Insights into the Evolution and Host Adaptation of the Monkeypox Virus from a Codon Usage Perspective: Focus on the Ongoing 2022 Outbreak
Abstract
1. Introduction
2. Results
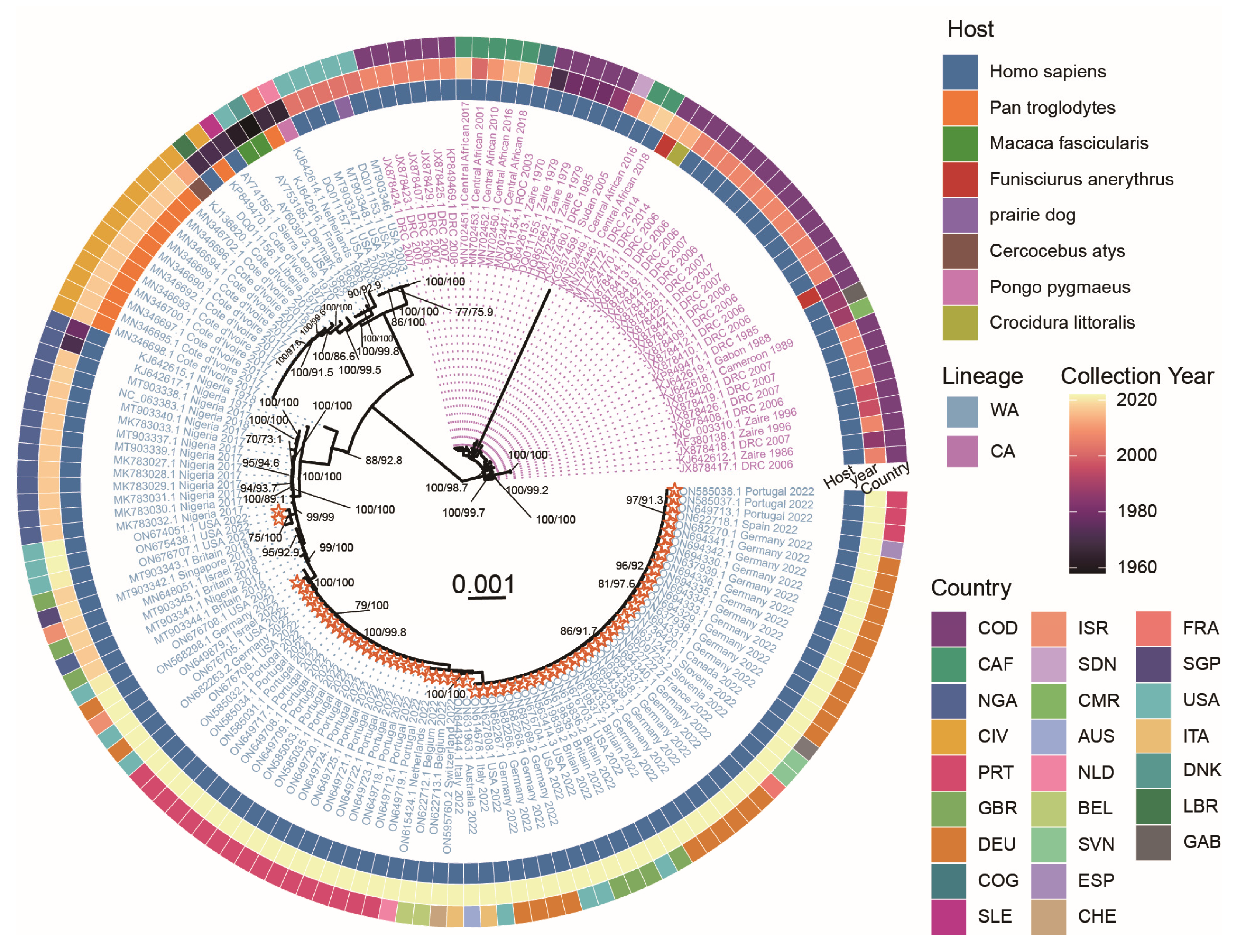
2.1. Phylogenetic Analysis of MPXV Strains of the 2022 Outbreak
2.2. MPXV Coding Sequences Are A-T Rich
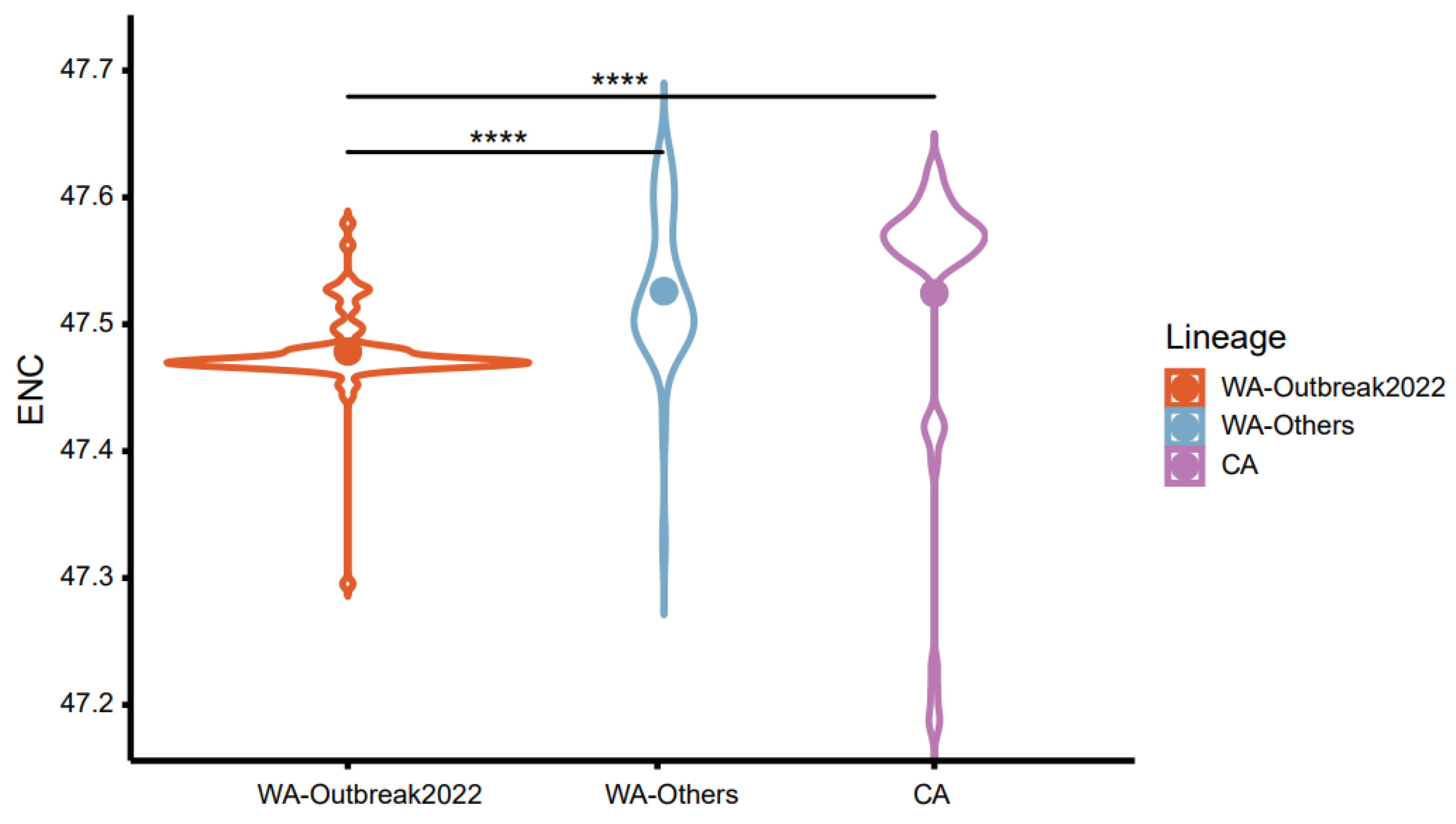
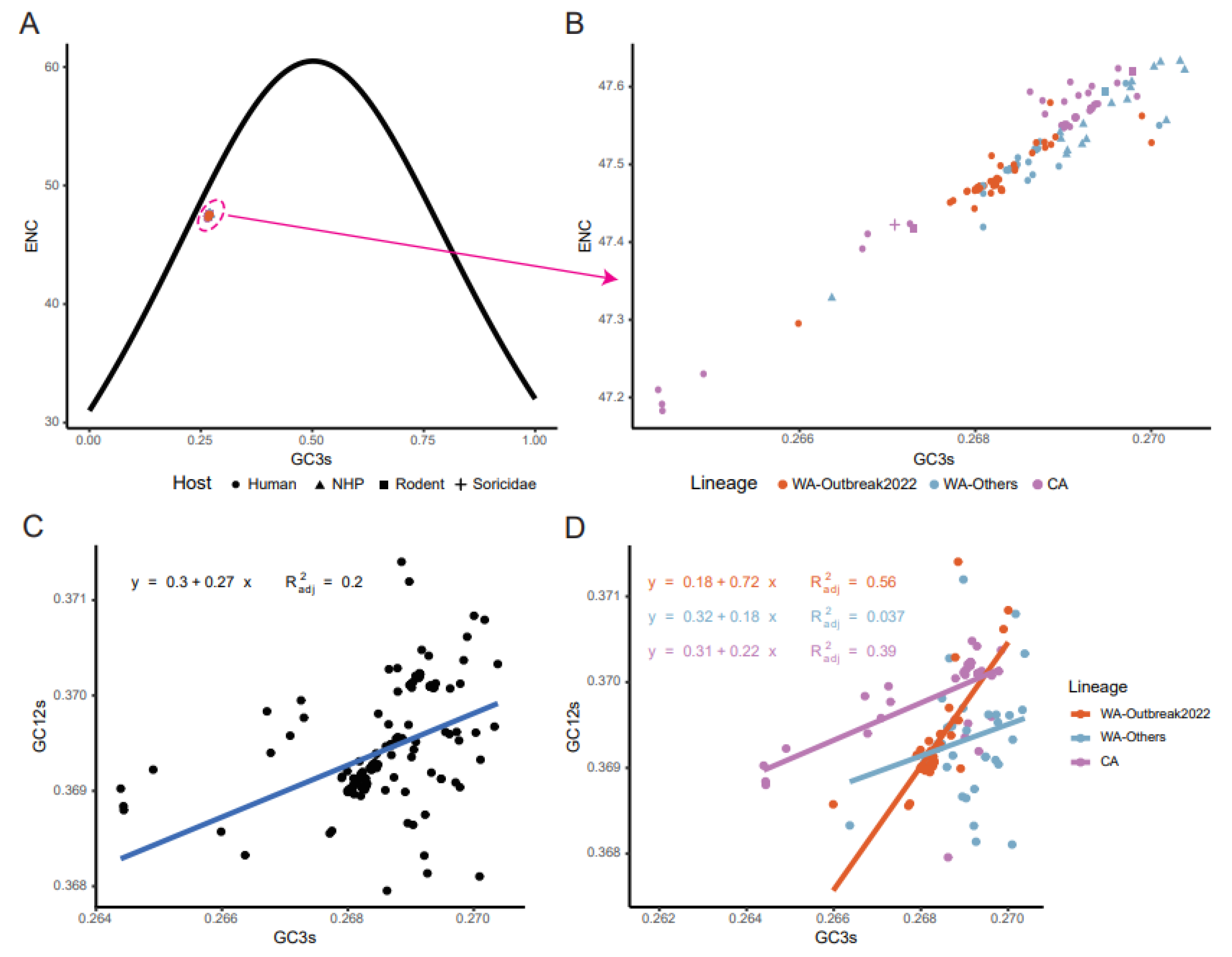
2.3. CUB of the MPXV Coding Sequences
2.4. Relative Synonymous Codon Usage (RSCU) of MPXV
2.5. Trends in Codon Usage Variation in MPXV Clades
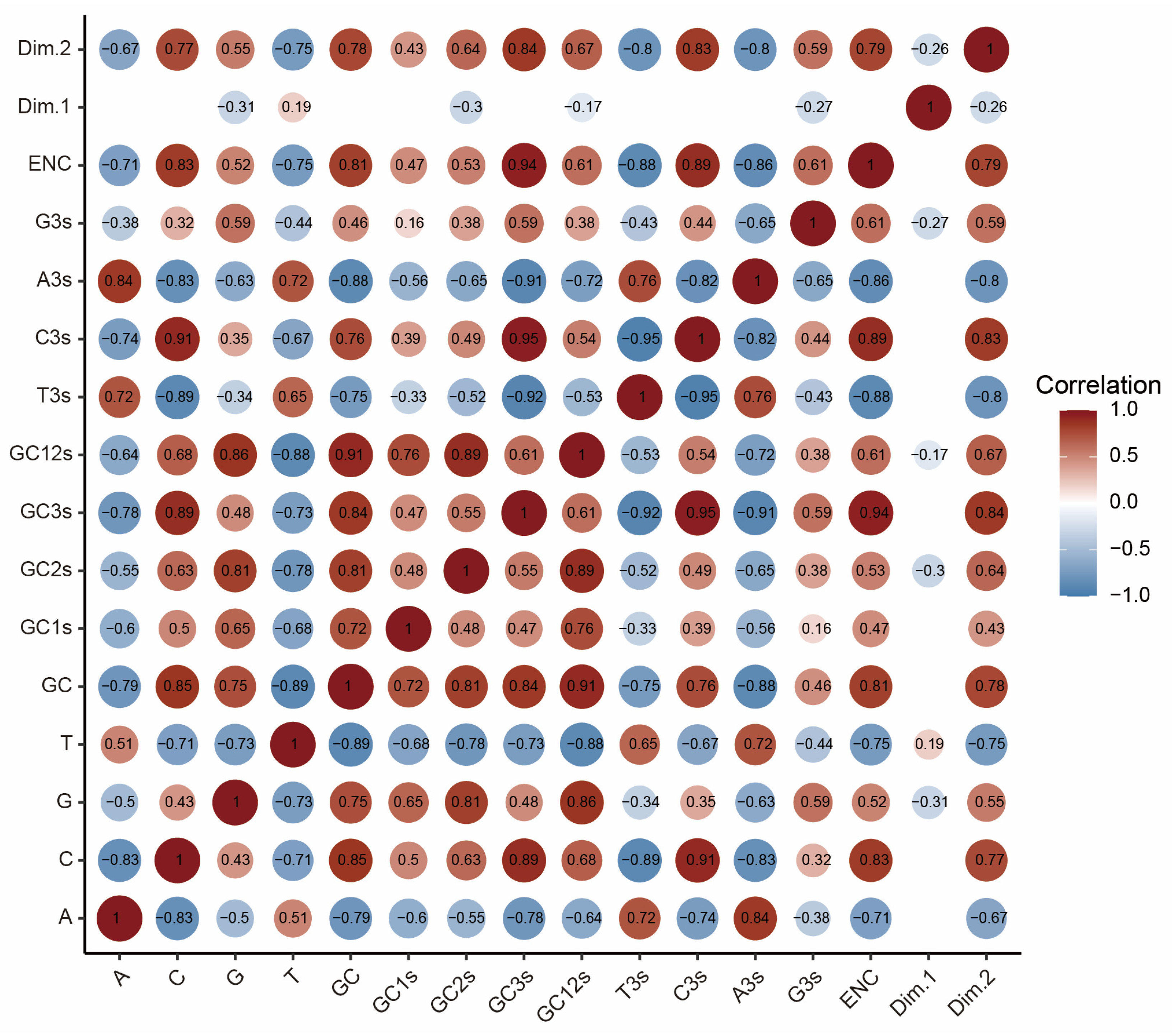
2.6. Mutation Pressure and Natural Selection Jointly Shaped Codon Usage Patterns of MPXV
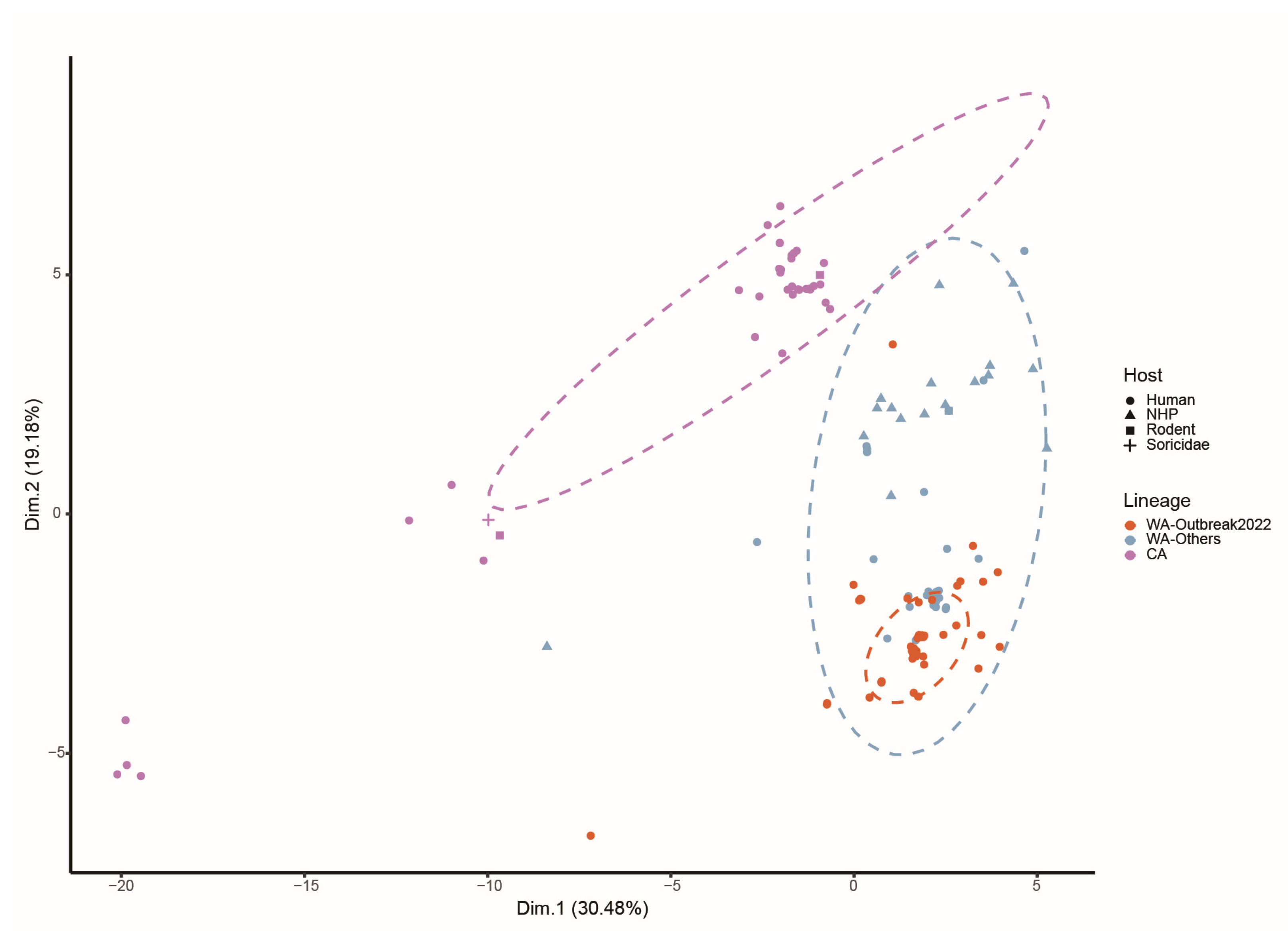
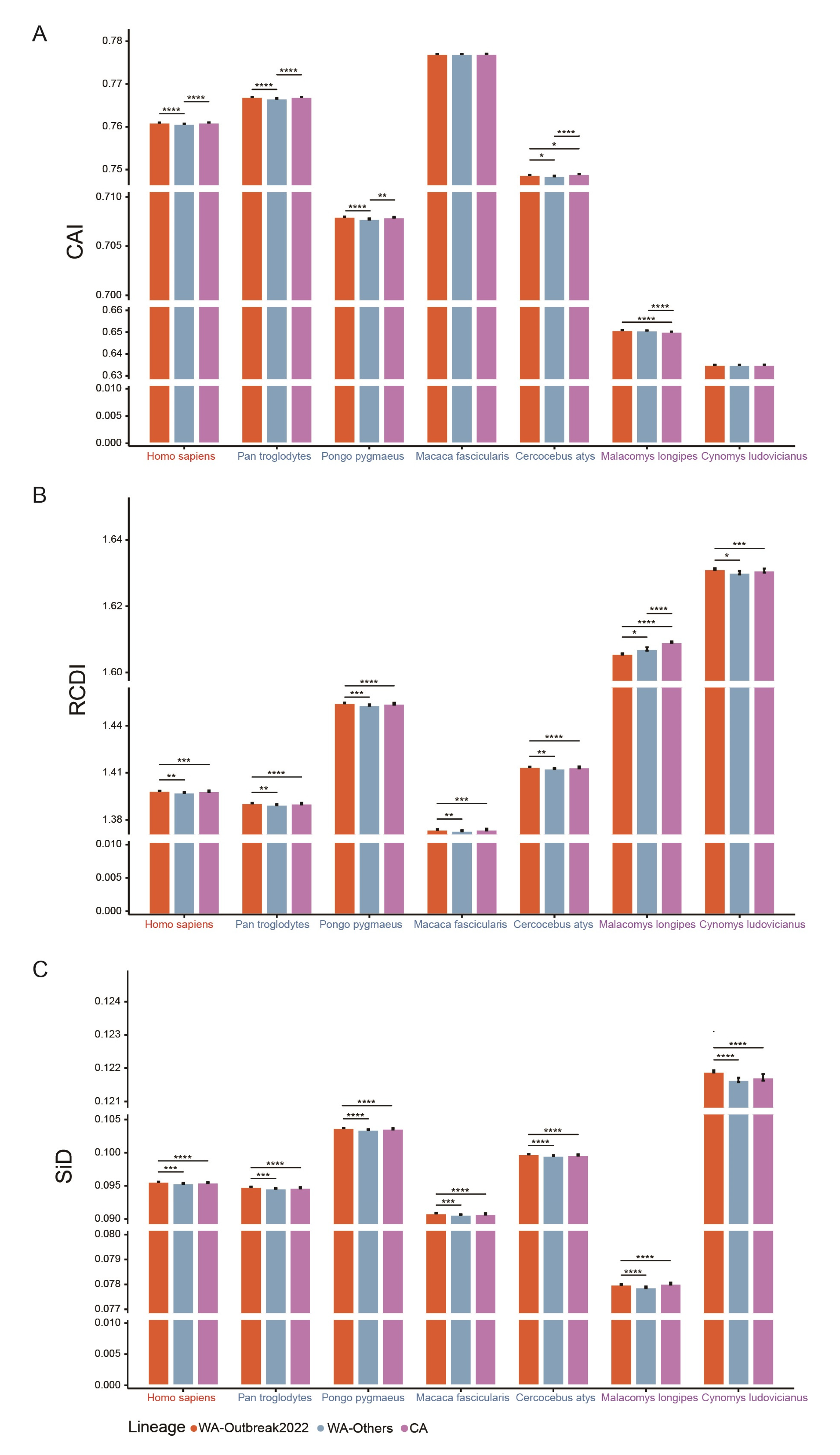
2.7. Distinct Patterns of MPXV Adaptation to Potential Hosts
2.8. Selection Pressure Driven by Hosts on MPXV
3. Discussion
4. Materials and Methods
4.1. Dataset
4.2. Recombination Analysis
4.3. Phylogenetic Analysis
4.4. Codon Usage Bias Analysis
4.4.1. Nucleotide Composition Analysis
4.4.2. The Effective Number of Codons (ENC) Analysis
4.4.3. The Relative Synonymous Codon Usage (RSCU) Analysis
4.4.4. Principal Component Analysis (PCA)
4.4.5. Neutrality Plot Analysis
4.4.6. Codon Adaption Index (CAI) Estimation
4.4.7. Relative Codon Deoptimization Index (RCDI) Computation
4.4.8. Similarity Index Analysis
4.4.9. Correlation and Statistics Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bunge, E.M.; Hoet, B.; Chen, L.; Lienert, F.; Weidenthaler, H.; Baer, L.R.; Steffen, R. The changing epidemiology of human monkeypox-A potential threat? A systematic review. PLoS Negl. Trop. Dis. 2022, 16, e0010141. [Google Scholar] [CrossRef] [PubMed]
- Thornhill, J.P.; Barkati, S.; Walmsley, S.; Rockstroh, J.; Antinori, A.; Harrison, L.B.; Palich, R.; Nori, A.; Reeves, I.; Habibi, M.S.; et al. Monkeypox Virus Infection in Humans across 16 Countries—April-June 2022. N. Engl. J. Med. 2022, 387, 679–691. [Google Scholar] [CrossRef]
- Karagoz, A.; Tombuloglu, H.; Alsaeed, M.; Tombuloglu, G.; AlRubaish, A.A.; Mahmoud, A.; Smajlović, S.; Ćordić, S.; Rabaan, A.A.; Alsuhaimi, E. Monkeypox (mpox) virus: Classification, origin, transmission, genome organization, antiviral drugs, and molecular diagnosis. J. Infect. Public Health 2023, 16, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Mauldin, M.R.; McCollum, A.M.; Nakazawa, Y.J.; Mandra, A.; Whitehouse, E.R.; Davidson, W.; Zhao, H.; Gao, J.; Li, Y.; Doty, J. Exportation of monkeypox virus from the African continent. J. Infect. Dis. 2022, 225, 1367–1376. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. 2022 Monkeypox Outbreak Global Map. Available online: https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html (accessed on 4 October 2022).
- Kugelman, J.R.; Johnston, S.C.; Mulembakani, P.M.; Kisalu, N.; Lee, M.S.; Koroleva, G.; McCarthy, S.E.; Gestole, M.C.; Wolfe, N.D.; Fair, J.N. Genomic variability of monkeypox virus among humans, Democratic Republic of the Congo. Emerg. Infect. Dis. 2014, 20, 232. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.I.O.; de Oliveira, J.S.; Kroon, E.G.; Trindade, G.d.S.; Drumond, B.P. Here, there, and everywhere: The wide host range and geographic distribution of zoonotic orthopoxviruses. Viruses 2020, 13, 43. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Yu, Y.; Xu, S. Human monkeypox infection threat: A comprehensive overview. PLoS Negl. Trop. Dis. 2023, 17, e0011246. [Google Scholar] [CrossRef]
- Wolff Sagy, Y.; Zucker, R.; Hammerman, A.; Markovits, H.; Arieh, N.G.; Abu Ahmad, W.; Battat, E.; Ramot, N.; Carmeli, G.; Mark-Amir, A.; et al. Real-world effectiveness of a single dose of mpox vaccine in males. Nat. Med. 2023, 29, 748–752. [Google Scholar] [CrossRef]
- Zhang, R.-R.; Wang, Z.-J.; Zhu, Y.-L.; Tang, W.; Zhou, C.; Zhao, S.-Q.; Wu, M.; Ming, T.; Deng, Y.-Q.; Chen, Q.; et al. Rational development of multicomponent mRNA vaccine candidates against mpox. Emerg. Microbes Infect 2023, 12, 2192815. [Google Scholar] [CrossRef]
- Zhang, N.; Cheng, X.; Zhu, Y.; Mo, O.; Yu, H.; Zhu, L.; Zhang, J.; Kuang, L.; Gao, Y.; Cao, R.; et al. Multi-valent mRNA vaccines against monkeypox enveloped or mature viron surface antigens demonstrate robust immune response and neutralizing activity. Sci. China Life Sci. 2023, 1–13. [Google Scholar] [CrossRef]
- Sang, Y.; Zhang, Z.; Liu, F.; Lu, H.; Yu, C.; Sun, H.; Long, J.; Cao, Y.; Mai, J.; Miao, Y.; et al. Monkeypox virus quadrivalent mRNA vaccine induces immune response and protects against vaccinia virus. Signal Transduct. Target. Ther. 2023, 8, 172. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Kaushik, R.; Tennakoon, C.; Uversky, V.N.; Mishra, A.; Sood, R.; Srivastava, P.; Tripathi, M.; Zhang, K.Y.J.; Bhatia, S. Evolutionary Signatures Governing the Codon Usage Bias in Coronaviruses and Their Implications for Viruses Infecting Various Bat Species. Viruses 2021, 13, 1847. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Guo, F.; Singh, B.; Gupta, S.; Paul, K.; Chen, X.; Sharma, N.R.; Jaishee, N.; Irwin, D.M.; Shen, Y. Base Composition and Host Adaptation of the SARS-CoV-2: Insight From the Codon Usage Perspective. Front. Microbiol. 2021, 12, 548275. [Google Scholar] [CrossRef]
- Malik, Y.S.; Ansari, M.I.; Kattoor, J.J.; Kaushik, R.; Sircar, S.; Subbaiyan, A.; Tiwari, R.; Dhama, K.; Ghosh, S.; Tomar, S.; et al. Evolutionary and codon usage preference insights into spike glycoprotein of SARS-CoV-2. Brief. Bioinform. 2021, 22, 1006–1022. [Google Scholar] [CrossRef] [PubMed]
- Sueoka, N. Directional mutation pressure and neutral molecular evolution. Proc. Natl. Acad. Sci. USA 1988, 85, 2653–2657. [Google Scholar] [CrossRef]
- Butt, A.M.; Nasrullah, I.; Qamar, R.; Tong, Y. Evolution of codon usage in Zika virus genomes is host and vector specific. Emerg. Microbes Infect. 2016, 5, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Nasrullah, I.; Butt, A.M.; Tahir, S.; Idrees, M.; Tong, Y. Genomic analysis of codon usage shows influence of mutation pressure, natural selection, and host features on Marburg virus evolution. BMC Evol. Biol. 2015, 15, 1–15. [Google Scholar] [CrossRef]
- Jolly, B.; Scaria, V. A distinct phylogenetic cluster of Monkeypox genomes suggests an early and cryptic spread of the virus. J. Infect. 2023, 86, e24–e26. [Google Scholar] [CrossRef]
- Gigante, C.M.; Korber, B.; Seabolt, M.H.; Wilkins, K.; Davidson, W.; Rao, A.K.; Zhao, H.; Hughes, C.M.; Minhaj, F.; Waltenburg, M.A. Multiple lineages of Monkeypox virus detected in the United States, 2021–2022. Science 2022, 378, 560–565. [Google Scholar] [CrossRef]
- Luna, N.; Ramírez, A.L.; Muñoz, M.; Ballesteros, N.; Patiño, L.H.; Castañeda, S.A.; Bonilla-Aldana, D.K.; Paniz-Mondolfi, A.; Ramírez, J.D. Phylogenomic analysis of the monkeypox virus (MPXV) 2022 outbreak: Emergence of a novel viral lineage? Travel Med. Infect. Dis. 2022, 49, 102402. [Google Scholar] [CrossRef]
- Isidro, J.; Borges, V.; Pinto, M.; Sobral, D.; Santos, J.D.; Nunes, A.; Mixão, V.; Ferreira, R.; Santos, D.; Duarte, S.; et al. Phylogenomic characterization and signs of microevolution in the 2022 multi-country outbreak of monkeypox virus. Nat. Med. 2022, 28, 1569–1572. [Google Scholar] [CrossRef]
- Karumathil, S.; Raveendran, N.T.; Ganesh, D.; Kumar NS, S.; Nair, R.R.; Dirisala, V.R. Evolution of synonymous codon usage bias in west African and central African strains of monkeypox virus. Evol. Bioinform. 2018, 14, 1176934318761368. [Google Scholar] [CrossRef] [PubMed]
- Sharp, P.M.; Li, W.H. An evolutionary perspective on synonymous codon usage in unicellular o rganisms. J. Mol. Evol. 1986, 24, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shang, J.; Weng, S.; Aliyari, S.R.; Ji, C.; Cheng, G.; Wu, A. Genomic annotation and molecular evolution of monkeypox virus outbreak in 2022. J. Med. Virol. 2023, 95, e28036. [Google Scholar] [CrossRef]
- Ata, G.; Wang, H.; Bai, H.; Yao, X.; Tao, S. Edging on Mutational Bias, Induced Natural Selection From Host and Natural Reservoirs Predominates Codon Usage Evolution in Hantaan Virus. Front. Microbiol. 2021, 12, 699788. [Google Scholar] [CrossRef]
- Si, F.; Jiang, L.; Yu, R.; Wei, W.; Li, Z. Study on the characteristic codon usage pattern in porcine epidemic diarrhea virus (PEDV) genomes and its host adaptation phenotype. Front. Microbiol. 2021, 12, 738082. [Google Scholar] [CrossRef]
- Butt, A.M.; Nasrullah, I.; Tong, Y. Genome-wide analysis of codon usage and influencing factors in chikungunya viruses. PLoS ONE 2014, 9, e90905. [Google Scholar] [CrossRef]
- Wright, F. The ‘effective number of codons’ used in a gene. Gene 1990, 87, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Seang, S.; Burrel, S.; Todesco, E.; Leducq, V.; Monsel, G.; Le Pluart, D.; Cordevant, C.; Pourcher, V.; Palich, R. Evidence of human-to-dog transmission of monkeypox virus. Lancet 2022, 400, 658–659. [Google Scholar] [CrossRef]
- Sharma, A.; Priyanka; Fahrni, M.L.; Choudhary, O.P. Monkeypox outbreak: New zoonotic alert after the COVID-19 pandemic. Int. J. Surg. 2022, 104, 106812. [Google Scholar] [CrossRef]
- Gomes, J.P.; Isidro, J.; Borges, V.; Pinto, M.; Sobral, D.; Santos, J.; Mixão, V.; Ferreira, R.; Nunes, A.; Santos, D. Multi-Country Outbreak of Monkeypox Virus: Phylogenomic Characterization and Signs of Microevolution. 2022. Available online: https://www.researchsquare.com/article/rs-1700947/v1 (accessed on 31 May 2022).
- Desingu, P.A.; Rubeni, T.P.; Sundaresan, N.R. Evolution of monkeypox virus from 2017 to 2022: In the light of point mutations. Front. Microbiol. 2022, 13, 1037598. [Google Scholar] [CrossRef] [PubMed]
- Sharp, P.M.; Li, W.-H. The codon adaptation index-a measure of directional synonymous codon usage bias, and its potential applications. Nucleic Acids Res. 1987, 15, 1281–1295. [Google Scholar] [CrossRef]
- Luo, W.; Roy, A.; Guo, F.; Irwin, D.M.; Shen, X.; Pan, J.; Shen, Y. Host adaptation and evolutionary analysis of Zaire ebolavirus: Insights from codon usage based investigations. Front. Microbiol. 2020, 11, 570131. [Google Scholar] [CrossRef] [PubMed]
- Nolen, L.D.; Osadebe, L.; Katomba, J.; Likofata, J.; Mukadi, D.; Monroe, B.; Doty, J.; Malekani, J.; Kabamba, J.; Bomponda, P.L. Introduction of monkeypox into a community and household: Risk factors and zoonotic reservoirs in the Democratic Republic of the Congo. Am. J. Trop. Med. Hyg. 2015, 93, 410. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.; Abubakar, I.; Ihekweazu, C.; Heymann, D.; Ntoumi, F.; Blumberg, L.; Asogun, D.; Mukonka, V.; Lule, S.A.; Bates, M. Monkeypox—Enhancing public health preparedness for an emerging lethal human zoonotic epidemic threat in the wake of the smallpox post-eradication era. Int. J. Infect. Dis. 2019, 78, 78–84. [Google Scholar] [CrossRef]
- Vivancos, R.; Anderson, C.; Blomquist, P.; Balasegaram, S.; Bell, A.; Bishop, L.; Brown, C.S.; Chow, Y.; Edeghere, O.; Florence, I. Community transmission of monkeypox in the United Kingdom, April to May 2022. Eurosurveillance 2022, 27, 2200422. [Google Scholar] [CrossRef]
- Minhaj, F.S.; Ogale, Y.P.; Whitehill, F.; Schultz, J.; Foote, M.; Davidson, W.; Hughes, C.M.; Wilkins, K.; Bachmann, L.; Chatelain, R. Monkeypox outbreak—Nine states, May 2022. Morb. Mortal. Wkly. Rep. 2022, 71, 764. [Google Scholar] [CrossRef]
- Duque, M.P.; Ribeiro, S.; Martins, J.V.; Casaca, P.; Leite, P.P.; Tavares, M.; Mansinho, K.; Duque, L.M.; Fernandes, C.; Cordeiro, R. Ongoing monkeypox virus outbreak, Portugal, 29 April to 23 May 2022. Eurosurveillance 2022, 27, 2200424. [Google Scholar]
- O’Toole, Á.; Rambaut, A. Initial Observations about Putative APOBEC3 Deaminase Editing Driving Short-Term Evolution of MPXV Since 2017. Available online: https://virological.org/t/initial-observations-about-putative-apobec3-deaminase-editing-driving-short-term-evolution-of-mpxv-since-2017/830 (accessed on 20 June 2022).
- Shean, R.C.; Makhsous, N.; Stoddard, G.D.; Lin, M.J.; Greninger, A.L. VAPiD: A lightweight cross-platform viral annotation pipeline and identification tool to facilitate virus genome submissions to NCBI GenBank. BMC Bioinform. 2019, 20, 1–8. [Google Scholar] [CrossRef]
- Martin, D.P.; Varsani, A.; Roumagnac, P.; Botha, G.; Maslamoney, S.; Schwab, T.; Kelz, Z.; Kumar, V.; Murrell, B. RDP5: A computer program for analyzing recombination in, and removing signals of recombination from, nucleotide sequence datasets. Virus Evol. 2021, 7, veaa087. [Google Scholar] [CrossRef]
- Diaz-Cánova, D.; Moens, U.L.; Brinkmann, A.; Nitsche, A.; Okeke, M.I. Genomic Sequencing and Analysis of a Novel Human Cowpox Virus With Mosaic Sequences From North America and Old World Orthopoxvirus. Front. Microbiol. 2022, 13, 868887. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; Von Haeseler, A.; Lanfear, R. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Hoang, D.T.; Chernomor, O.; Von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef]
- Anisimova, M.; Gil, M.; Dufayard, J.-F.; Dessimoz, C.; Gascuel, O. Survey of branch support methods demonstrates accuracy, power, and robustness of fast likelihood-based approximation schemes. Syst. Biol. 2011, 60, 685–699. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.; Von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Yu, G.; Smith, D.K.; Zhu, H.; Guan, Y.; Lam, T.T.Y. GGTREE: An R package for visualization and annotation of phylogenetic trees with their covariates and other associated data. Methods Ecol. Evol. 2017, 8, 28–36. [Google Scholar] [CrossRef]
- Zhou, J.; Xing, Y.; Zhou, Z.; Wang, S. A comprehensive analysis of Usutu virus (USUV) genomes revealed lineage-specific codon usage patterns and host adaptations. Front. Microbiol. 2023, 13, 967999. [Google Scholar] [CrossRef] [PubMed]
- Charif, D.; Lobry, J.R. SeqinR 1.0-2: A contributed package to the R project for statistical computing devoted to biological sequences retrieval and analysis. In Structural Approaches to Sequence Evolution; Springer: Berlin/Heidelberg, Germany, 2007; pp. 207–232. [Google Scholar]
- Peden, J. CodonW. 2005. Available online: https://codonw.sourceforge.net/ (accessed on 24 August 2020).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Sharp, P.M.; Li, W.-H. Codon usage in regulatory genes in Escherichia coli does not reflect selection for ‘rare’codons. Nucleic Acids Res. 1986, 14, 7737–7749. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Extrac and Visualize the Results of Multivariate Data Analyses. R Package Version 1.0.3. R Package Version 2020. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 12 April 2022).
- Puigbò, P.; Bravo, I.G.; Garcia-Vallve, S. CAIcal: A combined set of tools to assess codon usage adaptation. Biol. Direct. 2008, 3, 1–8. [Google Scholar] [CrossRef]
- Alexaki, A.; Kames, J.; Holcomb, D.D.; Athey, J.; Santana-Quintero, L.V.; Lam, P.V.N.; Hamasaki-Katagiri, N.; Osipova, E.; Simonyan, V.; Bar, H. Codon and codon-pair usage tables (CoCoPUTs): Facilitating genetic variation analyses and recombinant gene design. J. Mol. Biol. 2019, 431, 2434–2441. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.; Papamichail, D.; Coleman, J.R.; Skiena, S.; Wimmer, E. Reduction of the rate of poliovirus protein synthesis through large-sc ale codon deoptimization causes attenuation of viral virulence by lowe ring specific infectivity. J. Virol. 2006, 80, 9687–9696. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-H.; Zhang, J.; Sun, D.-J.; Ma, Q.; Chen, H.-T.; Ma, L.-N.; Ding, Y.-Z.; Liu, Y.-S. The distribution of synonymous codon choice in the translation initiat ion region of dengue virus. PLoS ONE 2013, 8, e77239. [Google Scholar] [CrossRef]
- Xu, S.; Chen, M.; Feng, T.; Zhan, L.; Zhou, L.; Yu, G. Use ggbreak to Effectively Utilize Plotting Space to Deal With Large Datasets and Outliers. Front. Genet. 2021, 12, 774846. [Google Scholar] [CrossRef]
- Kassambara, A. ggpubr:“ggplot2” Based Publication Ready Plots. R Package Version 0.4.0. 2020. Volume 438. Available online: https://rpkgs.datanovia.com/ggpubr/ (accessed on 11 October 2020).
- Kassambara, A. ggcorrplot: Visualization of a Correlation Matrix Using ‘ggplot2’. R Package Version 0.1.3. 2019, 3. Available online: https://CARN.R-project.org/package=ggcorrplot (accessed on 11 October 2020).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Wang, X.; Zhou, Z.; Wang, S. Insights into the Evolution and Host Adaptation of the Monkeypox Virus from a Codon Usage Perspective: Focus on the Ongoing 2022 Outbreak. Int. J. Mol. Sci. 2023, 24, 11524. https://doi.org/10.3390/ijms241411524
Zhou J, Wang X, Zhou Z, Wang S. Insights into the Evolution and Host Adaptation of the Monkeypox Virus from a Codon Usage Perspective: Focus on the Ongoing 2022 Outbreak. International Journal of Molecular Sciences. 2023; 24(14):11524. https://doi.org/10.3390/ijms241411524
Chicago/Turabian StyleZhou, Jianglin, Xuejun Wang, Zhe Zhou, and Shengqi Wang. 2023. "Insights into the Evolution and Host Adaptation of the Monkeypox Virus from a Codon Usage Perspective: Focus on the Ongoing 2022 Outbreak" International Journal of Molecular Sciences 24, no. 14: 11524. https://doi.org/10.3390/ijms241411524
APA StyleZhou, J., Wang, X., Zhou, Z., & Wang, S. (2023). Insights into the Evolution and Host Adaptation of the Monkeypox Virus from a Codon Usage Perspective: Focus on the Ongoing 2022 Outbreak. International Journal of Molecular Sciences, 24(14), 11524. https://doi.org/10.3390/ijms241411524






