Gut Microbiota, Metabolome, and Body Composition Signatures of Response to Therapy in Patients with Advanced Melanoma
Abstract
1. Introduction
2. Results
2.1. Demographic, Anthropometric, Body Composition, Physical Activity, Dietary, and Clinical Characteristics of the Study Population
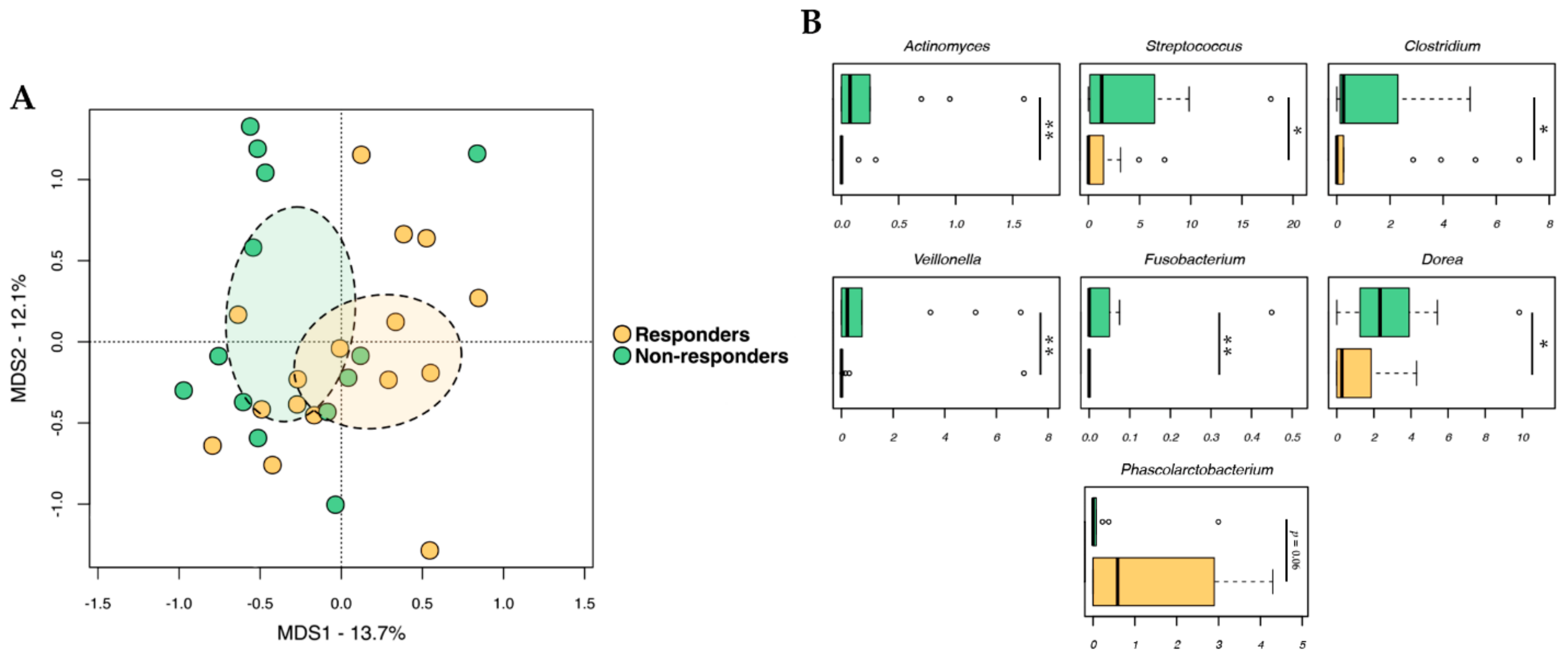
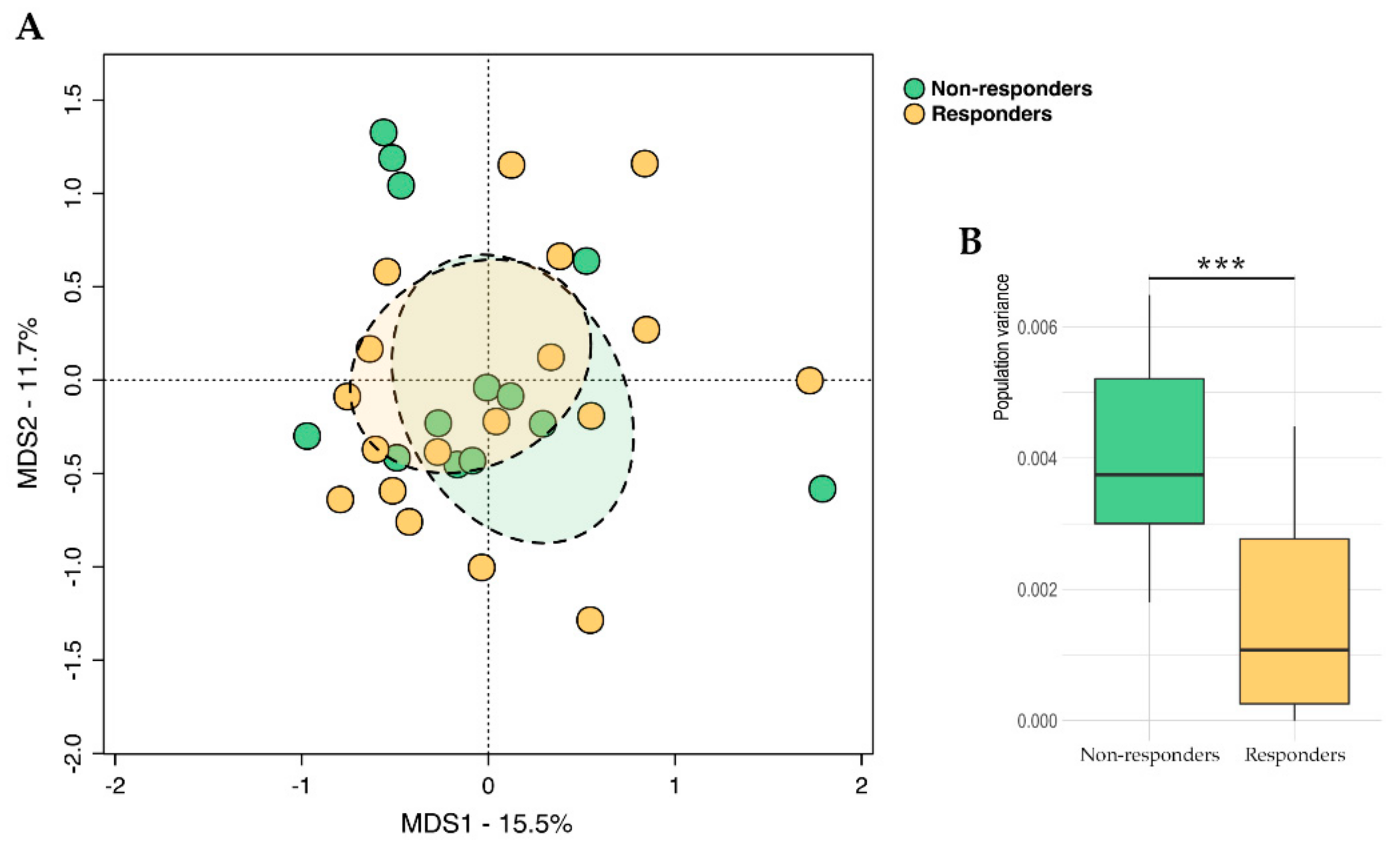
2.2. Gut Microbiota Profiling
2.3. Fecal Metabolomic Profile
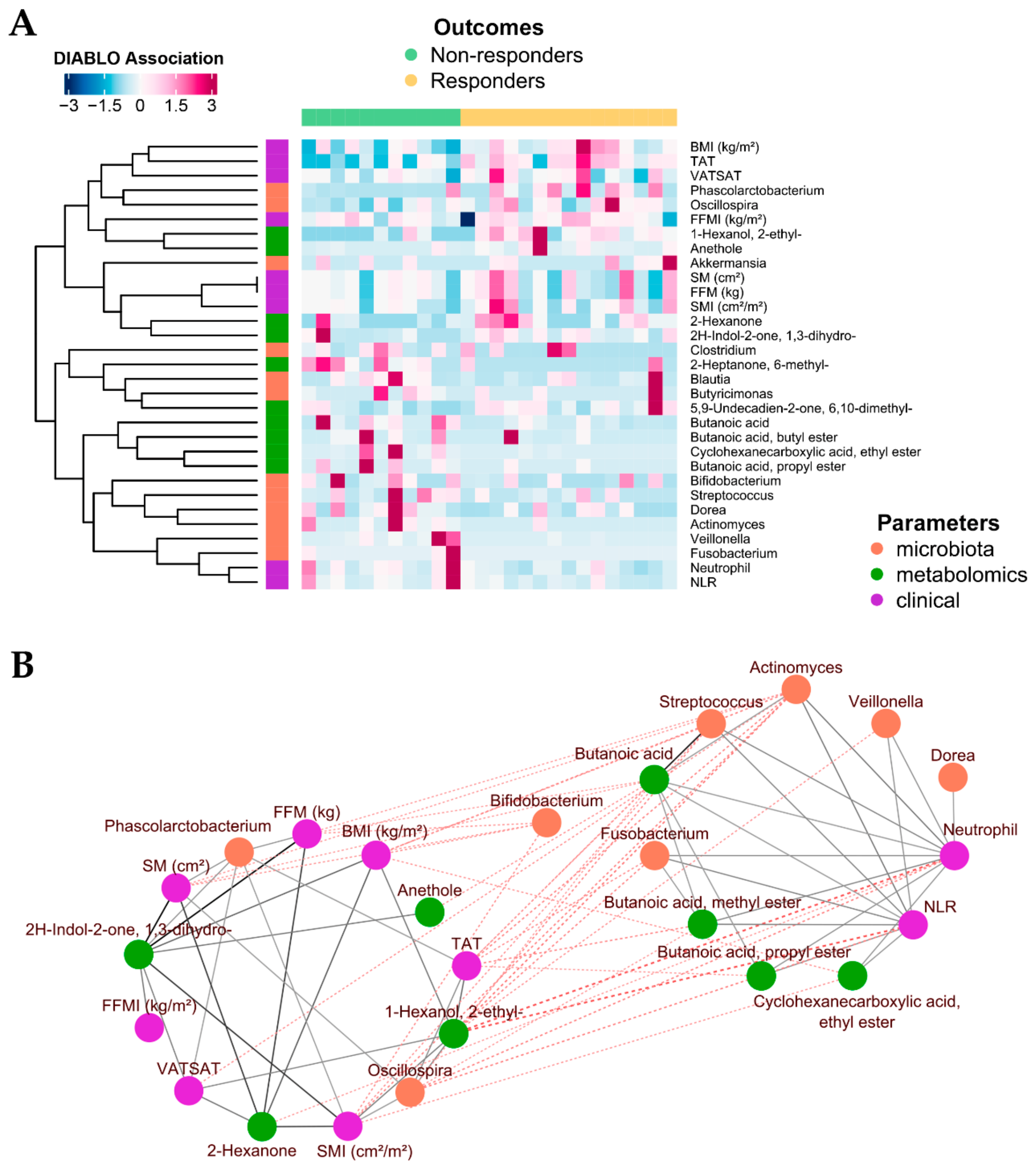
2.4. Integration of Omics (Microbiomics and Metabolomics) Data and Patient Metadata
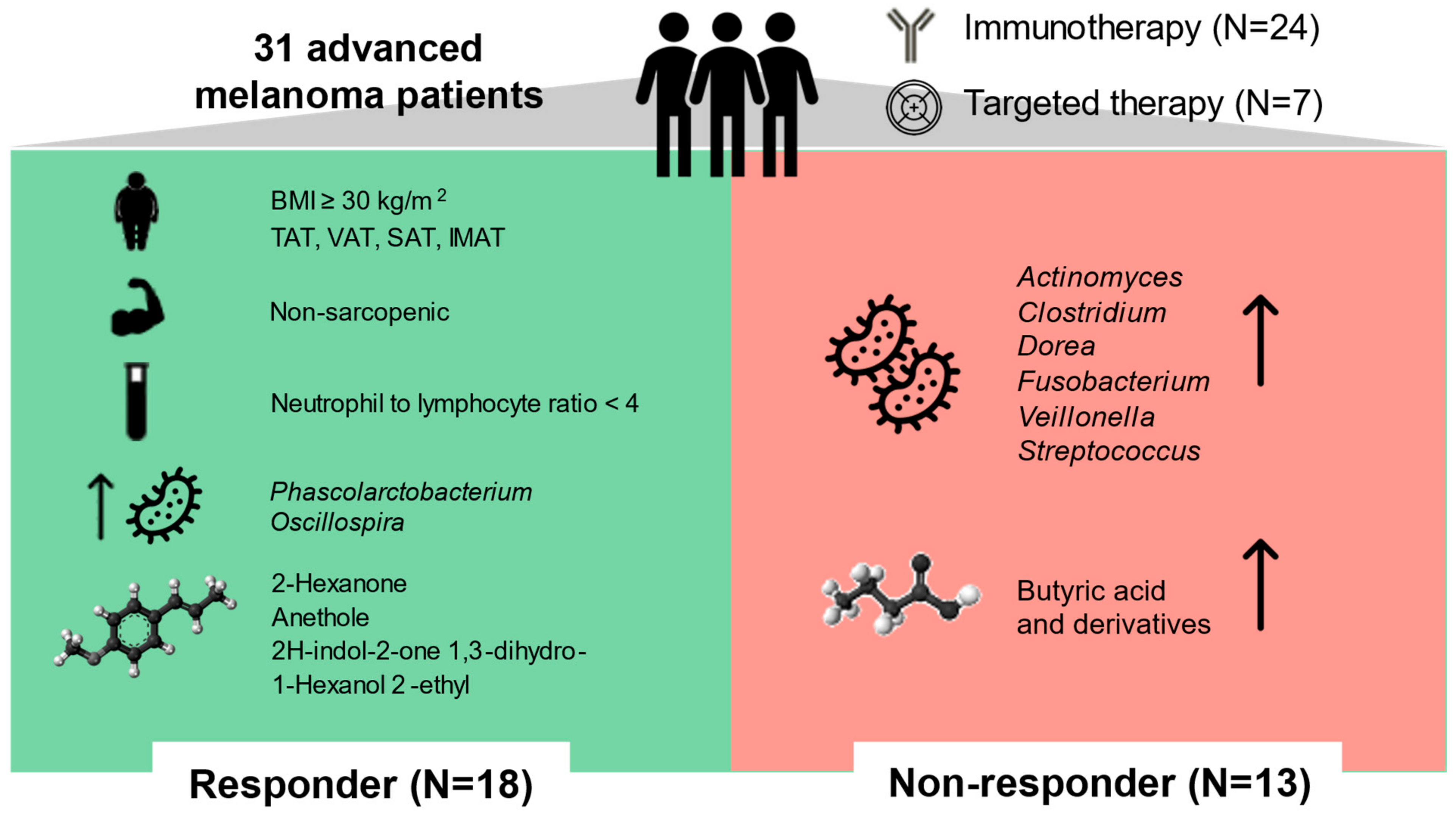
3. Discussion
4. Materials and Methods
4.1. Patient Enrollment and Sample Collection
4.2. Nutritional Status Assessment
4.3. Physical Activity and Dietary Questionnaires
4.4. Immune Profile
4.5. Microbial DNA Extraction and 16S rRNA Amplicon Sequencing
4.6. Fecal Metabolomics
4.7. Bioinformatic and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 2017, 377, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Schadendorf, D.; Long, G.V.; Stroiakovski, D.; Karaszewska, B.; Hauschild, A.; Levchenko, E.; Chiarion-Sileni, V.; Schachter, J.; Garbe, C.; Dutriaux, C.; et al. Three-year pooled analysis of factors associated with clinical outcomes across dabrafenib and trametinib combination therapy phase 3 randomised trials. Eur. J. Cancer 2017, 82, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Warner, A.B.; McQuade, J.L. Modifiable host factors in melanoma: Emerging evidence for obesity, diet, exercise, and the microbiome. Curr. Oncol. Rep. 2019, 21, 72. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Helmink, B.A.; Spencer, C.N.; Reuben, A.; Wargo, J.A. The influence of the gut microbiome on cancer, immunity, and cancer immunotherapy. Cancer Cell 2018, 33, 570–580. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Matson, V.; Fessler, J.; Bao, R.; Chongsuwat, T.; Zha, Y.; Alegre, M.L.; Luke, J.J.; Gajewski, T.F. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 2018, 359, 104–108. [Google Scholar] [CrossRef]
- Usyk, M.; Pandey, A.; Hayes, R.B.; Moran, U.; Pavlick, A.; Osman, I.; Weber, J.S.; Ahn, J. Bacteroides vulgatus and Bacteroides dorei predict immune-related adverse events in immune checkpoint blockade treatment of metastatic melanoma. Genome Med. 2021, 13, 160. [Google Scholar] [CrossRef]
- Guardamagna, M.; Berciano-Guerrero, M.A.; Villaescusa-González, B.; Perez-Ruiz, E.; Oliver, J.; Lavado-Valenzuela, R.; Rueda-Dominguez, A.; Barragán, I.; Queipo-Ortuño, M.I. Gut Microbiota and Therapy in Metastatic Melanoma: Focus on MAPK Pathway Inhibition. Int. J. Mol. Sci. 2022, 23, 11990. [Google Scholar] [CrossRef]
- McCulloch, J.A.; Davar, D.; Rodrigues, R.R.; Badger, J.H.; Fang, J.R.; Cole, A.M.; Balaji, A.K.; Vetizou, M.; Prescott, S.M.; Fernandes, M.R.; et al. Intestinal microbiota signatures of clinical response and immune-related adverse events in melanoma patients treated with anti-PD-1. Nat. Med. 2022, 28, 545–556. [Google Scholar] [CrossRef]
- Baruch, E.N.; Youngster, I.; Ben-Betzalel, G.; Ortenberg, R.; Lahat, A.; Katz, L.; Adler, K.; Dick-Necula, D.; Raskin, S.; Bloch, N.; et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science 2021, 371, 602–609. [Google Scholar] [CrossRef]
- Davar, D.; Dzutsev, A.K.; McCulloch, J.A.; Rodrigues, R.R.; Chauvin, J.M.; Morrison, R.M.; Deblasio, R.N.; Menna, C.; Ding, Q.; Pagliano, O.; et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science 2021, 371, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Spencer, C.N.; McQuade, J.L.; Gopalakrishnan, V.; McCulloch, J.A.; Vetizou, M.; Cogdill, A.P.; Khan, M.A.W.; Zhang, X.; White, M.G.; Peterson, C.B.; et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science 2021, 374, 1632–1640. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yuan, X.; Wang, M.; He, Z.; Li, H.; Wang, J.; Li, Q. Gut microbiota influence immunotherapy responses: Mechanisms and therapeutic strategies. J. Hematol. Oncol. 2022, 15, 47. [Google Scholar] [CrossRef] [PubMed]
- Limeta, A.; Ji, B.; Levin, M.; Gatto, F.; Nielsen, J. Meta-analysis of the gut microbiota in predicting response to cancer immunotherapy in metastatic melanoma. JCI Insight 2020, 5, e140940. [Google Scholar] [CrossRef]
- Sabel, M.S.; Lee, J.; Cai, S.; Englesbe, M.J.; Holcombe, S.; Wang, S. Sarcopenia as a prognostic factor among patients with stage III melanoma. Ann. Surg. Oncol. 2011, 18, 3579–3585. [Google Scholar] [CrossRef]
- Surov, A.; Meyer, H.J.; Wienke, A. Role of Sarcopenia in Advanced Malignant Cutaneous Melanoma Treated with Immunotherapy: A Meta-Analysis. Oncology 2022, 100, 498–504. [Google Scholar] [CrossRef]
- Ferrucci, P.F.; Ascierto, P.A.; Pigozzo, J.; Del Vecchio, M.; Maio, M.; Cappellini, G.C.A.; Guidoboni, M.; Queirolo, P.; Savoia, P.; Mandalà, M.; et al. Baseline neutrophils and derived neutrophil-to-lymphocyte ratio: Prognostic relevance in metastatic melanoma patients receiving ipilimumab. Ann. Oncol. 2016, 27, 732–738. [Google Scholar] [CrossRef]
- Anselmi, M.; Fontana, F.; Marzagalli, M.; Gagliano, N.; Sommariva, M.; Limonta, P. Melanoma Stem Cells Educate Neutrophils to Support Cancer Progression. Cancers 2022, 14, 3391. [Google Scholar] [CrossRef]
- Tomela, K.; Pietrzak, B.; Galus, Ł.; Mackiewicz, J.; Schmidt, M.; Mackiewicz, A.A.; Kaczmarek, M. Myeloid-Derived Suppressor Cells (MDSC) in Melanoma Patients Treated with Anti-PD-1 Immunotherapy. Cells 2023, 12, 789. [Google Scholar] [CrossRef]
- Gabrilovich, D.I. Myeloid-Derived Suppressor Cells. Cancer Immunol. Res. 2017, 5, 3–8. [Google Scholar] [CrossRef]
- Moses, K.; Brandau, S. Human neutrophils: Their role in cancer and relation to myeloid-derived suppressor cells. Semin. Immunol. 2016, 28, 187–196. [Google Scholar] [CrossRef]
- McQuade, J.L.; Daniel, C.R.; Hess, K.R.; Mak, C.; Wang, D.Y.; Rai, R.R.; Park, J.J.; Haydu, L.E.; Spencer, C.; Wongchenko, M.; et al. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: A retrospective, multicohort analysis. Lancet Oncol. 2018, 19, 310–322. [Google Scholar] [CrossRef]
- Makrakis, D.; Rounis, K.; Tsigkas, A.P.; Georgiou, A.; Galanakis, N.; Tsakonas, G.; Ekman, S.; Papadaki, C.; Monastirioti, A.; Kontogianni, M.; et al. Effect of body tissue composition on the outcome of patients with metastatic non-small cell lung cancer treated with PD-1/PD-L1 inhibitors. PLoS ONE 2023, 18, e0277708. [Google Scholar] [CrossRef] [PubMed]
- Martini, D.J.; Kline, M.R.; Liu, Y.; Shabto, J.M.; Williams, M.A.; Khan, A.I.; Lewis, C.; Collins, H.; Akce, M.; Kissick, H.T.; et al. Adiposity may predict survival in patients with advanced stage cancer treated with immunotherapy in phase 1 clinical trials. Cancer 2020, 126, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Hube, F.; Lietz, U.; Igel, M.; Jensen, P.B.; Tornqvist, H.; Joost, H.G.; Hauner, H. Difference in leptin mRNA levels between omental and subcutaneous abdominal adipose tissue from obese humans. Horm. Metab. Res. 1996, 28, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Aguilar, E.G.; Luna, J.I.; Dunai, C.; Khuat, L.T.; Le, C.T.; Mirsoian, A.; Minnar, C.M.; Stoffel, K.M.; Sturgill, I.R.; et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat. Med. 2019, 25, 141–151. [Google Scholar] [CrossRef]
- McQuade, J.L.; Daniel, C.R.; Davies, M.A. Body-mass index and metastatic melanoma outcomes—Authors’ reply. Lancet Oncol. 2018, 19, e227–e228. [Google Scholar] [CrossRef]
- Li, S.; Wang, T.; Tong, G.; Li, X.; You, D.; Cong, M. Prognostic Impact of Sarcopenia on Clinical Outcomes in Malignancies Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 726257. [Google Scholar] [CrossRef]
- Heidelberger, V.; Goldwasser, F.; Kramkimel, N.; Jouinot, A.; Huillard, O.; Boudou-Rouquette, P.; Chanal, J.; Arrondeau, J.; Franck, N.; Alexandre, J.; et al. Sarcopenic overweight is associated with early acute limiting toxicity of anti-PD1 checkpoint inhibitors in melanoma patients. Investig. New Drugs 2017, 35, 436–441. [Google Scholar] [CrossRef]
- Olekhnovich, E.I.; Ivanov, A.B.; Babkina, A.A.; Sokolov, A.A.; Ulyantsev, V.I.; Fedorov, D.E.; Ilina, E.N. Consistent Stool Metagenomic Biomarkers Associated with the Response to Melanoma Immunotherapy. mSystems 2023, 21, e0102322. [Google Scholar] [CrossRef]
- Tanoue, T.; Morita, S.; Plichta, D.R.; Skelly, A.N.; Suda, W.; Sugiura, Y.; Narushima, S.; Vlamakis, H.; Motoo, I.; Sugita, K.; et al. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature 2019, 565, 600–605. [Google Scholar] [CrossRef]
- Zhao, T.; Wang, X.; Fu, L.; Yang, K. Fusobacterium nucleatum: A new player in regulation of cancer development and therapeutic response. Cancer Drug Resist. 2022, 5, 436–450. [Google Scholar] [CrossRef]
- van Deuren, T.; Blaak, E.E.; Canfora, E.E. Butyrate to combat obesity and obesity-associated metabolic disorders: Current status and future implications for therapeutic use. Obes. Rev. 2022, 23, e13498. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Li, Q.; Wu, W.; Chen, H.; Fang, X.; Han, Y.; Xie, M.; Gao, H. In vitro fecal fermentation characteristics of bamboo shoot (Phyllostachys edulis) polysaccharide. Food Chem. X 2021, 11, 100129. [Google Scholar] [CrossRef]
- Conte, M.; Conte, G.; Martucci, M.; Monti, D.; Casarosa, L.; Serra, A.; Mele, M.; Franceschi, C.; Salvioli, S. The smell of longevity: A combination of Volatile Organic Compounds (VOCs) can discriminate centenarians and their offspring from age-matched subjects and young controls. Geroscience 2020, 42, 201–216. [Google Scholar] [CrossRef]
- Coutzac, C.; Jouniaux, J.M.; Paci, A.; Schmidt, J.; Mallardo, D.; Seck, A.; Asvatourian, V.; Cassard, L.; Saulnier, P.; Lacroix, L.; et al. Systemic short chain fatty acids limit antitumor effect of CTLA-4 blockade in hosts with cancer. Nat. Commun. 2020, 11, 2168. [Google Scholar] [CrossRef]
- Anand, S.; Kaur, H.; Mande, S.S. Comparative In silico Analysis of Butyrate Production Pathways in Gut Commensals and Pathogens. Front. Microbiol. 2016, 7, 1945. [Google Scholar] [CrossRef]
- Chen, B.J.; Takeshita, T.; Tajikara, T.; Asakawa, M.; Kageyama, S.; Shibata, Y.; Ayukawa, Y.; Yano, Y.; Yamashita, Y. Butyrate as a Potential Driver of a Dysbiotic Shift of the Tongue Microbiota. Msphere 2023, 8, e0049022. [Google Scholar] [CrossRef]
- Atlanta (GA): Agency for Toxic Substances and Disease Registry (US); 2020 Feb. Toxicological Profile for 2-Hexanone. CHAPTER 5. Available online: https://www.ncbi.nlm.nih.gov/books/NBK591912/ (accessed on 2 June 2022).
- Atlanta (GA): Agency for Toxic Substances and Disease Registry (US); 2020 Feb. Toxicological Profile for 2-Hexanone. CHAPTER 3. Available online: https://www.ncbi.nlm.nih.gov/books/NBK591916/ (accessed on 2 June 2022).
- Hidalgo, K.; Ratel, J.; Mercier, F.; Gauriat, B.; Bouchard, P.; Engel, E. Volatolomics in Bacterial Ecotoxicology, A Novel Method for Detecting Signatures of Pesticide Exposure? Front. Microbiol. 2019, 9, 3113. [Google Scholar] [CrossRef]
- Rees, C.A.; Burklund, A.; Stefanuto, P.H.; Schwartzman, J.D.; Hill, J.E. Comprehensive volatile metabolic fingerprinting of bacterial and fungal pathogen groups. J. Breath Res. 2018, 12, 026001. [Google Scholar] [CrossRef]
- Nissen, L.; Casciano, F.; Gianotti, A. Plant Volatiles of Lettuce and Chicory Cultivated in Aquaponics Are Associated to Their Microbial Community. Microorganisms 2021, 9, 580. [Google Scholar] [CrossRef] [PubMed]
- Tabaszewska, M.; Antoniewska, A.; Rutkowska, J.; Skoczylas, Ł.; Słupski, J.; Skoczeń-Słupska, R. Bioactive Components, Volatile Profile and In Vitro Antioxidative Properties of Taxus baccata L. Red Arils. Molecules 2021, 26, 4474. [Google Scholar] [CrossRef]
- Nam, J.H.; Lee, D.U. Foeniculum vulgare extract and its constituent, trans-anethole, inhibit UV-induced melanogenesis via ORAI1 channel inhibition. J. Dermatol. Sci. 2016, 84, 305–313. [Google Scholar] [CrossRef]
- Elkady, A.I. Anethole Inhibits the Proliferation of Human Prostate Cancer Cells via Induction of Cell Cycle Arrest and Apoptosis. Anticancer Agents Med. Chem. 2018, 18, 216–236. [Google Scholar] [CrossRef]
- Contant, C.; Rouabhia, M.; Loubaki, L.; Chandad, F.; Semlali, A. Anethole induces anti-oral cancer activity by triggering apoptosis, autophagy and oxidative stress and by modulation of multiple signaling pathways. Sci. Rep. 2021, 11, 13087. [Google Scholar] [CrossRef]
- Barone, M.; D’Amico, F.; Rampelli, S.; Brigidi, P.; Turroni, S. Age-related diseases, therapies and gut microbiome: A new frontier for healthy aging. Mech. Ageing Dev. 2022, 206, 111711. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Templeton, A.J.; McNamara, M.G.; Šeruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocaña, A.; Leibowitz-Amit, R.; Sonpavde, G.; Knox, J.J.; Tran, B.; et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2014, 106, dju124. [Google Scholar] [CrossRef]
- Physical status: The use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech. Rep. Ser. 1995, 854, 1–452.
- Prado, C.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Mourtzakis, M.; Prado, C.M.; Lieffers, J.R.; Reiman, T.; McCargar, L.J.; Baracos, V.E. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl. Physiol. Nutr. Metab. 2008, 33, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.H.; Macfarlane, D.J.; Lam, T.H.; Stewart, S.M. Validity of the international physical activity questionnaire short form (IPAQ-SF): A systematic review. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 115. [Google Scholar] [CrossRef]
- Pisani, P.; Faggiano, F.; Krogh, V.; Palli, D.; Vineis, P.; Berrino, F. Relative validity and reproducibility of a food frequency dietary questionnaire for use in the Italian EPIC centres. Int. J. Epidemiol. 1997, 26 (Suppl. S1), S152. [Google Scholar] [CrossRef]
- Società Italiana di Nutrizione Umana (SINU). Livelli di Assunzione di Riferimento di Nutrienti ed energia per la popolazione italiana. IV Revisione. Milano, Coordinamento editoriale SINU-INRAN. IV Revis. 2014. [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific opinion on dietary reference values for carbohydrates and dietary fibre. EFSA J. 2010, 8, 1462. [Google Scholar] [CrossRef]
- Agnoli, C.; Grioni, S.; Sieri, S.; Palli, D.; Masala, G.; Sacerdote, C.; Vineis, P.; Tumino, R.; Giurdanella, M.C.; Pala, V.; et al. Italian Mediterranean Index and risk of colorectal cancer in the Italian section of the EPIC cohort. Int. J. Cancer 2013, 132, 1404–1411. [Google Scholar] [CrossRef]
- Diks, A.M.; Bonroy, C.; Teodosio, C.; Groenland, R.J.; de Mooij, B.; de Maertelaere, E.; Neirynck, J.; Philippé, J.; Orfao, A.; van Dongen, J.J.M.; et al. Impact of blood storage and sample handling on quality of high dimensional flow cytometric data in multicenter clinical research. J. Immunol. Methods 2019, 475, 112616. [Google Scholar] [CrossRef] [PubMed]
- Lo Russo, G.; Sgambelluri, F.; Prelaj, A.; Galli, F.; Manglaviti, S.; Bottiglieri, A.; Di Mauro, R.M.; Ferrara, R.; Galli, G.; Signorelli, D.; et al. PEOPLE (NCT03447678), a first-line phase II pembrolizumab trial, in negative and low PD-L1 advanced NSCLC: Clinical outcomes and association with circulating immune biomarkers. ESMO Open 2022, 7, 100645. [Google Scholar] [CrossRef]
- Yu, Z.; Morrison, M. Improved extraction of PCR-quality community DNA from digesta and fecal samples. Biotechniques 2004, 36, 808–812. [Google Scholar] [CrossRef]
- D’Amico, F.; Perrone, A.M.; Rampelli, S.; Coluccelli, S.; Barone, M.; Ravegnini, G.; Fabbrini, M.; Brigidi, P.; De Iaco, P.; Turroni, S. Gut Microbiota Dynamics during Chemotherapy in Epithelial Ovarian Cancer Patients Are Related to Therapeutic Outcome. Cancers 2021, 13, 3999. [Google Scholar] [CrossRef]
- Masella, A.P.; Bartram, A.K.; Truszkowski, J.M.; Brown, D.G.; Neufeld, J.D. PANDAseq: Paired-end assembler for illumina sequences. BMC Bioinform. 2012, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 18, e2584. [Google Scholar] [CrossRef]
- Le Cao, K.A.; Rohart, F.; Gonzalez, I.; Dejean, S.; Gautier, B.; Bartolo, F.; Monget, P.; Coquery, J.; Yao, F.Z.; Liquet, B. mixOmics: Omics Data Integration Project. R Package Version 6.1.1. 2016. Available online: https://CRAN.R-project.org/package=mixOmics (accessed on 30 June 2022).
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]


| Characteristic | Overall (n = 31) | Responders (n = 18) | Non-Responders (n = 13) | p Value |
|---|---|---|---|---|
| Age (years), mean (SD) | 62 (11) | 62 (11) | 61 (12) | 0.5748 |
| Sex, n (%) | 0.4120 | |||
| Male | 23 (74) | 12 (52) | 11(48) | |
| Female | 8 (26) | 6 (75) | 2 (25) | |
| Stage, n (%) | 0.6207 | |||
| IIIC | 4 (13) | 3 (75) | 1 (25) | |
| IV | 27 (87) | 15(56) | 12 (44) | |
| ECOG performance status, n (%) | 0.1337 | |||
| 0 | 26 (84) | 17 (65) | 9 (35) | |
| 1–2 | 5 (16) | 1 (20) | 4 (80) | |
| Planned anti-PD-1 treatment, n (%) | 0.7777 | |||
| Nivolumab | 19 (61) | 12 (63) | 7 (37) | |
| Pembrolizumab | 5 (16) | 3 (60) | 2 (40) | |
| Targeted therapy, n (%) | 0.4130 | |||
| Dabrafenib and trametinib | 7 (23) | 3 (43) | 4 (57) | |
| NLR a, mean (SD) | 5.5 (7.5) | 2.8 (1.6) | 9.0 (10.4) | 0.0034 |
| NLR a | 0.0196 | |||
| <4, n (%) | 21 (70) | 15 (71) | 6 (29) | |
| ≥4, n (%) | 9 (30) | 2 (22) | 7 (78) | |
| Medication use b, n (%) | ||||
| Antibiotics a | 10 (33) | 5 (50) | 5 (50) | 0.4611 |
| Probiotics a | 6 (20) | 3 (50) | 3 (50) | 0.6599 |
| Proton-pump inhibitors | 7 (23) | 2 (29) | 5 (71) | 0.0994 |
| Corticosteroids | 6 (19) | 2 (33) | 4 (66) | 0.2076 |
| Characteristic | Overall (n = 31) | Responders (n = 18) | Non-Responders (n = 13) | p Value |
|---|---|---|---|---|
| Anthropometry | ||||
| Height (m), mean (SD) | 1.71 (0.1) | 1.72 (0.1) | 1.71 (0.1) | 0.6591 |
| Weight (kg), mean (SD) | 77.9 (17.4) | 84 (18.1) | 69.5 (12.5) | 0.0291 |
| BMI (kg/m2), mean (SD) BMI, n (%) | 26.5 (4.8) | 28.5 (4.9) | 23.8 (3.2) | 0.0073 0.0275 |
| <30 kg/m2 | 25 (81) | 12 (48) | 13 (52) | |
| ≥30 kg/m2 | 6 (19) | 6 (100) | 0 (0) | |
| CT a | ||||
| Sarcopenic | 14 (56) | 6 (43) | 8 (57) | 0.0029 |
| Non-sarcopenic | 11 (44) | 11 (100) | 0 (0) | |
| SM (cm2/m2), mean (SD) | 135.2 (29.4) | 139.5 (32.5) | 126.0 (20.0) | 0.3983 |
| TAT (cm2/m2), mean (SD) | 125.21 (118.63) | 149.18 (131.2) | 74.28 (62.1) | 0.0018 |
| VAT (cm2/m2), mean (SD) | 172.2 (123.4) | 207.8 (131.1) | 96.7 (57.7) | 0.0113 |
| SAT (cm2/m2), mean (SD) | 191.0 (89.4) | 225.3 (86.2) | 118.1 (38.7) | 0.0010 |
| IMAT (cm2/m2), mean (SD) | 12.4 (10.1) | 14.5 (10.7) | 8.0 (7.7) | 0.0510 |
| VATSAT, mean (SD) | 0.88 (0.45) | 0.93 (0.47) | 0.77 (0.41) | 0.3983 |
| FFM (kg), mean (SD) | 46.6 (8.8) | 47.9 (9.8) | 43.0 (5.9) | 0.4025 |
| Physical activity, n (%) b | 0.1516 | |||
| Low | 14 (45) | 6 (43) | 8 (57) | |
| Moderate | 11 (36) | 9 (82) | 2 (18) | |
| High | 6 (19) | 3 (50) | 3 (50) | |
| Fiber: EPIC FFQ c | 1.000 | |||
| <25 g/die | 17 (65) | 12 (71) | 5 (29) | |
| ≥25 g/die | 9 (35) | 6 (67) | 3 (33) | |
| Italian Mediterranean Index c | 0.0838 | |||
| 0–3 | 11 (42) | 10 (91) | 1 (9) | |
| 4–11 | 15 (58) | 8 (53) | 7 (47) |
| Metabolite | Non-Responders | Responders | p Value |
|---|---|---|---|
| Butanoic acid, methyl ester | 0.47 ± 0.1 | 0.013 ± 0.0063 | 0.002 |
| 1-Hexanol, 2-ethyl- | 0.034 ± 0.01 | 0.15 ± 0.015 | 0.003 |
| 2-Heptanone, 6-methyl- | 0.031 ± 0.0045 | 0.0069 ± 0.0025 | 0.006 |
| Cyclohexanecarboxylic acid, ethyl ester | 0.41 ± 0.12 | 0.0082 ± 0.0029 | 0.007 |
| Butanoic acid | 25 ± 4.3 | 5.7 ± 0.63 | 0.02 |
| Butanoic acid, propyl ester | 1.3 ± 0.36 | 0.1 ± 0.035 | 0.02 |
| 2H-Indol-2-one, 1,3-dihydro- | 0.11 ± 0.037 | 0.12 ± 0.011 | 0.02 |
| 2-Hexanone | 0.076 ± 0.022 | 0.18 ± 0.021 | 0.04 |
| 5,9-Undecadien-2-one, 6,10-dimethyl- | 0.13 ± 0.024 | 0.34 ± 0.047 | 0.04 |
| Anethole | 0.14 ± 0.05 | 0.86 ± 0.26 | 0.05 |
| Propanoic acid, ethyl ester | 0.12 ± 0.037 | 0.007 ± 0.0035 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vandoni, G.; D'Amico, F.; Fabbrini, M.; Mariani, L.; Sieri, S.; Casirati, A.; Di Guardo, L.; Del Vecchio, M.; Anichini, A.; Mortarini, R.; et al. Gut Microbiota, Metabolome, and Body Composition Signatures of Response to Therapy in Patients with Advanced Melanoma. Int. J. Mol. Sci. 2023, 24, 11611. https://doi.org/10.3390/ijms241411611
Vandoni G, D'Amico F, Fabbrini M, Mariani L, Sieri S, Casirati A, Di Guardo L, Del Vecchio M, Anichini A, Mortarini R, et al. Gut Microbiota, Metabolome, and Body Composition Signatures of Response to Therapy in Patients with Advanced Melanoma. International Journal of Molecular Sciences. 2023; 24(14):11611. https://doi.org/10.3390/ijms241411611
Chicago/Turabian StyleVandoni, Giulia, Federica D'Amico, Marco Fabbrini, Luigi Mariani, Sabina Sieri, Amanda Casirati, Lorenza Di Guardo, Michele Del Vecchio, Andrea Anichini, Roberta Mortarini, and et al. 2023. "Gut Microbiota, Metabolome, and Body Composition Signatures of Response to Therapy in Patients with Advanced Melanoma" International Journal of Molecular Sciences 24, no. 14: 11611. https://doi.org/10.3390/ijms241411611
APA StyleVandoni, G., D'Amico, F., Fabbrini, M., Mariani, L., Sieri, S., Casirati, A., Di Guardo, L., Del Vecchio, M., Anichini, A., Mortarini, R., Sgambelluri, F., Celano, G., Serale, N., De Angelis, M., Brigidi, P., Gavazzi, C., & Turroni, S. (2023). Gut Microbiota, Metabolome, and Body Composition Signatures of Response to Therapy in Patients with Advanced Melanoma. International Journal of Molecular Sciences, 24(14), 11611. https://doi.org/10.3390/ijms241411611









