6-Oxofurostane and (iso)Spirostane Types of Saponins in Smilax sieboldii: UHPLC-QToF-MS/MS and GNPS-Molecular Networking Approach for the Rapid Dereplication and Biodistribution of Specialized Metabolites
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of Conditions for UHPLC-QToF-MS
2.2. Unified Fragmentation Pattern with Isospirostane-Type Saponins
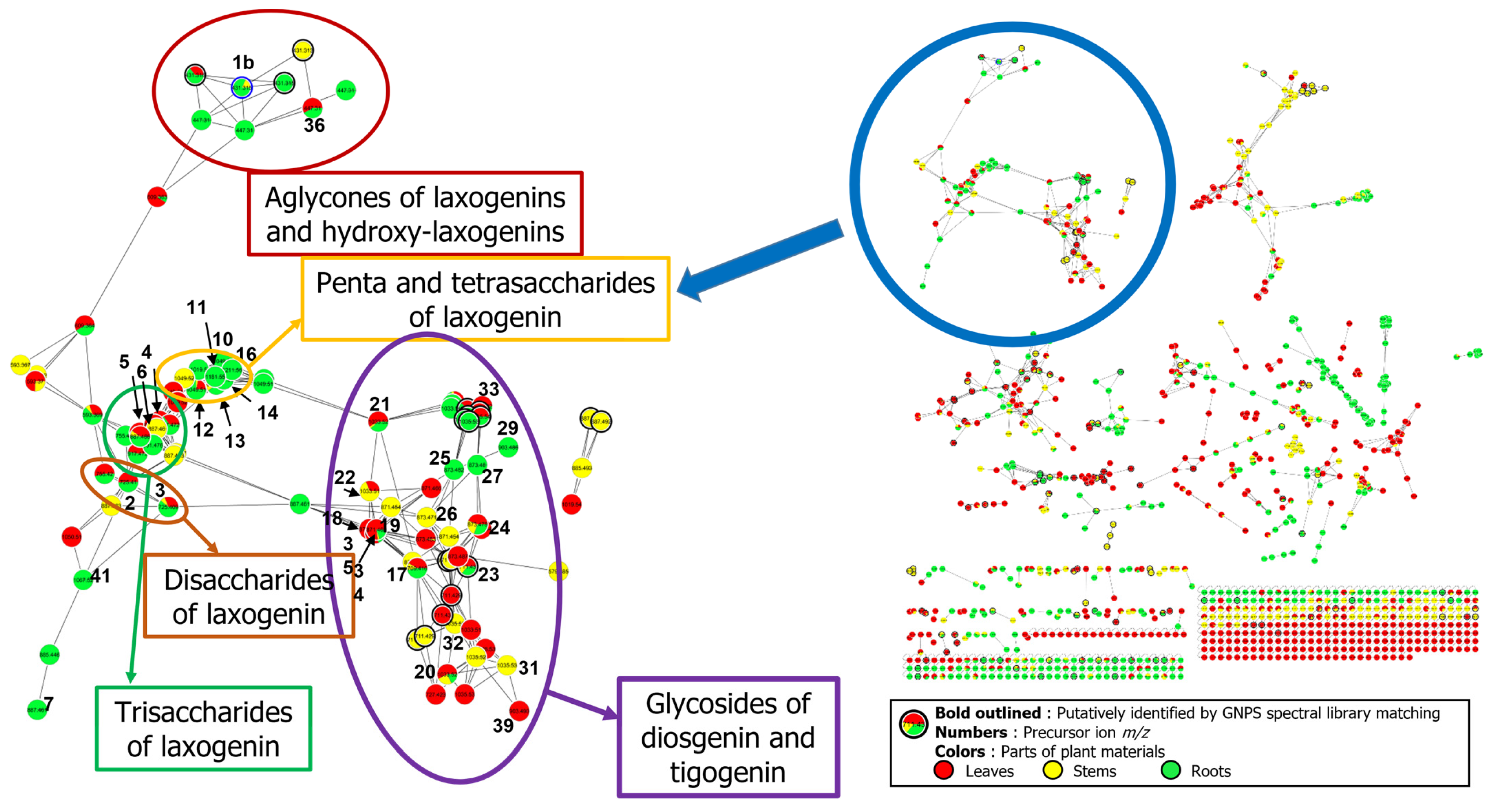
2.2.1. Cluster #1: Laxogenin-Type Saponins (1–16)
2.2.2. Cluster #2: Compounds 17–22
2.2.3. Cluster #3: Compounds 23–33
2.2.4. Cluster #4: Compounds 34–35
2.2.5. Cluster #5: Compounds 36–40
2.3. Biodistribution of Saponins within S. sieboldii Plant Parts
3. Materials and Methods
3.1. Standards and Chemicals
3.2. Plant Materials
3.3. Plant Sample and Standard Preparations
3.4. Ultra-High Performance Liquid Chromatography Coupled to Quadrupole Time of Flight-Mass Spectrometry (UHPLC-QToF-MS/MS)
3.5. Global Natural Products Social Molecular Networking (GNPS) Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ESI | Electrospray ionization |
| ESI-QToF | Electrospray ionization-quadrupole time of flight |
| GNPS | Global natural products social molecular networking |
| HPLC | High-performance liquid chromatography |
| LC | Liquid chromatography |
| LC-ESI-MS | Liquid chromatography-electrospray ionization-mass spectrometry |
| LC-MS | Liquid chromatography-mass spectrometry |
| MFE | Molecular features extractor |
| MS/MS | Tandem mass spectrometry |
| MW | Molecular weight |
| PTFE | Polytetrafluoroethylene |
| TIC | Total ion chromatogram |
| UHPLC | Ultra-high performance liquid chromatography |
| UV | Ultraviolet |
| MS | Mass spectrometry |
References
- Avula, B.; Chittiboyina, A.G.; Bae, J.Y.; Haider, S.; Wang, Y.H.; Wang, M.; Zhao, J.; Deuster, P.A.; Khan, I.A. The power of hyphenated chromatography-Time of flight mass spectrometry for unequivocal identification of spirostanes in bodybuilding dietary supplements. J. Pharm. Biomed. Anal. 2019, 67, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.H. The Medicinal Plant of Korea; Kyohaksa Press: Seoul, Republic of Korea, 2000; p. 542. [Google Scholar]
- Gao, Y.; Su, Y.; Qu, L.; Xu, S.; Meng, L.; Cai, S.Q.; Shou, C. Mitochondrial apoptosis contributes to the anticancer effect of Smilax glabra Roxb. Toxicol. Lett. 2012, 207, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yin, H.; Lan, Z.; Ma, S.; Zhang, C.; Yang, Z.; Li, P.; Lin, B. Anti-hyperuricemic and nephroprotective effects of Smilax china L. J. Ethnopharmacol. 2011, 135, 399–405. [Google Scholar] [CrossRef]
- Zou, W.; Zhou, H.; Hu, J.; Zhang, L.; Tang, Q.; Wen, X.; Xiao, Z.; Wang, W. Rhizoma Smilacis Glabrae inhibits pathogen-induced upper genital tract inflammation in rats through suppression of NF-κB pathway. J. Ethnopharmacol. 2017, 202, 103–113. [Google Scholar] [CrossRef]
- Tian, L.W.; Zhang, Z.; Long, H.L.; Zhang, Y.-J. Steroidal Saponins from the Genus Smilax and Their Biological Activities. Nat. Prod. Bioprospect. 2017, 7, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Woo, M.H.; Do, J.C.; Son, K.H. Five new spirostanol glycosides from the subterranean parts of Smilax sieboldii. J. Nat. Prod. 1992, 55, 1129–1135. [Google Scholar] [CrossRef]
- Nikaido, T.; Ohmoto, T.; Kubo, S.; Mimaki, Y.; Sashida, Y. Steroidal saponins from the rhizomes of Smilax sieboldii. Phytochemistry 1992, 31, 2445–2450. [Google Scholar] [CrossRef]
- Okanish, T.; Akahori, A.; Yasuda, F. Studies on the Steroidal components of domestic plants. XLVII. Constituents of the stem of Smilax sieboldii Miq. (1) The structure of Laxogenin. Chem. Pharm. Bull. 1965, 13, 545–550. [Google Scholar] [CrossRef]
- Kim, C.M.; Son, K.H.; Kim, S.H.; Kim, H.P. Steroidal sapogenin contents in some domestic plants. Arch. Pharm. Res. 1991, 14, 305–310. [Google Scholar] [CrossRef]
- Jung, Y.W.; Lee, J.A.; Lee, J.E.; Cha, E.; Choi, Y.-H.; Jeong, W.; Choi, C.W.; Oh, J.S.; Ahn, E.-K.; Hong, S.S. Anti-adipogenic activity of Secondary Metabolites isolated from Smilax sieboldii Miq. on 3T3-L1 Adipocytes. Int. J. Mol. Sci. 2023, 24, 8866. [Google Scholar] [CrossRef]
- Ryu, J.-H.; Ahn, H.; Kim, J.Y.; Kim, Y.-K. Inhibitory Activity of Plant Extracts on Nitric Oxide Synthesis in LPS-Activated Macrophages. Phytother. Res. 2003, 17, 485–489. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Avula, B.; Fu, X.; Wang, M.; Khan, I.A. Simultaneous determination of the absolute configuration of twelve monosaccharide enantiomers from natural products in a single injection by a UPLC-UV/MS method. Planta Med. 2012, 78, 834–837. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.H.; Ju, Y. Steroidal saponins from Smilax lebrunii. Phytochemistry 1992, 31, 3173–3175. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wang, N.-L.; Yao, X.-S.; Kitanaka, S. Steroidal saponins from the bulbs of Allium chinense. Stud. Plant Sci. 1999, 6, 212–219. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Xiang, L.; Huang, W.; He, X. Spirostanol saponins from Chinese onion (Allium chinense) exert pronounced anti-inflammatory and anti-proliferative activities. J. Funct. Foods 2016, 26, 208–219. [Google Scholar] [CrossRef]
- Wang, X.; Wu, G.Q. A New Steroidal Glycoside and Potential Anticancer Cytotoxic Activity of Compounds Isolated from the Bulbs of Lilium callosum. J. Chem. Res. 2014, 38, 577–579. [Google Scholar] [CrossRef]
- Kawano, K.; Sato, H.; Sakamura, S. Isolation and Structure of Furostanol Saponin in Asparagus Edible Shoots. Agric. Biol. Chem. 1977, 41, 1–8. [Google Scholar] [CrossRef]
- Matsuura, H.; Ushiroguchi, T.; Itakura, Y.; Fuwa, T. A Furostanol Glycoside from Allium chinense G. DON. Chem. Pharm. Bull. 1989, 37, 1390–1391. [Google Scholar] [CrossRef]
- Ju, Y.; Jia, Z.-J. Minor steroidal glycosides from the roots of Smilax lebrunii. Phytochemistry 1993, 31, 1193–1195. [Google Scholar] [CrossRef]
- Zhang, C.; Feng, S.; Zhang, L.; Ren, Z. A new cytotoxic steroidal saponin from the rhizomes and roots of Smilax scobinicaulis. Nat. Prod. Res. 2013, 27, 1255–1260. [Google Scholar] [CrossRef]
- Kang, L.; Zhao, Y.; Pang, X.; Yu, H.; Xiong, C.; Zhang, J.; Gao, Y.; Yu, K.; Liu, C.; Ma, B. Characterization and identification of steroidal saponins from the seeds of Trigonella foenum-graecum by ultra-high-performance liquid chromatography and hybrid time-of-flight mass spectrometry. J. Pharm. Biomed. Anal. 2013, 74, 257–267. [Google Scholar] [CrossRef]
- Dini, I.; Tenore, G.C.; Trimarco, E.; Dini, A. Furostanol saponins in Allium caepa L. var tropeana seeds. Food Chem. 2005, 93, 205–214. [Google Scholar] [CrossRef]
- Pang, X.; Cong, Y.; Yu, H.-S.; Kang, L.-P.; Feng, B.; Han, B.-X.; Zhao, Y.; Xiong, C.-Q.; Tan, D.-W.; Song, W.; et al. Spirostanol saponins derivated from the seeds of Trigonella foenum-graecum by β-glucosidase hydrolysis and their inhibitory effects on rat platelet aggregation. Planta Med. 2012, 78, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-L.; Gao, J.-M.; Zhu, W. Steroidal saponins from the rhizomes and roots of Smilax scobinicaulis. Phytochem. Lett. 2012, 5, 49–52. [Google Scholar] [CrossRef]
- Zhang, C.L.; Zhu, W.; Li, X.M.; Su, B.F.; Yan, X.Y. Antisepsis activity of the rhizomes of Smilax scobinicaulis. Linye Kexue 2006, 42, 69–73. [Google Scholar]
- Chambers, M.C.; Maclean, B.; Burke, R.; Amodei, D.; Ruderman, D.L.; Neumann, S.; Gatto, L.; Fischer, B.; Pratt, B.; Egertson, J.; et al. A Cross-platform Toolkit for Mass Spectrometry and Proteomics. Nat. Biotechnol. 2012, 30, 918–920. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef]


| # | Name | tR (min) | Molecular Formula | Mass | m/z [M-H]− | Error (ppm) | m/z [M+H]+ | Error (ppm) | Fragment Ions (50 eV) | S. sieboldii Plant Part | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leaf | Stem | Root | ||||||||||
| m/z 431 as an aglycone | ||||||||||||
| 1 | Laxogenin (Aglycone) | 32.3 | C27H42O4 | 430.3083 | - | - | 431.3161 (431.3156) | −1.2 | 413.3037 [M+H-H2O]+, 287.1998 [M+H-C8H16O2]+, 269.1894 [M+H-C8H16O2-H2O]+, 251.1789 [M+H-C8H16O2-2H2O]+ | + | + | + |
| 453.2983 (453.2975) [M+Na]+ | −1.8 | |||||||||||
| 2 | Unknown-1/2 (Smilaxin A/Laxogenin 3-O-α-L-arabinopyranosyl -(l→6)-β-D-glucopyranoside) | 22.4 | C38H60O13 | 724.4034 | 723.3958 (723.3961) 769.4012 (769.4016) [M+HCOO]− | 0.4 0.6 | 725.4099 (725.4107) | 1.1 | 593.3674 [M+H-Ara]+, 431.3159 [M+H-Ara-Glc]+, 413.3044 [M+H-Ara-Glc-H2O]+, 395.2931 [M+H-Ara-Glc-2H2O]+, 287.2000 [M+H -Ara-Glc-C8H16O2]+, 269.1889 [M+H-Ara-Glc-C8H16O2-H2O]+, 251.1784 [M+H-Ara-Glc-C8H16O2-2H2O]+ | + | + | + |
| 3 | 23.1 | + | + | + | ||||||||
| 742.4376 (742.4372) [M+NH4]+ | −0.5 | |||||||||||
| 747.3916 (747.3926) [M+Na]+ | 1.4 | |||||||||||
| 4 | Unknown-3–6 (Smilaxin B/Laxogenin 3-O-β-D-glucopyranosyl-(l→4)-O-[α-L-arabinopyranosyl-( l→6)]-β-D-glucopyranoside) | 13.1 | C44H70O18 | 886.4562 | 885.4483 (885.4489) 931.4554 (931.4544) [M+HCOO]− | 0.7 −1.1 | 887.4646 (887.4635) | −1.3 | 725.4094 [M+H-Glc]+, 593.3676 [M+H-Glc-Ara]+, 431.3150 [M+H-2Glc-Ara]+, 413.3035 [M+H-2Glc-Ara-H2O]+, 287.1988 [M+H-2Glc-Ara-C8H16O2]+, 269.1883 [M+H-2Glc-Ara-C8H16O2-H2O]+, 251.1764 [M+H-2Glc-Ara-C8H16O2-2H2O]+ | + | + | + |
| 5 | 14.0 | + | + | + | ||||||||
| 6 | 16.2 | + | + | + | ||||||||
| 7 | 22.6 | 904.4899 (904.4900) [M+NH4]+ | 0.2 | + | + | + | ||||||
| 8 | Unknown-7/8 (26-O-β-D-glucopyranosyl-3β, 22ξ, 26-trihydroxy-(25R)-5α-furostan-6-one 3-O-α- L-arabinopyranosyl-(l→6)]-β-D-glucopyranoside) | 12.9 | C44H72O19 | 904.4668 | 903.4579 (903.4595) 949.4645 (949.465) [M+HCOO]− | 1.8 0.5 | 887.4628 (887.4635) [M+H-H2O]+ | 0.8 | 755.4205 [M+H-H2O-Ara]+, 725.4087 [M+H-H2O-Glc]+, 593.3677 [M+H-H2O-Glc-Ara]+, 431.3157 [M+H-H2O-2Glc-Ara]+, 413.3051 [M+H -2Glc-Ara-2H2O]+, 287.2002 [M+H-H2O-2Glc-Ara-C8H16O2]+, 269.1894 [M+H-2Glc-Ara-C8H16O2-2H2O]+, 251.1790 [M+H-2Glc-Ara-C8H16O2-3H2O]+ | + | + | + |
| 9 | 13.4 | + | + | + | ||||||||
| 927.4570 (927.456) [M+Na]+ | −1.1 | |||||||||||
| 10 | Unknown-9–11 (26-O-β-D-glucopyranosyl- 3β, 22ξ, 26-trihydroxy-(25R)-5α-furostan-6-one 3-O-β-D-glucopyranosyl-(l→4)-O-[ α- L-arabinopyranosyl-(l→6)]-β-D-glucopyranoside) | 12.2 | C50H82O24 | 1066.5196 | 1065.5112 (1065.5123) 1111.5167 (1111.5178) [M+HCOO]− 578.2554 (578.258) [M+2HCOO]2− | 1.0 1.0 4.9 | 1049.5184 (1049.5163) [M+H-H2O]+ | −2.0 | 917.4723 [M+H-H2O-Ara]+, 887.4619 [M+H-H2O-Glc]+, 725.4108 [M+H-H2O-2Glc]+, 593.3680 [M+H-H2O-2Glc-Ara]+, 431.3162 [M+H-H2O-3Glc-Ara]+, 413.3046 [M+H-3Glc-Ara-2H2O]+, 395.2945 [M+H-3Glc-Ara-3H2O]+, 287.2005 [M+H-H2O-3Glc-Ara-C8H16O2]+, 269.1898 [M+H-3Glc-Ara-C8H16O2-2H2O]+, 251.1784 [M+H-3Glc-Ara-C8H16O2-3H2O]+ | ND | ND | + |
| 11 | 12.8 | 1084.5538 (1084.5544) [M+NH4]+ | −0.4 | + | + | + | ||||||
| 12 | 13.13 | 1089.5085 (1089.5088) [M+Na]+ | 0.3 | + | + | + | ||||||
| 13 | Unknown-12 | 12.3 | C55H90O28 | 1198.5619 | 1197.5523 (1197.5546) | 1.9 | 1181.5577 (1181.5586) [M+H-H2O]+ | 0.7 | 1067.5246 [M+H-Ara]+, 1049.5155 [M+H-H2O-Ara]+, 917.4684 [M+H-H2O-2Ara]+, 887.4642 [M+H-H2O-Ara -Glc]+, 755.4211 [M+H-H2O-2Ara -Glc]+, 593.3675 [M+H-H2O-2Ara -2Glc]+, 431.3149 [M+H-H2O-2Ara -3Glc]+, 413.3045 [M+H-2Ara -3Glc-2H2O]+, 395.2945 [M+H-2Ara -3Glc-3H2O]+, 287.2002 [M+H-H2O-3Glc-2Ara-C8H16O2]+, 269.1876 [M+H-3Glc-2Ara-C8H16O2-2H2O]+, 251.1793 [M+H-3Glc-2Ara-C8H16O2-3H2O]+ | ND | + | + |
| 1216.5950 (1216.5957) [M+NH4]+ | 0.6 | |||||||||||
| 1221.5507 (1221.5511) [M+Na]+ | 0.3 | |||||||||||
| 14 | Unknown-13–15 | 11.6 | C56H92O29 | 1228.5724 | 1227.5639 (1227.5652) | 1.0 | 1211.5693 (1211.5691) [M+H-H2O]+ | −0.1 | 1049.5142 [M+H-H2O-Glc]+, 917.4690 [M+H-H2O-Glc-Ara]+, 887.4606 [M+H-H2O-2Glc]+, 755.4214 [M+H-H2O-2Glc-Ara]+, 593.3672 [M+H-H2O-3Glc-Ara]+, 431.3149 [M+H-H2O-4Glc-Ara]+, 413.3048 [M+H-3Glc-Ara-2H2O]+, 287.1995 [M+H-H2O-3Glc-Ara-C8H16O2]+, 269.1887 [M+H-3Glc-Ara-C8H16O2-2H2O]+, 251.1788 [M+H-3Glc-Ara-C8H16O2-3H2O]+ | + | + | + |
| 15 | 12.4 | + | + | + | ||||||||
| 16 | 12.7 | + | + | + | ||||||||
| m/z 415 as an aglycone | ||||||||||||
| 17 | Unknown-16 | 26.05 | C38H60O12 | 708.4085 | 707.4010 (707.4012) 753.4058 (753.4067) [M+HCOO]− | 0.3 1.2 | 709.4170 (709.4158) | −1.8 | 577.3738 [M+H-Ara]+, 415.3204 [M+H-Glc-Ara]+, 397.3092 [M+H-Glc-Ara-H2O]+, 379.3018 [M+H-Glc-Ara-2H2O]+, 271.2044 [M+H-H2O-Glc-Ara- C8H16O2]+, 253.1945 [M+H-Glc-Ara C8H16O2-2H2O]+ | + | + | + |
726.4410 (726.4423) [M+NH4]+ | 1.8 | |||||||||||
731.3966 (731.3977) [M+Na]+ | 1.6 | |||||||||||
| 18 | Unknown-17/18 | 15.2 | C44H72O18 | 888.4719 | 887.4642 (887.4646) 933.4690 (933.4701) [M+HCOO]− | 0.4 1.2 | 871.4686 (871.4686) [M+H-H2O]+ | 0 | 739.4221 [M+H-H2O-Ara]+, 709.4157 [M+H-H2O-Glc]+, 577.3738 [M+H-H2O-Glc-Ara]+, 415.3204 [M+H-H2O-2Glc-Ara]+, 397.3097 [M+H-2Glc-Ara-2H2O]+, 379.2989 [M+H-2Glc-Ara-3H2O]+, 271.2044 [M+H-H2O-2Glc-Ara-C8H16O2]+, 253.1950 [M+H-2Glc-Ara-C8H16O2-2H2O]+ | + | + | + |
| 19 | 15.4 | + | + | + | ||||||||
| 20 | Unknown-19–21 | 14.2 | C50H82O23 | 1050.5247 | 1049.5158 (1049.5174) 1095.5219 (1095.5229) [M+HCOO]− | 1.5 0.9 | 1033.5236 (1033.5214) [M+H-H2O]+ | −2.1 | 901.4810 [M+H-H2O-Ara]+, 871.4686 [M+H-H2O-Glc]+, 739.4254 [M+H-H2O-Glc-Ara]+, 709.4142 [M+H-H2O-2Glc]+, 577.3738 [M+H-H2O-2Glc-Ara]+, 415.3204 [M+H-H2O-3Glc-Ara]+, 397.3087 [M+H-3Glc-Ara-2H2O]+, 379.2985 [M+H-3Glc-Ara-3H2O]+, 271.2042 [M+H-H2O-3Glc-Ara-C8H16O2]+, 253.1945 [M+H-3Glc-Ara-C8H16O2-2H2O]+ | t | + | t |
| 21 | 14.6 | + | + | + | ||||||||
| 22 | 14.8 | + | + | + | ||||||||
| 1073.5174 (1073.5139) [M+Na]+ | −3.3 | |||||||||||
| m/z 417 as an aglycone | ||||||||||||
| 23 | Unknown-22 | 26.5 | C38H62O12 | 710.4241 | 709.4163 (709.4169) 755.4212 (755.4223) [M+HCOO]− | 0.8 1.6 | 711.4299 (711.4314) | 2.1 | 579.3888 [M+H-Ara]+, 417.3317 [M+H-Glc-Ara]+, 399.3288 [M+H-Glc-Ara-H2O]+, 273.2201 [M+H-Glc-Ara-C8H16O2]+, 255.2095 [M+H-Glc-Ara-C8H16O2-H2O]+ | + | + | + |
| 733.4133 (733.4133) [M+Na]+ | 0.0 | |||||||||||
| 24 | Unknown-23–25 (Smilaxin C/Tigogenin 3-O-β-D-glucopyranosyl-(l→4)-O-[α-L-arabinopyranosyl-(l→6)]-β-D-glucopyranoside) | 15.6 | C44H72O17 | 872.477 | 871.4688 (871.4697) 917.4753 (917.4752) [M+HCOO]− | 1.0 −0.2 | 873.4834 (873.4842) | 1.0 | 741.4422 [M+H-Ara]+, 711.4335 [M+H-Glc]+, 579.3789 [M+H-Glc-Ara]+, 417.3346 [M+H-2Glc-Ara]+, 399.3222 [M+H-2Glc-Ara-H2O]+, 273.2186 [M+H-2Glc-Ara-C8H16O2]+, 255.2092 [M+H-2Glc-Ara-C8H16O2-H2O]+ | + | + | + |
| 25 | 15.1 | + | + | + | ||||||||
| 895.4652 (895.4662) [M+Na]+ | 1.1 | |||||||||||
| 26 | 25.4 | + | + | + | ||||||||
| 27 | Unknown-26/27 | 15.5 | C44H74O18 | 890.4875 | 889.4802 (889.4802) 935.4853 (935.4857) [M+HCOO]− | 0.0 0.5 | 873.4834 (873.4842) [M+H-H2O]+ | 1.0 | 711.4335 [M+H-H2O-Glc]+, 579.3789 [M+H-H2O-Glc-Ara]+, 417.3346 [M+H-H2O-2Glc-Ara]+, 399.3222 [M+H-2Glc-Ara-2H2O]+, 273.2186 [M+H-H2O-2Glc-Ara-C8H16O2]+, 255.2092 [M+H-2Glc-Ara-C8H16O2-2H2O]+ | + | ++ | + |
| 28 | 15.6 | + | ++ | + | ||||||||
| 29 | Unknown-28/29 (Sarsaparilloside C-furostane ring) | 16.1 | C45H76O19 | 920.4981 | 919.4906 (919.4908) 965.4931 (965.4963) [M+HCOO]− | 0.2 3.5 | 903.4943 (903.4948) [M+H-H2O]+ | 0.6 | 741.4372 [M+H-H2O-Glc]+, 579.3840 [M+H-H2O-2Glc]+, 417.3341 [M+H-H2O-3Glc]+, 399.3239 [M+H-3Glc-2H2O]+, 381.3138 [M+H-3Glc-3H2O]+, 273.2214 [M+H-H2O-3Glc -C8H16O2]+, 255.2099 [M+H-3Glc-C8H16O2-2H2O]+ | + | + | + |
| 30 | 16.2 | + | + | + | ||||||||
| 31 | Unknown-30–32 (Furostane-3,22,26-triol 3-O-[α-L-Arabinopyranosyl-(1→6)-[β-D-glucopyranosyl-(1→4)]-β-D-glucopyranoside], 26-O-β-D-glucopyranoside) | 14.4 | C50H84O23 | 1052.5403 | 1051.5333 (1051.5331) 1097.5389 (1097.5385) [M+HCOO]− | −0.2 −0.3 | 1035.5391 (1035.5371) [M+H-H2O]+ | −2.0 | 903.4945 [M+H-H2O-Ara]+, 873.4851 [M+H-H2O-Glc]+, 741.4401 [M+H-H2O-Glc-Ara]+, 729.3691 [M+H-Glc-Ara-2H2O]+, 711.4283 [M+H-H2O-2Glc]+, 579.3891 [M+H-H2O-2Glc-Ara]+, 417.3357 [M+H-H2O-3Glc-Ara]+, 399.3240 [M+H-3Glc-Ara-2H2O]+, 381.3159 [M+H-3Glc-Ara-3H2O]+, 273.2211 [M+H-H2O-3Glc-Ara-C8H16O2]+, 255.2098 [M+H-3Glc-Ara-C8H16O2-2H2O]+ | + | + | ND |
| 32 | 14.9 | + | + | + | ||||||||
| 33 | 15.03 | + | + | + | ||||||||
| m/z 433 as an aglycone | ||||||||||||
| 34 | Unknown-33/34 | 16.4 | C44H72O18 | 888.4719 | 887.4653 (887.4646) 933.4685 (933.4701) [M+HCOO]− | −0.8 1.8 | 889.4792 (889.4791) | −0.1 | 757.4344 [M+H-Ara]+, 727.4261 [M+H-Glc]+, 595.3829 [M+H-Glc-Ara]+, 433.3306 [M+H-2Glc-Ara]+, 415.3190 [M+H-2Glc-Ara-H2O]+, 397.3067 [M+H-2Glc-Ara-2H2O]+, 255.2096 [M+H-2Glc-Ara-2H2O-C8H14O2]+ | t | t | + |
| 35 | 20 | + | t | + | ||||||||
| 911.4622 (911.4611) [M+Na]+ | −1.3 | |||||||||||
| m/z 447 as an aglycone | ||||||||||||
| 36 | Unknown-35 (Sieboldigenin, Aglycone) | 23.7 | C27H42O5 | 446.3032 | - | - | 447.3112 (447.3105) | −1.6 | 429.2996 [M+H-H2O]+, 287.1993 [M+H-H2O-C8H14O2]+, 269.1882 [M+H-C8H14O2-2H2O]+, 251.1775 [M+H-C8H14O2-3H2O]+ | ND | t | + |
| 469.2921 (469.2924) [M+Na]+ | 0.8 | |||||||||||
| 37 | Unknown-36/37 (Sieboldin B) | 16.7 | C38H60O14 | 740.3938 | 739.3901 (739.391) 785.3966 (785.3965) [M+HCOO]− | 1.3 −0.1 | 741.4063 (741.4056) | −1.0 | 609.3629 [M+H-Ara]+, 579.3542 [M+H-Glc]+, 447.3106 [M+H-Ara-Glc]+, | + | + | + |
| 38 | 17.4 | 763.3873 (763.3875) [M+Na]+ | 0.3 | 609.3626 [M+H-Ara]+, 447.3097 [M+H-Ara-Glc]+, 429.2990 [M+H-Ara-Glc-H2O]+, 411.2890 [M+H-Ara-Glc-2H2O]+, 287.1995 [M+H-Ara-Glc-H2O-C8H14O2]+, 269.1892 [M+H-Ara-Glc-2H2O-C8H14O2]+, 251.1785 [M+H-Ara-Glc-3H2O-C8H14O2]+ | + | + | + | |||||
| 39 | Unknown-38/39 (Sieboldin A-(3β, 27-dihydroxy-(25S)-5α-spirostan-6-one 3-O-β-D-glucopyranosyl-(l→4)-O-[α-L-arabinopyranosyl-(l→6)]-β-D-glucopyranoside)) | 16.13 | C44H70O19 | 902.4511 | 901.4435 (901.4439) 947.4495 (947.4493) [M+HCOO]− | 0.4 −0.2 | 903.4536 (903.4584) | 5.3 | 771.4178 [M+H-Ara]+, 741.4070 [M+H-Glc]+, 609.3633 [M+H-Glc-Ara]+, 579.3526 [M+H-2Glc]+, 447.3099 [M+H-2Glc-Ara]+, 429.3001 [M+H-2Glc-Ara-H2O]+, 287.1999 [M+H-2Glc-Ara-H2O-C8H14O2]+ | + | + | + |
| 925.4407 (925.4404) [M+Na]+ | −0.4 | |||||||||||
| 40 | 16.8 | + | + | + | ||||||||
| m/z 449 as an aglycone | ||||||||||||
| 41 | Unknown-40 (tetra-glycoside of 3, 6, 27-trihydroxy furostane-type saponin) | 12.3 | C50H84O25 | 1084.5302 | 1083.5229 (1083.5229) 1129.5257 (1129.5284) [M+HCOO]− | 0.0 2.5 | 1067.5268 (1067.5269) [M+H-H2O]+ | 0.1 | 935.4892 [M+H-H2O-Ara]+, 905.4713 [M+H-H2O-Glc]+, 773.4279 [M+H-H2O-Glc-Ara]+, 743.4184 [M+H-H2O-2Glc]+, 725.4059 [M+H-2Glc-2H2O]+, 611.3740 [M+H-H2O-2Glc-Ara]+, 593.3687 [M+H-2Glc-Ara-2H2O]+, 449.3214 [M+H-H2O-3Glc-Ara]+, 431.3148 [M+H-3Glc-Ara-2H2O]+, 413.3029 [M+H-3Glc-Ara-3H2O]+, 395.2903 [M+H-3Glc-Ara-4H2O]+, 287.1994 [M+H-3Glc-Ara-2H2O-C8H16O2]+, 269.1873 [M+H-3Glc-Ara-3H2O-C8H16O2]+, 251.1770 [M+H-3Glc-Ara-4H2O-C8H16O2]+ | + | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avula, B.; Bae, J.-Y.; Ahn, J.; Katragunta, K.; Wang, Y.-H.; Wang, M.; Kwon, Y.; Khan, I.A.; Chittiboyina, A.G. 6-Oxofurostane and (iso)Spirostane Types of Saponins in Smilax sieboldii: UHPLC-QToF-MS/MS and GNPS-Molecular Networking Approach for the Rapid Dereplication and Biodistribution of Specialized Metabolites. Int. J. Mol. Sci. 2023, 24, 11487. https://doi.org/10.3390/ijms241411487
Avula B, Bae J-Y, Ahn J, Katragunta K, Wang Y-H, Wang M, Kwon Y, Khan IA, Chittiboyina AG. 6-Oxofurostane and (iso)Spirostane Types of Saponins in Smilax sieboldii: UHPLC-QToF-MS/MS and GNPS-Molecular Networking Approach for the Rapid Dereplication and Biodistribution of Specialized Metabolites. International Journal of Molecular Sciences. 2023; 24(14):11487. https://doi.org/10.3390/ijms241411487
Chicago/Turabian StyleAvula, Bharathi, Ji-Yeong Bae, Jongmin Ahn, Kumar Katragunta, Yan-Hong Wang, Mei Wang, Yongsoo Kwon, Ikhlas A. Khan, and Amar G. Chittiboyina. 2023. "6-Oxofurostane and (iso)Spirostane Types of Saponins in Smilax sieboldii: UHPLC-QToF-MS/MS and GNPS-Molecular Networking Approach for the Rapid Dereplication and Biodistribution of Specialized Metabolites" International Journal of Molecular Sciences 24, no. 14: 11487. https://doi.org/10.3390/ijms241411487
APA StyleAvula, B., Bae, J.-Y., Ahn, J., Katragunta, K., Wang, Y.-H., Wang, M., Kwon, Y., Khan, I. A., & Chittiboyina, A. G. (2023). 6-Oxofurostane and (iso)Spirostane Types of Saponins in Smilax sieboldii: UHPLC-QToF-MS/MS and GNPS-Molecular Networking Approach for the Rapid Dereplication and Biodistribution of Specialized Metabolites. International Journal of Molecular Sciences, 24(14), 11487. https://doi.org/10.3390/ijms241411487








