Assessing the Impact of Polyethylene Nano/Microplastic Exposure on Human Vaginal Keratinocytes
Abstract
1. Introduction
2. Results
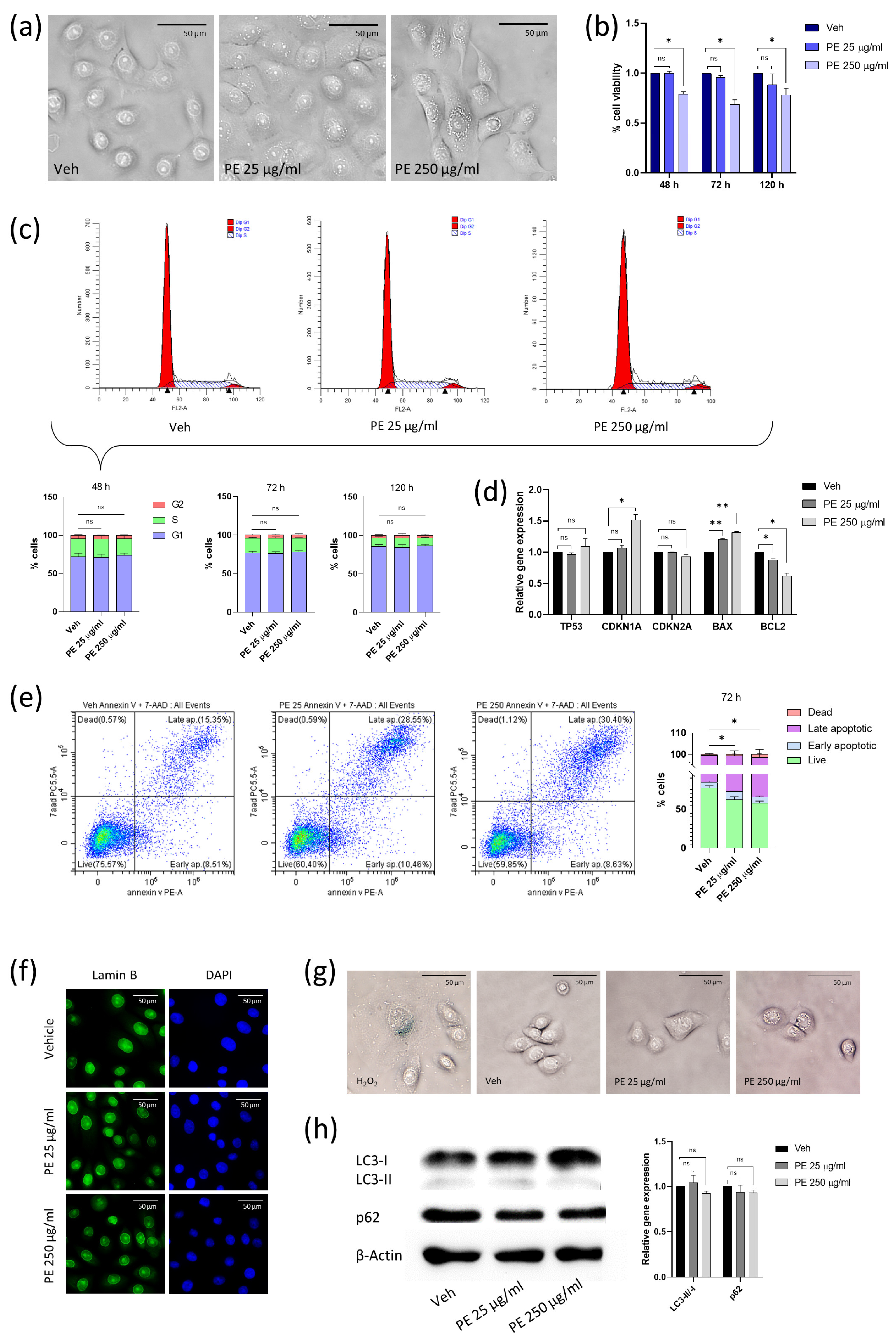
2.1. Acute Exposure to PE N/MPLs Affects Vaginal Keratinocyte Morphology and Vitality
2.2. Cellular Uptake of N/MPLs during Acute Exposure to PE Particles Is Directly Proportional to PE N/MPL Concentration and Protein Corona Formation
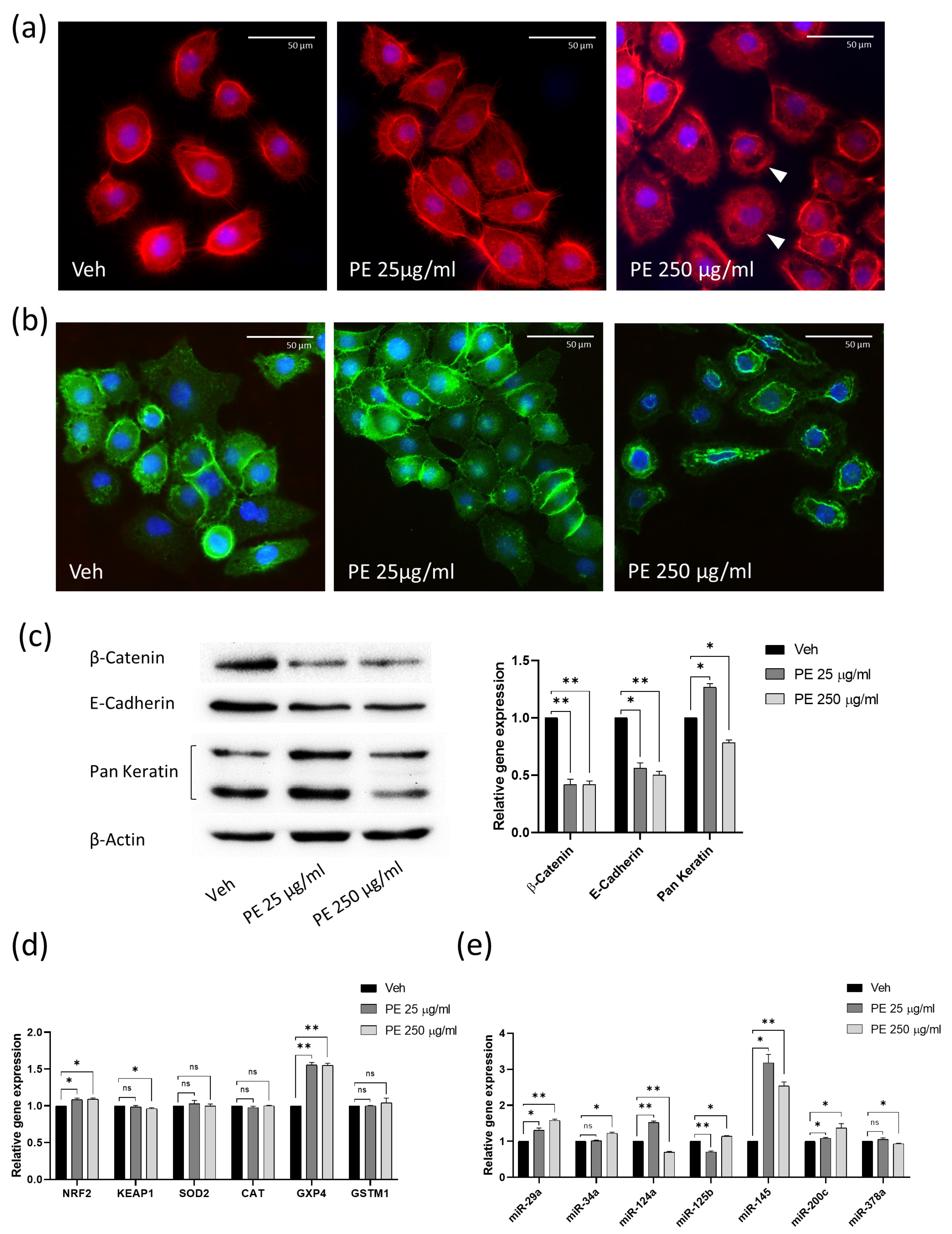
2.3. PE N/MPL Acute Exposure Impacts on Vaginal Keratinocyte Cytoskeleton Structure, Redox Homeostasis, and Cell Stress
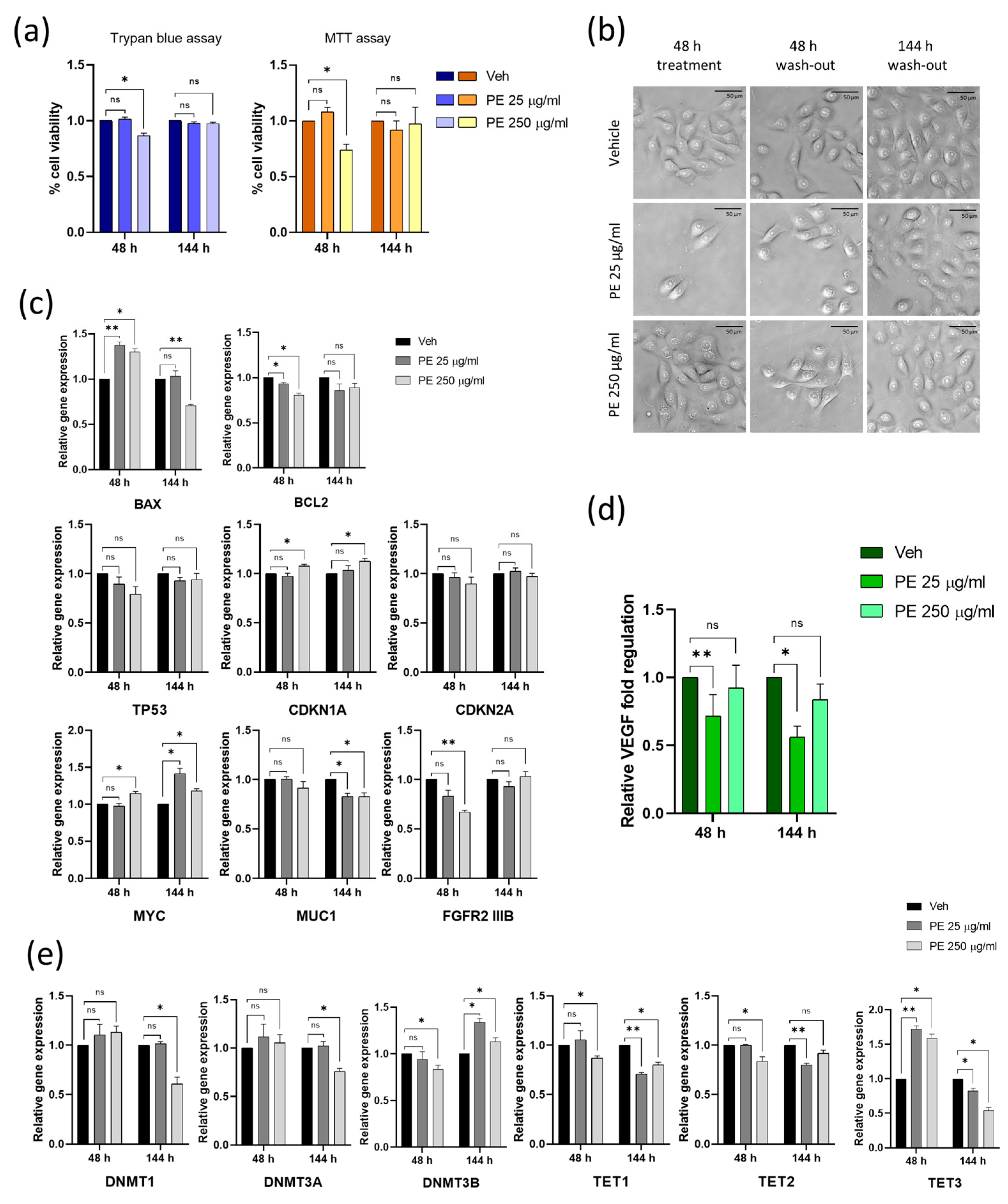
2.4. Vaginal Keratinocytes Could Recover from the Effects of Discontinuous PE N/MPL Exposure, Which Leaves a Mark on Epigenetic Enzymes Expression Profile
2.5. Chronic Exposure to PE N/MPLs Allows Keratinocytes to Adapt to PE Particle Toxicity Overtime
3. Discussion
4. Materials and Methods
4.1. Nano/Microplastics and Reagents
4.2. Cell Cultures and Treatments
4.3. Cell Viability Assays
4.4. Cell Cycle Analysis
4.5. Apoptosis Analysis
4.6. Quantitative Real-Time PCR (RT-qPCR)
4.7. Senescence Analysis by β-Galactosidase Staining
4.8. Immunofluorescence
4.9. Western Blot Analysis
4.10. Electron Microscopy
4.11. Multiplex Cytokine Analyses of Cell Culture Supernatants
4.12. Colony Formation Assay
4.13. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Llorca, M.; Farré, M. Current Insights into Potential Effects of Micro-Nanoplastics on Human Health by in-vitro Tests. Front. Toxicol. 2021, 3, 752140. [Google Scholar] [CrossRef]
- Andrady, A.L.; Neal, M.A. Applications and societal benefits of plastics. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1977–1984. [Google Scholar] [CrossRef]
- Hartmann, N.B.; Hüffer, T.; Thompson, R.C.; Hassellöv, M.; Verschoor, A.; Daugaard, A.E.; Rist, S.; Karlsson, T.; Brennholt, N.; Cole, M.; et al. Are We Speaking the Same Language? Recommendations for a Definition and Categorization Framework for Plastic Debris. Environ. Sci. Technol. 2019, 53, 1039–1047. [Google Scholar] [CrossRef]
- Park, H.; Park, B. Review of Microplastic Distribution, Toxicity, Analysis Methods, and Removal Technologies. Water 2021, 13, 2736. [Google Scholar] [CrossRef]
- Carbery, M.; O’Connor, W.; Palanisami, T. Trophic transfer of microplastics and mixed contaminants in the marine food web and implications for human health. Environ. Int. 2018, 115, 400–409. [Google Scholar] [CrossRef]
- Zaki, M.R.M.; Aris, A.Z. An overview of the effects of nanoplastics on marine organisms. Sci. Total Environ. 2022, 831, 154757. [Google Scholar] [CrossRef] [PubMed]
- Halappanavar, S.; Mallach, G. Adverse outcome pathways and in vitro toxicology strategies for microplastics hazard testing. Curr. Opin. Toxicol. 2021, 28, 52–61. [Google Scholar] [CrossRef]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases 2007, 2, MR17–MR71. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, P.M.; Twayana, K.S.; Ravanan, P.; Thomas, J.; Mukherjee, A.; Jenkins, D.F.; Chandrasekaran, N. Prospects on the nano-plastic particles internalization and induction of cellular response in human keratinocytes. Part. Fibre Toxicol. 2021, 18, 35. [Google Scholar] [CrossRef]
- Hirt, N.; Body-Malapel, M. Immunotoxicity and intestinal effects of nano- and microplastics: A review of the literature. Part. Fibre Toxicol. 2020, 17, 57. [Google Scholar] [CrossRef]
- Munoz, L.P.; Baez, A.G.; Purchase, D.; Jones, H.; Garelick, H. Release of microplastic fibres and fragmentation to billions of nanoplastics from period products: Preliminary assessment of potential health implications. Environ. Sci. Nano 2022, 9, 606–620. [Google Scholar] [CrossRef]
- Ramsperger, A.; Bergamaschi, E.; Panizzolo, M.; Fenoglio, I.; Barbero, F.; Peters, R.; Undas, A.; Purker, S.; Giese, B.; Lalyer, C.R.; et al. Nano- and microplastics: A comprehensive review on their exposure routes, translocation, and fate in humans. NanoImpact 2022, 29, 100441. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Ding, N.; Meza-Wilson, E.; Manuradha Devasurendra, A.; Godwin, C.; Kyun Park, S.; Batterman, S. Volatile organic compounds in feminine hygiene products sold in the US market: A survey of products and health risks. Environ. Int. 2020, 144, 105740. [Google Scholar] [CrossRef] [PubMed]
- Leslie, H.A.; van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef]
- Hannas, B.R.; Howdeshell, K.L.; Furr, J.; Gray, L.E. In utero phthalate effects in the female rat: A model for MRKH syndrome. Toxicol. Lett. 2013, 223, 315–321. [Google Scholar] [CrossRef]
- Schmidt, A.; da Silva Brito, W.A.; Singer, D.; Mühl, M.; Berner, J.; Saadati, F.; Wolff, C.; Miebach, L.; Wende, K.; Bekeschus, S. Short- and long-term polystyrene nano- and microplastic exposure promotes oxidative stress and divergently affects skin cell architecture and Wnt/beta-catenin signaling. Part. Fibre Toxicol. 2023, 20, 3. [Google Scholar] [CrossRef]
- Tian, X.; Liu, Z.; Niu, B.; Zhang, J.; Tan, T.K.; Lee, S.R.; Zhao, Y.; Harris, D.C.; Zheng, G. E-cadherin/β-catenin complex and the epithelial barrier. J. Biomed. Biotechnol. 2011, 2011, 567305. [Google Scholar] [CrossRef]
- Ramms, L.; Fabris, G.; Windoffer, R.; Schwarz, N.; Springer, R.; Zhou, C.; Lazar, J.; Stiefel, S.; Hersch, N.; Schnakenberg, U.; et al. Keratins as the main component for the mechanical integrity of keratinocytes. Proc. Natl. Acad. Sci. USA 2013, 110, 18513–18518. [Google Scholar] [CrossRef]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Weaver, K.; Skouta, R.A.-O. The Selenoprotein Glutathione Peroxidase 4: From Molecular Mechanisms to Novel Therapeutic Opportunities. Biomedicines 2022, 10, 891. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.-Y. The Role of MicroRNAs in Epidermal Barrier. Int. J. Mol. Sci. 2020, 21, 5781. [Google Scholar] [CrossRef]
- Harmanci, D.; Erkan, E.P.; Kocak, A.; Akdogan, G.G. Role of the microRNA-29 family in fibrotic skin diseases. Biomed. Rep. 2017, 6, 599–604. [Google Scholar] [CrossRef]
- Zhang, G.; Song, K.; Yan, H. MicroRNA-124 represses wound healing by targeting SERP1 and inhibiting the Wnt/β-catenin pathway. Adv. Clin. Exp. Med. 2019, 28, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.A.-O.; Qiao, M.; Li, R.H.; Zhao, X.T.; Wang, X.Y.; Sun, Q. Downregulation of miR-145-5p contributes to hyperproliferation of keratinocytes and skin inflammation in psoriasis. Br. J. Dermatol. 2019, 180, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Magenta, A.; D’Agostino, M.; Sileno, S.; Di Vito, L.; Uras, C.; Abeni, D.; Martino, F.; Barillà, F.; Madonna, S.; Albanesi, C.; et al. The Oxidative Stress-Induced miR-200c Is Upregulated in Psoriasis and Correlates with Disease Severity and Determinants of Cardiovascular Risk. Oxid. Med. Cell. Longev. 2019, 2019, 8061901. [Google Scholar] [CrossRef]
- Xia, P.; Pasquali, L.; Gao, C.; Srivastava, A.; Khera, N.; Freisenhausen, J.C.; Luo, L.; Rosén, E.; van Lierop, A.; Homey, B.; et al. miR-378a regulates keratinocyte responsiveness to interleukin-17A in psoriasis*. Br. J. Dermatol. 2022, 187, 211–222. [Google Scholar] [CrossRef]
- Watt, F.M.; Frye, M.; Benitah, S.A. MYC in mammalian epidermis: How can an oncogene stimulate differentiation? Nat. Rev. Cancer 2008, 8, 234–242. [Google Scholar] [CrossRef]
- Hjelm, B.E.; Berta, A.N.; Nickerson, C.A.; Arntzen, C.J.; Herbst-Kralovetz, M.M. Development and Characterization of a Three-Dimensional Organotypic Human Vaginal Epithelial Cell Model1. Biol. Reprod. 2010, 82, 617–627. [Google Scholar] [CrossRef]
- Marchese, C.; Sorice, M.; De Stefano, C.; Frati, L.; Torrisi, M.R. Modulation of keratinocyte growth factor receptor expression in human cultured keratinocytes. Cell Growth Differ. 1997, 8, 989–997. [Google Scholar]
- Nagy, N.; Bata-Csörgő, Z.; Kopasz, N.; Szeg, C.; Pivarcsi, A.; Koreck, A.; Dobozy, A.; Kemény, L.; Széll, M. The expression of keratinocyte growth factor receptor (FGFR2-IIIb) correlates with the high proliferative rate of HaCaT keratinocytes. Exp. Dermatol. 2006, 15, 596–605. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Wang, Y.H.; Duramad, O.; Hori, T.; Delespesse, G.J.; Watanabe, N.; Qin, F.X.F.; Yao, Z.; Cao, W.; Liu, Y.J. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J. Exp. Med. 2005, 202, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, M.; Ziegler, S.F. Cutting edge: Identification of the thymic stromal lymphopoietin-responsive dendritic cell subset critical for initiation of type 2 contact hypersensitivity. J. Immunol. 2013, 191, 4903–4907. [Google Scholar] [CrossRef] [PubMed]
- Savinko, T.; Matikainen, S.; Saarialho-Kere, U.; Lehto, M.; Wang, G.; Lehtimäki, S.; Karisola, P.; Reunala, T.; Wolff, H.; Lauerma, A.; et al. IL-33 and ST2 in atopic dermatitis: Expression profiles and modulation by triggering factors. J. Investig. Dermatol. 2012, 132, 1392–1400. [Google Scholar] [CrossRef]
- Dienz, O.; Rincon, M. The effects of IL-6 on CD4 T cell responses. Clin. Immunol. 2009, 130, 27–33. [Google Scholar] [CrossRef]
- Kennedy-Crispin, M.; Billick, E.; Mitsui, H.; Gulati, N.; Fujita, H.; Gilleaudeau, P.; Sullivan-Whalen, M.; Johnson-Huang, L.M.; Suárez-Fariñas, M.; Krueger, J.G. Human keratinocytes’ response to injury upregulates CCL20 and other genes linking innate and adaptive immunity. J. Investig. Dermatol. 2012, 132, 105–113. [Google Scholar] [CrossRef]
- Homey, B.; Alenius, H.; Müller, A.; Soto, H.; Bowman, E.P.; Yuan, W.; McEvoy, L.; Lauerma, A.I.; Assmann, T.; Bünemann, E.; et al. CCL27–CCR10 interactions regulate T cell–mediated skin inflammation. Nat. Med. 2002, 8, 157–165. [Google Scholar] [CrossRef]
- Detmar, M. Molecular regulation of angiogenesis in the skin. J. Investig. Dermatol. 1996, 106, 207–208. [Google Scholar] [CrossRef]
- Detmar, M. The role of VEGF and thrombospondins in skin angiogenesis. J. Dermatol. Sci. 2000, 1, S78–S84. [Google Scholar] [CrossRef]
- Lyko, F. The DNA methyltransferase family: A versatile toolkit for epigenetic regulation. Nat. Rev. Genet. 2018, 19, 81–92. [Google Scholar] [CrossRef]
- Rasmussen, K.D.; Helin, K.A.-O. Role of TET enzymes in DNA methylation, development, and cancer. Genes Dev. 2016, 30, 733–750. [Google Scholar] [CrossRef]
- Jin, B.; Robertson, K.D. DNA methyltransferases, DNA damage repair, and cancer. Adv. Exp. Med. Biol. 2013, 754, 3–29. [Google Scholar] [PubMed]
- Yee, M.S.-L.; Hii, L.-W.; Looi, C.K.; Lim, W.-M.; Wong, S.-F.; Kok, Y.-Y.; Tan, B.-K.; Wong, C.-Y.; Leong, C.-O. Impact of Microplastics and Nanoplastics on Human Health. Nanomaterials 2021, 11, 496. [Google Scholar] [CrossRef]
- Anderson, D.J.; Marathe, J.; Pudney, J. The structure of the human vaginal stratum corneum and its role in immune defense. Am. J. Reprod. Immunol. 2014, 71, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Heddagaard, F.E.; Møller, P. Hazard assessment of small-size plastic particles: Is the conceptual framework of particle toxicology useful? Food Chem. Toxicol. 2020, 136, 111106. [Google Scholar] [CrossRef]
- Hu, M.; Palić, D. Micro- and nano-plastics activation of oxidative and inflammatory adverse outcome pathways. Redox Biol. 2020, 37, 101620. [Google Scholar] [CrossRef]
- Walczak, A.P.; Kramer, E.; Hendriksen, P.J.; Tromp, P.; Helsper, J.P.; van der Zande, M.; Rietjens, I.M.; Bouwmeester, H. Translocation of differently sized and charged polystyrene nanoparticles in in vitro intestinal cell models of increasing complexity. Nanotoxicology 2015, 9, 453–461. [Google Scholar] [CrossRef]
- Nasser, F.; Lynch, I. Secreted protein eco-corona mediates uptake and impacts of polystyrene nanoparticles on Daphnia magna. J. Proteom. 2016, 137, 45–51. [Google Scholar] [CrossRef]
- Kuhn, D.A.; Vanhecke, D.; Michen, B.; Blank, F.; Gehr, P.; Petri-Fink, A.; Rothen-Rutishauser, B. Different endocytotic uptake mechanisms for nanoparticles in epithelial cells and macrophages. Beilstein J. Nanotechnol. 2014, 5, 1625–1636. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, T.; Varela, J.; Lynch, I.; Salvati, A.; Dawson, K.A. Effects of Transport Inhibitors on the Cellular Uptake of Carboxylated Polystyrene Nanoparticles in Different Cell Lines. PLoS ONE 2011, 6, e24438. [Google Scholar] [CrossRef]
- Xu, D.; Ma, Y.; Han, X.; Chen, Y. Systematic toxicity evaluation of polystyrene nanoplastics on mice and molecular mechanism investigation about their internalization into Caco-2 cells. J. Hazard. Mater. 2021, 417, 126092. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; He, G.; Jiang, H.; Pan, K.; Liu, W. Nanoplastics induces oxidative stress and triggers lysosome-associated immune-defensive cell death in the earthworm Eisenia fetida. Environ. Int. 2023, 174, 107899. [Google Scholar] [CrossRef]
- Rasool, S.; Geethakumari, A.M.; Biswas, K.H. Role of Actin Cytoskeleton in E-cadherin-Based Cell–Cell Adhesion Assembly and Maintenance. J. Indian Inst. Sci. 2021, 101, 51–62. [Google Scholar] [CrossRef]
- Christgen, M.; Bartels, S.; van Luttikhuizen, J.L.; Bublitz, J.; Rieger, L.U.; Christgen, H.; Stark, H.; Sander, B.; Lehmann, U.; Steinemann, D.; et al. E-cadherin to P-cadherin switching in lobular breast cancer with tubular elements. Mod. Pathol. 2020, 33, 2483–2498. [Google Scholar] [CrossRef] [PubMed]
- Fleury, J.-B.; Baulin, V.A. Microplastics destabilize lipid membranes by mechanical stretching. Proc. Natl. Acad. Sci. USA 2021, 118, e2104610118. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Sun, Z.; Wang, X.J.; Jiang, T.; Huang, Z.; Fang, D.; Zhang, D.D. Direct interaction between Nrf2 and p21(Cip1/WAF1) upregulates the Nrf2-mediated antioxidant response. Mol. Cell 2009, 34, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Banikazemi, Z.; Farshadi, M.; Rajabi, A.; Homayoonfal, M.; Sharifi, N.; Sharafati Chaleshtori, R. Nanoplastics: Focus on the role of microRNAs and long non-coding RNAs. Chemosphere 2022, 308, 136299. [Google Scholar] [CrossRef]
- Qu, M.; Luo, L.; Yang, Y.; Kong, Y.; Wang, D. Nanopolystyrene-induced microRNAs response in Caenorhabditis elegans after long-term and lose-dose exposure. Sci. Total Environ. 2019, 697, 134131. [Google Scholar] [CrossRef]
- Barguilla, I.; Domenech, J.; Ballesteros, S.; Rubio, L.; Marcos, R.; Hernández, A. Long-term exposure to nanoplastics alters molecular and functional traits related to the carcinogenic process. J. Hazard. Mater. 2022, 438, 129470. [Google Scholar] [CrossRef]
- Waikel, R.L.; Kawachi, Y.; Waikel, P.A.; Wang, X.J.; Roop, D.R. Deregulated expression of c-Myc depletes epidermal stem cells. Nat. Genet. 2001, 28, 165–168. [Google Scholar] [CrossRef]
- Kufe, D.A.-O. Emergence of MUC1 in Mammals for Adaptation of Barrier Epithelia. Cancers 2022, 14, 4805. [Google Scholar] [CrossRef]
- Rossiter, H.; Barresi, C.; Pammer, J.; Rendl, M.; Haigh, J.; Wagner, E.F.; Tschachler, E. Loss of Vascular Endothelial Growth Factor A Activity in Murine Epidermal Keratinocytes Delays Wound Healing and Inhibits Tumor Formation. Cancer Res. 2004, 64, 3508–3516. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.K.; Capell, B.C. Methyltransferases in the Pathogenesis of Keratinocyte Cancers. Cancers 2021, 13, 3402. [Google Scholar] [CrossRef] [PubMed]
- Moltrasio, C.; Romagnuolo, M.; Marzano, A.V. Epigenetic Mechanisms of Epidermal Differentiation. Int. J. Mol. Sci. 2022, 23, 4874. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.; Liu, N.; Zhu, K.; Zhang, S.; Duan, X.; Huang, Y.; Jin, Z.; Jaypaul, H.; Wu, Y.; et al. TET2 is involved in DNA hydroxymethylation, cell proliferation and inflammatory response in keratinocytes. Mol. Med. Rep. 2020, 21, 1941–1949. [Google Scholar] [CrossRef] [PubMed]
- Ghaffarinia, A.; Ayaydin, F.; Póliska, S.A.-O.X.; Manczinger, M.; Bolla, B.S.; Flink, L.A.-O.; Balogh, F.; Veréb, Z.; Bozó, R.A.-O.; Szabó, K.; et al. Psoriatic Resolved Skin Epidermal Keratinocytes Retain Disease-Residual Transcriptomic and Epigenomic Profiles. Int. J. Mol. Sci. 2023, 24, 4556. [Google Scholar] [CrossRef] [PubMed]
- Domenech, J.; de Britto, M.; Velázquez, A.; Pastor, S.A.-O.; Hernández, A.; Marcos, R.A.-O.X.; Cortés, C. Long-Term Effects of Polystyrene Nanoplastics in Human Intestinal Caco-2 Cells. Biomolecules 2021, 11, 1442. [Google Scholar] [CrossRef] [PubMed]
- Visalli, G.; Facciolà, A.; Pruiti Ciarello, M.; De Marco, G.; Maisano, M.; Di Pietro, A. Acute and Sub-Chronic Effects of Microplastics (3 and 10 µm) on the Human Intestinal Cells HT-29. Int. J. Environ. Res. Public Health 2021, 18, 5833. [Google Scholar] [CrossRef]
- Wang, K.; Liu, H.; Hu, Q.; Wang, L.; Liu, J.; Zheng, Z.; Zhang, W.; Ren, J.; Zhu, F.; Liu, G.-H. Epigenetic regulation of aging: Implications for interventions of aging and diseases. Signal Transduct. Target. Ther. 2022, 7, 374. [Google Scholar] [CrossRef]
- Mahajan, G.; Doherty, E.; To, T.; Sutherland, A.; Grant, J.; Junaid, A.; Gulati, A.; LoGrande, N.; Izadifar, Z.; Timilsina, S.S.; et al. Vaginal microbiome-host interactions modeled in a human vagina-on-a-chip. Microbiome 2022, 10, 201. [Google Scholar] [CrossRef]
- Han, Y.; Liu, Z.; Chen, T. Role of Vaginal Microbiota Dysbiosis in Gynecological Diseases and the Potential Interventions. Front. Microbiol. 2021, 12, 643422. [Google Scholar] [CrossRef]
- van Ruissen, F.; Le, M.; Carroll, J.M.; van der Valk, P.G.M.; Schalkwijk, J. Differential Effects of Detergents on Keratinocyte Gene Expression. J. Investig. Dermatol. 1998, 110, 358–363. [Google Scholar] [CrossRef]
- Anastasiadou, E.; Messina, E.; Sanavia, T.; Labruna, V.; Ceccarelli, S.; Megiorni, F.; Gerini, G.; Pontecorvi, P.; Camero, S.; Perniola, G.; et al. Calcineurin Gamma Catalytic Subunit PPP3CC Inhibition by miR-200c-3p Affects Apoptosis in Epithelial Ovarian Cancer. Genes 2021, 12, 1400. [Google Scholar] [CrossRef]
- D’Amici, S.; Ceccarelli, S.; Vescarelli, E.; Romano, F.; Frati, L.; Marchese, C.; Angeloni, A. TNFα Modulates Fibroblast Growth Factor Receptor 2 Gene Expression through the pRB/E2F1 Pathway: Identification of a Non-Canonical E2F Binding Motif. PLoS ONE 2013, 8, e61491. [Google Scholar] [CrossRef]
- Pontecorvi, P.; Banki, M.A.; Zampieri, C.; Zalfa, C.; Azmoon, P.; Kounnas, M.Z.; Marchese, C.; Gonias, S.L.; Mantuano, E. Fibrinolysis protease receptors promote activation of astrocytes to express pro-inflammatory cytokines. J. Neuroinflamm. 2019, 16, 257. [Google Scholar] [CrossRef] [PubMed]
- Anastasiadou, E.; Ceccarelli, S.; Messina, E.; Gerini, G.; Megiorni, F.; Pontecorvi, P.; Camero, S.; Onesti, M.G.; Trivedi, P.; Faenza, M.; et al. MiR-200c-3p maintains stemness and proliferative potential in adipose-derived stem cells by counteracting senescence mechanisms. PLoS ONE 2021, 16, e0257070. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, S.; Gerini, G.; Megiorni, F.; Pontecorvi, P.; Messina, E.; Camero, S.; Anastasiadou, E.; Romano, E.; Onesti, M.G.; Napoli, C.; et al. Inhibiting DNA methylation as a strategy to enhance adipose-derived stem cells differentiation: Focus on the role of Akt/mTOR and Wnt/β-catenin pathways on adipogenesis. Front. Cell Dev. Biol. 2022, 10, 926180. [Google Scholar] [CrossRef] [PubMed]
- Pontecorvi, P.; Megiorni, F.; Camero, S.; Ceccarelli, S.; Bernardini, L.; Capalbo, A.; Anastasiadou, E.; Gerini, G.; Messina, E.; Perniola, G.; et al. Altered Expression of Candidate Genes in Mayer-Rokitansky-Küster-Hauser Syndrome May Influence Vaginal Keratinocytes Biology: A Focus on Protein Kinase X. Biology 2021, 10, 450. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Megiorni, F.; Camero, S.; Pontecorvi, P.; Camicia, L.; Marampon, F.; Ceccarelli, S.; Anastasiadou, E.; Bernabò, N.; Perniola, G.; Pizzuti, A.; et al. OTX015 Epi-Drug Exerts Antitumor Effects in Ovarian Cancer Cells by Blocking GNL3-Mediated Radioresistance Mechanisms: Cellular, Molecular and Computational Evidence. Cancers 2021, 13, 1519. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pontecorvi, P.; Ceccarelli, S.; Cece, F.; Camero, S.; Lotti, L.V.; Niccolai, E.; Nannini, G.; Gerini, G.; Anastasiadou, E.; Scialis, E.S.; et al. Assessing the Impact of Polyethylene Nano/Microplastic Exposure on Human Vaginal Keratinocytes. Int. J. Mol. Sci. 2023, 24, 11379. https://doi.org/10.3390/ijms241411379
Pontecorvi P, Ceccarelli S, Cece F, Camero S, Lotti LV, Niccolai E, Nannini G, Gerini G, Anastasiadou E, Scialis ES, et al. Assessing the Impact of Polyethylene Nano/Microplastic Exposure on Human Vaginal Keratinocytes. International Journal of Molecular Sciences. 2023; 24(14):11379. https://doi.org/10.3390/ijms241411379
Chicago/Turabian StylePontecorvi, Paola, Simona Ceccarelli, Fabrizio Cece, Simona Camero, Lavinia Vittoria Lotti, Elena Niccolai, Giulia Nannini, Giulia Gerini, Eleni Anastasiadou, Elena Sofia Scialis, and et al. 2023. "Assessing the Impact of Polyethylene Nano/Microplastic Exposure on Human Vaginal Keratinocytes" International Journal of Molecular Sciences 24, no. 14: 11379. https://doi.org/10.3390/ijms241411379
APA StylePontecorvi, P., Ceccarelli, S., Cece, F., Camero, S., Lotti, L. V., Niccolai, E., Nannini, G., Gerini, G., Anastasiadou, E., Scialis, E. S., Romano, E., Venneri, M. A., Amedei, A., Angeloni, A., Megiorni, F., & Marchese, C. (2023). Assessing the Impact of Polyethylene Nano/Microplastic Exposure on Human Vaginal Keratinocytes. International Journal of Molecular Sciences, 24(14), 11379. https://doi.org/10.3390/ijms241411379











