Production of Plant-Derived Japanese Encephalitis Virus Multi-Epitope Peptide in Nicotiana benthamiana and Immunological Response in Mice
Abstract
1. Introduction
2. Results
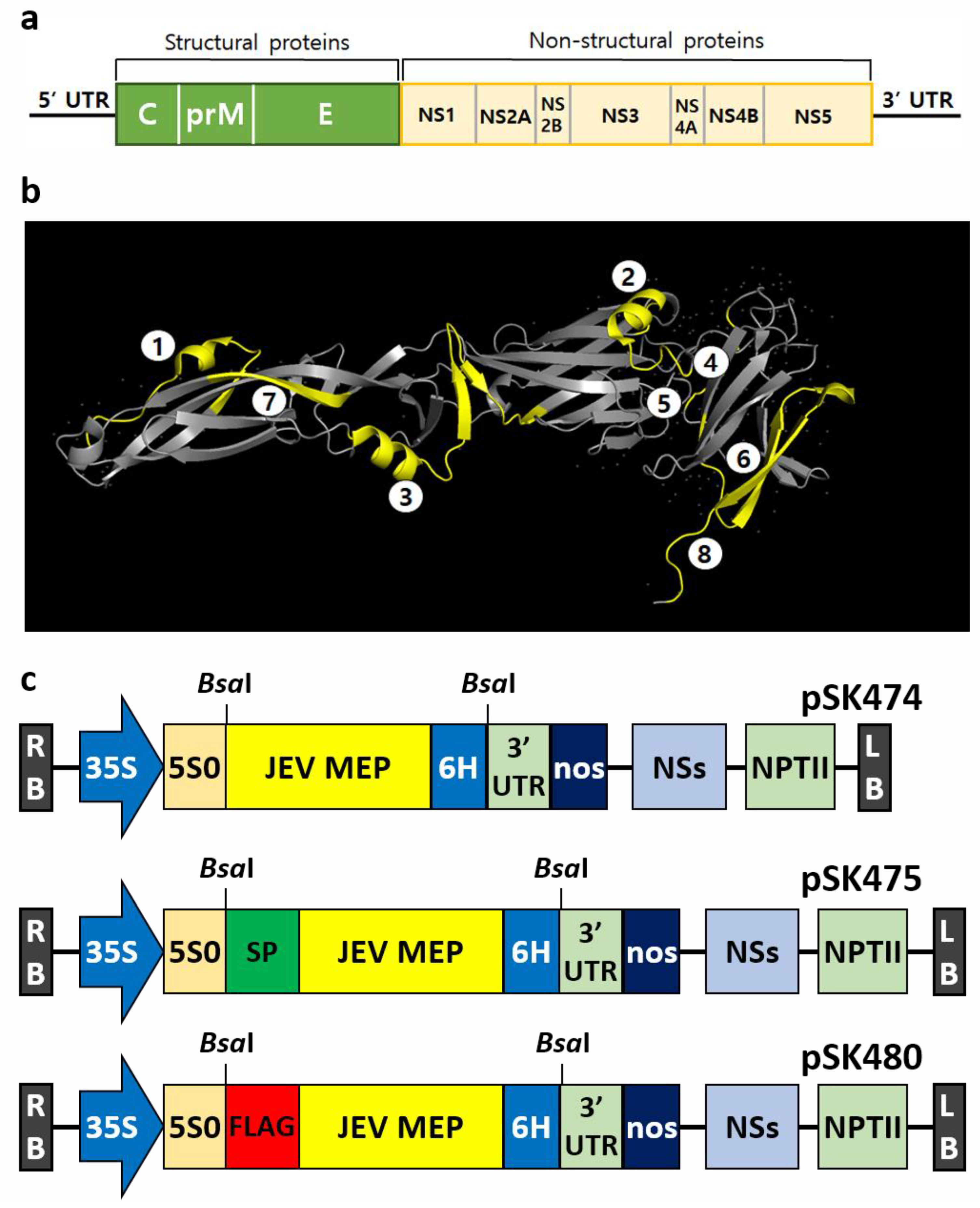
2.1. Vector Construction for N. benthamiana Expression System
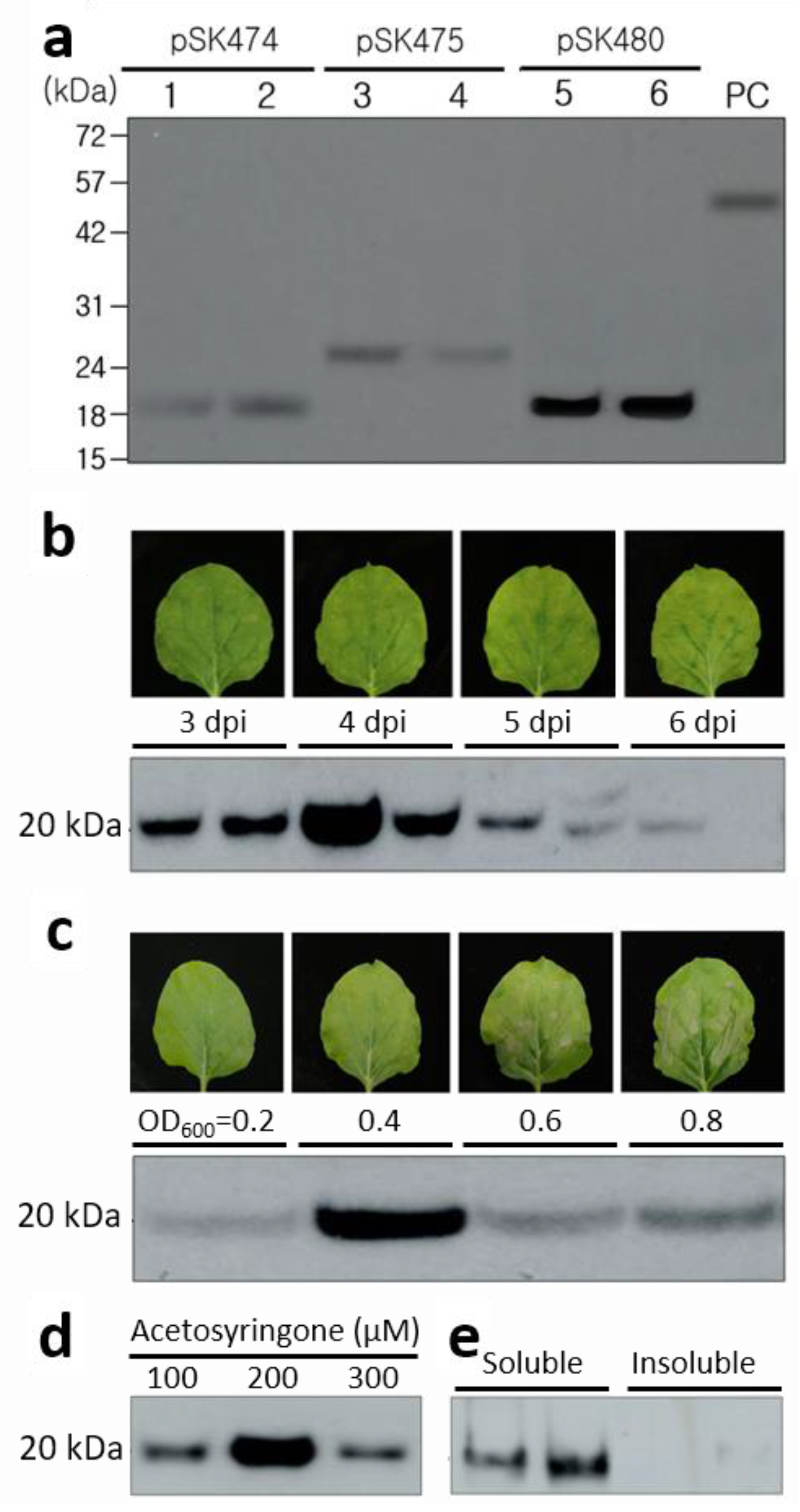
2.2. Expression of JEV-MEP
2.3. Optimization of JEV-MEP Expression in N. benthamiana
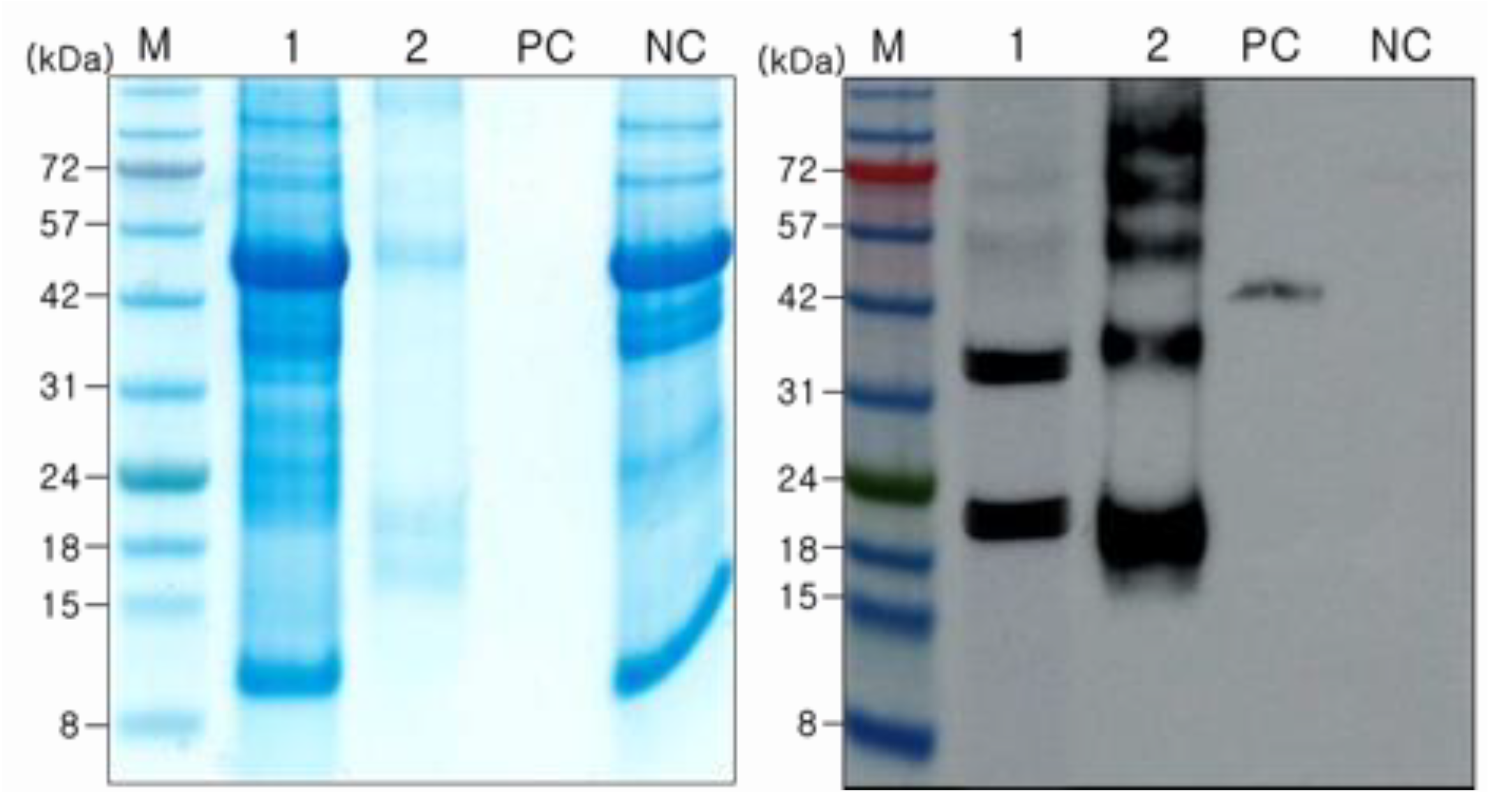
2.4. Purification of JEV-MEP
2.5. Induction of Protective Immunity against JEV by Vaccination with Purified JEV-MEP
3. Discussion
3.1. Expression and Multimerization of JEV-MEP
3.2. Immunogenicity of JEV-MEP
4. Materials and Methods
4.1. Plasmid Construction for Expression of JEV-MEP
4.2. E. coli and A. tumefaciens Transformation
4.3. Transient Expression of JEV in N. benthamiana Leaves
4.4. Immunoblotting Assay
4.5. Protein Purification
4.6. Analysis of JEV-MEP Specific Antibody in the Mouse by ELISA
4.7. Titration of Neutralizing Antibody against JEV
4.8. Quantification and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, A.R.; Song, J.M.; Seo, S.U. Emerging Japanese Encephalitis Virus Genotype V in Republic of Korea. J. Microbiol. Biotechnol. 2022, 32, 955–959. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.I.; Lee, Y.M. Japanese encephalitis: The virus and vaccines. Hum. Vaccin. Immunother. 2014, 10, 263–279. [Google Scholar] [CrossRef] [PubMed]
- Hegde, N.R.; Gore, M.M. Japanese encephalitis vaccines: Immunogenicity, protective efficacy, effectiveness, and impact on the burden of disease. Hum. Vaccin. Immunother. 2017, 13, 1320–1337. [Google Scholar] [CrossRef] [PubMed]
- Unni, S.K.; Ruzek, D.; Chhatbar, C.; Mishra, R.; Johri, M.K.; Singh, S.K. Japanese encephalitis virus: From genome to infectome. Microbes Infect. 2011, 13, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Kuhn, R.J.; Rossmann, M.G. A structural perspective of the flavivirus life cycle. Nat. Rev. Microbiol. 2005, 3, 13–22. [Google Scholar] [CrossRef]
- Stiasny, K.; Heinz, F.X. Flavivirus membrane fusion. J. Gen. Virol. 2006, 87, 2755–2766. [Google Scholar] [CrossRef]
- Seif, S.A.; Morita, K.; Matsuo, S.; Hasebe, F.; Igarashi, A. Finer mapping of neutralizing epitope(s) on the C-terminal of Japanese encephalitis virus E-protein expressed in recombinant Escherichia coli system. Vaccine 1995, 13, 1515–1521. [Google Scholar] [CrossRef]
- Kolaskar, A.S.; Kulkarni-Kale, U. Prediction of three-dimensional structure and mapping of conformational epitopes of envelope glycoprotein of Japanese encephalitis virus. Virology 1999, 261, 31–42. [Google Scholar] [CrossRef]
- Sette, A.; Fikes, J. Epitope-based vaccines: An update on epitope identification, vaccine design and delivery. Curr. Opin. Immunol. 2003, 15, 461–470. [Google Scholar] [CrossRef]
- Wei, J.C.; Huang, Y.Z.; Zhong, D.K.; Kang, L.; Ishag, H.; Mao, X.; Cao, R.B.; Zhou, B.; Chen, P.Y. Design and evaluation of a multi-epitope peptide against Japanese encephalitis virus infection in BALB/c mice. Biochem. Biophys. Res. Commun. 2010, 396, 787–792. [Google Scholar] [CrossRef]
- Plesner, A.M.; Ronne, T. Allergic mucocutaneous reactions to Japanese encephalitis vaccine. Vaccine 1997, 15, 1239–1243. [Google Scholar] [CrossRef] [PubMed]
- Shlim, D.R.; Solomon, T. Japanese encephalitis vaccine for travelers: Exploring the limits of risk. Clin. Infect. Dis. 2002, 35, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.K.; Yadav, N.; Khurana, S.M.P. Chapter 26—Vaccines: Present status and applications. In Animal Biotechnology, 2nd ed.; Verma, A.S., Singh, A., Eds.; Academic Press: Boston, MA, USA, 2020; pp. 523–542. [Google Scholar] [CrossRef]
- Peyret, H.; Steele, J.F.C.; Jung, J.W.; Thuenemann, E.C.; Meshcheriakova, Y.; Lomonossoff, G.P. Producing Vaccines against Enveloped Viruses in Plants: Making the Impossible, Difficult. Vaccines 2021, 9, 780. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.W.; Zahmanova, G.; Minkov, I.; Lomonossoff, G.P. Plant-based expression and characterization of SARS-CoV-2 virus-like particles presenting a native spike protein. Plant. Biotechnol. J. 2022, 20, 1363–1372. [Google Scholar] [CrossRef]
- Ward, B.J.; Makarkov, A.; Seguin, A.; Pillet, S.; Trepanier, S.; Dhaliwall, J.; Libman, M.D.; Vesikari, T.; Landry, N. Efficacy, immunogenicity, and safety of a plant-derived, quadrivalent, virus-like particle influenza vaccine in adults (18–64 years) and older adults (>/=65 years): Two multicentre, randomised phase 3 trials. Lancet 2020, 396, 1491–1503. [Google Scholar] [CrossRef]
- Ward, B.J.; Seguin, A.; Couillard, J.; Trepanier, S.; Landry, N. Phase III: Randomized observer-blind trial to evaluate lot-to-lot consistency of a new plant-derived quadrivalent virus like particle influenza vaccine in adults 18–49 years of age. Vaccine 2021, 39, 1528–1533. [Google Scholar] [CrossRef]
- Hager, K.J.; Perez Marc, G.; Gobeil, P.; Diaz, R.S.; Heizer, G.; Llapur, C.; Makarkov, A.I.; Vasconcellos, E.; Pillet, S.; Riera, F.; et al. Efficacy and Safety of a Recombinant Plant-Based Adjuvanted COVID-19 Vaccine. N. Engl. J. Med. 2022, 386, 2084–2096. [Google Scholar] [CrossRef]
- Peyret, H.; Brown, J.K.M.; Lomonossoff, G.P. Improving plant transient expression through the rational design of synthetic 5’ and 3’ untranslated regions. Plant. Methods 2019, 15, 108. [Google Scholar] [CrossRef]
- Krachmarova, E.; Tileva, M.; Lilkova, E.; Petkov, P.; Maskos, K.; Ilieva, N.; Ivanov, I.; Litov, L.; Nacheva, G. His-FLAG Tag as a Fusion Partner of Glycosylated Human Interferon-Gamma and Its Mutant: Gain or Loss? BioMed. Res. Int. 2017, 2017, 3018608. [Google Scholar] [CrossRef]
- Kaur, R.; Vrati, S. Development of a recombinant vaccine against Japanese encephalitis. J. Neurovirol. 2003, 9, 421–431. [Google Scholar] [CrossRef]
- Saito, A.; Hizukuri, Y.; Matsuo, E.-i.; Chiba, S.; Mori, H.; Nishimura, O.; Ito, K.; Akiyama, Y. Post-liberation cleavage of signal peptides is catalyzed by the site-2 protease (S2P) in bacteria. Proc. Natl. Acad. Sci. USA 2011, 108, 13740–13745. [Google Scholar] [CrossRef] [PubMed]
- Sojikul, P.; Buehner, N.; Mason, H.S. A plant signal peptide-hepatitis B surface antigen fusion protein with enhanced stability and immunogenicity expressed in plant cells. Proc. Natl. Acad. Sci. USA 2003, 100, 2209–2214. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Tan, S.; Berry, J.O.; Li, P.; Ren, N.; Li, S.; Yang, G.; Wang, W.B.; Qi, X.T.; Yin, L.P. An Uncleaved Signal Peptide Directs the Malus xiaojinensis Iron Transporter Protein Mx IRT1 into the ER for the PM Secretory Pathway. Int. J. Mol. Sci. 2014, 15, 20413–20433. [Google Scholar] [CrossRef]
- Magnelli, P.E.; Bielik, A.M.; Guthrie, E.P. Identification and characterization of protein glycosylation using specific endo- and exoglycosidases. J. Vis. Exp. 2011, 58, e3749. [Google Scholar] [CrossRef]
- Norkunas, K.; Harding, R.; Dale, J.; Dugdale, B. Improving agroinfiltration-based transient gene expression in Nicotiana benthamiana. Plant. Methods 2018, 14, 71. [Google Scholar] [CrossRef]
- Siriwattananon, K.; Manopwisedjaroen, S.; Kanjanasirirat, P.; Budi Purwono, P.; Rattanapisit, K.; Shanmugaraj, B.; Smith, D.R.; Borwornpinyo, S.; Thitithanyanont, A.; Phoolcharoen, W. Development of Plant-Produced Recombinant ACE2-Fc Fusion Protein as a Potential Therapeutic Agent Against SARS-CoV-2. Front. Plant Sci. 2020, 11, 604663. [Google Scholar] [CrossRef]
- Shukla, A.A.; Gupta, P.; Han, X. Protein aggregation kinetics during Protein A chromatography. Case study for an Fc fusion protein. J. Chromatogr. A 2007, 1171, 22–28. [Google Scholar] [CrossRef]
- Gil, D.; Schrum, A.G. Strategies to stabilize compact folding and minimize aggregation of antibody-based fragments. Adv. Biosci. Biotechnol. 2013, 4, 73–84. [Google Scholar] [CrossRef]
- Wu, H.; Kroe-Barrett, R.; Singh, S.; Robinson, A.S.; Roberts, C.J. Competing aggregation pathways for monoclonal antibodies. FEBS Lett. 2014, 588, 936–941. [Google Scholar] [CrossRef]
- Yang, C.; Gao, X.; Gong, R. Engineering of Fc Fragments with Optimized Physicochemical Properties Implying Improvement of Clinical Potentials for Fc-Based Therapeutics. Front. Immunol. 2018, 8, 1860. [Google Scholar] [CrossRef]
- Raso, S.W.; Abel, J.; Barnes, J.M.; Maloney, K.M.; Pipes, G.; Treuheit, M.J.; King, J.; Brems, D.N. Aggregation of granulocyte-colony stimulating factor in vitro involves a conformationally altered monomeric state. Protein Sci. 2005, 14, 2246–2257. [Google Scholar] [CrossRef] [PubMed]
- Mechtcheriakova, I.A.; Eldarov, M.A.; Nicholson, L.; Shanks, M.; Skryabin, K.G.; Lomonossoff, G.P. The use of viral vectors to produce hepatitis B virus core particles in plants. J. Virol. Methods 2006, 131, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Ramessar, K.; Rademacher, T.; Sack, M.; Stadlmann, J.; Platis, D.; Stiegler, G.; Labrou, N.; Altmann, F.; Ma, J.; Stoger, E.; et al. Cost-effective production of a vaginal protein microbicide to prevent HIV transmission. Proc. Natl. Acad. Sci. USA 2008, 105, 3727–3732. [Google Scholar] [CrossRef] [PubMed]
- Castells-Graells, R.; Lomonossoff, G.P. Plant-based production can result in covalent cross-linking of proteins. Plant. Biotechnol. J. 2021, 19, 1095–1097. [Google Scholar] [CrossRef]
- Lomonossoff, G.P.; D’Aoust, M.A. Plant-produced biopharmaceuticals: A case of technical developments driving clinical deployment. Science 2016, 353, 1237–1240. [Google Scholar] [CrossRef]
- Belhaj, K.; Chaparro-Garcia, A.; Kamoun, S.; Patron, N.J.; Nekrasov, V. Editing plant genomes with CRISPR/Cas9. Curr. Opin. Biotechnol. 2015, 32, 76–84. [Google Scholar] [CrossRef]
- Medialdea-Carrera, R.; Levy, F.; Castanha, P.; Carvalho de Sequeira, P.; Brasil, P.; Lewis-Ximenez, L.L.; Turtle, L.; Solomon, T.; Bispo de Filippis, A.M.; Brown, D.W.; et al. A Systematic Evaluation of IgM and IgG Antibody Assay Accuracy in Diagnosing Acute Zika Virus Infection in Brazil: Lessons Relevant to Emerging Infections. J. Clin. Microbiol. 2021, 59, e0289320. [Google Scholar] [CrossRef]
- Dhanze, H.; Kumar, M.S.; Singh, V.; Gupta, M.; Bhilegaonkar, K.N.; Kumar, A.; Mishra, B.P.; Singh, R.K. Detection of recent infection of Japanese encephalitis virus in swine population using IgM ELISA: A suitable sentinel to predict infection in humans. J. Immunol. Methods 2020, 486, 112848. [Google Scholar] [CrossRef]
- Litzba, N.; Klade, C.S.; Lederer, S.; Niedrig, M. Evaluation of serological diagnostic test systems assessing the immune response to Japanese encephalitis vaccination. PLoS Negl. Trop. Dis. 2010, 4, e883. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, N. On the way to commercializing plant cell culture platform for biopharmaceuticals: Present status and prospect. Pharm. Bioprocess. 2014, 2, 499–518. [Google Scholar] [CrossRef]
- Bardor, M.; Faveeuw, C.; Fitchette, A.C.; Gilbert, D.; Galas, L.; Trottein, F.; Faye, L.; Lerouge, P. Immunoreactivity in mammals of two typical plant glyco-epitopes, core alpha(1,3)-fucose and core xylose. Glycobiology 2003, 13, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Piron, R.; Santens, F.; De Paepe, A.; Depicker, A.; Callewaert, N. Using GlycoDelete to produce proteins lacking plant-specific N-glycan modification in seeds. Nat. Biotechnol. 2015, 33, 1135–1137. [Google Scholar] [CrossRef] [PubMed]
- van Ree, R.; Cabanes-Macheteau, M.; Akkerdaas, J.; Milazzo, J.P.; Loutelier-Bourhis, C.; Rayon, C.; Villalba, M.; Koppelman, S.; Aalberse, R.; Rodriguez, R.; et al. Beta(1,2)-xylose and alpha(1,3)-fucose residues have a strong contribution in IgE binding to plant glycoallergens. J. Biol. Chem. 2000, 275, 11451–11458. [Google Scholar] [CrossRef] [PubMed]
- Shaaltiel, Y.; Tekoah, Y. Plant specific N-glycans do not have proven adverse effects in humans. Nat. Biotechnol. 2016, 34, 706–708. [Google Scholar] [CrossRef]
- Santos, R.B.; Abranches, R.; Fischer, R.; Sack, M.; Holland, T. Putting the Spotlight Back on Plant Suspension Cultures. Front. Plant Sci. 2016, 7, 297. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, J.-W.; Park, P.-G.; Lee, W.-K.; Shin, J.-H.; Jang, M.-H.; Seo, E.-H.; An, T.; Kim, Y.B.; Moon, M.H.; Choi, S.-K.; et al. Production of Plant-Derived Japanese Encephalitis Virus Multi-Epitope Peptide in Nicotiana benthamiana and Immunological Response in Mice. Int. J. Mol. Sci. 2023, 24, 11643. https://doi.org/10.3390/ijms241411643
Jung J-W, Park P-G, Lee W-K, Shin J-H, Jang M-H, Seo E-H, An T, Kim YB, Moon MH, Choi S-K, et al. Production of Plant-Derived Japanese Encephalitis Virus Multi-Epitope Peptide in Nicotiana benthamiana and Immunological Response in Mice. International Journal of Molecular Sciences. 2023; 24(14):11643. https://doi.org/10.3390/ijms241411643
Chicago/Turabian StyleJung, Jae-Wan, Pil-Gu Park, Won-Kyung Lee, Jun-Hye Shin, Mi-Hwa Jang, Eun-Hye Seo, Timothy An, Young Beom Kim, Myeong Hee Moon, Seuk-Keun Choi, and et al. 2023. "Production of Plant-Derived Japanese Encephalitis Virus Multi-Epitope Peptide in Nicotiana benthamiana and Immunological Response in Mice" International Journal of Molecular Sciences 24, no. 14: 11643. https://doi.org/10.3390/ijms241411643
APA StyleJung, J.-W., Park, P.-G., Lee, W.-K., Shin, J.-H., Jang, M.-H., Seo, E.-H., An, T., Kim, Y. B., Moon, M. H., Choi, S.-K., Yun, J. S., Hong, K.-J., & Kim, S.-R. (2023). Production of Plant-Derived Japanese Encephalitis Virus Multi-Epitope Peptide in Nicotiana benthamiana and Immunological Response in Mice. International Journal of Molecular Sciences, 24(14), 11643. https://doi.org/10.3390/ijms241411643




