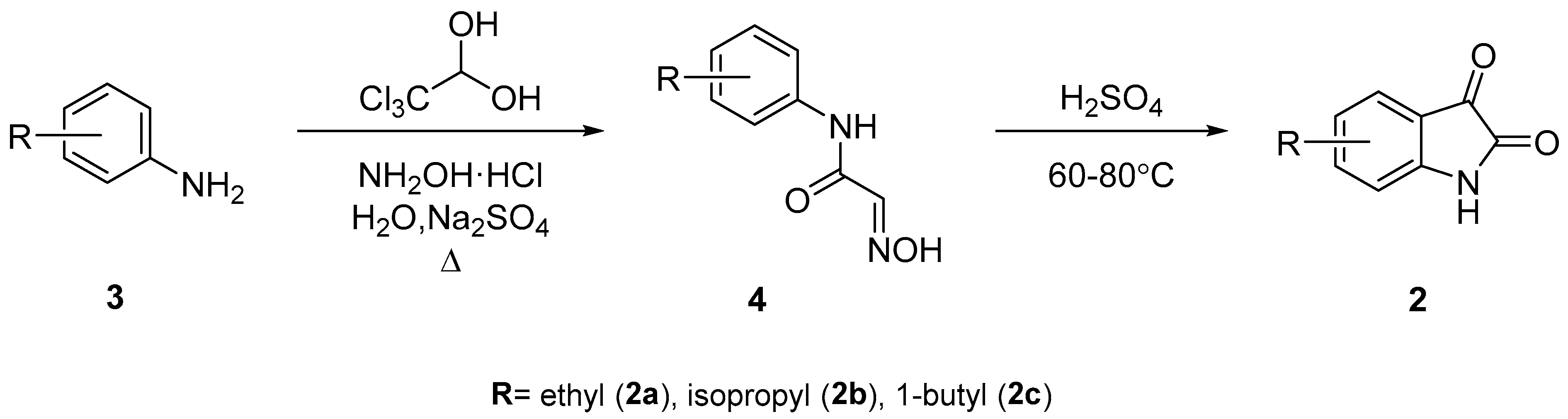
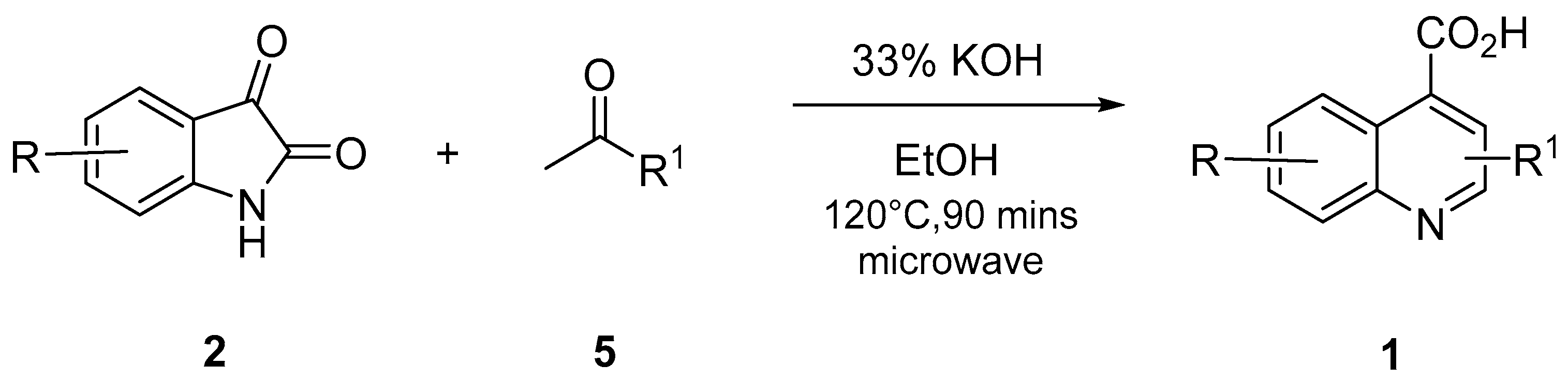
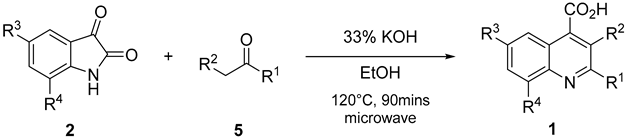
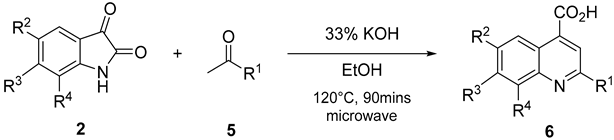
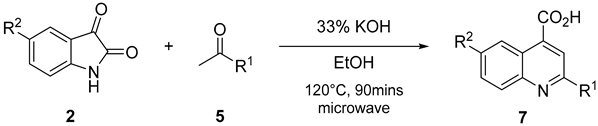
3.2.2. General Approach for the Synthesis of QCA 1, 6, and 7
A mixture of substituted isatin
2 (1 mmol), ketone
5 (1.0 mmol), and 33% potassium hydroxide (0.1 mL per reactant mmol of isatin) in 2–10 mL ethanol was placed in a closed Teflon vessel and irradiated on a microwave instrument for 90 min at 120 °C. The reaction mixture was dried in vacuo and washed thrice with diethyl ether. The obtained aqueous layer was cooled at 0–10 °C, and glacial acetic acid was slowly added until a pH of 4 was attained. The precipitates that formed were filtered and washed with cold ethanol. The obtained precipitates were purified by recrystallization, using ethanol and water (V
EtOH:V
water = 1:1), or by silica gel column chromatography, using dichloromethane (DCM) and methanol (MeOH) (V
DCM:V
MeOH = 15:1), to yield compounds
1a–1p, 6a–6r, and
7a–7n (
Supplementary Figures S1–S54).
2-pentylquinoline-4-carboxylic acid (1a): Neon yellow powder, yield 51.0%, Rf = 0.27 (5:1 EtOAc-MeOH). 1H NMR (600 MHz, MeOH-d4) δ 8.44 (s, 1H), 7.96 (d, J = 7.9 Hz, 1H), 7.69 (t, 1H), 7.65–7.48 (m, 2H) 2.94 (t, 2H), 1.90–1.73 (m, 2H), 1.51–1.30 (m, 4H), 0.89 (t, 3H). 13C NMR (151 MHz, MeOH-d4) δ 173.9, 162.9, 147.5, 146.6, 129.5, 127.05, 126.4, 125.9, 123.6, 119.2, 38.1, 31.4, 29.5, 22.9, 13.0.
2-phenethylquinoline-4-carboxylic acid (1b): Beige crystals, yield 92.0%, Rf = 0.25 (6:1 EtOAc-MeOH). 1H NMR (600 MHz, DMSO-d6) δ 7.57 (dd, J = 8.5, 1.0 Hz, 1H), 7.03–6.98 (m, 1H), 6.81 (s 1H), 6.74 (ddd, J = 8.3, 6.8, 1.4 Hz, 1H), 6.59 (ddd, J = 8.4, 6.9, 1.3 Hz, 1H), 6.26–6.18 (m, 3H), 6.15–6.08 (m, 1H), 2.26–2.20 (m, 2H), 2.06 (dd, J = 9.4, 6.7 Hz, 2H. 13C NMR (151 MHz, DMSO-d6) δ 168.2, 162.0, 148.6, 141.8, 136.8, 130.2, 129.6, 129.0, 128.8, 127.6, 126.4, 125.9, 123.5, 122.9, 40.2, 35.1.
2-benzyl-3-phenylquinoline-4-carboxylic acid (1c): Beige powder, yield 50.0%, Rf = 0.15 (6:1 EtOAc-MeOH). 1H NMR (600 MHz, DMSO-d6) δ 8.04 (d, J = 8.4 Hz,1H), 7.84–7.72 (m,2H), 7.62 (t, J = 7.4 Hz,1H), 7.47–7.31 (m,3H), 7.14 (d, J = 6.2 Hz,2H), 7.07 (dq, J = 14.0, 6.9 Hz,3H), 6.78 (d, J = 7.1 Hz,2H), 4.09 (s,2H). 13C NMR (151 MHz, DMSO-d6) δ 168.5, 159.7, 146.9, 139.3, 137.0, 130.5, 130.2, 130.1, 129.4, 129.0, 128.7, 128.6, 128.5, 128.4, 127.8, 126.5, 125.6, 122.3, 42.8.
2-benzhydrylquinoline-4-carboxylic acid (1d): Beige crystals; yield 63.0%, Rf = 0.36 (6:1 EtOAc-MeOH). 1H NMR (600 MHz, DMSO-d6) δ 8.62 (dd, J = 8.6, 1.1 Hz, 1H), 8.01–7.98 (m, 1H), 7.79 (s, 1H), 7.76 (ddd, J = 8.4, 6.9, 1.4 Hz, 1H), 7.64 (ddd, J = 8.4, 6.9, 1.3 Hz, 1H), 7.30–7.27 (m, 4H), 7.26–7.23 (m, 4H), 7.22–7.18 (m, 2H), 5.97 (s, 1H). 13C NMR (151 MHz, DMSO- d6) δ 168.0, 163.2, 148.6, 142.8, 137.2, 130.5, 130.0, 129.7, 129.0, 128.2, 127.1, 126.0, 123.6, 123.5, 56.6. HRMS (ESI): calculated [M + H+]+ 340.1338, found 340.1334.
2,6-dimethylquinoline-4-carboxylic acid (1e): White flakes, yield 24.9%, Rf = 0.14 (6:1 EtOAc-MeOH). 1H NMR (600 MHz, CDCl3) δ 8.15 (s, 1H), 7.62 (d, J = 8.6 Hz, 1H), 7.48 (d, J = 1.6 Hz, 1H), 7.36 (d, J = 8.6 Hz, 1H), 2.50 (s, 3H), 2.26 (s, 3H).13C NMR (151 MHz, CDCl3) δ 168.8, 157.0, 144.2, 141.9, 138.0, 133.3, 129.4, 125.2, 123.8, 122.3, 22.9, 21.4.
6-chloro-2-methylquinoline-4-carboxylic acid (1f): Pale yellow powder, yield 76.0%, Rf = 0.14 (6:1 EtOAc-MeOH). 1H NMR (600 MHz, DMSO-d6) δ 8.69 (s, 1H), 7.93 (s, 1H), 7.84 (s,1H), 7.71 (s, 1H), 2.64 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 167.7, 159.9, 147.1, 135.8, 132.0, 131.4, 130.5, 124.9, 124.6, 124.2, 25.2.
2-phenylquinoline-4-carboxylic acid (1g): Yellow powder, yield 88.0%, Rf = 0.45 (5:1 EtOAc-MeOH). 1H NMR (600 MHz, MeOH-d4) δ 8.57 (d, J = 8.4 Hz, 1H), 8.18 (s, 1H), 8.10 (m, 3H), 7.78–7.73 (m, 1H), 7.61–7.41 (m, 4H). 13C NMR (151 MHz, MeOH-d4) δ 171.6, 157.4, 148.5, 144.0, 139.1, 129.7, 129.4, 128.6, 127.4, 126.7, 126.1, 124.1, 118.1.
2-([1,1’-biphenyl]-4-yl)quinoline-4-carboxylic acid (1h): Beige powder, yield 79.7%, Rf = 0.21 (6:1 EtOAc-MeOH). 1H NMR (600 MHz, DMSO-d6) δ 7.60 (dd, J = 8.5, 0.9 Hz, 1H), 7.46 (s, 1H), 7.36–7.32 (m, 2H), 7.12 (d, J = 8.1 Hz, 1H), 6.83–6.78 (m, 3H), 6.70 (dt, J = 2.8, 1.7 Hz, 2H), 6.64 (ddd, J = 8.3, 6.9, 1.3 Hz, 1H), 6.46–6.42 (m, 1H), 6.37–6.33 (m, 2H). 13C NMR (151 MHz, DMSO-d6) δ 168.2, 155.8, 148.9, 141.9, 139.9, 138.2, 137.4, 130.8, 130.2, 129.6, 128.4, 128.3, 127.7, 127.3, 125.9, 124.0, 119.5.
2-(naphthalen-1-yl)quinoline-4-carboxylic acid (1i): Beige powder, yield 54.5%, Rf = 0.38 (6:1 EtOAc-MeOH). 1H NMR (600 MHz, DMSO-d6) δ 8.78 (d, J = 2.2 Hz, 1H), 8.77 (d, J = 2.4 Hz, 1H), 8.59 (s, 1H), 8.41 (dd, J = 8.6, 1.8 Hz, 1H), 8.14 (d, J = 9.0 Hz, 1H), 8.12–8.08 (m, 1H), 8.05 (d, J = 8.6 Hz, 1H), 7.97–7.93 (m, 1H), 7.80 (dd, J = 9.0, 2.5 Hz, 1H), 7.61–7.54 (m, 2H). 13C NMR (151 MHz, DMSO-d6) δ 168.2, 156.7, 147.4, 139.9, 135.6, 134.2, 133.5, 132.4, 132.1, 131.0, 129.4, 129.1, 128.1, 127.8, 127.6, 127.2, 125.1, 124.9, 120.5.
2-(naphthalen-2-yl)quinoline-4-carboxylic acid (1j): Neon yellow powder, yield 70.0%, Rf = 0.26 (6:1 EtOAc-MeOH). 1H NMR (600 MHz, DMSO-d6) δ 8.91 (d, J = 1.2 Hz, 1H), 8.70 (dd, J = 8.5, 0.8 Hz, 1H), 8.68 (s, 1H), 8.53 (dd, J = 8.6, 1.8 Hz, 1H), 8.24 (d, J = 7.9 Hz, 1H), 8.19–8.15 (m, 1H), 8.12 (d, J = 8.7 Hz, 1H), 8.03–8.00 (m, 1H), 7.89 (ddd, J = 8.3, 6.8, 1.4 Hz, 1H), 7.74 (ddd, J = 8.3, 6.8, 1.3 Hz, 1H), 7.64–7.60 (m, 2H). 13C NMR (151 MHz, DMSO-d6) δ 168.3, 156.2, 149.0, 138.3, 135.8, 134.2, 133.6, 130.8, 130.3, 129.4, 129.0, 128.3, 128.1, 127.7, 127.6, 127.1, 126.02, 125.0, 124.0, 124.0, 119.8.
2-(anthracen-2-yl)quinoline-4-carboxylic acid (1k): Brown powder, yield 16.0%, Rf = 0.15 (6:1 EtOAc-MeOH). 1H NMR (600 MHz, DMSO-d6) δ 9.03 (s, 1H), 8.76 (s, 1H), 8.68 (s, 1H), 8.65 (d, J = 8.4 Hz,1H), 8.59 (s, 1H), 8.48 (d, J = 9.0 Hz,1H), 8.20 (dd, J = 15.3, 8.6 Hz,2H), 8.13–8.04 (m,2H), 7.84 (t, J = 7.5 Hz,1H), 7.68 (t, J = 7.6 Hz,1H), 7.56–7.45 (m,2H). 13C NMR (151 MHz, DMSO-d6) δ 168.3, 156.1, 149.0, 138.5, 135.2, 132.5, 132.1, 131.9, 131.6, 130.8, 130.3, 129.3, 128.8, 128.9, 128.3, 128.3, 128.1, 126.7, 126.5, 126.4, 126.1, 124.5, 124.1, 119.8.
2-(phenanthren-3-yl)quinoline-4-carboxylic acid (1l): Yellow powder, yield 33.0%, Rf = 0.15 (6:1 EtOAc-MeOH). 1H NMR (600 MHz, DMSO-d6) δ 9.64 (s,1H), 9.10 (d, J = 8.3 Hz,1H), 8.73 (s,1H), 8.61–8.55 (m,2H), 8.21 (d, J = 8.3 Hz,1H), 8.14 (d, J = 8.3 Hz,1H), 8.01 (d, J = 8.4 Hz,1H), 7.91 (s,2H), 7.86–7.80 (m,1H), 7.78–7.71 (m,1H), 7.67 (ddd, J = 8.1, 4.0, 1.9 Hz,2H). 13C NMR (151 MHz, DMSO-d6) δ 168.6, 156.4, 148.9, 136.9, 133.0, 132.4, 130.7, 130.6, 130.3, 130.3, 129.8, 129.2, 128.6, 128.1, 127.7, 127.0, 126.2, 126.1, 126.1, 124.1, 123.9, 123.9, 122.3, 119.6.
2-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)quinoline-4-carboxylic acid (1m): Bright yellow powder, yield 18.6%, Rf = 0.19 (6:1 EtOAc-MeOH). 1H NMR (600 MHz, DMSO-d6) δ 8.56 (dd, J = 8.6, 1.0 Hz, 1H), 8.33 (s, 1H), 8.07 (d, J = 7.9 Hz, 1H), 7.80–7.76 (m, 2H), 7.75 (dd, J = 8.5, 2.2 Hz, 1H), 7.62 (ddd, J = 8.3, 6.8, 1.3 Hz, 1H), 6.99 (d, J = 8.4 Hz, 1H), 4.32–4.25 (m, 4H) 13C NMR (151 MHz, DMSO-d6) δ 168.2, 155.6, 148.8, 145.9, 144.3, 138.2, 131.7, 130.6, 130.1, 127.9, 125.9, 123.7, 121.0, 119.2, 118.0, 116.3, 64.9, 64.6.
2-(4-methoxyphenyl)quinoline-4-carboxylic acid (1n): Beige powder, 58.0%; 1H NMR (600 MHz,) δ 8.60 (dd, J = 15.7, 8.5 Hz, 1H), 8.05 (dd, J = 14.7, 8.4 Hz, 1H), 7.77 (dd, J = 11.1, 8.8 Hz, 2H), 7.65–7.62 (m, 1H), 7.25 (dd, J = 13.8, 8.4 Hz, 2H), 6.90–6.81 (m, 2H), 3.69 (d, J = 13.6 Hz, 3H). 13C NMR (151 MHz, DMSO-d6) δ 168.1, 161.9, 158.4, 148.6, 137.4, 131.5, 131.1, 130.9, 130.3, 129.7, 127.7, 125.9, 122.6, 114.6, 55.5.
2-(4-fluoro-3-nitrophenyl)quinoline-4-carboxylic acid (1o): Brown solid, yield 49.1%, Rf = 0.11 (6:1 EtOAc-MeOH). 1H NMR (600 MHz, DMSO-d6) δ 8.71 (d, J = 2.4 Hz, 1H), 8.59 (dd, J = 8.5, 0.9 Hz, 1H), 8.34 (dd, J = 8.8, 2.3 Hz, 1H), 8.18 (s, 1H), 8.01 (d, J = 8.1 Hz, 1H), 7.70 (ddd, J = 8.3, 6.8, 1.4 Hz, 1H), 7.53 (ddd, J = 8.2, 6.8, 1.2 Hz, 1H), 7.28 (d, J = 8.8 Hz, 1H). 13C NMR (151 MHz, DMSO-d6) δ 169.4, 156.4, 154.4, 148.7, 138.1, 138,1, 133.4, 130.1, 129.6, 128.1, 127.1, 126.8, 124.5, 124.4, 121.3, 117.1. HRMS (ESI): calculated [M + H+]+ 313.0625, found 313.0627
2-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-8-fluoroquinoline-4-carboxylic acid (1p): Yellow powder, yield 62.0%, Rf = 0.15 (6:1 EtOAc-MeOH). 1H NMR (600 MHz, MeOH-d4)δ 8.47 (d, J = 8.6 Hz), 8.34 (s), 7.70 (d, J = 2.1 Hz), 7.64 (dd, J = 8.5, 2.1 Hz), 7.41 (td, J = 8.1, 5.3 Hz), 7.36–7.31 (m), 6.92 (d, J = 8.4 Hz), 3.57 (s). 13C NMR (151 MHz, CDCl3) δ 168.3, 158.9, 157.24, 156.3, 145.6, 144.0, 136.6, 132.0, 126.8, 121.4, 121.0, 117.7, 116.7, 116.4, 113.9, 113.8, 64.7, 64.3. HRMS (ESI): calculated [M + H+]+ 326.0829, found 326.0843
2-([1,1’-biphenyl]-4-yl)-6-chloroquinoline-4-carboxylic acid (6a): Neon yellow powder; 28.0% yield, Rf = 0.46 (6:1 EtOAc-MeOH). 1H NMR (600 MHz, DMSO-d6) δ 8.73 (t, J = 2.2 Hz, 1H), 8.55–8.53 (m, 1H), 8.36–8.31 (m, 2H), 8.13 (ddd, J = 8.9, 4.2, 1.5 Hz, 1H), 7.82 (dt, J = 5.3, 4.2 Hz, 3H), 7.75–7.70 (m, 2H), 7.50–7.44 (m, 2H), 7.38 (dd, J = 11.0, 3.7 Hz, 1H). 13C NMR (151 MHz, DMSO-d6) δ 167.6, 156.3, 147.5, 142.2, 139.8, 136.9, 136.6, 132.8, 132.4, 131.2, 129.6, 128.5, 128.3, 127.7, 127.2, 124.9, 124.8, 121.0.
2-([1,1’-biphenyl]-4-yl)-8-chloroquinoline-4-carboxylic acid (6b): Neon yellow powder, yield 15.5%, Rf = 0.24 (6:1 EtOAc-MeOH). 1H NMR (600 MHz, DMSO-d6) δ 8.52 (dd, J = 8.5, 0.9 Hz, 1H), 8.48 (s, 1H), 8.39 (d, J = 8.4 Hz, 1H), 7.96 (dd, J = 7.5, 1.0 Hz, 1H), 7.82 (d, J = 8.4 Hz, 2H), 7.71 (d, J = 7.4 Hz, 2H), 7.57 (dd, J = 8.4, 7.6 Hz, 1H), 7.45 (t, J = 7.7 Hz, 1H), 7.36 (t, J = 7.4 Hz, 1H). 13C NMR (151 MHz, DMSO-d6) δ 172.6, 168.2, 156.1, 144.6, 142.3, 141.0, 139.8, 137.1, 133.5, 130.7, 129.6, 128.50, 128.4, 127.9, 127.8, 127.3, 125.6, 119.6. HRMS (ESI): calculated [M + H+]+ 359.8090, found 360.0785
2-([1,1’-biphenyl]-4-yl)-6-fluoroquinoline-4-carboxylic acid (6c): Neon yellow powder, yield 75.0%, Rf = 0.13 (6:1 EtOAc-MeOH). 1H NMR (600 MHz, DMSO-d6) δ 8.60 (s, 1H), 8.51–8.43 (m, 1H), 8.38 (d, J = 8.3 Hz, 2H), 8.25 (dd, J = 9.1, 5.8 Hz, 1H), 7.87 (t, J = 7.2 Hz, 2H), 7.77 (d, J = 7.5 Hz, 2H), 7.51 (t, J = 7.6 Hz, 3H), 7.41 (t, J = 7.2 Hz, 1H). 13C NMR (151 MHz, DMSO- d6) δ 167.2, 159.7, 154.9, 145.9, 141.6, 139.3, 136.6, 132.6, 129.0, 127.9, 127.7, 127.4, 127.2, 127.1, 126.7, 124.5, 124.4, 120.4, 120.3, 120.2, 109.3, 109.1. HRMS (ESI): calculated [M + H+]+ 344.1087, found 344.1084
2-([1,1’-biphenyl]-4-yl)-8-fluoroquinoline-4-carboxylic acid (6d): Pale yellow powder, yield 82.3%, Rf = 0.39 (6:1 EtOAc-MeOH). 1H NMR (600 MHz, DMSO-d6) δ 8.59 (d, J = 3.5 Hz, 1H), 8.49–8.43 (m, 1H), 8.42–8.37 (m, 2H), 7.90–7.84 (m, 2H), 7.76 (td, J = 5.3, 1.5 Hz, 2H), 7.72–7.63 (m, 2H), 7.53–7.47 (m, 2H), 7.44–7.38 (m, 1H) 13C NMR (151 MHz, DMSO-d6) δ 167.8, 160.5, 155.6, 150.5, 141.4, 139.4, 137.5, 137.0, 129.1, 127.9, 127.7, 127.2, 126.8, 126.6, 120.5, 118.7, 116.7, 108.0, 55.6. HRMS (ESI): calculated [M + H+]+ 344.1087, found 344.1087
2-([1,1’-biphenyl]-4-yl)-6-bromoquinoline-4-carboxylic acid (6e): Brown solid, yield 61.6%, Rf = 0.38 (6:1 EtOAc-MeOH). 1H NMR (600 MHz, DMSO-d6) δ 9.03 (s, 1H), 8.78 (d, J = 6.0 Hz, 3H), 8.60 (s, 1H), 8.49–8.44 (m, 1H), 8.22 (dd, J = 13.7, 9.0 Hz, 2H), 8.10 (d, J = 8.2 Hz, 2H), 7.87 (dd, J = 8.9, 2.3 Hz, 1H), 7.58–7.53 (m, 2H). 13C NMR (151 MHz, DMSO-d6) δ 167.7, 156.6, 147.5, 134.8, 133.6, 133.0, 132.6, 132.4, 132.1, 131.9, 131.5, 131.3, 129.4, 128.8, 128.7, 128.4, 126.8, 126.5, 125.0, 124.9, 124.3, 121.3.
2-([1,1’-biphenyl]-4-yl)-8-bromoquinoline-4-carboxylic acid (6f): Beige powder, yield 87.6%, Rf = 0.26 (6:1 EtOAc-MeOH). 1H NMR (600 MHz, DMSO-d6) δ 8.64 (dd, J = 8.3, 1.0 Hz), 8.32–8.28 (m), 8.04 (s), 7.97 (d, J = 8.3 Hz), 7.84–7.80 (m), 7.74 (d, J = 7.6 Hz), 7.64 (ddd, J = 8.2, 7.0, 1.3 Hz), 7.47 (ddd, J = 9.3, 6.6, 1.4 Hz), 7.39–7.33 (m).13C NMR (151 MHz, DMSO- d6) δ 173.9, 169.6, 155.6, 151.2, 151.1, 150.1, 148.8, 148.7, 141.3, 140.1, 138.7, 129.6, 129.4, 128.3, 128.3, 128.1, 128.0, 127.6, 127.2, 125.8, 125.4, 116.3.
2-([1,1’-biphenyl]-4-yl)-6-iodoquinoline-4-carboxylic acid (6g): Yellow powder, yield 31.7%, Rf = 0.10 (6:1 EtOAc-MeOH). 1H NMR (600 MHz, DMSO-d6) δ 8.64 (d, J = 8.8 Hz, 1H), 8.50 (s, 1H), 8.39 (d, J = 8.5 Hz, 2H), 8.18 (d, J = 8.2 Hz, 1H), 7.86 (dd, J = 19.3, 8.4 Hz, 3H), 7.77 (d, J = 7.9 Hz, 2H), 7.70 (t, J = 7.7 Hz, 1H), 7.51 (t, J = 7.7 Hz, 2H), 7.41 (t, J = 7.4 Hz, 1H). 13C NMR (151 MHz, DMSO-d6) δ 167.7, 155.4, 148.5, 141.6, 139.4, 138.0, 136.9, 130.4, 129.8, 129.1, 128.0, 127.9, 127.3, 127.0, 126.9, 126.8, 125.5, 123.5, 119.0, 21.8, 18.6, 14.0. HRMS (ESI): calculated [M + H+]+ 452.0147, found 452.0143
2-([1,1’-biphenyl]-4-yl)-6-nitroquinoline-4-carboxylic acid (6h): Dark brown powder, yield 38.3%, Rf = 0.20 (6:1 EtOAc-MeOH). 1H NMR (600 MHz, DMSO-d6) δ 8.33 (s, 1H), 8.28 (dt, J = 8.7, 1.6 Hz, 2H), 7.87 (d, 1H), 7.83–7.81 (m, 2H), 7.75 (dt, J = 8.3, 1.3 Hz, 2H), 7.68 (d, J = 2.6 Hz, 1H), 7.51–7.48 (m, 2H), 7.41–7.38 (m, 1H), 7.26 (dd, J = 9.0, 2.6 Hz, 1H). 13C NMR (151 MHz, DMSO-d6) δ 168.8, 149.6, 149.2, 143.7, 140.8, 140.1, 138.1, 133.9, 131.4, 129.6, 128.2, 127.6, 127.4, 127.2, 126.7, 122.8, 119.6, 103.0. HRMS (ESI): calculated [M + H+]+ 371.1032, found 371.1032
2-([1,1’-biphenyl]-4-yl)-7-methoxyquinoline-4-carboxylic acid(6i): Bright yellow powder, 59.2% (586mg); Rf = 0.31 (6:1 EtOAc-MeOH, Yellow spot with vanillin–sulfuric acid). 1H NMR (600 MHz, DMSO-d6) δ 8.57 (d, J = 9.3 Hz, 1H), 8.41–8.37 (m, 2H), 8.35 (s, 1H), 7.90–7.85 (m, 2H), 7.80–7.76 (m, 2H), 7.56 (d, J = 2.7 Hz, 1H), 7.54–7.49 (m, 2H), 7.45–7.39 (m, 1H), 7.35 (dd, J = 9.3, 2.7 Hz, 1H), 3.98 (s, 3H). 13C NMR (151 MHz, DMSO-D6) δ 167.8, 160.5, 155.6, 150.5, 141.4, 139.4, 137.5, 137.0, 129.1, 127.9, 127.7, 127.2, 126.8, 126.6, 120.5, 118.7, 116.7, 108.0, 55.6. HRMS (ESI): calculated [M + H+]+ 356.1287, found 356.1282
6-chloro-2-(phenanthren-3-yl)quinoline-4-carboxylic acid (6j): Yellow powder, yield 36.6%, Rf = 0.31 (6:1 EtOAc-MeOH). 1H NMR (600 MHz, DMSO- d6) δ 9.20 (s, 1H), 8.63 (d, J = 13.5 Hz, 2H), 8.42 (s, 1H), 8.07 (d, J = 6.9 Hz, 1H), 7.93 (d, J = 7.9 Hz, 1H), 7.77 (d, J = 7.3 Hz, 1H), 7.64 (d, J = 6.2 Hz, 1H), 7.53 (s, 2H), 7.49–7.41 (m, 2H), 7.36 (d, J = 6.4 Hz, 2H). 13C NMR (151 MHz, DMSO-d6) δ 167.8, 156.9, 147.5, 137.2, 136.3, 133.2, 132.9, 132.4, 132.4, 131.2, 130.5, 130.4, 129.8, 129.2, 128.7, 127.7, 127.7, 126.9, 126.0, 124.8, 124.8, 123.9, 122.5, 121.5. HRMS (ESI): calculated [M + H+]+ 384.0791, found 384.0801
2-(anthracen-2-yl)-6-chloroquinoline-4-carboxylic acid (6k): Yellow solid, yield 19.8%. Rf = 0.38 (6:1 EtOAc-MeOH). 1H NMR (600 MHz, DMSO-d6) δ 8.96 (s, 1H), 8.73 (s, 1H), 8.62 (s, 1H), 8.56 (s, 1H), 8.46–8.43 (m, 1H), 8.40 (s, 1H), 8.18 (d, J = 9.0 Hz, 1H), 7.65 (dd, J = 8.6, 1.8 Hz, 1H), 7.53–7.46 (m, 2H). 13C NMR (151 MHz, DMSO-d6) δ 168.4, 156.4, 148.9, 138.9, 136.8, 133.1, 132.4, 130.8, 130.6, 130.5, 130.4, 129.8, 129.2, 128.6, 128.3, 127.7, 127.0, 126.1, 125.9, 123.9, 122.4, 120.0. HRMS (ESI): calculated [M + H+]+ 384.0791, found 384.0793
6-chloro-2-(naphthalen-1-yl)quinoline-4-carboxylic acid (6l): Bright yellow crystals, yield 93.0%, Rf = 0.39 (6:1 EtOAc-MeOH). 1H NMR (600 MHz, DMSO-d6) δ 8.91 (d, J = 2.3 Hz, 1H), 8.26 (s, 1H), 8.18 (d, J = 9.0 Hz, 1H), 8.14–8.04 (m, 3H), 7.89 (dd, J = 9.0, 2.4 Hz, 1H), 7.79 (dd, J = 7.1, 1.3 Hz, 1H), 7.66 (dd, J = 8.2, 7.0 Hz, 1H), 7.60–7.52 (m, 2H). 13C NMR (151 MHz, DMSO-d6) δ 166.9, 158.9, 146.9, 136.9, 135.5, 133.5, 132.8, 131.9, 130.7, 130.4, 129.6, 128.5, 128.2, 127.0, 126.2, 125.5, 125.1, 125.0, 124.4, 124.2.
6-chloro-2-(naphthalen-2-yl)quinoline-4-carboxylic acid (6m): Bright yellow crystals, yield 96.6%, Rf = 0.39 (6:1 EtOAc-MeOH). 1H NMR (600 MHz, DMSO-d6) δ 8.78 (d, J = 2.2 Hz, 1H), 8.77 (d, J = 2.4 Hz, 1H), 8.59 (s, 1H), 8.41 (dd, J = 8.6, 1.8 Hz, 1H), 8.14 (d, J = 9.0 Hz, 1H), 8.12–8.08 (m, 1H), 8.05 (d, J = 8.6 Hz, 1H), 7.97–7.93 (m, 1H), 7.80 (dd, J = 9.0, 2.5 Hz, 1H), 7.61–7.54 (m, 2H). 13C NMR (151 MHz, DMSO-d6) δ 168.2, 156.7, 147.4, 139.9, 135.6, 134.2, 133.5, 132.4, 132.1, 131.0, 129.4, 129.1, 128.1, 127.8, 127.6, 127.2, 125.1, 124.9, 120.5.
8-chloro-2-(naphthalen-2-yl)quinoline-4-carboxylic acid (6n): Yellow solid yield 16.5%, Rf = 0.38 (6:1 EtOAc-MeOH). 1H NMR (600 MHz, DMSO-d6) δ 8.90 (s, 1H), 8.72 (s, 1H), 8.57 (ddd, J = 15.8, 8.6, 1.3 Hz, 2H), 8.11 (dd, J = 15.3, 9.0 Hz, 2H), 8.06–8.02 (m, 1H), 8.01–7.97 (m, 1H), 7.66 (s, 1H), 7.60 (d, J = 4.1 Hz, 2H). 13C NMR (151 MHz, DMSO-d6) δ 168.0, 156.5, 144.7, 139.4, 135.4, 134.3, 133.7, 133.5, 130.9, 129.5, 129.2, 128.3, 128.2, 128.0, 127.9, 127.3, 125.5, 125.4, 124.9, 120.4. HRMS (ESI): calculated [M + H+]+ 334.0635, found 334.0626
2-(anthracen-2-yl)-8-chloroquinoline-4-carboxylic acid (6o): Yellow solid, yield 28.9%. Rf = 0.40 (6:1 EtOAc-MeOH). 1H NMR (600 MHz, DMSO-d6) δ 9.69 (s, 1H), 9.14 (d, J = 8.3 Hz, 1H), 8.83 (s, 1H), 8.65–8.61 (m, 2H), 8.27 (d, J = 8.3 Hz, 1H), 8.18 (d, J = 8.3 Hz, 1H), 8.04 (d, J = 8.5 Hz, 1H), 7.94 (s, 2H), 7.88 (s, 1H), 7.78 (t, J = 8.1 Hz, 1H), 7.72 (dt, J = 14.0, 7.3 Hz, 2H). 13C NMR (151 MHz, DMSO-d6) δ 167.7, 167.6, 156.6, 151.6, 147.5, 134.8, 133.2, 133.0, 132.3, 132.1, 131.5, 131.3, 130.8, 129.4, 128.8, 128.7, 128.4, 126.5, 125.8, 125.0, 124.3, 123.9, 121.3, 79.7. HRMS (ESI): calculated [M + H+]+ 384.0791, found 384.0790
8-fluoro-2-(naphthalen-1-yl)quinoline-4-carboxylic acid (6p): Yellow powder, yield 7.5%, Rf = 0.22 (6:1 EtOAc-MeOH). 1H NMR (600 MHz, DMSO-d6) δ 8.50–8.46 (m), 8.16 (dd, J = 25.3, 8.7 Hz), 8.14 (d, J = 8.8 Hz), 8.05 (d, J = 8.1 Hz), 7.97 (d, J = 2.4 Hz), 7.72 (t, J = 7.5 Hz), 7.49 (d, J = 7.9 Hz). 13C NMR (151 MHz, DMSO-d6) δ 168.5, 159.05, 157.0, 155.2, 140.9, 134.0, 131.0, 129.9, 129.6, 129.0, 128.6, 127.4, 126.7, 126.0, 125.7, 123.5, 123.0, 117.8, 114.3, 110.4, 99.0. HRMS (ESI): calculated [M + H+]+ 318.0930, found 318.0931
6-bromo-2-(naphthalen-2-yl)quinoline-4-carboxylic acid (6q): Brown solid, yield 26.8%, Rf = 0.65 (6:1 EtOAc-MeOH). 1H NMR (600 MHz, DMSO-d6) δ 8.89 (s, 1H), 8.70–8.67 (m, 1H), 8.67 (s, 1H), 8.51 (dd, J = 8.6, 1.8 Hz, 1H), 8.22 (d, J = 8.1 Hz, 1H), 8.15 (dd, J = 6.0, 3.4 Hz, 1H), 8.09 (d, J = 8.6 Hz, 1H), 7.99 (dq, J = 6.4, 3.2 Hz, 1H), 7.86 (dd, J = 8.3, 1.3 Hz, 1H), 7.76–7.69 (m, 1H), 7.60 (dt, J = 6.3, 3.5 Hz, 2H). 13C NMR (151 MHz, DMSO-d6) δ 168.2, 156.2, 149.0, 138.3, 135.8, 134.2, 133.6, 130.8, 130.3, 129.4, 129.0, 128.3, 128.1, 127.7, 127.6, 127.2, 126.0, 125.0, 124.0, 119.9.
6-bromo-2-(phenanthren-3-yl)quinoline-4-carboxylic acid (6r): Brown solid yield 86.0%. Rf = 0.16 (6:1 EtOAc-MeOH). 1H NMR (600 MHz, DMSO-d6) δ 8.66 (d, J = 8.0 Hz, 1H), 8.52 (s, 1H), 8.41 (d, J = 8.4 Hz, 2H), 8.19 (d, J = 8.3 Hz, 1H), 7.87 (dd, J = 15.6, 8.4 Hz, 3H), 7.79–7.75 (m, 2H), 7.73–7.69 (m, 1H), 7.51 (t, J = 7.7 Hz, 2H), 7.42 (t, J = 7.4 Hz, 1H). 13C NMR (151 MHz, DMSO-d6) δ 168.2, 155.9, 149.0, 142.1, 139.9, 138.3, 137.4, 130.8, 130.3, 129.6, 128.5, 128.3, 128.3, 127.7, 127.3, 126.0, 124.0, 119.6. HRMS (ESI): calculated [M + H+]+ 428.0286, found 428.0285
2-([1,1’-biphenyl]-4-yl)-6-methylquinoline-4-carboxylic acid (7a): Beige powder, yield 63.8%, Rf = 0.33 (6:1 EtOAc-MeOH). 1H NMR (600 MHz, DMSO-d6) δ 8.47 (s, 1H), 8.43, (s, 1H), 8.39–8.34 (m, 2H), 8.07 (d, J = 8.5 Hz, 1H), 7.88–7.83 (m, 2H), 7.79–7.74 (m, 2H), 7.69 (dd, J = 8.7, 2.1 Hz, 1H), 7.53–7.47 (m, 2H), 7.44–7.38 (m, 1H), 2.54 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 168.3, 154.9, 147.7, 141.8, 139.9, 138.0, 137.5, 137.4, 132.9, 130.1, 129.6, 128.4, 128.15, 127.7, 127.2, 124.6, 124.0, 119.5, 22.2.
2-([1,1’-biphenyl]-4-yl)-6-ethylquinoline-4-carboxylic acid (7b): Yellow powder, yield 57.5%, Rf = 0.10 (6:1 EtOAc-MeOH). 1H NMR (600 MHz, DMSO-d6) δ 8.58 (s), 8.26 (d, J = 8.2 Hz), 7.93 (d, J = 8.5 Hz), 7.66 (d, J = 7.5 Hz), 7.55 (d, J = 9.9 Hz), 7.44 (t, J = 7.7 Hz), 7.35 (t, J = 7.3 Hz), 2.76–2.59 (m), 1.18 (t, J = 7.3 Hz). 13C NMR (151 MHz, DMSO- d6) δ 171.0, 154.7, 147.7, 146.9, 142.0, 141.2, 140.0, 138.3, 130.7, 129.6, 129.5, 128.3, 128.0, 128.0, 127.8, 127.5, 127.1, 125.4, 125.3, 125.3, 125.1, 117.7, 29.1, 16.1. HRMS (ESI): calculated [M + H+]+ 354.1494, found 354.1491
2-([1,1’-biphenyl]-4-yl)-6-isopropylquinoline-4-carboxylic acid (7c): Beige powder, yield 39.8%, Rf = 0.15 (6:1 EtOAc-MeOH). 1H NMR (600 MHz, MeOH-d4) δ 8.61 (s, 1H), 8.45 (s, 1H), 8.26 (d, J = 8.3 Hz, 2H), 8.13 (d, J = 8.7 Hz, 1H), 7.83 (d, J = 8.3 Hz, 2H), 7.78 (dd, J = 8.7, 2.0 Hz, 1H), 7.72 (d, J = 7.3 Hz, 2H), 7.48 (t, J = 7.7 Hz, 2H), 7.38 (t, J = 7.4 Hz, 1H), 3.19–3.12 (m, 1H), 1.67 (d, J = 15.6 Hz, 1H). 13C NMR (151 MHz, MeOH-d4) δ 170.4, 169.8, 157.1, 149.9, 149.0, 143.9, 141.6, 138.7, 131.2, 130.2, 130.1, 130.0, 129.1, 128.8, 128.5, 128.2, 128.2, 128.0, 127.9, 125.5, 123.2, 121.1, 35.8, 24.2. HRMS (ESI): calculated [M + H+]+ 368.1651, found 368.1650
2-([1,1’-biphenyl]-4-yl)-6-butylquinoline-4-carboxylic acid (7d): Yellow powder, yield 34.5%, Rf = 0.20 (6:1 EtOAc-MeOH). 1H NMR (600 MHz, MeOH-d4) δ 8.40 (s, 1H), 8.26 (s, 1H), 8.23 (d, J = 8.2 Hz, 1H), 7.82 (d, J = 8.2 Hz, 1H), 7.75 (d, J = 8.5 Hz, 1H), 7.30 (t, J = 7.3 Hz, 1H), 2.87–2.81 (m, 1H), 1.78–1.70 (m, 1H), 1.48–1.39 (m, 1H), 0.98 (t, J = 7.4 Hz, 1H). 13C NMR (151 MHz, MeOH-d4) δ 157.3, 147.3, 143.7, 141.7, 141.1, 137.1, 132.7, 130.1, 130.1, 130.0, 129.8, 129.4, 129.1, 128.8, 128.5, 128.2, 128.2, 128.0, 127.9, 127.0, 125.6, 36.9, 34.7, 26.7, 23.4, 14.3. HRMS (ESI): calculated [M + H+]+ 382.1807, found 382.1802
6-methyl-2-(naphthalen-2-yl)quinoline-4-carboxylic acid (7e): Yellow solid, yield 28.0%, Rf = 0.11 (6:1 EtOAc-MeOH. 1H NMR (600 MHz, DMSO-d6) δ 8.80 (s, 1H), 8.56 (s, 1H), 8.45–8.38 (m, 2H), 8.11–8.01 (m, 3H), 7.96–7.91 (m, 1H), 7.65 (d, J = 8.6 Hz, 1H), 7.56–7.51 (m, 2H). 13C NMR (151 MHz, DMSO-d6) δ 168.3, 155.2, 147.7, 138.0, 137.5, 135.9, 134.1, 133.6, 132.9, 130.1, 129.4, 129.0, 128.1, 127.6, 127.3, 127.1, 124.9, 124.7, 124.0, 119.8, 22.2.
6-methyl-2-(naphthalen-1-yl)quinoline-4-carboxylic acid (7f): Beige powder, yield 7.5%, Rf = 0.17 (6:1 EtOAc-MeOH). 1H NMR (600 MHz, DMSO-d6) δ 8.52 (s, 1H), 8.12 (d, J = 8.5 Hz, 1H), 8.09–7.91 (m, 4H), 7.76–7.70 (m, 1H), 7.70–7.61 (m, 2H), 7.59–7.54 (m, 1H), 7.54–7.48 (m, 1H), 2.56 (d, J = 3.8 Hz, 3H). 13C NMR (151 MHz, DMSO-d6) δ 168.7, 157.8, 147.5, 138.2, 137.5, 134.0, 132.5, 131.1, 129.8, 129.6, 129.0, 128.4, 127.3, 126.6, 126.0, 125.8, 125.3, 125.2, 124.1, 123.1, 22.2.
6-ethyl-2-(naphthalen-2-yl)quinoline-4-carboxylic acid (7g): Brown powder, yield 25.2%, Rf = 0.13 (6:1 EtOAc-MeOH). 1H NMR (600 MHz, DMSO-d6) δ 8.83 (s), 8.63 (s), 8.60 (s), 8.49–8.40 (m), 8.05 (d, J = 8.6 Hz), 7.86–7.81 (m), 7.72 (d, J = 8.6 Hz), 7.65–7.60 (m), 2.81 (dd, J = 15.1, 7.5 Hz), 1.26 (t, J = 7.6 Hz). 13C NMR (151 MHz, DMSO-d6) δ 168.3, 155.3, 147.9, 145.5, 144.1, 137.6, 135.8, 134.1, 133.6, 132.7, 131.8, 130.1, 128.8, 128.2, 127.3, 126.5, 124.9, 124.0, 123.7, 68.7, 29.1, 15.9. HRMS (ESI): calculated [M + H+]+ 328.1338, found 328.1335
6-isopropyl-2-(naphthalen-2-yl)quinoline-4-carboxylic acid (7h): White powder, yield 45.6%, Rf = 0.13 (6:1 EtOAc-MeOH). 1H NMR (600 MHz, MeOH-d4) δ 8.58 (s, 1H), 8.31–8.24 (m, 2H), 8.14 (s, 1H), 8.07 (d, J = 8.7 Hz, 1H), 7.93 (dd, J = 9.1, 4.8 Hz, 2H), 7.69 (ddd, J = 10.8, 8.7, 1.9 Hz, 2H), 7.50 (d, J = 4.4 Hz, 1H), 6.91 (dd, J = 8.0, 1.7 Hz, 2H), 6.69 (d, J = 8.1 Hz, 1H), 2.82–2.71 (m, 3H), 1.37 (d, J = 7.0 Hz, 6H). 13C NMR (151 MHz, MeOH-d4) δ 150.1, 148.7, 138.2, 135.3, 130.7, 129.8, 129.7, 129.5, 128.9, 128.7, 128.3, 127.9, 127.5, 126.1, 124.2, 124.0, 119.4, 118.5, 118.1, 35.7, 34.8, 24.7, 24.3. HRMS (ESI): calculated [M + H+]+ 342.1494, found 342.1504
6-1-butyl-2-(naphthalen-2-yl)quinoline-4-carboxylic acid (7i): Yellow powder, yield 33.3%, Rf = 0.13 (6:1 EtOAc-MeOH). 1H NMR (600 MHz, DMSO-d6) δ 8.75 (s, 1H), 8.44 (dd, J = 8.7, 1.3 Hz, 2H), 8.17 (s, 1H), 8.13 (d, J = 7.4 Hz, 2H), 8.05 (d, J = 8.6 Hz, 1H), 7.89–7.81 (m, 2H), 7.67 (t, J = 6.9 Hz, 1H), 7.62 (t, J = 7.4 Hz, 1H), 2.76 (t, J = 7.7 Hz, 2H), 1.65 (dt, J = 15.1, 7.6 Hz, 2H), 1.40–1.33 (m, 3H), 0.93 (t, J = 7.4 Hz, 3H). 13C NMR (151 MHz, DMSO-d6) δ 133.3, 133.1, 130.3, 129.5, 128.8, 128.8, 128.6, 128.2, 128.2, 127.7, 127.6, 127.5, 126.9, 126.7, 126.4, 126.2, 125.9, 124.6, 123.5, 123.2, 35.2, 33.1, 21.9, 13.8. HRMS (ESI): calculated [M + H+]+ 356.1651, found 356.1658
6-methyl-2-(phenanthren-3-yl)quinoline-4-carboxylic acid (7j): Yellow solid, yield 78.6%, Rf = 0.16 (6:1 EtOAc-MeOH) 1H NMR (600 MHz, CDCl3) δ 9.53 (d, J = 1.3 Hz, 1H), 8.91 (d, J = 8.2 Hz, 1H), 8.85 (d, J = 2.3 Hz, 1H), 8.64 (s, 1H), 8.40 (d, J = 1.7 Hz, 1H), 8.24 (d, J = 8.9 Hz, 1H), 8.05 (dd, J = 8.3, 5.0 Hz 1H), 7.93 (d, J = 7.0 Hz, 1H), 7.85–7.77 (m, 2H), 7.74 (ddd, J = 8.3, 5.2, 1.8 Hz, 2H), 7.65 (td, J = 7.4, 7.0, 1.0 Hz, 1H), 4.60 (q, J = 7.2 Hz, 2H), 1.55 (t, J = 7.2 Hz, 3H). 13C NMR (151 MHz, CDCl3) δ 166.1, 157.1, 147.9, 136.4, 135.2, 134.0, 133.1, 132.4, 131.9, 131.0, 130.7 130.6, 129.4, 128.8, 128.3, 127.0, 127.0, 126.6, 125.5, 124.8, 123.0, 122.2, 121.4, 62.3, 14.5. HRMS (ESI): calculated [M + H+]+ 364.1338, found 364.1339
2-(anthracen-2-yl)-6-methylquinoline-4-carboxylic acid (7k): Yellow solid, yield 59.6%, Rf = 0.15 (6:1 EtOAc-MeOH). 1H NMR (600 MHz, DMSO-d6) δ 8.96 (s, 1H), 8.73 (s, 1H), 8.62 (s, 1H), 8.56 (s, 1H), 8.46–8.43 (m, 1H), 8.40 (s, 1H), 8.18 (d, J = 9.0 Hz, 1H), 7.65 (dd, J = 8.6, 1.8 Hz, 1H), 7.53–7.46 (m, 2H). 13C NMR (151 MHz, DMSO-d6) δ 168.4, 156.4, 148.9, 138.9, 136.8, 133.1, 132.4, 130.8, 130.6, 130.5, 130.4, 129.8, 129.2, 128.6, 128.3, 127.7, 127.0, 126.1, 125.9, 123.9, 122.4, 120.0. HRMS (ESI): calculated [M + H+]+ 364.1338, found 364.1338
6-ethyl-2-(phenanthren-3-yl)quinoline-4-carboxylic acid (7l): Yellow powder, yield 14.1%, Rf = 0.10 (6:1 EtOAc-MeOH). 1H NMR (600 MHz, DMSO-d6) δ 9.55 (s), 9.00 (d, J = 8.3 Hz), 8.51 (s), 8.37 (s), 8.08 (d, J = 8.3 Hz), 8.03–7.96 (m), 7.87 (s), 7.71 (t, J = 7.5 Hz), 7.64 (t, J = 7.4 Hz), 2.75 (q, J = 7.6 Hz), 1.24 (t, J = 7.6 Hz). 13C NMR (151 MHz, DMSO-d6) δ 170.7, 155.3, 147.7, 141.5, 138.0, 132.6, 132.4, 130.5, 130.3, 129.5, 129.1, 128.1, 127.6, 127.6, 127.1, 127.0, 126.1, 126.0, 125.8, 125.6, 125.3, 123.7, 121.6, 79.5, 29.1, 16.1. HRMS (ESI): calculated [M + H+]+ 378.1494, found 378.1492
6-isopropyl-2-(phenanthren-3-yl)quinoline-4-carboxylic acid (7m): Yellow powder, yield 51.0%, Rf = 0.18 (6:1 EtOAc-MeOH). 1H NMR (600 MHz, DMSO-d6) δ 9.59 (s, 1H), 9.05 (d, J = 8.3 Hz, 1H), 8.65 (s, 1H), 8.52 (dd, J = 8.3, 1.5 Hz, 1H), 8.39 (s, 1H), 8.16 (dd, J = 12.7, 8.5 Hz, 3H), 8.01 (d, J = 8.4 Hz, 1H), 7.91 (s, 2H), 7.76 (t, J = 7.6 Hz, 1H), 7.69 (t, J = 7.4 Hz, 1H), 1.30 (d, J = 6.9 Hz, 6H), 1.25–1.14 (m, 2H). 13C NMR (151 MHz, DMSO-d6) δ 155.7, 148.5, 147.9, 136.9, 132.9, 132.3, 130.5, 130.4, 130.4, 130.2, 129.8, 129.2, 128.6, 127.8, 126.9, 126.0, 124.0, 123.7, 122.0, 119.6, 79.4, 56.7, 34.3, 33.9, 24.1, 22.2, 18.8, 14.4. HRMS (ESI): calculated [M + H+]+ 392.1651, found 392.1654
6-butyl-2-(phenanthren-3-yl)quinoline-4-carboxylic acid (7n): Yellow powder, yield 37.3%, Rf = 0.20 (6:1 EtOAc-MeOH). 1H NMR (600 MHz, MeOH-d4) δ 9.48 (s, 1H), 8.95 (d, J = 8.3 Hz, 1H), 8.33 (dd, J = 8.2, 1.4 Hz, 1H), 8.26 (s, 1H), 8.07 (dd, J = 20.4, 8.4 Hz, 2H), 7.94 (d, J = 8.1 Hz, 1H), 7.82 (s, 2H), 7.74–7.70 (m, 1H), 7.64 (dd, J = 7.9, 2.6 Hz, 2H), 2.85–2.81 (m, 2H), 1.73 (dt, J = 15.2, 7.6 Hz, 3H), 1.43 (dq, J = 14.3, 7.2 Hz, 3H), 0.97 (t, J = 7.4 Hz, 4H). 13C NMR (151 MHz, MeOH-d4) δ 158.2, 148.7, 142.7, 138.9, 134.1, 133.8, 132.5, 132.4, 132.0, 131.7, 130.2, 129.7, 129.6, 129.0, 128.1, 128.0, 127.5, 126.9, 126.2, 125.4, 124.0, 123.2, 118.6, 36.9, 34.7, 23.5, 23.3, 15.4, 14.3. HRMS (ESI): calculated [M + H+]+ 406.1807, found 406.1808