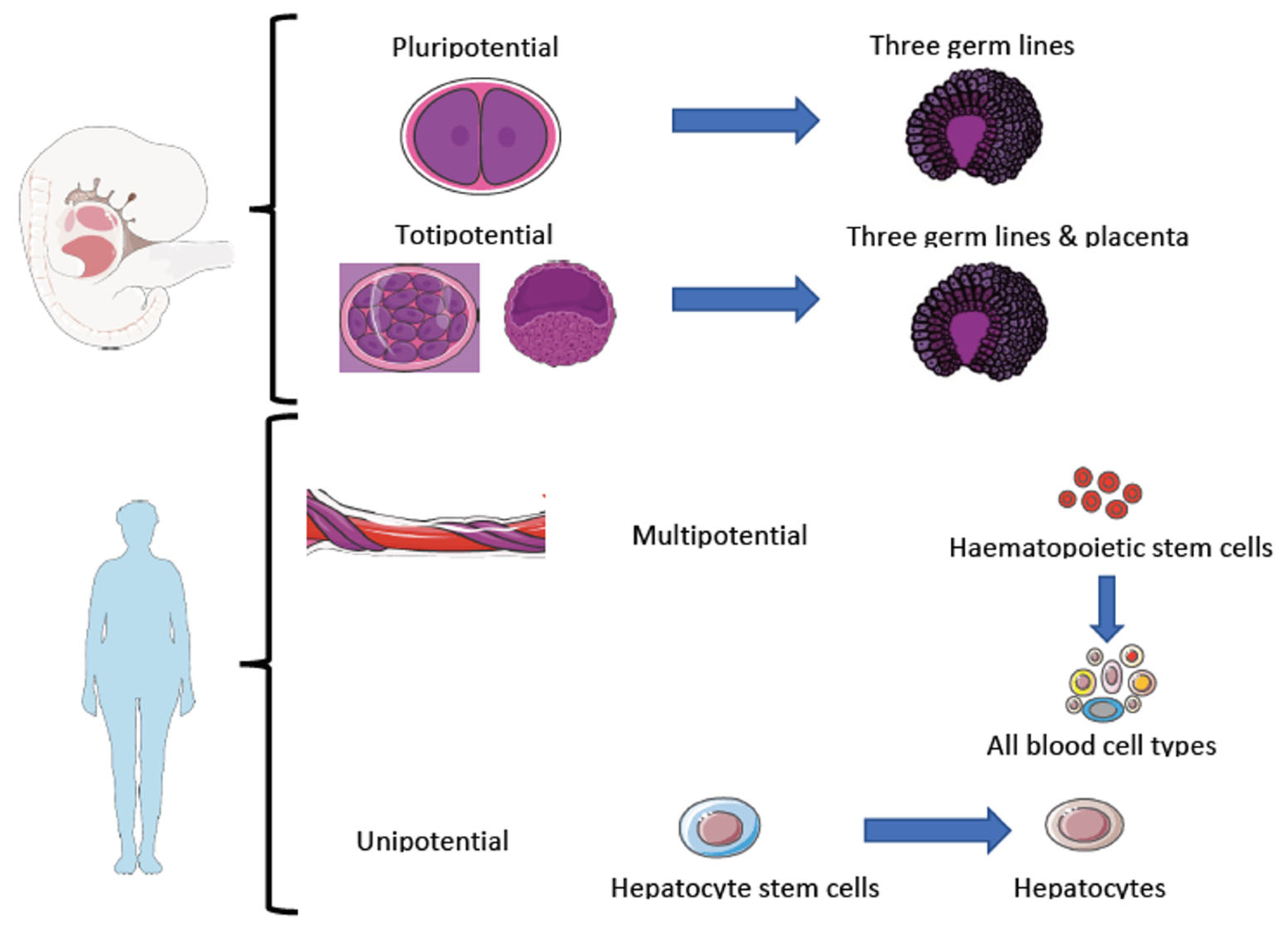
1.6.2. Stem Cells
- Neural progenitor cells:
- Embryonic cells:
- Hematopoietic cells:
- Mesenchymal cells:
- Bone marrow mesenchymal stem cells.
- Umbilical cord mesenchymal stem cells:
- Adipose-derived mesenchymal stem cells:
1.7. Objectives
1.7.1. Main Objective
1.7.2. Specific Objectives
- To analyze changes in AIS (ASIA Impairment Scale) grade.
- To study changes in ASIA sensory and motor scores.
- To evaluate changes in neurophysiological and urodynamic parameters.
- To identify changes in neuroimaging tests.
- To test for the existence of adverse effects of MSC therapy.
2. Methods
2.1. Search Strategy
2.2. Eligibility Criteria
- Type of study: randomized and non-randomized clinical trials, cohort studies, case-control studies, and case series.
- Papers are available in English and Spanish until February 2023.
- Human studies.
- Traumatic spinal cord injury at any level and any AIS grade.
- Intervention: MSC therapy.
- Results: improvement in ASIA sensory and motor scores and grade on the AIS scale. Neurophysiological studies (somatosensory and motor evoked potentials, electromyography). Neuroimaging changes (magnetic resonance imaging). Urodynamic studies. Adverse effects.
- Meta-analysis and systematic reviews.
- Narrative reviews and expert opinions.
- MSC studies were performed exclusively on animals and in vitro.
- Non-mesenchymal stem cell studies in humans
- Non-traumatic spinal cord injuries.
2.3. Analyzed Variables
- Sociodemographic variables: age, gender, level of injury, and extent of spinal cord injury according to the AIS scale.
- Study variables: author, year, type of study, randomization, control group, sample size, level of evidence, and methodological quality.
- Therapy-related variables: type of stem cells, route of administration, and dose administered.
- Efficacy outcome variables: improvement in AIS grade, improvement in ASIA sensory and motor scores, neurophysiological studies, urodynamic studies, neuroimaging studies, and adverse events.
2.4. Quality Assessment
| Reason 1 | Studies by Moviglia G et al., Zamani H et al., Oraee-Yazdani S et al. used cell types other than MSCs [64,69,70] |
| Reason 2 | The study by Xie Z et al. was published in Chinese [71] |
| Reason 3 | The studies by Oliveri R et al., Jeon SR P et al., Deng L et al., Yang Z et al., Shang Z et al., Sarveazad A et al., Lu Y et al., Shang Z et al.; were conducted in animals [72,73,74,75,76,77,78,79] |
| Reason 4 | Studies by Zhang D et al., Chen X et al., Khan S et al., Xu P et al., Yousefifard M et al., Muthu S et al., Chen W et al., Tang Q et al., Johnson L et al., Kvistad C et al., Xu X et al., Liu S et al. were meta-analyses or systematic reviews [11,80,81,82,83,84,85,86,87,88,89,90] |
| Reason 5 | Studies by Xiao Z et al., Li Z et al., Zhao Y et al., Deng W et al. combined stem cells with other therapies [91,92,93,94] |
| Reason 6 | Study by Vaquero J et al. included patients with syringomyelia [95]. |

3. Results and Discussion
3.1. Overview of Studies Included in the Systematic Review
3.2. Outcomes following Cell Therapy
3.2.1. AIS Grade
3.2.2. ASIA Sensory Score
3.2.3. ASIA Motor Score
3.2.4. Neurophysiological Studies
3.2.5. Urodynamic Studies
3.2.6. Neuroimaging Studies (MRI Findings)
3.2.7. Severe Adverse Events
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kooijmans, H.; Post, M.W.M.; Stam, H.J.; van der Woude, L.H.V.; Spijkerman, D.C.M.; Snoek, G.J.; Bongers-Janssen, H.M.H.; van Koppenhagen, C.F.; Twisk, J.W.; ALLRISC Group; et al. Effectiveness of a Self-Management Intervention to Promote an Active Lifestyle in Persons with Long-Term Spinal Cord Injury: The HABITS Randomized Clinical Trial. Neurorehabilit. Neural Repair 2017, 31, 991–1004. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Tetreault, L.; Kalsi-Ryan, S.; Nouri, A.; Fehlings, M.G. Global prevalence and incidence of traumatic spinal cord injury. Clin. Epidemiol. 2014, 6, 309–331. [Google Scholar] [PubMed]
- Lee, B.B.; Cripps, R.A.; Fitzharris, M.; Wing, P.C. The global map for traumatic spinal cord injury epidemiology: Update 2011, global incidence rate. Spinal Cord 2014, 52, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Montoto-Marqués, A.; Ferreiro-Velasco, M.E.; Salvador-De La Barrera, S.; Balboa-Barreiro, V.; Rodriguez-Sotillo, A.; Meijide-Failde, R. Epidemiology of traumatic spinal cord injury in Galicia, Spain: Trends over a 20-year period. Spinal Cord 2017, 55, 588–594. [Google Scholar] [CrossRef]
- Galeiras Vázquez, R.; Ferreiro Velasco, M.E.; Mourelo Fariña, M.; Montoto Marqués, A.; Salvador de la Barrera, S. Actualización en lesión medular aguda postraumática: Parte 1. Med. Intensiva 2017, 41, 237–247. [Google Scholar] [CrossRef]
- Gaitán Pérez, N.; Montoto Marqués, A. Disfunción Vesical e Intestinal en La Lesión Medular; Méderic Ediciones, S.L.: Barcelona, Spain, 2019; pp. 1–25. [Google Scholar]
- Ahuja, C.S.; Wilson, J.R.; Nori, S.; Kotter, M.R.N.; Druschel, C.; Curt, A.; Fehlings, M.G. Traumatic spinal cord injury. Nat. Rev. Dis. Primers 2017, 3, 17018. [Google Scholar] [CrossRef]
- Venkatesh, K.; Ghosh, S.K.; Mullick, M.; Manivasagam, G.; Sen, D. Spinal cord injury: Pathophysiology, treatment strategies, associated challenges, and future implications. Cell Tissue Res. 2019, 377, 125–151. [Google Scholar] [CrossRef]
- Padilla-Zambrano, H.; Ramos-Villegas, Y.; Raphael Alvis-Miranda, H.; Joaquin, A.F.; Rafael Moscote-Salazar, L.; Rafael Moscote, L. Fisiopatología del trauma raquimedular. Pathophysiology of spinal trauma. Rev. Mex. Neurocienc. 2017, 18, 46–53. [Google Scholar]
- Tator, C.H.; Fehlings, M.G. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J. Neurosurg. 1991, 75, 15–26. [Google Scholar] [CrossRef]
- Muthu, S.; Jeyaraman, M.; Gulati, A.; Arora, A. Current evidence on mesenchymal stem cell therapy for traumatic spinal cord injury: Systematic review and meta-analysis. Cytotherapy 2020, 23, 186–197. [Google Scholar] [CrossRef]
- Sekhon, L.H.S.; Fehlings, M.G. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine 2001, 26 (Suppl. S24), S2–S12. [Google Scholar] [CrossRef]
- Acevedo González, J.C.; Varón, L.F.; Berbeo Calderón, M.E.; Feo Lee, O.; Díaz Orduz, R. Avances fisiopatológicos para el entendimiento de la lesión medular traumática: Revisión bibliográfica. Rev. Colomb. Ortop. Traumatol. 2008, 22, 272–281. [Google Scholar]
- Oyinbo, C.A. Secondary injury mechanisms in traumatic spinal cord injury: A nugget of this multiply cascade. Acta Neurobiol. Exp. 2011, 71, 281–299. [Google Scholar]
- Herrmann, J.E.; Imura, T.; Song, B.; Qi, J.; Ao, Y.; Nguyen, T.K.; Korsak, R.A.; Takeda, K.; Akira, S.; Sofroniew, M.V. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J. Neurosci. 2008, 28, 7231–7243. [Google Scholar] [CrossRef]
- Ahuja, C.S.; Mothe, A.; Khazaei, M.; Badhiwala, J.H.; Gilbert, E.A.; van der Kooy, D.; Morshead, C.M.; Tator, C.; Fehlings, M.G. The leading edge: Emerging neuroprotective and neuroregenerative cell-based therapies for spinal cord injury. Stem Cells Transl. Med. 2020, 9, 1509–1530. [Google Scholar] [CrossRef]
- Eckert, M.J.; Martin, M.J. Trauma: Spinal Cord Injury. Surg. Clin. N. Am. 2017, 97, 1031–1045. [Google Scholar] [CrossRef]
- Galvagno, S.M.; Nahmias, J.T.; Young, D.A. Advanced Trauma Life Support® Update 2019: Management and Applications for Adults and Special Populations. Anesthesiol. Clin. 2019, 37, 13–32. [Google Scholar] [CrossRef]
- Theodore, N.; Hadley, M.N.; Aarabi, B.; Dhall, S.S.; Gelb, D.E.; Hurlbert, R.J.; Rozzelle, C.J.; Ryken, T.C.; Walters, B.C. Prehospital cervical spinal immobilization after trauma. Neurosurgery 2013, 72 (Suppl. S2), 22–34. [Google Scholar] [CrossRef]
- Kreinest, M.; Gliwitzky, B.; Schüler, S.; Grützner, P.A.; Münzberg, M. Development of a new Emergency Medicine Spinal Immobilization Protocol for trauma patients and a test of applicability by German emergency care providers. Scand. J. Trauma Resusc. Emerg. Med. 2016, 24, 71. [Google Scholar] [CrossRef]
- Hoffman, J.R.; Mower, W.R.; Wolfson, A.B.; Todd, K.H.; Zucker, M.I.; National Emergency X-Radiography Utilization Study Group. Validity of a set of clinical criteria to rule out injury to the cervical spine in patients with blunt trauma. N. Engl. J. Med. 2000, 343, 94–99. [Google Scholar] [CrossRef]
- Stiell, I.G.; Wells, G.A.; Vandemheen, K.L.; Clement, C.M.; Lesiuk, H.; de Maio, V.J.; Laupacis, A.; Schull, M.; McKnight, D.; Verbeek, R.; et al. The Canadian C-spine rule for radiography in alert and stable trauma patients. JAMA 2001, 286, 1841–1848. [Google Scholar] [CrossRef] [PubMed]
- American Spinal Injury Association. International Standards for Neurological Classification of Spinal Cord Injury. Richmond (VA): ASIA. 2019. Available online: https://asia-spinalinjury.org/international-standards-neurological-classification-sci-isncsci-worksheet/ (accessed on 18 June 2023).
- Kirshblum, S.; Snider, B.; Rupp, R.; Read, M.S. International Standards Committee of ASIA and ISCoS. Updates of the International Standards for Neurologic Classification of Spinal Cord Injury: 2015 and 2019. Phys. Med. Rehabil. Clin. N. Am. 2020, 31, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Walters, B.C.; Hadley, M.N.; Hurlbert, R.J.; Aarabi, B.; Dhall, S.S.; Gelb, D.E.; Harrigan, M.R.; Rozelle, C.J.; Ryken, T.C.; Theodore, N. Guidelines for the management of acute cervical spine and spinal cord injuries: 2013 update. Neurosurgery 2013, 60 (Suppl. S1), 82–91. [Google Scholar] [CrossRef] [PubMed]
- Outcomes following traumatic spinal cord injury: Clinical practice guidelines for health-care professionals. J. Spinal Cord Med. 2000, 23, 289–316. [CrossRef]
- El Tecle, N.E.; Dahdaleh, N.S.; Bydon, M.; Ray, W.Z.; Torner, J.C.; Hitchon, P.W. The natural history of complete spinal cord injury: A pooled analysis of 1162 patients and a meta-analysis of modern data. J. Neurosurg. Spine 2018, 28, 436–443. [Google Scholar] [CrossRef]
- Fawcett, J.W.; Curt, A.; Steeves, J.D.; Coleman, W.P.; Tuszynski, M.H.; Lammertse, D.; Bartlett, P.F.; Blight, A.R.; Dietz, V.; Ditunno, J.; et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: Spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord 2007, 45, 190–205. [Google Scholar] [CrossRef]
- Wilson, J.R.; Cadotte, D.W.; Fehlings, M.G. Clinical predictors of neurological outcome, functional status, and survival after traumatic spinal cord injury: A systematic review. J. Neurosurg. Spine 2012, 17 (Suppl. S1), 11–26. [Google Scholar] [CrossRef]
- Montoto Marqués, A. Lesión Medular Traumática en Galicia (1995–2014): Estudio de la Evolución Epidemiológica y Resultados Clínico-Funcionales. Ph.D. Thesis, Universidade da Coruña, A Coruña, Spain, 2018. [Google Scholar]
- Mourelo Fariña, M.; Salvador de la Barrera, S.; Montoto Marqués, A.; Ferreiro Velasco, M.E.; Galeiras Vázquez, R. Actualización en lesión medular aguda postraumática. Parte 2 Actualización en lesión medular aguda postraumática. Parte Med. Intensiv. 2017, 41, 306–315. [Google Scholar] [CrossRef]
- Como, J.J.; Diaz, J.J.; Dunham, C.M.; Chiu, W.C.; Duane, T.M.; Capella, J.M.; Holevar, M.R.; Khwaja, K.A.; Mayglothling, J.A.; Shapiro, M.B.; et al. Practice management guidelines for identification of cervical spine injuries following trauma: Update from the eastern association for the surgery of trauma practice management guidelines committee. J. Trauma 2009, 67, 651–659. [Google Scholar] [CrossRef]
- Griffin, J.M.; Bradke, F. Therapeutic repair for spinal cord injury: Combinatory approaches to address a multifaceted problem. EMBO Mol. Med. 2020, 12, e11505. [Google Scholar] [CrossRef]
- Roquilly, A.; Vigué, B.; Boutonnet, M.; Bouzat, P.; Buffenoir, K.; Cesareo, E.; Chauvin, A.; Court, C.; Cook, F.; de Crouy, A.C.; et al. French recommendations for the management of patients with spinal cord injury or at risk of spinal cord injury. Anaesth. Crit. Care Pain Med. 2020, 39, 279–289. [Google Scholar] [CrossRef]
- Readdy, W.J.; Chan, A.K.; Matijakovich, D.J.; Dhall, S.S. A review and update on the guidelines for the acute non-operative management of cervical spinal cord injury. J. Neurosurg. Sci. 2015, 59, 119–128. [Google Scholar]
- Fehlings, M.G.; Tetreault, L.A.; Wilson, J.R.; Aarabi, B.; Anderson, P.; Arnold, P.M.; Brodke, D.S.; Burns, A.S.; Chiba, K.; Dettori, J.R.; et al. A Clinical Practice Guideline for the Management of Patients with Acute Spinal Cord Injury and Central Cord Syndrome: Recommendations on the Timing (≤24 Hours Versus >24 Hours) of Decompressive Surgery. Glob. Spine J. 2017, 7 (Suppl. S3), 195S–202S. [Google Scholar] [CrossRef]
- Wilson, J.R.; Tetreault, L.A.; Kwon, B.K.; Arnold, P.M.; Mroz, T.E.; Shaffrey, C.; Harrop, J.S.; Chapman, J.R.; Casha, S.; Skelly, A.C.; et al. Timing of Decompression in Patients With Acute Spinal Cord Injury: A Systematic Review. Glob. Spine J. 2017, 7 (Suppl. S3), 95S–115S. [Google Scholar] [CrossRef]
- Varma, A.K.; Das, A.; Wallace, G.; Barry, J.; Vertegel, A.A.; Ray, S.K.; Banik, N.L. Spinal cord injury: A review of current therapy, future treatments, and basic science frontiers. Neurochem. Res. 2013, 38, 895–905. [Google Scholar] [CrossRef]
- Gant, K.L.; Guest, J.D.; Palermo, A.E.; Vedantam, A.; Jimsheleishvili, G.; Bunge, M.B.; Brooks, A.E.; Anderson, K.D.; Thomas, C.K.; Santamaria, A.J.; et al. Phase 1 Safety Trial of Autologous Human Schwann Cell Transplantation in Chronic Spinal Cord Injury. J. Neurotrauma 2022, 39, 285–299. [Google Scholar] [CrossRef]
- Anderson, K.D.; Guest, J.D.; Dietrich, W.D.; Bartlett Bunge, M.; Curiel, R.; Dididze, M.; Green, B.A.; Khan, A.; Pearse, D.D.; Saraf-Lavi, E.; et al. Safety of Autologous Human Schwann Cell Transplantation in Subacute Thoracic Spinal Cord Injury. J Neurotrauma 2017, 34, 2950–2963. [Google Scholar] [CrossRef]
- Oraee-Yazdani, S.; Hafizi, M.; Atashi, A.; Ashrafi, F.; Seddighi, A.S.; Hashemi, S.M.; Seddighi, A.; Soleimani, M.; Zali, A. Cotransplantation of autologous bone marrow mesenchymal stem cells and Schwann cells through cerebral spinal fluid for the treatment of patients with chronic spinal cord injury: Safety and possible outcome. Spinal Cord 2016, 54, 102–109. [Google Scholar] [CrossRef]
- Chang, J.; Qian, Z.; Wang, B.; Cao, J.; Zhang, S.; Jiang, F.; Kong, R.; Yu, X.; Cao, X.; Yang, L.; et al. Transplantation of A2 type astrocytes promotes neural repair and remyelination after spinal cord injury. Cell Commun. Signal. 2023, 21, 37. [Google Scholar] [CrossRef]
- Valori, C.h.F.; Possenti, A.; Brambilla, L.; Rossi, D. Challenges and Opportunities of Targeting Astrocytes to Halt Neurodegenerative Disorders. Cells 2021, 10, 2019. [Google Scholar] [CrossRef]
- Hernández Ramírez, P. Medicina regenerativa y células madre. Mecanismos de acción de las células madre adultas. Rev. Cuba. Hematol. Inmunol. Hemoter. 2009, 25, 1–15. [Google Scholar]
- Shende, P.; Subedi, M. Pathophysiology, mechanisms and applications of mesenchymal stem cells for the treatment of spinal cord injury. Biomed. Pharmacother. 2017, 91, 693–706. [Google Scholar] [CrossRef] [PubMed]
- Mata-Miranda, M.; Vázquez-Zapién, G.J.; Sánchez-Monroy, V. Generalidades y Aplicaciones de Las Células Madre. Perinatol. Reprod. Hum. 2013, 27, 194–199. Available online: http://www.medigraphic.com/inper (accessed on 18 June 2023).
- Kolios, G.; Moodley, Y. Introduction to stem cells and regenerative medicine. Respiration 2013, 85, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Szymoniuk, M.; Litak, J.; Sakwa, L.; Dryla, A.; Zezuliński, W.; Czyżewski, W.; Kamieniak, P.; Blicharski, T. Molecular Mechanisms and Clinical Application of Multipotent Stem Cells for Spinal Cord Injury. Cells 2022, 12, 120. [Google Scholar] [CrossRef]
- Waddington, C.H. The Strategy of the Genes; Allen & Unwin: Crows Nest, Australia, 1956; p. 1. [Google Scholar]
- Weismann, A. The Germ-Plasm: A Theory of Heredity; Scribner’s: New York, NY, USA, 1983. [Google Scholar]
- Yamanaka, S. Induction of pluripotent stem cells from mouse fibroblasts by four transcription factors. Cell Prolif. 2008, 41 (Suppl. S1), 51–56. [Google Scholar] [CrossRef]
- Ming, G.L.; Song, H. Adult Neurogenesis in the Mammalian Brain: Significant Answers and Significant Questions. Neuron 2011, 70, 687–702. [Google Scholar] [CrossRef]
- Shao, A.; Tu, S.; Lu, J.; Zhang, J. Crosstalk between stem cell and spinal cord injury: Pathophysiology and treatment strategies. Stem Cell Res. Ther. 2019, 10, 238. [Google Scholar] [CrossRef]
- Levi, A.D.; Anderson, K.D.; Okonkwo, D.O.; Park, P.; Bryce, T.N.; Kurpad, S.N.; Aarabi, B.; Hsieh, J.; Gant, K. Clinical Outcomes from a Multi-Center Study of Human Neural Stem Cell Transplantation in Chronic Cervical Spinal Cord Injury. J. Neurotrauma 2019, 36, 891–902. [Google Scholar] [CrossRef]
- Verfaillie, C.M.; Pera, M.F.; Lansdorp, P.M. Stem cells: Hype and reality. Hematol. Am. Soc. Hematol. Educ. Program 2002, 2002, 369–391. [Google Scholar] [CrossRef]
- Bryukhovetskiy, A.S.; Bryukhovetskiy, I.S. Effectiveness of repeated transplantations of hematopoietic stem cells in spinal cord injury. World J. Transplant. 2015, 5, 110–128. [Google Scholar] [CrossRef]
- Deda, H.; Inci, M.C.; Kurekçi, A.; Kayihan, K.; Özgün, E.; Ustunsoy, G.; Kocabay, S. Treatment of chronic spinal cord injured patients with autologous bone marrow-derived hematopoietic stem cell transplantation: 1-year follow-up. Cytotherapy 2008, 10, 565–574. [Google Scholar] [CrossRef]
- Han, D.; Wu, C.; Xiong, Q.; Zhou, L.; Tian, Y. Anti-inflammatory Mechanism of Bone Marrow Mesenchymal Stem Cell Transplantation in Rat Model of Spinal Cord Injury. Cell Biochem. Biophys. 2015, 71, 1341–1347. [Google Scholar] [CrossRef]
- Hofstetter, C.P.; Schwarz, E.J.; Hess, D.; Widenfalk, J.; el Manira, A.; Prockop, D.J.; Olson, L. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc. Natl. Acad. Sci. USA 2002, 99, 2199–2204. [Google Scholar] [CrossRef]
- Cofano, F.; Boido, M.; Monticelli, M.; Zenga, F.; Ducati, A.; Vercelli, A.; Garbossa, D. Mesenchymal Stem Cells for Spinal Cord Injury: Current Options, Limitations, and Future of Cell Therapy. Int. J. Mol. Sci. 2019, 20, 2698. [Google Scholar] [CrossRef]
- Xia, Y.; Zhu, J.; Yang, R.; Wang, H.; Li, Y.; Fu, C. Mesenchymal stem cells in the treatment of spinal cord injury: Mechanisms, current advances and future challenges. Front. Immunol. 2023, 14, 1141601. [Google Scholar] [CrossRef]
- Kouroupis, D.; Sanjurjo-Rodriguez, C.; Jones, E.; Correa, D. Mesenchymal Stem Cell Functionalization for Enhanced Therapeutic Applications. Tissue Eng. Part B Rev. 2019, 25, 55–77. [Google Scholar] [CrossRef]
- Huang, L.; Fu, C.; Xiong, F.; He, C.; Wei, Q. Stem Cell Therapy for Spinal Cord Injury. Cell Transplant. 2021, 30, 963689721989266. [Google Scholar] [CrossRef]
- Oraee-Yazdani, S.; Akhlaghpasand, M.; Golmohammadi, M.; Hafizi, M.; Zomorrod, M.S.; Kabir, N.M.; Oraee-Yazdani, M.; Ashrafi, F.; Zali, A.; Soleimani, M. Combining cell therapy with human autologous Schwann cell and bone marrow-derived mesenchymal stem cell in patients with subacute complete spinal cord injury: Safety considerations and possible outcomes. Stem Cell Res. Ther. 2021, 12, 445. [Google Scholar] [CrossRef]
- Kaner, T.; Karadag, T.; Cirak, B.; Erken, H.A.; Karabulut, A.; Kiroglu, Y.; Akkaya, S.; Acar, F.; Coskun, E.; Genc, O.; et al. The effects of human umbilical cord blood transplantation in rats with experimentally induced spinal cord injury. J. Neurosurg. Spine 2010, 13, 543–551. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Moseley, A.M.; Herbert, R.D.; Sherrington, C.; Maher, C.G. Evidence for physiotherapy practice: A survey of the Physiotherapy Evidence Database (PEDro). Aust. J. Physiother. 2002, 48, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Yepes-Nuñez, J.J.; Urrútia, G.; Romero-García, M.; Alonso-Fernández, S. Declaración PRISMA 2020: Una guía actualizada para la publicación de revisiones sistemáticas. Rev. Esp. Cardiol. 2021, 74, 790–799. [Google Scholar]
- Moviglia, G.A.; Fernandez Viña, R.; Brizuela, J.A.; Saslavsky, J.; Vrsalovic, F.; Varela, G.; Bastos, F.; Farina, P.; Etchegaray, G.; Barbieri, M.; et al. Combined protocol of cell therapy for chronic spinal cord injury. Report on the electrical and functional recovery of two patients. Cytotherapy 2006, 8, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Zamani, H.; Soufizomorrod, M.; Oraee-Yazdani, S.; Naviafar, D.; Akhlaghpasand, M.; Seddighi, A.; Soleimani, M. Safety and feasibility of autologous olfactory ensheathing cell and bone marrow mesenchymal stem cell co-transplantation in chronic human spinal cord injury: A clinical trial. Spinal Cord 2022, 60, 63–70. [Google Scholar] [CrossRef]
- Xie, Z.W.; Cui, G.X.; Li, Y.Z.; Li, B.W.; Zhu, S.W.; Song, C.Z.; Shi, Q.; Hou, H.S.; Shen, B.J. Curative effect of autologous mesenchymal stem cell transplantation on spinal cord injury. J. Clin. Rehabil. Tissue Eng. Res. 2007, 11, 1277–1279. [Google Scholar]
- Oliveri, R.S.; Bello, S.; Biering-Sørensen, F. Mesenchymal stem cells improve locomotor recovery in traumatic spinal cord injury: Systematic review with meta-analyses of rat models. Neurobiol. Dis. 2014, 62, 338–353. [Google Scholar] [CrossRef]
- Jeon, S.R.; Park, J.H.; Lee, J.H.; Kim, D.Y.; Kim, H.S.; Sung, I.Y.; Choi, G.H.; Jeon, M.H.; Kim, G.G. Treatment of spinal cord injury with bone marrow-derived, cultured autologous mesenchymal stem cells. Tissue Eng. Regen. Med. 2010, 7, 316–322. [Google Scholar]
- Deng, L.; Lv, J.Q.; Sun, L. Experimental treatments to attenuate blood spinal cord barrier rupture in rats with traumatic spinal cord injury: A meta-analysis and systematic review. Front. Pharmacol. 2022, 13, 950368. [Google Scholar] [CrossRef]
- Yang, Z.; Rao, J.; Liang, Z.; Xu, X.; Lin, F.; Lin, Y.; Wang, C.; Chen, C. Efficacy of miRNA-modified mesenchymal stem cell extracellular vesicles in spinal cord injury: A systematic review of the literature and network meta-analysis. Front. Neurosci. 2022, 16, 989295. [Google Scholar] [CrossRef]
- Shang, Z.; Wang, M.; Zhang, B.; Wang, X.; Wanyan, P. Subacute traumatic spinal cord injury: A systematic review and network meta-analysis of therapeutic strategies based on bone marrow mesenchymal stromal cells in animal models. Cytotherapy 2022, 24, 1181–1189. [Google Scholar] [CrossRef]
- Sarveazad, A.; Toloui, A.; Moarrefzadeh, A.; Nafchi, H.G.; Neishaboori, A.M.; Yousefifard, M. Mesenchymal Stem Cell-Conditioned Medium Promotes Functional Recovery Following Spinal Cord Injury: A Systematic Review and Meta-analysis. Spine Surg. Relat. Res. 2022, 6, 433–442. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, W.; Tian, Z.; Liang, Q.; Liu, C.; Wu, Y.; Zhang, L.; Rong, L. The optimal transplantation strategy of umbilical cord mesenchymal stem cells in spinal cord injury: A systematic review and network meta-analysis based on animal studies. Stem Cell Res. Ther. 2022, 13, 441. [Google Scholar] [CrossRef]
- Shang, Z.; Wang, R.; Li, D.; Chen, J.; Zhang, B.; Wang, M.; Wang, X.; Wanyan, P. Spinal Cord Injury: A Systematic Review and Network Meta-Analysis of Therapeutic Strategies Based on 15 Types of Stem Cells in Animal Models. Front. Pharmacol. 2022, 13, 819861. [Google Scholar] [CrossRef]
- Zhang, D.; He, X. A Meta-analysis of the motion function through the therapy of spinal cord injury with intravenous transplantation of bone marrow mesenchymal stem cells in rats. PLoS ONE 2014, 9, e93487. [Google Scholar] [CrossRef]
- Chen, X.; Xue, B.; Li, Y.; Song, C.; Jia, P.; Ren, X.; Zang, W.; Wang, J. Meta-analysis of stem cell transplantation for reflex hypersensitivity after spinal cord injury. Neuroscience 2017, 363, 66–75. [Google Scholar] [CrossRef]
- Khan, S.; Mafi, P.; Mafi, R.; Khan, W. A Systematic Review of Mesenchymal Stem Cells in Spinal Cord Injury, Intervertebral Disc Repair and Spinal Fusion. Curr. Stem Cell Res. Ther. 2018, 13, 316–323. [Google Scholar] [CrossRef]
- Xu, P.; Yang, X. The Efficacy and Safety of Mesenchymal Stem Cell Transplantation for Spinal Cord Injury Patients: A Meta-Analysis and Systematic Review. Cell Transplant. 2019, 28, 36–46. [Google Scholar] [CrossRef]
- Yousefifard, M.; Maleki, S.N.; Askarian-Amiri, S.; Vaccaro, A.R.; Chapman, J.R.; Fehlings, M.G.; Hosseini, M.; Rahimi-Movaghar, V. A combination of mesenchymal stem cells and scaffolds promotes motor functional recovery in spinal cord injury: A systematic review and meta-analysis. J. Neurosurg. Spine 2020, 32, 269–284. [Google Scholar] [CrossRef]
- Chen, W.C.; Liu, W.F.; Bai, Y.Y.; Zhou, Y.Y.; Zhang, Y.; Wang, C.M.; Lin, S.; He, H.F. Transplantation of mesenchymal stem cells for spinal cord injury: A systematic review and network meta-analysis. J. Transl. Med. 2021, 19, 178. [Google Scholar] [CrossRef]
- Tang, Q.R.; Xue, H.; Zhang, Q.; Guo, Y.; Xu, H.; Liu, Y.; Liu, J.M. Evaluation of the Clinical Efficacy of Stem Cell Transplantation in the Treatment of Spinal Cord Injury: A Systematic Review and Meta-analysis. Cell Transplant. 2021, 30, 9636897211067804. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.D.V.; Pickard, M.R.; Johnson, W.E.B. The comparative effects of mesenchymal stem cell transplantation therapy for spinal cord injury in humans and animal models: A systematic review and meta-analysis. Biology 2021, 10, 230. [Google Scholar] [CrossRef] [PubMed]
- Kvistad, C.E.; Kråkenes, T.; Gjerde, C.; Mustafa, K.; Rekand, T.; Bø, L. Safety and Clinical Efficacy of Mesenchymal Stem Cell Treatment in Traumatic Spinal Cord Injury, Multiple Sclerosis and Ischemic Stroke—A Systematic Review and Meta-Analysis. Front. Neurol. 2022, 13, 891514. [Google Scholar] [CrossRef]
- Xu, X.; Liang, Z.; Lin, Y.; Rao, J.; Lin, F.; Yang, Z.; Wang, R.; Chen, C. Comparing the Efficacy and Safety of Cell Transplantation for Spinal Cord Injury: A Systematic Review and Bayesian Network Meta-Analysis. Front. Cell. Neurosci. 2022, 16, 860131. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, H.; Wang, H.; Huang, J.; Yang, Y.; Li, G.; Yu, K.; Yang, L. A Comparative Study of Different Stem Cell Transplantation for Spinal Cord Injury: A Systematic Review and Network Meta-Analysis. World Neurosurg. 2022, 159, e232–e243. [Google Scholar] [CrossRef]
- Xiao, Z.; Tang, F.; Zhao, Y.; Han, G.; Yin, N.; Li, X.; Chen, B.; Han, S.; Jiang, X.; Yun, C.; et al. Significant Improvement of Acute Complete Spinal Cord Injury Patients Diagnosed by a Combined Criteria Implanted with NeuroRegen Scaffolds and Mesenchymal Stem Cells. Cell Transplant. 2018, 27, 907–915. [Google Scholar] [CrossRef]
- Li, Z.Y.; Bu, X.Y.; Zhang, S.X.; Liang, Q.H.; Li, T.P.; Chen, S.L.; Li, L.Y.; Zhao, Y.W.; Zhai, Y.P.; Zhang, Y.F. Autologous bone marrow mesenchymal stem cells in combination with peripheral nerve transplantion for treating spinal cord injury. J. Clin. Rehabil. Tissue Eng. Res. 2008, 12, 3041–3044. [Google Scholar]
- Zhao, Y.; Tang, F.; Xiao, Z.; Han, G.; Wang, N.; Yin, N.; Chen, B.; Jiang, X.; Yun, C.; Han, W.; et al. Clinical Study of NeuroRegen Scaffold Combined With Human Mesenchymal Stem Cells for the Repair of Chronic Complete Spinal Cord Injury. Cell Transplant. 2017, 26, 891–900. [Google Scholar] [CrossRef]
- Deng, W.S.; Ma, K.; Liang, B.; Liu, X.Y.; Xu, H.Y.; Zhang, J.; Shi, H.Y.; Sun, H.T.; Chen, X.Y.; Zhang, S. Collagen scaffold combined with human umbilical cord-mesenchymal stem cells transplantation for acute complete spinal cord injury. Neural Regen. Res. 2020, 15, 1686–1700. [Google Scholar]
- Vaquero, J.; Zurita, M.; Rico, M.A.; Aguayo, C.; Fernandez, C.; Rodriguez-Boto, G.; Marin, E.; Tapiador, N.; Sevilla, M.; Carballido, J.; et al. Cell therapy with autologous mesenchymal stromal cells in post-traumatic syringomyelia. Cytotherapy 2018, 20, 796–805. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 29, 372. [Google Scholar]
- Syková, E.; Homola, A.; Mazanec, R.; Lachmann, H.; Langkramer Konrádová, S.I.; Kobylka, P.; Pádr, R.; Neuwirth, J.; Komrska, V.; Vávra, V.; et al. Autologous Bone Marrow Transplantation in Patients With Subacute and Chronic Spinal Cord Injury. Cell Transplant. 2006, 15, 675–687. [Google Scholar] [CrossRef]
- Geffner, L.F.; Santacruz, P.; Izurieta, M.; Flor, L.; Maldonado, B.; Auad, A.H.; Montenegro, X.; Gonzalez, R.; Silva, F. Administration of Autologous Bone Marrow Stem Cells Into Spinal Cord Injury Patients Via Multiple Routes Is Safe and Improves Their Quality of Life: Comprehensive Case Studies. Cell Transplant. 2008, 17, 1277–1293. [Google Scholar] [CrossRef]
- Pal, R.; Venkataramana, N.K.; Bansal, A.; Balaraju, S.; Jan, M.; Chandra, R.; Dixit, A.; Rauthan, A.; Murgod, U.; Totey, S. Ex vivo-expanded autologous bone marrow-derived mesenchymal stromal cells in human spinal cord injury/paraplegia: A pilot clinical study. Cytotherapy 2009, 11, 897–911. [Google Scholar] [CrossRef]
- Bhanot, Y.; Rao, S.; Ghosh, D.; Balaraju, S.; Radhika, C.R.; Satish Kumar, K.V. Autologous mesenchymal stem cells in chronic spinal cord injury. Br. J. Neurosurg. 2011, 25, 516–522. [Google Scholar] [CrossRef]
- Ra, J.C.; Shin, I.S.; Kim, S.H.; Kang, S.K.; Kang, B.C.; Lee, H.Y.; Kim, Y.J.; Jo, J.Y.; Yoon, E.J.; Choi, H.J.; et al. Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans. Stem Cells Dev. 2011, 20, 1297–1308. [Google Scholar] [CrossRef]
- Karamouzian, S.; Nematollahi-Mahani, S.N.; Nakhaee, N.; Eskandary, H. Clinical safety and primary efficacy of bone marrow mesenchymal cell transplantation in subacute spinal cord injured patients. Clin. Neurol. Neurosurg. 2012, 114, 935–939. [Google Scholar] [CrossRef]
- Dai, G.; Liu, X.; Zhang, Z.; Yang, Z.; Dai, Y.; Xu, R. Transplantation of autologous bone marrow mesenchymal stem cells in the treatment of complete and chronic cervical spinal cord injury. Brain Res. 2013, 1533, 73–79. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, X.; Hua, R.; Dai, G.; Wang, X.; Gao, J.; An, Y. Clinical observation of umbilical cord mesenchymal stem cell transplantation in treatment for sequelae of thoracolumbar spinal cord injury. J. Transl. Med. 2014, 12, 253. [Google Scholar] [CrossRef]
- El-Kheir, W.A.; Gabr, H.; Awad, M.R.; Ghannam, O.; Barakat, Y.; Farghali, H.A.M.A.; El Maadawi, Z.M.; Ewes, I.; Sabaawy, H.E. Autologous bone marrow-derived cell therapy combined with physical therapy induces functional improvement in chronic spinal cord injury patients. Cell Transplant. 2014, 23, 729–745. [Google Scholar] [CrossRef]
- Mendonça, M.V.P.; Larocca, T.F.; Souza, B.S.D.F.; Villarreal, C.F.; Silva, L.F.M.; Matos, A.C.; Novaes, M.A.; Bahia, C.M.; de Oliveira Melo Martinez, A.C.; Kaneto, C.M.; et al. Safety and neurological assessments after autologous transplantation of bone marrow mesenchymal stem cells in subjects with chronic spinal cord injury. Stem Cell Res. Ther. 2014, 5, 126. [Google Scholar] [CrossRef] [PubMed]
- Hur, J.W.; Cho, T.H.; Park, D.H.; Lee, J.B.; Park, J.Y.; Chung, Y.G. Intrathecal transplantation of autologous adipose-derived mesenchymal stem cells for treating spinal cord injury: A human trial. J. Spinal Cord Med. 2016, 39, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.K.; Choi, K.H.; Yoo, J.Y.; Kim, D.Y.; Kim, S.J.; Jeon, S.R. A Phase III Clinical Trial Showing Limited Efficacy of Autologous Mesenchymal Stem Cell Therapy for Spinal Cord Injury. Neurosurgery 2016, 78, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Satti, H.S.; Waheed, A.; Ahmed, P.; Ahmed, K.; Akram, Z.; Aziz, T.; Satti, T.M.; Shahbaz, N.; Khan, M.A.; Malik, S.A. Autologous mesenchymal stromal cell transplantation for spinal cord injury: A Phase I pilot study. Cytotherapy 2016, 18, 518–522. [Google Scholar] [CrossRef]
- Thakkar, U.; Vanikar, A.; Trivedi, H.; Shah, V.; Dave, S.; Dixit, S.; Tiwari, B.B.; Shah, H.H. Infusion of autologous adipose tissue derived neuronal differentiated mesenchymal stem cells and hematopoietic stem cells in post-traumatic paraplegia offers a viable therapeutic approach. Adv. Biomed. Res. 2016, 5, 51. [Google Scholar]
- Vaquero, J.; Zurita, M.; Rico, M.A.; Bonilla, C.; Aguayo, C.; Montilla, J.; Bustamante, S.; Carballido, J.; Marin, E.; Martinez, F.; et al. An approach to personalized cell therapy in chronic complete paraplegia: The Puerta de Hierro phase I/II clinical trial. Cytotherapy 2016, 18, 1025–1036. [Google Scholar] [CrossRef]
- Larocca, T.F.; Macêdo, C.T.; Souza, B.S.d.F.; Andrade-Souza, Y.M.; Villarreal, C.F.; Matos, A.C.; Silva, D.N.; da Silva, K.; Moura de Souza, M.L.E.; da Silva Paixa, D.; et al. Image-guided percutaneous intralesional administration of mesenchymal stromal cells in subjects with chronic complete spinal cord injury: A pilot study. Cytotherapy 2017, 19, 1189–1196. [Google Scholar] [CrossRef]
- Vaquero, J.; Zurita, M.; Rico, M.A.; Bonilla, C.; Aguayo, C.; Fernández, C.; Tapiador, N.; Sevilla, M.; Morejon, C.; Montilla, J.; et al. Repeated subarachnoid administrations of autologous mesenchymal stromal cells supported in autologous plasma improve quality of life in patients suffering incomplete spinal cord injury. Cytotherapy 2017, 19, 349–359. [Google Scholar] [CrossRef]
- Vaquero, J.; Zurita, M.; Rico, M.A.; Aguayo, C.; Bonilla, C.; Marin, E.; Tapiador, N.; Sevilla, M.; Vázquez, D.; Carballido, J.; et al. Intrathecal administration of autologous mesenchymal stromal cells for spinal cord injury: Safety and efficacy of the 100/3 guideline. Cytotherapy 2018, 20, 806–819. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, L.; Sun, W.; Li, W.; Wang, K. Therapeutic effect of mesenchymal stem cell in spinal cord injury. Int. J. Clin. Exp. Med. 2020, 13, 1979–1986. [Google Scholar]
- Albu, S.; Kumru, H.; Coll, R.; Vives, J.; Vallés, M.; Benito-Penalva, J.; Rodriguez, L.; Codinach, M.; Hernández, J.; Navarro, X.; et al. Clinical effects of intrathecal administration of expanded Wharton jelly mesenchymal stromal cells in patients with chronic complete spinal cord injury: A randomized controlled study. Cytotherapy 2021, 23, 146–156. [Google Scholar] [CrossRef]
- Honmou, O.; Yamashita, T.; Morita, T.; Oshigiri, T.; Hirota, R.; Iyama, S.; Kato, J.; Sasaki, Y.; Ishiai, S.; Ito, Y.M.; et al. Intravenous infusion of auto serum-expanded autologous mesenchymal stem cells in spinal cord injury patients: 13 case series. Clin. Neurol. Neurosurg. 2021, 203, 106565. [Google Scholar] [CrossRef]
- Yang, Y.; Pang, M.; Du, C.; Liu, Z.Y.; Chen, Z.H.; Wang, N.X.; Zhang, L.M.; Chen, Y.Y.; Mo, J.; Dong, J.W.; et al. Repeated subarachnoid administrations of allogeneic human umbilical cord mesenchymal stem cells for spinal cord injury: A phase 1/2 pilot study. Cytotherapy 2021, 23, 57–64. [Google Scholar] [CrossRef]
- Castillo-Melendez, M.; Yawno, T.; Jenkin, G.; Miller, S.L. Stem cell therapy to protect and repair the developing brain: A review of mechanisms of action of cord blood and amnion epithelial derived cells. Front. Neurosci. 2013, 7, 194. [Google Scholar] [CrossRef]
- Evaniew, N.; Sharifi, B.; Waheed, Z.; Fallah, N.; Ailon, T.; Dea, N.; Paquette, S.; Charest-Morin, R.; Street, J.; Fisher, C.G.; et al. The influence of neurological examination timing within hours after acute traumatic spinal cord injuries: An observational study. Spinal Cord 2020, 58, 247–254. [Google Scholar] [CrossRef]
- Burns, A.; Lee, B.S.; Ditunno, J.F., Jr.; Tessler, A. Patient selection for clinical trials: The reliability of the early spinal cord injury examination. J. Neurotrauma 2023, 18, 299–305. [Google Scholar] [CrossRef]
- Cunningham, C.J.; Viskontas, M.; Janowicz, J.; Sani, Y.; Hakansson, M.E.; Heidari, A.; Huang, W.; Bo, X. The potential of gene therapies for spinal cord injury repair: A systematic review and meta-analysis of pre-clinical studies. Neural Regen. Res. 2023, 18, 299–305. [Google Scholar] [CrossRef]
- Gong, W.; Zhang, T.; Che, M.; Wang, Y.; He, C.; Liu, L.; Lv, Z.; Xiao, C.; Wang, H.; Zhang, S. Recent advances in nanomaterials for the treatment of spinal cord injury. Mater. Today Bio 2023, 18, 100524. [Google Scholar] [CrossRef]
- Wagner, F.B.; Mignardot, J.B.; Goff-Mignardot, C.G.; Demesmaeker, R.; Komi, S.; Capogrosso, M.; Rowald, A.; Seáñez, I.; Caban, M.; Pirondini, E.; et al. Targeted neurotechnology restores walking in humans with spinal cord injury. Nature 2018, 563, 65–71. [Google Scholar] [CrossRef]
- Rowald, A.; Komi, S.; Demesmaeker, R.; Baaklini, E.; Hernandez-Charpak, S.D.; Paoles, E.; Montanaro, H.; Antonino Cassara, A.; Becce, F.; Lloyd, B.; et al. Activity-dependent spinal cord neuromodulation rapidly restores trunk and leg motor functions after complete paralysis. Nat. Med. 2022, 28, 260–271. [Google Scholar] [CrossRef]
| Warning Signs and Symptoms of Spinal Cord Injury |
|---|
| -Limb weakness or paralysis |
| -Trunk or limb sensory disorders |
| -Difficulties with language skills (hypophonia) |
| -Abdominal breathing |
| -Hypotension and paradoxical bradycardia |
| -Elbow flexion position |
| -Spinal cord pain or deformity |
| -Paresthesias or electric shock feeling |
| -Absence of pain when predictably painful lesions exist |
| -Priapism |
| A = Complete. no sensory or motor function is preserved in the sacral segments S4–S5 |
| B = Sensory incomplete. Sensory but not motor function is preserved below the neurological level and includes the sacral segments S4-S5 (light touch or pin-prick at S4–S5 or deep anal pressure) AND no motor function is preserved more than three levels below the motor level on either side of the body |
| C = Motor incomplete. Motor function is preserved below the neurological level AND more than half of the key muscle functions below the neurological level of injury have a muscle grade less than 3 (grades 0–2) |
| D = Motor incomplete. Motor function is preserved below the neurological level AND at least half (half or more) of the key muscle functions below the neurological level of injury have a muscle grade ≥3 |
| E = Normal. If sensation and motor function as tested with the ISNCSCI are graded as normal in all segments AND the patient has prior deficits, then the AIS grade is E. Someone without an initial SCI does not receive an AIS grade |
| Article | Journal | Type of Study | Randomi-Sation | Control Group | Methodological Quality (PEDro Scale) |
|---|---|---|---|---|---|
| Syková E et al. [97], 2006 | Cell Transplantion | Clinical trial | NO | NO | 3 |
| Geffner L et al. [98], 2008 | Cell Transplantion | Clinical trial | NO | NO | 4 |
| Pal R et al. [99], 2009 | Cytotherapy | Clinical trial | NO | NO | 3 |
| Bhanot Y et al. [100], 2011 | British Journal of Neurosurgery | Clinical trial | NO | NO | 4 |
| Ra J et al. [101], 2011 | Stem Cells and Development | Clinical trial | NO | NO | 3 |
| Karamouzian S et al. [102], 2012 | Clinical Neurology and Neurosurgery | Clinical trial | NO | YES | 3 |
| Dai G et al. [103], 2013 | Brain Research | Clinical trial | YES | YES | 5 |
| Cheng H et al. [104], 2014 | Journal of Translational Medicine | Clinical trial | YES | YES | 8 |
| El-Kheir W et al. [105], 2014 | Cell Transplantion | Clinical trial | YES | YES | 7 |
| Mendonça M et al. [106], 2014 | Stem Cell Research and Therapy | Clinical trial | NO | NO | 6 |
| Hur J et al. [107], 2016 | Journal of Spinal Cord Medicine | Clinical trial | NO | NO | 4 |
| Oh S et al. [108], 2016 | Neurosurgery | Clinical trial | NO | NO | 6 |
| Satti H et al. [109], 2016 | Cytotherapy | Clinical trial | NO | NO | 4 |
| Thakkar U et al. [110], 2016 | Advanced Biomedical Research | Clinical trial | NO | NO | 4 |
| Vaquero J et al. [111], 2016 | Cytotherapy | Clinical trial | NO | NO | 5 |
| Larocca T et al. [112], 2017 | Cytotherapy | Clinical trial | NO | NO | 4 |
| Vaquero J et al. [113], 2017 | Cytotherapy | Clinical trial | NO | NO | 4 |
| Vaquero J et al. [114], 2018 | Cytotherapy | Clinical trial | NO | NO | 5 |
| Yang Yalin Z et al. [115], 2020 | International Journal of Clinical and Experimental Medicine | Clinical trial | YES | YES | 8 |
| Albu S et al. [116], 2021 | Cytotherapy | Clinical trial | YES | NO | 9 |
| Honmou O et al. [117], 2021 | Clinical Neurology and Neurosurgery | Case Series | NO | NO | |
| Yang Y et al. [118], 2021 | Cytotherapy | Clinical trial | NO | NO | 6 |
| Article | Age | Gender | Sample Size | Type of TSCI | Injury Level | Type of Mesenchymal Stem Cell | Route of Administration | |
|---|---|---|---|---|---|---|---|---|
| Men | Women | |||||||
| Syková E et al. [97], 2006 | 19–41 | 16 | 4 | 20 | 13 Chronic 7 Subacute | 12 Cervical 8 Thoracic | Autologous bone marrow | Intra-arterial Intravenous |
| Geffner L et al. [98], 2008 | 28–44 | 7 | 1 | 8 | 4 Chronic 4 Acute | Thoracic | Autologous bone marrow | Intralesional Intrathecal Intravenous |
| Pal R et al. [99], 2009 | 17–56 | 27 | 3 | 30 | 10 Subacute Chronic | 7 Cervical 23 Thoracic | Autologous bone marrow | Intrathecal |
| Bhanot Y et al. [100] 2011 | 18–52 | 10 | 3 | 13 | Chronic | 5 Cervical 8 Thoracic | Autologous bone marrow | Intralesional Intrathecal |
| Ra J et al. [101], 2011 | N.D. | 8 | 0 | 8 | Chronic | Unspecified | Autologous adipose tissue | Intravenous |
| Karamouzian S et al. [102], 2012 | 23–48 | 24 | 7 | 31 | Subacute | Thoracic | Autologous bone marrow | Intrathecal |
| Dai G et al. [103], 2013 | 22–54 | 28 | 12 | 40 | Chronic | 40 Cervical | Autologous bone marrow | Intrathecal |
| Cheng H et al. [104], 2014 | 27–43 | Unspecified | 10 | Chronic | 10 Dorsal-lumbar | Allogeneic umbilical cord cells | Intralesional | |
| El-Kheir W et al. [105], 2014 | 16–45 | 61 | 9 | 70 | Chronic | 53 Thoracic 17 Cervical | Autologous bone marrow | Intrathecal |
| Mendonça M et al. [106], 2014 | 18–65 | 10 | 4 | 14 | Chronic | 14 Dorsal-lumbar | Autologous bone marrow | Intrathecal |
| Hur J et al. [107], 2016 | 20–66 | 12 | 2 | 14 | 10 Chronic 4 Subacute | 6 Cervical 7 Thoracic 1 Lumbar | Autologous adipose tissue | Intrathecal |
| Oh S et al. [108], 2016 | 18–65 | Unspecified | 16 | Chronic | Cervical | Autologous bone marrow | Intralesional Intrathecal | |
| Satti H et al. [109], 2016 | 24–38 | Unspecified | 9 | 6 Chronic 3 Subacute | Thoracic | Autologous bone marrow | Intrathecal | |
| Thakkar U et al. [110], 2016 | 9–42 | 8 | 2 | 10 | Chronic | 6 Dorsal 3 Dorsal-lumbar 1 Lumbar | Autologous adipose tissue | Intrathecal |
| Vaquero J et al. [111], 2016 | 32–50 | 9 | 3 | 12 | Chronic | Thoracic | Autologous bone marrow | Intralesional Intrathecal |
| Larocca T et al. [112], 2017 | 36–52 | 5 | 0 | 5 | Chronic | Thoracic | Autologous bone marrow | Intrathecal |
| Vaquero J et al. [113], 2017 | 33–51 | 8 | 2 | 10 | Chronic | 5 Cervical 2 Thoracic 3 Lumbar | Autologous bone marrow | Intrathecal |
| Vaquero J et al. [114], 2018 | 28–62 | 7 | 4 | 11 | Chronic | 4 Cervical 4 Thoracic 3 Dorsal-lumbar | Autologous bone marrow | Intrathecal |
| Yang Yalin et al. [115], 2020 | 27–43 | 53 | 15 | 68 | Chronic | 44 Cervical 24 Thoracic | Autologous bone marrow | Intrathecal |
| Albu S et al. [116], 2021 | 25–47 | 7 | 3 | 10 | Chronic | Thoracic | Allogeneic umbilical cord cells | Intrathecal |
| Honmou O et al. [117], 2021 | 21–66 | 12 | 1 | 13 | Acute | Cervical | Autologous bone marrow | Intravenous |
| Yang Y et al. [118], 2021 | 18–65 | 33 | 8 | 41 | Chronic | 24 Cervical 7 Thoracic 10 Dorsal-lumbar | Allogeneic umbilical cord cells | Intrathecal |
| AIS * Grade | AIS Grade Improvement | ASIA ** Sensory Score Improvement | ASIA Motor Score Improvement | Image Test (MRI) | Improvement in Neurophysiological Studies | Improvement in Urodynamic Studies | Serious Adverse Effects | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | Yes | No | Yes | No | Yes | No | |||||
| Syková E et al. [97], 2006 | 15 | 4 | 1 | 0 | 3 | 17 | 6 | 14 | 6 | 14 | Gliosis | 4 Improve in SSEPs *** 3 Improve in MEPs **** | Not done | No |
| Geffner L et al. [98], 2008 | 5 | 1 | 2 | 0 | 6 | 2 | 8 | 0 | 8 | 0 | Reduction of cavity and lesion hyperintensity | Not done | Yes | No |
| Pal R et al. [99], 2009 | 24 | 0 | 6 | 0 | No improvement | Non-significant improvement | Non-significant improvement | No significant changes | No significant changes | 3 Improved sphincter control | No | |||
| Bhanot Y et al. [100], 2011 | Unspecified | 1 | 12 | 3 | 9 | No improvement | Not done | Not done | 1 Improved bladder fullness sensation | Mild side effects | ||||
| Ra J et al. [101], 2011 | Unspecified | Unspecified | Unspecified | Unspecified | No significant changes | Not done | Not done | No | ||||||
| Karamouzian S et al. [102], 2012 | Unspecified | 5 | 11 | Significant improvement | Significant improvement | Not done | Not done | Not done | No | |||||
| Dai G et al. [103], 2013 | 40 | 0 | 0 | 0 | 9 | 11 | Significant improvement | Significant improvement | No significant changes | Yes | Reduction in residual urine volume | No | ||
| Cheng H et al. [104], 2014 | 10 | 0 | 0 | 0 | Unspecified | Non-significant improvement | Non-significant improvement | Not done | Not done | Yes | No | |||
| El-Kheir W et al. [105], 2014 | 25 | 45 | 0 | 0 | 17 | 33 | Significant improvement | Significant improvement | Gliosis and reduced lesion hyperintensity | Yes | Not done | No | ||
| Mendonça M et al. [106], 2014 | 14 | 0 | 0 | 0 | 7 | 7 | 8 | 6 | 14 | 0 | No significant changes | Improve in SSEPs | Yes | No |
| Oh S et al. [107], 2016 | 1 | 15 | 0 | 0 | No improvement | Unspecified | 2 | 14 | 5 Increase in medullary diameter 1 Disappearance of cavity 3 Reduction of cavity | Yes | Unspecified | 8 Mild adverse events | ||
| Satti H et al. [108], 2016 | 9 | 0 | 0 | 0 | Unspecified | Unspecified | Unspecified | No significant changes | Not done | Unspecified | 3 Mild adverse events | |||
| Hur J et al. [109], 2016 | 12 | 1 | 0 | 1 | No improvement | 10 | 4 | 5 | 9 | No significant changes | Minimum SSEPs change | Unspecified | 4 Mild adverse events | |
| Thakkar et al. [110], 2016 | 10 | 0 | 0 | 0 | 10 | 0 | Unspecified | Unspecified | Not done | Not done | 5 Improved bladder fullness sensation | Mild adverse events | ||
| Vaquero J et al. [111], 2016 | 12 | 0 | 0 | 0 | 4 | 8 | 12 | 0 | 9 | 3 | 5 Reduction or disappearance of lesion hyperintensity | Yes | Improvement in bladder capacity and compliance and in the detrusor muscle pressure | 69 Mild to moderate adverse events (22 related to surgery) |
| Larocca T et al. [112], 2017 | 5 | 0 | 0 | 0 | 1 | 4 | 3 | 2 | No improvement | Not done | Not done | 2 Improved sphincter control, 1 Bladder filling sensation | No | |
| Vaquero J et al. [113], 2017 | 0 | 4 | 5 | 1 | Significant improvement | Significant improvement | Significant improvement | No significant changes | Yes | Improvement in bladder capacity and compliance and in the detrusor muscle pressure | Acute non-treatment-related bronchitis | |||
| Vaquero J et al. [114], 2018 | 3 | 4 | 3 | 1 | 3 | 8 | Significant improvement | Significant improvement | No significant changes | Yes | Improvement in bladder capacity and compliance and in the detrusor muscle pressure | No | ||
| Yang Yalin et al. [115], 2020 | 42 | 16 | 10 | 0 | Unspecified | Significant improvement | Significant improvement | Not done | Not done | Not done | 64 Mild adverse events | |||
| Albu S et al. [116], 2021 | 10 | 0 | 0 | 0 | No improvement | Significant improvement | No improvement | Not done | No significant changes | No significant changes | No | |||
| Honmou O et al. [117], 2021 | 6 | 5 | 2 | 0 | 12 | 1 | Unspecified | Unspecified | Not done | Not done | Unspecified | No | ||
| Yang Y et al. [118], 2021 | Unspecified | Unspecified | Significant improvement | Significant improvement | Unspecified | Not done | Reduction in residual urine volume | 81 Mild adverse events | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montoto-Meijide, R.; Meijide-Faílde, R.; Díaz-Prado, S.M.; Montoto-Marqués, A. Mesenchymal Stem Cell Therapy in Traumatic Spinal Cord Injury: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 11719. https://doi.org/10.3390/ijms241411719
Montoto-Meijide R, Meijide-Faílde R, Díaz-Prado SM, Montoto-Marqués A. Mesenchymal Stem Cell Therapy in Traumatic Spinal Cord Injury: A Systematic Review. International Journal of Molecular Sciences. 2023; 24(14):11719. https://doi.org/10.3390/ijms241411719
Chicago/Turabian StyleMontoto-Meijide, Rodrigo, Rosa Meijide-Faílde, Silvia María Díaz-Prado, and Antonio Montoto-Marqués. 2023. "Mesenchymal Stem Cell Therapy in Traumatic Spinal Cord Injury: A Systematic Review" International Journal of Molecular Sciences 24, no. 14: 11719. https://doi.org/10.3390/ijms241411719
APA StyleMontoto-Meijide, R., Meijide-Faílde, R., Díaz-Prado, S. M., & Montoto-Marqués, A. (2023). Mesenchymal Stem Cell Therapy in Traumatic Spinal Cord Injury: A Systematic Review. International Journal of Molecular Sciences, 24(14), 11719. https://doi.org/10.3390/ijms241411719






