Bacterial Biofilm Formation on Biomaterials and Approaches to Its Treatment and Prevention
Abstract
1. Introduction
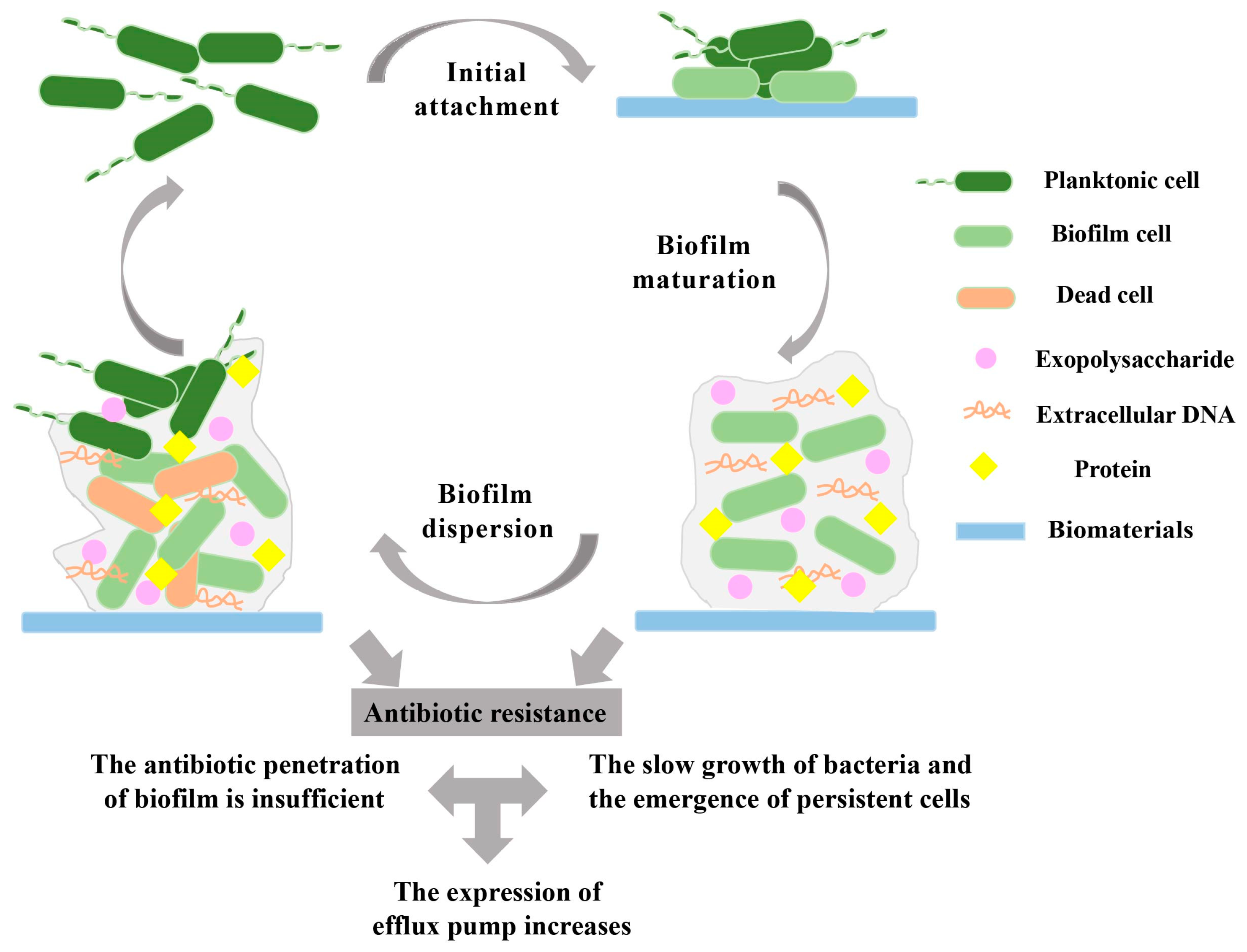
2. Biofilm Formation on Biomaterials
2.1. Biofilm Initial Attachment
2.2. Biofilm Maturation
2.3. Biofilm Dispersion
3. Mechanisms of Antibiotic-Resistant Biofilms
3.1. Prevention of Antibiotic Penetration by Biofilms
3.2. Presence of Slow-Growing or Persistent Cells in Biofilms
3.3. Increased Expression of Efflux Pumps in Biofilms
4. Clinical Characteristics of Biomaterial-Associated Biofilms
- (1)
- Persistent infection (more than 7 days): For example, central venous catheter-associated biofilms and almost all indwelling central venous catheters are colonized by microorganisms that can produce biofilms. Common bacteria isolated from catheter biofilms are coagulase-negative Staphylococcus [105]. Colonization and biofilm formation on catheter surfaces by these microorganisms can occur within 24 h of insertion and are pervasive and persistent [106]. In one study, short-term (<10 days) catheters had greater biofilm formation on the external surface; long-term catheters (>30 days) had more biofilm formation on the catheter inner lumen [42].
- (2)
- Inflammation: An example is a biofilm infection associated with mechanical heart valves. Infectious endocarditis (IE) is a disease with substantial morbidity and mortality today due to the increasing use of implantable devices, such as prosthetic heart valves [107]. Microbes initially adhere to the prosthetic valve surface via fibronectin and polysaccharides and grow in a platelet–fibrin matrix to form an intact biofilm [108]. Because biofilms formed on artificial surfaces promote inflammatory responses and hypercoagulability, the presence of foreign tissue needs to be maintained through inflammatory and thrombotic processes [109]. In addition, prosthetic joint infection (PJI), a serious complication after joint replacement, causes inflammation [110]. According to the symptoms that appear after implantation, PJIs can be divided into three categories: early infection, delayed infection, and late infection [111]. Both early and delayed infections are often the result of surgical contamination and are considered the most common cause of biomaterial-related biofilm infections. These infections are often associated with local and systemic symptoms, in addition to triggering an inflammatory response that is accompanied by increases in laboratory markers of inflammation, such as C-reactive protein, erythrocyte sedimentation rate, and white blood cell count levels [112,113,114]. Therefore, in addition to routine methods for detecting biofilm-associated infections, blood and tissue cultures can be used to detect PJI infections at an early stage [113,115].
- (3)
- Failure of antibiotic treatment and recurrence of infection: It is the formation of biofilms on the surface of biomaterials that leads to the development of drug resistance in the treatment of related infections [116]. The formation of a biofilm matrix can maintain temporary dormancy of the cells, which may lead to repeated infections [117]. For example, the treatment of PJI requires complex therapeutic strategies, and it is the long-term infection problems that lead to multiple surgical revisions and long-term antimicrobial therapy [110].
| Application | Biomaterials | Infection-Causing Agents | References |
|---|---|---|---|
| Orthopedic devices | Stainless steel, cobalt-based alloys, titanium, silicone, polyethylene, polypropylene, polymethyl methacrylate, aluminum oxide, and calcium phosphates | S. epidermidis, S. aureus, and Staphylococcus hominis | [118] |
| Prosthetic heart valves | Titanium, graphite, pyrolytic carbon, polyethylene, polypropylene, polyamide, and diamond-like carbon | S. aureus, S. epidermidis, Candida albicans, and Aspergillus | [119] |
| Dental implants | Acrylic resin, titanium and its alloys, zirconia, silver and silver nanoparticles, and ZnO | Gram-negative anaerobic bacteria (Porphiromonas gengivalis, Actinobacillus actinomycetemcomitans, Fusobacterium nucleatum, and Veillonella spp.) | [120] |
| Urologic devices | Polytetrafluoroethylene, rubber, polyurethane, polyamide, silicones, and polyhydroxyalkonates | S. aureus and S. epidermis | [121] |
| Bone allografts | Calcium phosphate ceramic, calcium sulphate, hydroxyapatite, bioactive glasses, and magnesium | Hepatitis, tuberculosis, and human immunodeficiency virus | [122,123] |
| Contact lenses and corneal implants | Silicone hydrogel and polymethylmethacrylate | P. aeruginosa and Gram-positive cocci | [124] |
| Breast implants | Silicone gel within silicone rubber envelopes and inflatable saline | S. aureus, Enterococcus spp., S. epidermidis, and P. acnes | [125,126,127] |
5. Treatment of Biofilms on the Surface of Biomaterials
5.1. Antibacterial Coatings
5.1.1. Active Coatings
5.1.2. Passive Coatings
5.2. Modification of the Surface of Medical Implants
5.2.1. Physical Modifications
5.2.2. Chemical Modification
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Huang, M.; Melander, C.; Kjellerup, B.V. Dispersal and inhibition of biofilms associated with infections. J. Appl. Microbiol. 2020, 128, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Assefa, M.; Amare, A. Biofilm-associated multi-drug resistance in hospital-acquired infections: A review. Infect. Drug Resist. 2022, 15, 5061–5068. [Google Scholar] [CrossRef]
- Tran, H.M.; Tran, H.; Booth, M.A.; Fox, K.E.; Nguyen, T.H.; Tran, N.; Tran, P.A. Nanomaterials for treating bacterial biofilms on implantable medical devices. Nanomaterials 2020, 10, 2253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; de Boer, L.; Stockhammer, O.W.; Grijpma, D.W.; Spaink, H.P.; Zaat, S.A.J. A zebrafish embryo model for in vivo visualization and intravital analysis of biomaterial-associated staphylococcus aureus Infection. J. Vis. Exp. 2019, 143, 58523. [Google Scholar]
- Rosman, C.W.K.; van Dijl, J.M.; Sjollema, J. Interactions between the foreign body reaction and Staphylococcus aureus biomaterial-associated infection. Winning strategies in the derby on biomaterial implant surfaces. Crit. Rev. Microbiol. 2022, 48, 624–640. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Rodriguez, J.A.; Shaqour, B.; Guarch-Pérez, C.; Choińska, E.; Riool, M.; Verleije, B.; Beyers, K.; Costantini, V.J.A.; Święszkowski, W.; Zaat, S.A.J.; et al. A niclosamide-releasing hot-melt extruded catheter prevents Staphylococcus aureus experimental biomaterial-associated infection. Sci. Rep. 2022, 12, 12329. [Google Scholar] [CrossRef]
- Gebreyohannes, G.; Nyerere, A.; Bii, C.; Sbhatu, D.B. Challenges of intervention, treatment, and antibiotic resistance of biofilm-forming microorganisms. Heliyon 2019, 5, e02192. [Google Scholar] [CrossRef]
- Cao, H.; Qiao, S.; Qin, H.; Jandt, K.D. Antibacterial designs for implantable medical devices: Evolutions and challenges. J. Funct. Biomater. 2022, 13, 86. [Google Scholar] [CrossRef]
- Premkumar, A.; Kolin, D.A.; Farley, K.X.; Wilson, J.M.; McLawhorn, A.S.; Cross, M.B.; Sculco, P.K. Projected economic burden of periprosthetic joint infection of the hip and knee in the united states. J. Arthroplast. 2021, 36, 1484–1489. [Google Scholar] [CrossRef]
- Luo, Y.; Yang, Q.; Zhang, D.; Yan, W. Mechanisms and control strategies of antibiotic resistance in pathological biofilms. J. Microbiol. Biotechnol. 2021, 31, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Gupta, A.; Upadhye, V.; Singh, S.C.; Sinha, R.P.; Häder, D.P. Therapeutic strategies against biofilm infections. Life 2023, 13, 172. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yao, H.; Zhao, X.; Ge, C. Biofilm formation and control of foodborne pathogenic bacteria. Molecules 2023, 28, 2432. [Google Scholar] [CrossRef] [PubMed]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef]
- Wang, D.; Fletcher, G.C.; On, S.L.W.; Palmer, J.S.; Gagic, D.; Flint, S.H. Biofilm formation, sodium hypochlorite susceptibility and genetic diversity of Vibrio parahaemolyticus. Int. J. Food Microbiol. 2023, 385, 110011. [Google Scholar] [CrossRef]
- Sauer, K.; Stoodley, P.; Goeres, D.M.; Hall-Stoodley, L.; Burmølle, M.; Stewart, P.S.; Bjarnsholt, T. The biofilm life cycle: Expanding the conceptual model of biofilm formation. Nat. Rev. Microbiol. 2022, 20, 608–620. [Google Scholar] [CrossRef]
- Öztürk, F.Y.; Darcan, C.; Kariptaş, E. The determination, monitoring, molecular mechanisms and formation of biofilm in E. coli. Braz. J. Microbiol. 2023, 54, 259–277. [Google Scholar] [CrossRef]
- Rabin, N.; Zheng, Y.; Opoku-Temeng, C.; Du, Y.; Bonsu, E.; Sintim, H.O. Biofilm formation mechanisms and targets for developing antibiofilm agents. Future Med. Chem. 2015, 7, 493–512. [Google Scholar] [CrossRef]
- De Silva, L.; Heo, G.J. Biofilm formation of pathogenic bacteria isolated from aquatic animals. Arch. Microbiol. 2022, 205, 36. [Google Scholar] [CrossRef]
- Abebe, G.M. The role of bacterial biofilm in antibiotic resistance and food contamination. Int. J. Microbiol. 2020, 2020, 1705814. [Google Scholar] [CrossRef]
- Gupta, P.; Sarkar, S.; Das, B.; Bhattacharjee, S.; Tribedi, P. Biofilm, pathogenesis and prevention—A journey to break the wall: A review. Arch. Microbiol. 2016, 198, 1–15. [Google Scholar] [CrossRef]
- Stoica, P.; Chifiriuc, M.C.; Rapa, M.; Lazăr, V. Overview of biofilm-related problems in medical devices. In Biofilms and Implantable Medical Devices; Elsevier: Amsterdam, The Netherlands, 2017; pp. 3–23. [Google Scholar]
- Li, P.; Zong, W.; Zhang, Z.; Lv, W.; Ji, X.; Zhu, D.; Du, X.; Wang, S. Effects and molecular mechanism of flagellar gene flgK on the motility, adhesion/invasion, and desiccation resistance of Cronobacter sakazakii. Food Res. Int. 2023, 164, 112418. [Google Scholar] [CrossRef] [PubMed]
- Nedeljković, M.; Sastre, D.E.; Sundberg, E.J. Bacterial flagellar filament: A supramolecular multifunctional nanostructure. Int. J. Mol. Sci. 2021, 22, 7521. [Google Scholar] [CrossRef]
- Kolenda, R.; Ugorski, M.; Grzymajlo, K. Everything you always wanted to know about salmonella type 1 fimbriae, but were afraid to ask. Front. Microbiol. 2019, 10, 1017. [Google Scholar] [CrossRef] [PubMed]
- Puri, D.; Fang, X.; Allison, K.R. Evidence of a possible multicellular life cycle in Escherichia coli. iScience 2023, 26, 105795. [Google Scholar] [CrossRef]
- Azara, E.; Longheu, C.M.; Attene, S.; Sanna, S.; Sale, M.; Addis, M.F.; Tola, S. Comparative profiling of agr locus, virulence, and biofilm-production genes of human and ovine non-aureus staphylococci. BMC Vet. Res. 2022, 18, 212. [Google Scholar] [CrossRef]
- Mack, D.; Fischer, W.; Krokotsch, A.; Leopold, K.; Hartmann, R.; Egge, H.; Laufs, R. The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear beta-1,6-linked glucosaminoglycan: Purification and structural analysis. J. Bacteriol. 1996, 178, 175–183. [Google Scholar] [CrossRef]
- Lv, Z.T.; Qian, C.; Liu, Y.N.; Lv, Y.H.; Liu, X.W. Optical tracking of surfactant-tuned bacterial adhesion: A single-cell imaging study. Appl. Environ. Microbiol. 2022, 88, e0162622. [Google Scholar] [CrossRef] [PubMed]
- Pellielo, G.; Agyapong, E.D.; Pinton, P.; Rimessi, A. Control of mitochondrial functions by Pseudomonas aeruginosa in cystic fibrosis. Int. Rev. Cell Mol. Biol. 2023, 377, 19–43. [Google Scholar]
- Mariappan, V.; Thimma, J.; Vellasamy, K.M.; Shankar, E.M.; Vadivelu, J. Adhesion and invasion attributes of Burkholderia pseudomallei are dependent on airway surface liquid and glucose concentrations in lung epithelial cells. Environ. Microbiol. Rep. 2018, 10, 217–225. [Google Scholar] [CrossRef]
- Wales, A.D.; Woodward, M.J.; Pearson, G.R. Attaching-effacing bacteria in animals. J. Comp. Pathol. 2005, 132, 1–26. [Google Scholar] [CrossRef]
- Pierrat, X.; Wong, J.P.H.; Al-Mayyah, Z.; Persat, A. The mammalian membrane microenvironment regulates the sequential attachment of bacteria to host cells. mBio 2021, 12, e0139221. [Google Scholar] [CrossRef] [PubMed]
- Federle, M.J.; Bassler, B.L. Interspecies communication in bacteria. J. Clin. Investig. 2003, 112, 1291–1299. [Google Scholar] [CrossRef]
- Xiao, Y.; Zou, H.; Li, J.; Song, T.; Lv, W.; Wang, W.; Wang, Z.; Tao, S. Impact of quorum sensing signaling molecules in gram-negative bacteria on host cells: Current understanding and future perspectives. Gut Microbes. 2022, 14, 2039048. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Cheng, J.; Wang, Y.; Shen, X. The Pseudomonas Quinolone Signal (PQS): Not just for quorum sensing anymore. Front. Cell Infect. Microbiol. 2018, 8, 230. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bian, Z.; Wang, Y. Biofilm formation and inhibition mediated by bacterial quorum sensing. Appl. Microbiol. Biotechnol. 2022, 106, 6365–6381. [Google Scholar] [CrossRef] [PubMed]
- Cooke, A.C.; Florez, C.; Dunshee, E.B.; Lieber, A.D.; Terry, M.L.; Light, C.J.; Schertzer, J.W. Pseudomonas Quinolone Signal-induced outer membrane vesicles enhance biofilm dipersion in Pseudomonas aeruginosa. mSphere 2020, 5, e01109-20. [Google Scholar] [CrossRef]
- Allesen-Holm, M.; Barken, K.B.; Yang, L.; Klausen, M.; Webb, J.S.; Kjelleberg, S.; Molin, S.; Givskov, M.; Tolker-Nielsen, T. A characterization of DNA release in Pseudomonas aeruginosa cultures and biofilms. Mol. Microbiol. 2006, 59, 1114–1128. [Google Scholar] [CrossRef]
- Fu, H.; Wang, J.; Liu, Q.; Ding, L.; Ren, H. The role of immobilized quorum sensing strain in promoting biofilm formation of Moving Bed Biofilm Reactor during long-term stable operation. Environ. Res. 2022, 215, 114159. [Google Scholar] [CrossRef]
- Yin, R.; Cheng, J.; Wang, J.; Li, P.; Lin, J. Treatment of Pseudomonas aeruginosa infectious biofilms: Challenges and strategies. Front. Microbiol. 2022, 13, 955286. [Google Scholar] [CrossRef]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Parsek, M.R.; Singh, P.K. Bacterial biofilms: An emerging link to disease pathogenesis. Annu. Rev. Microbiol. 2003, 57, 677–701. [Google Scholar] [CrossRef]
- Wimpenny, J.; Manz, W.; Szewzyk, U. Heterogeneity in biofilms. FEMS Microbiol. Rev. 2000, 24, 661–671. [Google Scholar] [CrossRef]
- Schilcher, K.; Horswill, A.R. Staphylococcal Biofilm Development: Structure, Regulation, and Treatment Strategies. Microbiol. Mol. Biol. Rev. 2020, 84, e00026-19. [Google Scholar] [CrossRef] [PubMed]
- Morelli, K.A.; Kerkaert, J.D.; Cramer, R.A. Aspergillus fumigatus biofilms: Toward understanding how growth as a multicellular network increases antifungal resistance and disease progression. PLoS Pathog. 2021, 17, e1009794. [Google Scholar] [CrossRef]
- Sauer, K.; Cullen, M.C.; Rickard, A.H.; Zeef, L.A.; Davies, D.G.; Gilbert, P. Characterization of nutrient-induced dispersion in Pseudomonas aeruginosa PAO1 biofilm. J. Bacteriol. 2004, 186, 7312–7326. [Google Scholar] [CrossRef] [PubMed]
- Kostakioti, M.; Hadjifrangiskou, M.; Hultgren, S.J. Bacterial biofilms: Development, dispersal, and therapeutic strategies in the dawn of the postantibiotic era. Cold Spring Harb. Perspect. Med. 2013, 3, a010306. [Google Scholar] [CrossRef]
- Shen, D.; Langenheder, S.; Jürgens, K. Dispersal modifies the diversity and composition of active bacterial communities in response to a salinity disturbance. Front. Microbiol. 2018, 9, 2188. [Google Scholar] [CrossRef]
- Wille, J.; Coenye, T. Biofilm dispersion: The key to biofilm eradication or opening Pandora’s box? Biofilm 2020, 2, 100027. [Google Scholar] [CrossRef]
- Fleming, D.; Rumbaugh, K.P. Approaches to dispersing medical biofilms. Microorganisms 2017, 5, 15. [Google Scholar] [CrossRef]
- Oppenheimer-Shaanan, Y.; Steinberg, N.; Kolodkin-Gal, I. Small molecules are natural triggers for the disassembly of biofilms. Trends Microbiol. 2013, 21, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Veerachamy, S.; Yarlagadda, T.; Manivasagam, G.; Yarlagadda, P.K. Bacterial adherence and biofilm formation on medical implants: A review. Proc. Inst. Mech. Eng. H 2014, 228, 1083–1099. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.Z.; Wang, D.; Liu, Y.; Zhang, Z.; Wozniak, D.J. Regulation of biofilm exopolysaccharide biosynthesis and degradation in Pseudomonas aeruginosa. Annu. Rev. Microbiol. 2022, 76, 413–433. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, I.W. Polysaccharases for microbial exopolysaccharides. Carbohydr. Polym. 1999, 38, 319–328. [Google Scholar] [CrossRef]
- Kalia, M.; Resch, M.D.; Cherny, K.E.; Sauer, K. The alginate and motility regulator AmrZ is essential for the regulation of the dispersion response by Pseudomonas aeruginosa biofilms. mSphere 2022, 7, e0050522. [Google Scholar] [CrossRef]
- Colvin, K.M.; Irie, Y.; Tart, C.S.; Urbano, R.; Whitney, J.C.; Ryder, C.; Howell, P.L.; Wozniak, D.J.; Parsek, M.R. The Pel and Psl polysaccharides provide Pseudomonas aeruginosa structural redundancy within the biofilm matrix. Environ. Microbiol. 2012, 14, 1913–1928. [Google Scholar] [CrossRef]
- Wang, S.T.; Niu, Y.T.; Zhang, H.; Li, P.X.; Zhang, N.M.; Cheng, J.L.; Lin, J.S. An engineered bacterium for the targeted delivery of proteins to destroy Pseudomonas aeruginosa biofilms. Acta Microbiol. Sin. 2021, 61, 2726–2748. [Google Scholar]
- Tsagkari, E.; Connelly, S.; Liu, Z.; McBride, A.; Sloan, W.T. The role of shear dynamics in biofilm formation. NPJ Biofilms Microbiomes 2022, 8, 33. [Google Scholar] [CrossRef]
- Choi, Y.C.; Morgenroth, E. Monitoring biofilm detachment under dynamic changes in shear stress using laser-based particle size analysis and mass fractionation. Water Sci. Technol. 2003, 47, 69–76. [Google Scholar] [CrossRef]
- Hwang, G.; Klein, M.I.; Koo, H. Analysis of the mechanical stability and surface detachment of mature Streptococcus mutans biofilms by applying a range of external shear forces. Biofouling 2014, 30, 1079–1091. [Google Scholar] [CrossRef]
- Oulahal-Lagsir, N.; Martial-Gros, A.; Bonneau, M.; Blum, L.J. “Escherichia coli-milk” biofilm removal from stainless steel surfaces: Synergism between ultrasonic waves and enzymes. Biofouling 2003, 19, 159–168. [Google Scholar] [PubMed]
- Oulahal, N.; Martial-Gros, A.; Bonneau, M.; Blum, L.J. Combined Effect of Chelating Agents and Ultrasound on Biofilm Removal from Stainless Steel Surfaces. Application to Escherichia coli Milk and Staphylococcus aureus Milk Biofilms; Cambridge University Press: Cambridge, UK, 2004; Volume 1, pp. 65–73. [Google Scholar]
- Nigri, G.R.; Tsai, S.; Kossodo, S.; Waterman, P.; Fungaloi, P.; Hooper, D.C.; Doukas, A.G.; LaMuraglia, G.M. Laser-induced shock waves enhance sterilization of infected vascular prosthetic grafts. Lasers Surg. Med. 2001, 29, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Staphylococcal infections: Mechanisms of biofilm maturation and detachment as critical determinants of pathogenicity. Annu. Rev. Med. 2013, 64, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Ceri, H.; Olson, M.E.; Stremick, C.; Read, R.R.; Morck, D.; Buret, A. The calgary biofilm device: New technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J. Clin. Microbiol. 1999, 37, 1771–1776. [Google Scholar] [CrossRef] [PubMed]
- Michael; Waturangi, D.E. Antibiofilm activity from endophyte bacteria, Vibrio cholerae strains, and actinomycetes isolates in liquid and solid culture. BMC Microbiol. 2023, 23, 83. [Google Scholar] [CrossRef] [PubMed]
- Pokharel, K.; Dawadi, B.R.; Shrestha, L.B. Role of biofilm in bacterial infection and antimicrobial resistance. JNMA J. Nepal. Med. Assoc. 2022, 60, 836–840. [Google Scholar] [CrossRef]
- Cascioferro, S.; Carbone, D.; Parrino, B.; Pecoraro, C.; Giovannetti, E.; Cirrincione, G.; Diana, P. Therapeutic strategies to counteract antibiotic resistance in MRSA biofilm-associated infections. ChemMedChem 2021, 16, 65–80. [Google Scholar] [CrossRef]
- Thomsen, K.; Høiby, N.; Jensen, P.; Ciofu, O.; Moser, C. Immune response to biofilm growing pulmonary Pseudomonas aeruginosa infection. Biomedicines 2022, 10, 2064. [Google Scholar] [CrossRef]
- Güneş, B.; Akçelik, N. The role of eDNA in biofilm structure of Enterococcus faecalis and investigation of the efficiency of enzyme and antibiotic application in biofilm eradication. Mikrobiyol. Bul. 2022, 56, 606–619. [Google Scholar]
- Sheng, Y.; Chen, Z.; Wu, W.; Lu, Y. Engineered organic nanoparticles to combat biofilms. Drug Discov. Today 2023, 28, 103455. [Google Scholar] [CrossRef]
- Tian, C.; Yuan, M.; Tao, Q.; Xu, T.; Liu, J.; Huang, Z.; Wu, Q.; Pan, Y.; Zhao, Y.; Zhang, Z. Discovery of novel resistance mechanisms of Vibrio parahaemolyticus biofilm against aminoglycoside antibiotics. Antibiotics 2023, 12, 638. [Google Scholar] [CrossRef]
- Mulcahy, H.; Charron-Mazenod, L.; Lewenza, S. Extracellular DNA chelates cations and induces antibiotic resistance in Pseudomonas aeruginosa biofilms. PLoS Pathog. 2008, 4, e1000213. [Google Scholar] [CrossRef] [PubMed]
- Ciofu, O.; Rojo-Molinero, E.; Macià, M.D.; Oliver, A. Antibiotic treatment of biofilm infections. Apmis 2017, 125, 304–319. [Google Scholar] [CrossRef]
- Hoiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.M.A.; Alnasser, S.M.; Abd-Elhafeez, H.H.; Alotaibi, M.; Batiha, G.E.; Younis, W. detection of β-Lactamase resistance and biofilm genes in Pseudomonas species isolated from chickens. Microorganisms 2022, 10, 1975. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Moreau, A.; Khodaparast, S.; Perazzo, A.; Feng, J.; Fei, C.; Mao, S.; Mukherjee, S.; Košmrlj, A.; Wingreen, N.S.; et al. Bacterial biofilm material properties enable removal and transfer by capillary peeling. Adv. Mater. 2018, 30, e1804153. [Google Scholar] [CrossRef]
- Spoering, A.L.; Lewis, K. Biofilms and planktonic cells of Pseudomonas aeruginosa have similar resistance to killing by antimicrobials. J. Bacteriol. 2001, 183, 6746–6751. [Google Scholar] [CrossRef]
- Mah, T.F. Biofilm-specific antibiotic resistance. Future Microbiol. 2012, 7, 1061–1072. [Google Scholar] [CrossRef]
- Stewart, P.S. Mechanisms of antibiotic resistance in bacterial biofilms. Int. J. Med. Microbiol. 2002, 292, 107–113. [Google Scholar] [CrossRef]
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef]
- Pamp, S.J.; Gjermansen, M.; Johansen, H.K.; Tolker-Nielsen, T. Tolerance to the antimicrobial peptide colistin in Pseudomonas aeruginosa biofilms is linked to metabolically active cells, and depends on the pmr and mexAB-oprM genes. Mol. Microbiol. 2008, 68, 223–240. [Google Scholar] [CrossRef] [PubMed]
- Gaurav, A.; Bakht, P.; Saini, M.; Pandey, S.; Pathania, R. Role of bacterial efflux pumps in antibiotic resistance, virulence, and strategies to discover novel efflux pump inhibitors. Microbiology 2023, 169, 001333. [Google Scholar] [CrossRef]
- Seukep, A.J.; Mbuntcha, H.G.; Kuete, V.; Chu, Y.; Fan, E.; Guo, M.Q. What approaches to thwart bacterial efflux pumps-mediated resistance? Antibiotics 2022, 11, 1287. [Google Scholar] [CrossRef] [PubMed]
- Başaran, S.N.; Öksüz, L. The role of efflux pumps ın antıbıotıc resıstance of gram negatıve rods. Arch. Microbiol. 2023, 205, 192. [Google Scholar] [CrossRef] [PubMed]
- Mudde, S.E.; Schildkraut, J.A.; Ammerman, N.C.; de Vogel, C.P.; de Steenwinkel, J.E.M.; van Ingen, J.; Bax, H.I. Unraveling antibiotic resistance mechanisms in Mycobacterium abscessus: The potential role of efflux pumps. J. Glob. Antimicrob. Resist. 2022, 31, 345–352. [Google Scholar] [CrossRef]
- Barnabas, V.; Kashyap, A.; Raja, R.; Newar, K.; Rai, D.; Dixit, N.M.; Mehra, S. The extent of antimicrobial resistance due to efflux pump regulation. ACS Infect. Dis. 2022, 8, 2374–2388. [Google Scholar] [CrossRef]
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef]
- Uddin, T.M.; Chakraborty, A.J.; Khusro, A.; Zidan, B.R.M.; Mitra, S.; Emran, T.B.; Dhama, K.; Ripon, M.K.H.; Gajdács, M.; Sahibzada, M.U.K.; et al. Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects. J. Infect. Public Health 2021, 14, 1750–1766. [Google Scholar] [CrossRef]
- Grossman, T.H. Tetracycline antibiotics and resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025387. [Google Scholar] [CrossRef]
- Venkatesan, N.; Perumal, G.; Doble, M. Bacterial resistance in biofilm-associated bacteria. Future Microbiol. 2015, 10, 1743–1750. [Google Scholar] [CrossRef]
- Shi, K.; Cao, M.; Li, C.; Huang, J.; Zheng, S.; Wang, G. Efflux proteins MacAB confer resistance to arsenite and penicillin/macrolide-type antibiotics in Agrobacterium tumefaciens 5A. World J. Microbiol. Biotechnol. 2019, 35, 115. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, L.; Haghshenas, M.R.; Mirzaei, B.; Khalili, Y.; Goli, H.R. Role of MexAB-OprM efflux pump in the emergence of multidrug-resistant clinical isolates of Pseudomonas aeruginosa in Mazandaran province of Iran. Mol. Biol. Rep. 2023, 50, 2603–2609. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Espada, R.; Shahrour, H.; Pitts, B.; Stewart, P.S.; Sánchez-Gómez, S.; Martínez-de-Tejada, G. A permeability-increasing drug synergizes with bacterial efflux pump inhibitors and restores susceptibility to antibiotics in multi-drug resistant Pseudomonas aeruginosa strains. Sci. Rep. 2019, 9, 3452. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.J.; Bayer, A.S.; Mishra, N.N.; Meehl, M.; Ledala, N.; Yeaman, M.R.; Xiong, Y.Q.; Cheung, A.L. The Staphylococcus aureus two-component regulatory system, GraRS, senses and confers resistance to selected cationic antimicrobial peptides. Infect. Immun. 2012, 80, 74–81. [Google Scholar] [CrossRef]
- Cho, J.; Costa, S.K.; Wierzbicki, R.M.; Rigby, W.F.C.; Cheung, A.L. The extracellular loop of the membrane permease VraG interacts with GraS to sense cationic antimicrobial peptides in Staphylococcus aureus. PLoS Pathog. 2021, 17, e1009338. [Google Scholar] [CrossRef]
- Hiron, A.; Falord, M.; Valle, J.; Débarbouillé, M.; Msadek, T. Bacitracin and nisin resistance in Staphylococcus aureus: A novel pathway involving the BraS/BraR two-component system (SA2417/SA2418) and both the BraD/BraE and VraD/VraE ABC transporters. Mol. Microbiol. 2011, 81, 602–622. [Google Scholar] [CrossRef]
- Arii, K.; Kawada-Matsuo, M.; Oogai, Y.; Noguchi, K.; Komatsuzawa, H. Single mutations in BraRS confer high resistance against nisin A in Staphylococcus aureus. Microbiologyopen 2019, 8, e791. [Google Scholar] [CrossRef]
- Babushkina, I.V.; Bondarenko, A.S.; Ulyanov, V.Y.; Mamonova, I.A. Biofilm formation by gram-negative bacteria during implant-associated infection. Bull. Exp. Biol. Med. 2020, 169, 365–368. [Google Scholar] [CrossRef]
- Barros, J.; Monteiro, F.J.; Ferraz, M.P. Bioengineering approaches to fight against orthopedic biomaterials related-infections. Int. J. Mol. Sci. 2022, 23, 11658. [Google Scholar] [CrossRef]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019, 83, 37–54. [Google Scholar] [CrossRef]
- Høiby, N.; Bjarnsholt, T.; Moser, C.; Bassi, G.L.; Coenye, T.; Donelli, G.; Hall-Stoodley, L.; Holá, V.; Imbert, C.; Kirketerp-Møller, K.; et al. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin. Microbiol. Infect. 2015, 21 (Suppl. 1), S1–S25. [Google Scholar] [CrossRef] [PubMed]
- Wi, Y.M.; Patel, R. Understanding biofilms and novel approaches to the diagnosis, prevention, and treatment of medical devicea-Associated infections. Infect. Dis. Clin. N. Am. 2018, 32, 915–929. [Google Scholar] [CrossRef] [PubMed]
- Lebeaux, D.; Fernández-Hidalgo, N.; Chauhan, A.; Lee, S.; Ghigo, J.M.; Almirante, B.; Beloin, C. Management of infections related to totally implantable venous-access ports: Challenges and perspectives. Lancet Infect. Dis. 2014, 14, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Gominet, M.; Compain, F.; Beloin, C.; Lebeaux, D. Central venous catheters and biofilms: Where do we stand in 2017? Apmis 2017, 125, 365–375. [Google Scholar] [CrossRef]
- Mangner, N.; Woitek, F.; Haussig, S.; Schlotter, F.; Stachel, G.; Höllriegel, R.; Wilde, J.; Lindner, A.; Holzhey, D.; Leontyev, S.; et al. Incidence, predictors, and outcome of patients developing infective endocarditis following transfemoral transcatheter aortic valve replacement. J. Am. Coll. Cardiol. 2016, 67, 2907–2908. [Google Scholar] [CrossRef]
- Elgharably, H.; Hussain, S.T.; Shrestha, N.K.; Blackstone, E.H.; Pettersson, G.B. Current hypotheses in cardiac surgery: Biofilm in infective endocarditis. Semin. Thorac. Cardiovasc. Surg. 2016, 28, 56–59. [Google Scholar] [CrossRef]
- Nappi, F.; Iervolino, A.; Singh, S.S.A. The new challenge for heart endocarditis: From conventional prosthesis to new devices and platforms for the treatment of structural heart disease. Biomed. Res. Int. 2021, 2021, 7302165. [Google Scholar] [CrossRef]
- Izakovicova, P.; Borens, O.; Trampuz, A. Periprosthetic joint infection: Current concepts and outlook. EFORT Open Rev. 2019, 4, 482–494. [Google Scholar] [CrossRef]
- Kamihata, S.; Ando, W.; Nakahara, I.; Enami, H.; Takashima, K.; Uemura, K.; Hamada, H.; Sugano, N. Optimizing vancomycin release from novel carbon fiber-reinforced polymer implants with small holes: Periprosthetic joint infection treatment. J. Artif. Organs 2023, online ahead of print. [Google Scholar] [CrossRef]
- Belgiovine, C.; Pellegrino, L.; Bulgarelli, A.; Lauta, F.C.; Di Claudio, A.; Ciceri, R.; Cancellara, A.; Calcaterra, F.; Mavilio, D.; Grappiolo, G.; et al. Interaction of bacteria, immune cells, and surface topography in periprosthetic joint infections. Int. J. Mol. Sci. 2023, 24, 9028. [Google Scholar] [CrossRef]
- Gbejuade, H.O.; Lovering, A.M.; Webb, J.C. The role of microbial biofilms in prosthetic joint infections. Acta Orthop. 2015, 86, 147–158. [Google Scholar] [CrossRef]
- Osmon, D.R.; Berbari, E.F.; Berendt, A.R.; Lew, D.; Zimmerli, W.; Steckelberg, J.M.; Rao, N.; Hanssen, A.; Wilson, W.R. Diagnosis and management of prosthetic joint infection: Clinical practice guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 2013, 56, e1–e25. [Google Scholar] [CrossRef] [PubMed]
- Jacqueline, C.; Caillon, J. Impact of bacterial biofilm on the treatment of prosthetic joint infections. J. Antimicrob. Chemother. 2014, 69 (Suppl. 1), i37–i40. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Misba, L.; Khan, A.U. Antibiotics versus biofilm: An emerging battleground in microbial communities. Antimicrob. Resist. Infect. Control. 2019, 8, 76. [Google Scholar] [CrossRef]
- Khan, F.; Pham, D.T.N.; Tabassum, N.; Oloketuyi, S.F.; Kim, Y.M. Treatment strategies targeting persister cell formation in bacterial pathogens. Crit. Rev. Microbiol. 2020, 46, 665–688. [Google Scholar] [CrossRef] [PubMed]
- Filipović, U.; Dahmane, R.G.; Ghannouchi, S.; Zore, A.; Bohinc, K. Bacterial adhesion on orthopedic implants. Adv. Colloid. Interface Sci. 2020, 283, 102228. [Google Scholar] [CrossRef]
- Ivanovic, B.; Trifunovic, D.; Matic, S.; Petrovic, J.; Sacic, D.; Tadic, M. Prosthetic valve endocarditis—A trouble or a challenge? J. Cardiol. 2019, 73, 126–133. [Google Scholar] [CrossRef]
- Alves, C.H.; Russi, K.L.; Rocha, N.C.; Bastos, F.; Darrieux, M.; Parisotto, T.M.; Girardello, R. Host-microbiome interactions regarding peri-implantitis and dental implant loss. J. Transl. Med. 2022, 20, 425. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, Z.; Li, S.; Yuan, X. Antimicrobial strategies for urinary catheters. J. Biomed. Mater. Res. A 2019, 107, 445–467. [Google Scholar] [CrossRef]
- Ng, V.Y. Risk of disease transmission with bone allograft. Orthopedics 2012, 35, 679–681. [Google Scholar] [CrossRef]
- Trobos, M.; Johansson, M.L.; Jonhede, S.; Peters, H.; Hoffman, M.; Omar, O.; Thomsen, P.; Hultcrantz, M. The clinical outcome and microbiological profile of bone-anchored hearing systems (BAHS) with different abutment topographies: A prospective pilot study. Eur. Arch. Otorhinolaryngol. 2018, 275, 1395–1408. [Google Scholar] [CrossRef] [PubMed]
- Zare, M.; Ghomi, E.R.; Venkatraman, P.D.; Ramakrishna, S. Silicone-based biomaterials for biomedical applications: Antimicrobial strategies and 3D printing technologies. J. Appl. Polym. Sci. 2021, 138, 50969. [Google Scholar] [CrossRef]
- Elbourne, A.; Chapman, J.; Gelmi, A.; Cozzolino, D.; Crawford, R.J.; Truong, V.K. Bacterial-nanostructure interactions: The role of cell elasticity and adhesion forces. J. Colloid. Interface Sci. 2019, 546, 192–210. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Hou, J.; van der Mei, H.C.; Busscher, H.J.; Ren, Y. Emergent properties in Streptococcus mutans biofilms are controlled through adhesion force sensing by initial colonizers. mBio 2019, 10, e01908-19. [Google Scholar] [CrossRef]
- Viljoen, A.; Mignolet, J.; Viela, F.; Mathelié-Guinlet, M.; Dufrêne, Y.F. How microbes use force to control adhesion. J. Bacteriol. 2020, 202, e00125-20. [Google Scholar] [CrossRef]
- Sousa, V.; Mardas, N.; Spratt, D.; Hassan, I.A.; Walters, N.J.; Beltrán, V.; Donos, N. The effect of microcosm biofilm decontamination on surface topography, chemistry, and biocompatibility dynamics of implant titanium surfaces. Int. J. Mol. Sci. 2022, 23, 10033. [Google Scholar] [CrossRef]
- Folliero, V.; Franci, G.; Dell’Annunziata, F.; Giugliano, R.; Foglia, F.; Sperlongano, R.; De Filippis, A.; Finamore, E.; Galdiero, M. Evaluation of antibiotic resistance and biofilm production among clinical strain isolated from medical devices. Int. J. Microbiol. 2021, 2021, 9033278. [Google Scholar] [CrossRef]
- Tran, P.A.; Hocking, D.M.; O’Connor, A.J. In situ formation of antimicrobial silver nanoparticles and the impregnation of hydrophobic polycaprolactone matrix for antimicrobial medical device applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 47, 63–69. [Google Scholar] [CrossRef]
- Haidari, H.; Kopecki, Z.; Bright, R.; Cowin, A.J.; Garg, S.; Goswami, N.; Vasilev, K. Ultrasmall AgNP-impregnated biocompatible hydrogel with highly effective biofilm elimination properties. ACS Appl. Mater. Interfaces 2020, 12, 41011–41025. [Google Scholar] [CrossRef]
- Ivanova, K.; Fernandes, M.M.; Mendoza, E.; Tzanov, T. Enzyme multilayer coatings inhibit Pseudomonas aeruginosa biofilm formation on urinary catheters. Appl. Microbiol. Biotechnol. 2015, 99, 4373–4385. [Google Scholar] [CrossRef]
- Rodríguez López, A.L.; Lee, M.R.; Ortiz, B.J.; Gastfriend, B.D.; Whitehead, R.; Lynn, D.M.; Palecek, S.P. Preventing S. aureus biofilm formation on titanium surfaces by the release of antimicrobial β-peptides from polyelectrolyte multilayers. Acta Biomater. 2019, 93, 50–62. [Google Scholar] [CrossRef]
- Zou, Y.; Liu, C.; Zhang, H.; Wu, Y.; Lin, Y.; Cheng, J.; Lu, K.; Li, L.; Zhang, Y.; Chen, H.; et al. Three lines of defense: A multifunctional coating with anti-adhesion, bacteria-killing and anti-quorum sensing properties for preventing biofilm formation of Pseudomonas aeruginosa. Acta Biomater. 2022, 151, 254–263. [Google Scholar] [CrossRef]
- Saravanan, S.; Vimalraj, S.; Thanikaivelan, P.; Banudevi, S.; Manivasagam, G. A review on injectable chitosan/beta glycerophosphate hydrogels for bone tissue regeneration. Int. J. Biol. Macromol. 2019, 121, 38–54. [Google Scholar] [CrossRef]
- Robert, B.; Chenthamara, D.; Subramaniam, S. Fabrication and biomedical applications of arabinoxylan, pectin, chitosan, soy protein, and silk fibroin hydrogels via laccase-ferulic acid redox chemistry. Int. J. Biol. Macromol. 2022, 201, 539–556. [Google Scholar] [CrossRef] [PubMed]
- Jalil, S.A.; Akram, M.; Bhat, J.A.; Hayes, J.J.; Singh, S.C.; ElKabbash, M.; Guo, C. Creating superhydrophobic and antibacterial surfaces on gold by femtosecond laser pulses. Appl. Surf. Sci. 2020, 506, 144952. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, A.; Kumar, A.; Sreedharan, S.; Muralidharan, G.; Pramanik, A.; Nandi, D.; Sen, P. Fabrication of low-cost flexible superhydrophobic antibacterial surface with dual-scale roughness. ACS Biomater. Sci. Eng. 2018, 4, 2213–2223. [Google Scholar] [CrossRef] [PubMed]
- Petrini, M.; Giuliani, A.; Di Campli, E.; Di Lodovico, S.; Iezzi, G.; Piattelli, A.; D’Ercole, S. The bacterial anti-adhesive activity of double-etched titanium (DAE) as a dental implant surface. Int. J. Mol. Sci. 2020, 21, 8315. [Google Scholar] [CrossRef]
- D’Ercole, S.; Cellini, L.; Pilato, S.; Di Lodovico, S.; Iezzi, G.; Piattelli, A.; Petrini, M. Material characterization and Streptococcus oralis adhesion on Polyetheretherketone (PEEK) and titanium surfaces used in implantology. J. Mater. Sci. Mater. Med. 2020, 31, 84. [Google Scholar] [CrossRef]
- Hasan, J.; Jain, S.; Padmarajan, R.; Purighalla, S.; Sambandamurthy, V.K.; Chatterjee, K. Multi-scale surface topography to minimize adherence and viability of nosocomial drug-resistant bacteria. Mater. Des. 2018, 140, 332–344. [Google Scholar] [CrossRef]
- May, R.M.; Hoffman, M.G.; Sogo, M.J.; Parker, A.E.; O’Toole, G.A.; Brennan, A.B.; Reddy, S.T. Micro-patterned surfaces reduce bacterial colonization and biofilm formation in vitro: Potential for enhancing endotracheal tube designs. Clin. Transl. Med. 2014, 3, 1–9. [Google Scholar] [CrossRef]
- Xu, B.; Wei, Q.; Mettetal, M.R.; Han, J.; Rau, L.; Tie, J.; May, R.M.; Pathe, E.T.; Reddy, S.T.; Sullivan, L.; et al. Surface micropattern reduces colonization and medical device-associated infections. J. Med. Microbiol. 2017, 66, 1692–1698. [Google Scholar] [CrossRef]
- May, R.M.; Magin, C.M.; Mann, E.E.; Drinker, M.C.; Fraser, J.C.; Siedlecki, C.A.; Brennan, A.B.; Reddy, S.T. An engineered micropattern to reduce bacterial colonization, platelet adhesion and fibrin sheath formation for improved biocompatibility of central venous catheters. Clin. Transl. Med. 2015, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Rostami, S.; Puza, F.; Ucak, M.; Ozgur, E.; Gul, O.; Ercan, U.K.; Garipcan, B. Bifunctional sharkskin mimicked chitosan/graphene oxide membranes: Reduced biofilm formation and improved cytocompatibility. Appl. Surf. Sci. 2021, 544, 148828. [Google Scholar] [CrossRef]
- He, M.W.Q.; Zhao, W. A self-defensive bilayer hydrogel coating with bacteria triggered switching from cell adhesion to antibacterial adhesion. Polym. Chem. 2017, 8, 5344–5353. [Google Scholar] [CrossRef]
- Park, J.S.; Ryu, E.J.; Li, L.; Choi, B.K.; Kim, B.M. New bicyclic brominated furanones as potent autoinducer-2 quorum-sensing inhibitors against bacterial biofilm formation. Eur. J. Med. Chem. 2017, 137, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Olivares, E.; Badel-Berchoux, S.; Provot, C.; Prévost, G.; Bernardi, T.; Jehl, F. Clinical impact of antibiotics for the treatment of Pseudomonas aeruginosa biofilm infections. Front. Microbiol. 2019, 10, 2894. [Google Scholar] [CrossRef]
- Srinivasan, R.; Santhakumari, S.; Poonguzhali, P.; Geetha, M.; Dyavaiah, M.; Lin, X. Bacterial biofilm inhibition: A focused rview on recent therapeutic strategies for combating the biofilm mediated infections. Front. Microbiol. 2021, 12, 676458. [Google Scholar] [CrossRef]
- Cloutier, M.; Mantovani, D.; Rosei, F. Antibacterial coatings: Challenges, perspectives, and opportunities. Trends Biotechnol. 2015, 33, 637–652. [Google Scholar] [CrossRef]
- Olmo, A.D.; Rubio, R.; Martinez, S.; Perez-Alvarez, L.; Vilela, V.J.C. Antibacterial coatings for improving the performance of biomaterials. Coatings 2020, 10, 139. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver nanoparticles and their antibacterial applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Romanò, C.L.; Scarponi, S.; Gallazzi, E.; Romanò, D.; Drago, L. Antibacterial coating of implants in orthopaedics and trauma: A classification proposal in an evolving panorama. J. Orthop. Surg. Res. 2015, 10, 157. [Google Scholar] [CrossRef] [PubMed]
- Kuehl, R.; Brunetto, P.S.; Woischnig, A.K.; Varisco, M.; Rajacic, Z.; Vosbeck, J.; Terracciano, L.; Fromm, K.M.; Khanna, N. Preventing implant-associated infections by silver coating. Antimicrob. Agents Chemother. 2016, 60, 2467–2475. [Google Scholar] [CrossRef] [PubMed]
- Haktaniyan, M.; Bradley, M. Polymers showing intrinsic antimicrobial activity. Chem. Soc. Rev. 2022, 51, 8584–8611. [Google Scholar] [CrossRef] [PubMed]
- Asma, S.T.; Imre, K.; Morar, A.; Herman, V.; Acaroz, U.; Mukhtar, H.; Arslan-Acaroz, D.; Shah, S.R.A.; Gerlach, R. An Overview of Biofilm Formation-Combating Strategies and Mechanisms of Action of Antibiofilm Agents. Life 2022, 12, 1110. [Google Scholar] [CrossRef] [PubMed]
- Leng, C.; Hung, H.C.; Sun, S.; Wang, D.; Li, Y.; Jiang, S.; Chen, Z. Probing the surface hydration of nonfouling zwitterionic and PEG materials in contact with proteins. ACS Appl. Mater. Interfaces 2015, 7, 16881–16888. [Google Scholar] [CrossRef]
- Liu, C.; Faria, A.F.; Ma, J.; Elimelech, M. Mitigation of biofilm development on thin-film composite membranes functionalized with zwitterionic polymers and silver nanoparticles. Environ. Sci. Technol. 2017, 51, 182–191. [Google Scholar] [CrossRef]
- Costa, B.; Martínez-de-Tejada, G.; Gomes, P.A.C.; Martins, M.C.L.; Costa, F. Antimicrobial peptides in the battle against orthopedic implant-related infections: A review. Pharmaceutics 2021, 13, 1918. [Google Scholar] [CrossRef]
- Barbosa, M.; Costa, F.; Monteiro, C.; Duarte, F.; Martins, M.C.L.; Gomes, P. Antimicrobial coatings prepared from Dhvar-5-click-grafted chitosan powders. Acta Biomater. 2019, 84, 242–256. [Google Scholar] [CrossRef]
- Guo, S.; Zhu, X.; Loh, X.J. Controlling cell adhesion using layer-by-layer approaches for biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70, 1163–1175. [Google Scholar] [CrossRef]
- Ahmad, D.; van den Boogaert, I.; Miller, J.; Presswell, R.; Jouhara, H. Hydrophilic and hydrophobic materials and their applications. Energy Sources Part A Recovery Util. Environ. Eff. 2018, 40, 2686–2725. [Google Scholar] [CrossRef]
- Drago, L.; Cappelletti, L.; De Vecchi, E.; Pignataro, L.; Torretta, S.; Mattina, R. Antiadhesive and antibiofilm activity of hyaluronic acid against bacteria responsible for respiratory tract infections. APMIS 2014, 122, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Nemani, S.K.; Annavarapu, R.K.; Mohammadian, B.; Raiyan, A.; Sojoudi, H. Surface modification: Surface modification of polymers: Methods and applications. Adv. Mater. Interfaces 2018, 5, 1870121. [Google Scholar] [CrossRef]
- Al-Gunaid, T.A.; Krupa, I.; Ouederni, M.; Krishnamoorthy, S.K.; Popelka, A. Enhancement of adhesion characteristics of low-density polyethylene using atmospheric plasma iitiated-grafting of polyethylene glycol. Polymers 2021, 13, 1309. [Google Scholar] [CrossRef] [PubMed]
- Sterzenbach, T.; Helbig, R.; Hannig, C.; Hannig, M. Bioadhesion in the oral cavity and approaches for biofilm management by surface modifications. Clin. Oral. Investig. 2020, 24, 4237–4260. [Google Scholar] [CrossRef]
- Nemani, S.K.; Annavarapu, R.K.; Mohammadian, B.; Raiyan, A.; Heil, J.; Haque, M.A.; Abdelaal, A.; Sojoudi, H. Surface Modification of Polymers: Methods and Applications. Adv. Mater. Interfaces 2018, 5, 1801247. [Google Scholar] [CrossRef]
- Klodzinska, S.N.; Wan, F.; Jumaa, H.; Sternberg, C.; Rades, T.; Nielsen, H.M. Utilizing nanoparticles for improving anti-biofilm effects of azithromycin: A head-to-head comparison of modified hyaluronic acid nanogels and coated poly (lactic-co-glycolic acid) nanoparticles. J. Colloid. Interface Sci. 2019, 555, 595–606. [Google Scholar] [CrossRef]
- Yadav, J.; Kumari, R.M.; Verma, V.; Nimesh, S. Recent development in therapeutic strategies targeting Pseudomonas aeruginosa biofilms—A review. Mater. Today Proc. 2021, 46, 245. [Google Scholar] [CrossRef]
- Encinas, N.; Pantoja, M.; Abenojar, J.; Martínez, M.A. Control of wettability of polymers by surface roughness modification. J. Adhes. Sci. Technol. 2010, 24, 1869–1883. [Google Scholar] [CrossRef]
- Epperlein, N.; Menzel, F.; Schwibbert, K.; Koter, R.; Bonse, J.; Sameith, J.; Krueger, J.; Toepel, J. Influence of femtosecond laser produced nanostructures on biofilm growth on steel. Appl. Surf. Sci. 2017, 418, 420–424. [Google Scholar] [CrossRef]
- Gillett, A.; Waugh, D.; Lawrence, J.; Swainson, M.; Dixon, R. Laser surface modification for the prevention of biofouling by infection causing Escherichia coli. J. Laser Appl. 2016, 28, 022503. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Q.; Long, M.; Han, R.; Cao, K.; Zhang, S.; Feng, D.; Jia, T.; Sun, Z.; Qiu, J.; et al. Femtosecond laser-induced periodic structures: Mechanisms, techniques, and applications. Opto-Electron. Sci. 2022, 1, 220005. [Google Scholar] [CrossRef]
- Monteiro, D.R.; de Souza Batista, V.E.; Caldeirão, A.C.M.; Jacinto, R.C.; Pessan, J.P. Oral prosthetic microbiology: Aspects related to the oral microbiome, surface properties, and strategies for controlling biofilms. Biofouling 2021, 37, 353–371. [Google Scholar] [CrossRef] [PubMed]
- Giner, L.; Mercadé, M.; Torrent, S.; Punset, M.; Pérez, R.A.; Delgado, L.M.; Gil, F.J. Double acid etching treatment of dental implants for enhanced biological properties. J. Appl. Biomater. Funct. Mater. 2018, 16, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Siddiqui, F. Applications of polyetheretherketone (PEEK) in oral implantology and prosthodontics. J. Prosthodont. Res. 2016, 60, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, Y.; Cao, Y.; Li, G.; Liao, Y. Influence of surface roughness on contact angle hysteresis and spreading work. Colloid Polym. Sci. 2020, 298, 1107–1112. [Google Scholar] [CrossRef]
- Wu, S.; Altenried, S.; Zogg, A.; Zuber, F.; Maniura-Weber, K.; Ren, Q. Role of the surface nanoscale roughness of stainless steel on bacterial adhesion and microcolony formation. ACS Omega 2018, 3, 6456–6464. [Google Scholar] [CrossRef]
- Kozmos, M.; Virant, P.; Rojko, F.; Abram, A.; Rudolf, R.; Raspor, P.; Zore, A.; Bohinc, K. Bacterial adhesion of Streptococcus mutans to dental material surfaces. Molecules 2021, 26, 1152. [Google Scholar] [CrossRef]
- Lu, A.; Gao, Y.; Jin, T.; Luo, X.; Zeng, Q.; Shang, Z. Effects of surface roughness and texture on the bacterial adhesion on the bearing surface of bio-ceramic joint implants: An in vitro study. Ceram. Int. 2020, 46, 6550–6559. [Google Scholar] [CrossRef]
- Matos, G.R.M. Surface roughness of dental implant and osseointegration. J. Maxillofac. Oral. Surg. 2021, 20, 1–4. [Google Scholar] [CrossRef]
- Nazarov, D.V.; Smirnov, V.M.; Zemtsova, E.G.; Yudintceva, N.M.; Shevtsov, M.A.; Valiev, R.Z. Enhanced osseointegrative properties of ultra-fine-grained titanium implants modified by chemical etching and atomic layer deposition. ACS Biomater. Sci. Eng. 2018, 4, 3268–3281. [Google Scholar] [CrossRef]
- Wardhono, E.Y.; Pinem, M.P.; Susilo, S.; Siom, B.J.; Sudrajad, A.; Pramono, A.; Meliana, Y.; Guénin, E. Modification of physio-mechanical properties of chitosan-based films via physical treatment approach. Polymers 2022, 14, 5216. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Seenivasan, M.K.; Inbarajan, A. A literature review on biofilm formation on silicone and poymethyl methacrylate used for maxillofacial prostheses. Cureus 2021, 13, e20029. [Google Scholar] [CrossRef] [PubMed]
- Desrousseaux, C.; Sautou, V.; Descamps, S.; Traoré, O. Modification of the surfaces of medical devices to prevent microbial adhesion and biofilm formation. J. Hosp. Infect. 2013, 85, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Renner, L.D.; Weibel, D.B. Physicochemical regulation of biofilm formation. MRS Bull. 2011, 36, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Xiong, F.Z.; Zhao, X.X.; Liao, Y.H. Effect of material surface characteristics on biofilm formation and its application. Microbiol. China 2018, 45, 155–165. [Google Scholar]
- Freitas, S.C.; Correa-Uribe, A.; Martins, M.C.L.; Pelaez-Vargas, A. Self-assembled monolayers for dental implants. Int. J. Dent. 2018, 2018, 4395460. [Google Scholar] [CrossRef]
- Somasundaram, S. Silane coatings of metallic biomaterials for biomedical implants: A preliminary review. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 2901–2918. [Google Scholar] [CrossRef]
- Nicosia, C.; Huskens, J. Reactive self-assembled monolayers: From surface functionalization to gradient formation. Mater. Horiz. 2014, 1, 32–45. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Kim, K.D.; Chun, S.C. Antibacterial Activity of Chitosan Nanoparticles: A Review. Processes 2020, 8, 1173. [Google Scholar] [CrossRef]
- Tan, W.-S.; Law, J.W.-F.; Law, L.N.-S.; Letchumanan, V.; Chan, K.-G. Insights into quorum sensing (QS): QS-regulated biofilm and inhibitors. Prog. Microbes Mol. Biol. 2020, 3, 3. [Google Scholar] [CrossRef]
- Sionov, R.V.; Steinberg, D. Targeting the holy triangle of quorum sensing, biofilm formation, and antibiotic resistance in pathogenic bacteria. Microorganisms 2022, 10, 1239. [Google Scholar] [CrossRef]
- Vashistha, A.; Sharma, N.; Nanaji, Y.; Kumar, D.; Singh, G.; Barnwal, R.P.; Yadav, A.K. Quorum sensing inhibitors as Therapeutics: Bacterial biofilm inhibition. Bioorganic Chem. 2023, 136, 106551. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, Y.; Ge, Y.; Zhu, X.; Pan, J. Regulatory mechanisms and promising applications of quorum sensing-inhibiting agents in control of bacterial biofilm Formation. Front. Microbiol. 2020, 11, 589640. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, S.; Hachicha, M.; Boukraa, M.; Soulère, L.; Efrit, M.L.; Queneau, Y. Heterocyclic chemistry applied to the design of N-Acyl homoserine lactone analogues as bacterial quorum sensing signals mimics. Molecules 2021, 26, 5135. [Google Scholar] [CrossRef] [PubMed]
- Kolenbrander, P.E.; Palmer, R.J., Jr.; Periasamy, S.; Jakubovics, N.S. Oral multispecies biofilm development and the key role of cell-cell distance. Nat. Rev. Microbiol. 2010, 8, 471–480. [Google Scholar] [CrossRef]
| Types | Mechanisms | Materials Preparation | References | |
|---|---|---|---|---|
| Antibacterial coatings | Active coatings | Fight infection by releasing antimicrobial agents | Antibiotic or silver compound coatings, enzymes, antimicrobial peptides | [53,130,131,132,133] |
| Passive coatings | Prevent bacteria from attaching using hydrophilic materials | Hyaluronic acid, hydrogel coatings | [134,135,136] | |
| Modification of the surface of medical implants | Physical modification | Affect cell adhesion by changing the surface roughness and generating hydrophobic surfaces | Femtosecond laser-induced surfaces, superhydrophobic non-planar 3D surfaces, double-etched suface, polyetheretherketone, nano- and shark micropattern surfaces | [137,138,139,140,141,142,143,144,145] |
| Chemical modification | Regulate the formation of biofilms by changing the chemical properties of the material surface | Chitosan hydrogel films, surface-immobilized brominated furanones | [146,147] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Yin, R.; Cheng, J.; Lin, J. Bacterial Biofilm Formation on Biomaterials and Approaches to Its Treatment and Prevention. Int. J. Mol. Sci. 2023, 24, 11680. https://doi.org/10.3390/ijms241411680
Li P, Yin R, Cheng J, Lin J. Bacterial Biofilm Formation on Biomaterials and Approaches to Its Treatment and Prevention. International Journal of Molecular Sciences. 2023; 24(14):11680. https://doi.org/10.3390/ijms241411680
Chicago/Turabian StyleLi, Panxin, Rui Yin, Juanli Cheng, and Jinshui Lin. 2023. "Bacterial Biofilm Formation on Biomaterials and Approaches to Its Treatment and Prevention" International Journal of Molecular Sciences 24, no. 14: 11680. https://doi.org/10.3390/ijms241411680
APA StyleLi, P., Yin, R., Cheng, J., & Lin, J. (2023). Bacterial Biofilm Formation on Biomaterials and Approaches to Its Treatment and Prevention. International Journal of Molecular Sciences, 24(14), 11680. https://doi.org/10.3390/ijms241411680






