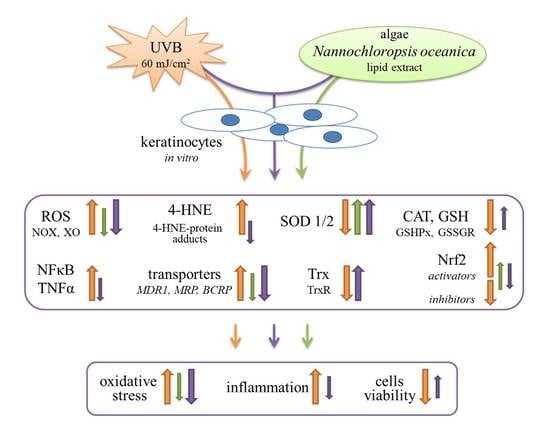
Prevention of UVB Induced Metabolic Changes in Epidermal Cells by Lipid Extract from Microalgae Nannochloropsis oceanica
Abstract
1. Introduction
2. Results
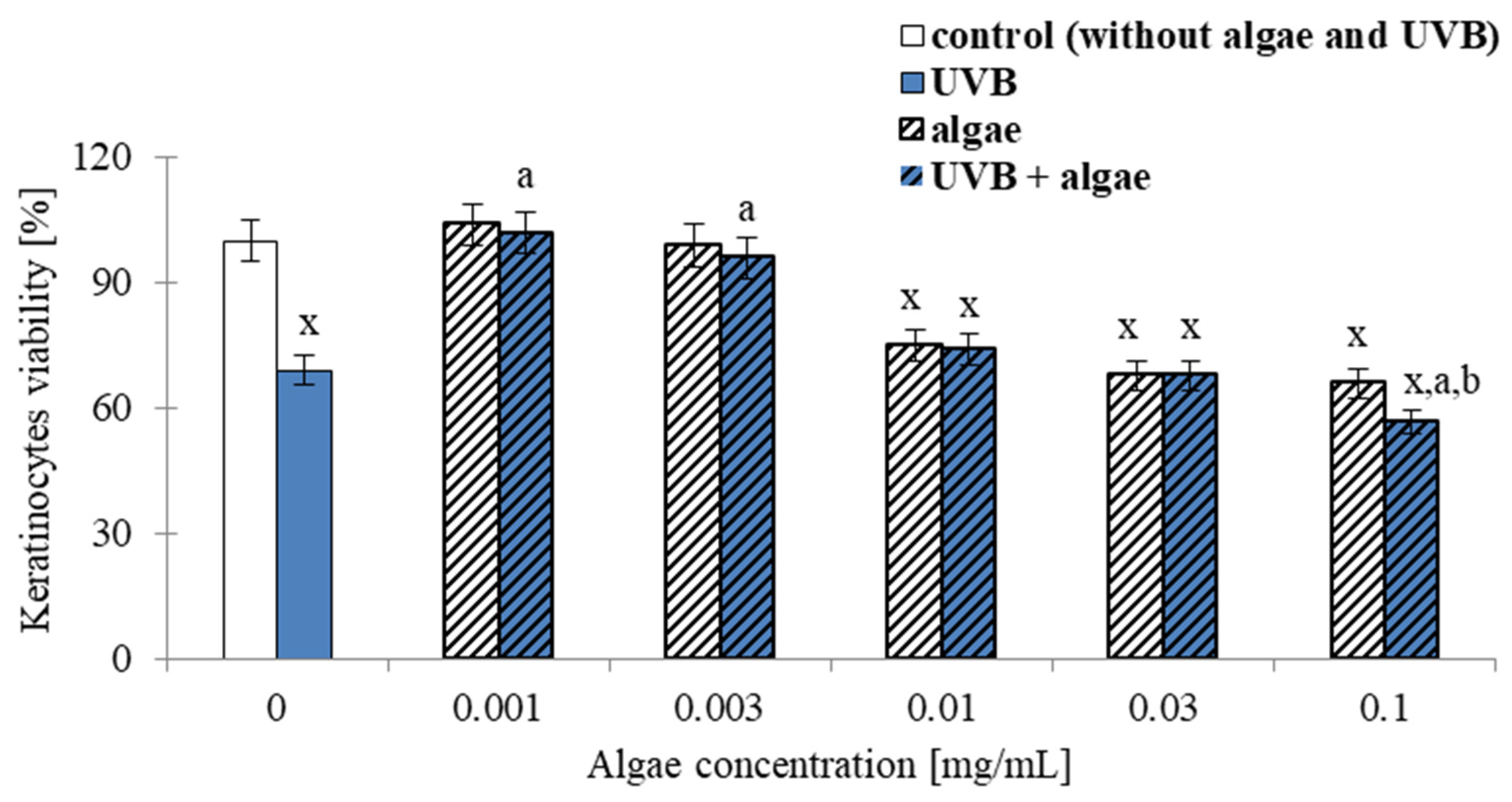
2.1. Viability of Keratinocytes
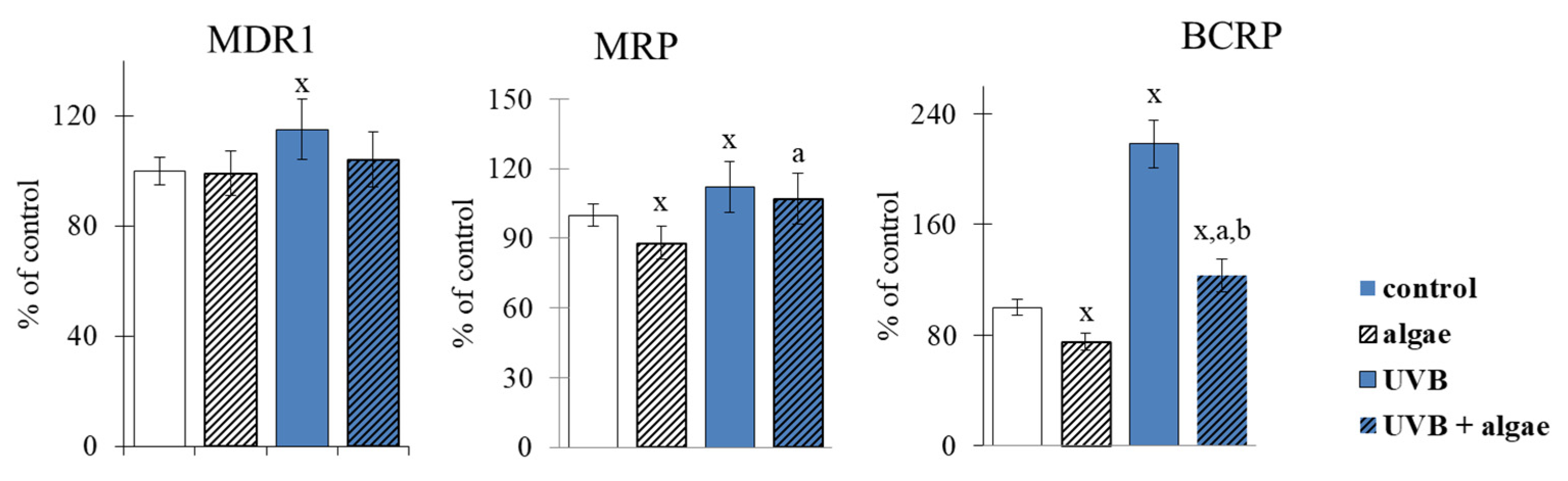
2.2. Keratinocyte Membrane Transporters
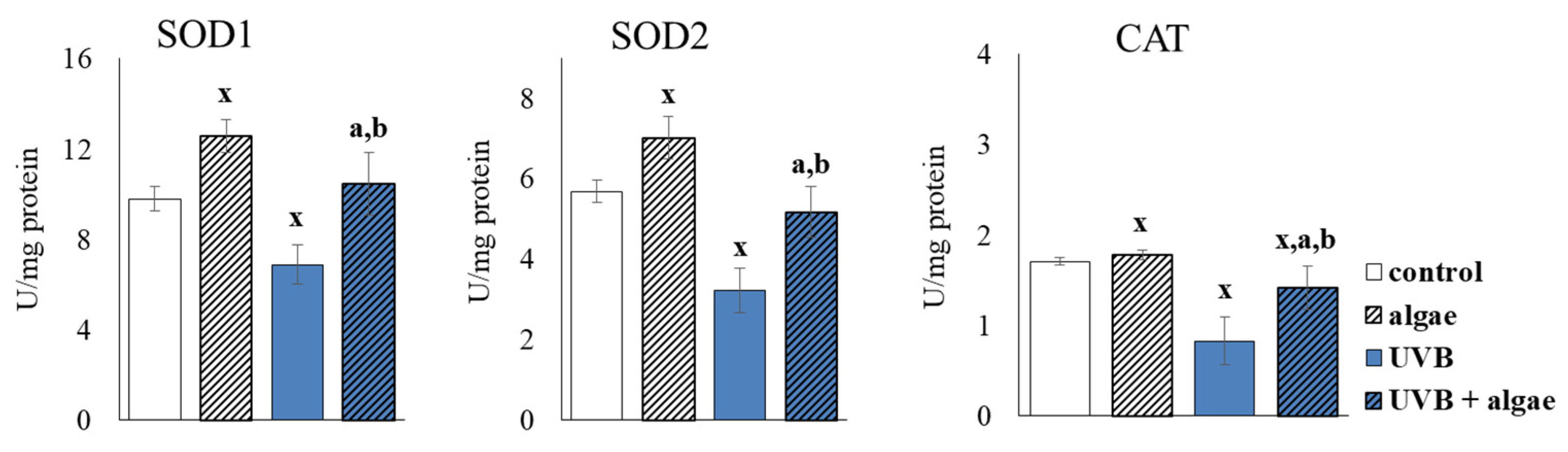
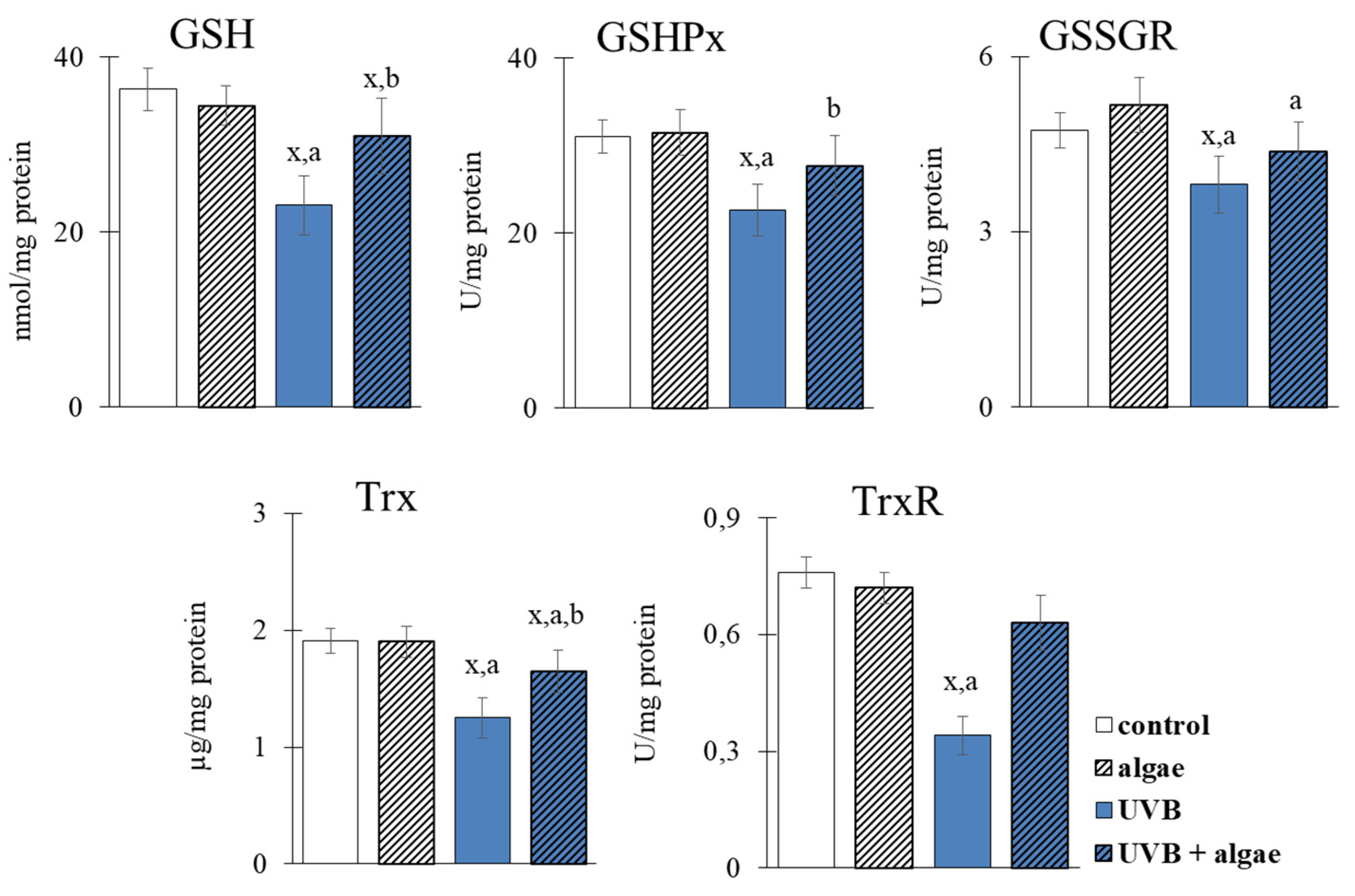
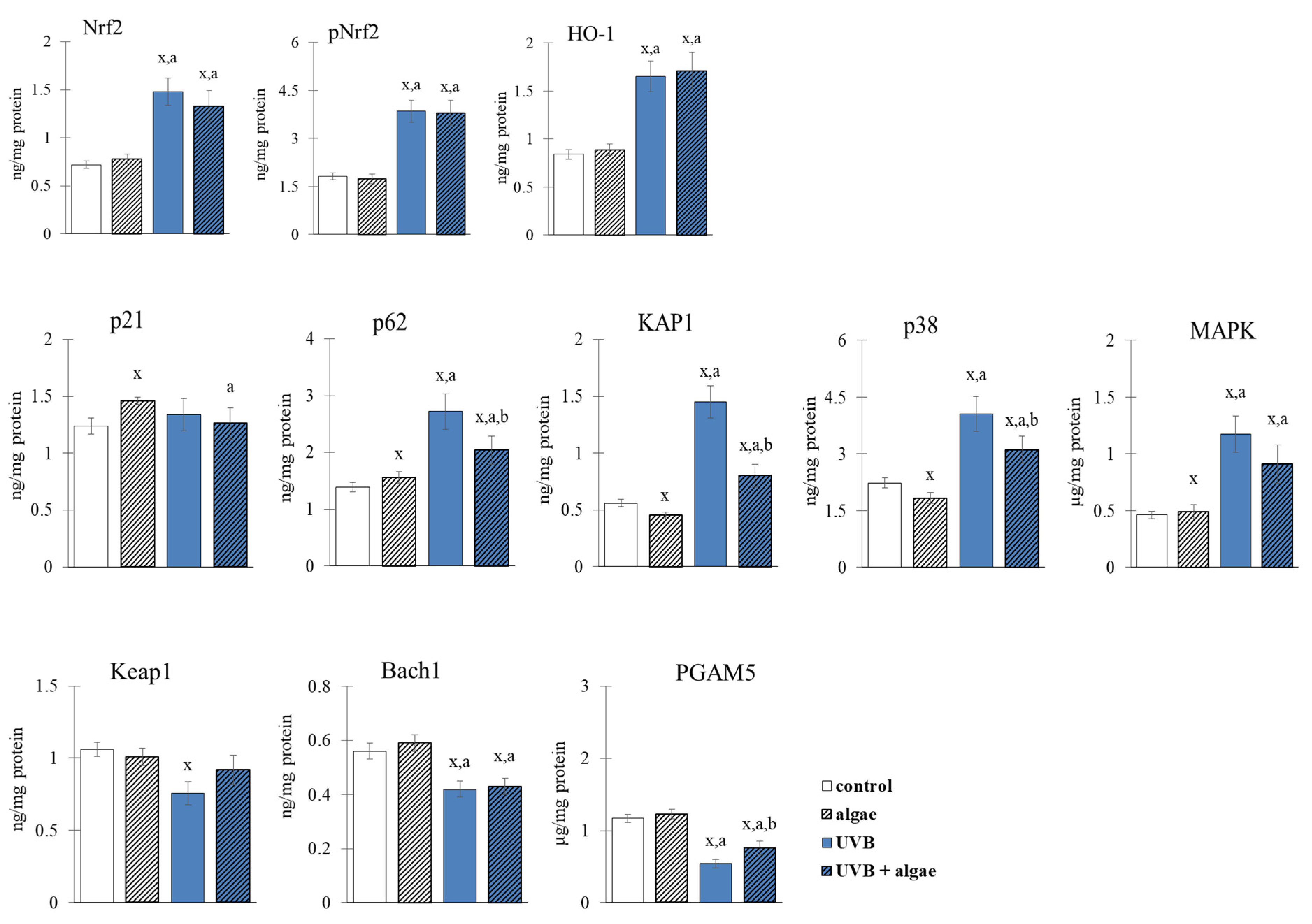
2.3. Redox Balance of Keratinocytes
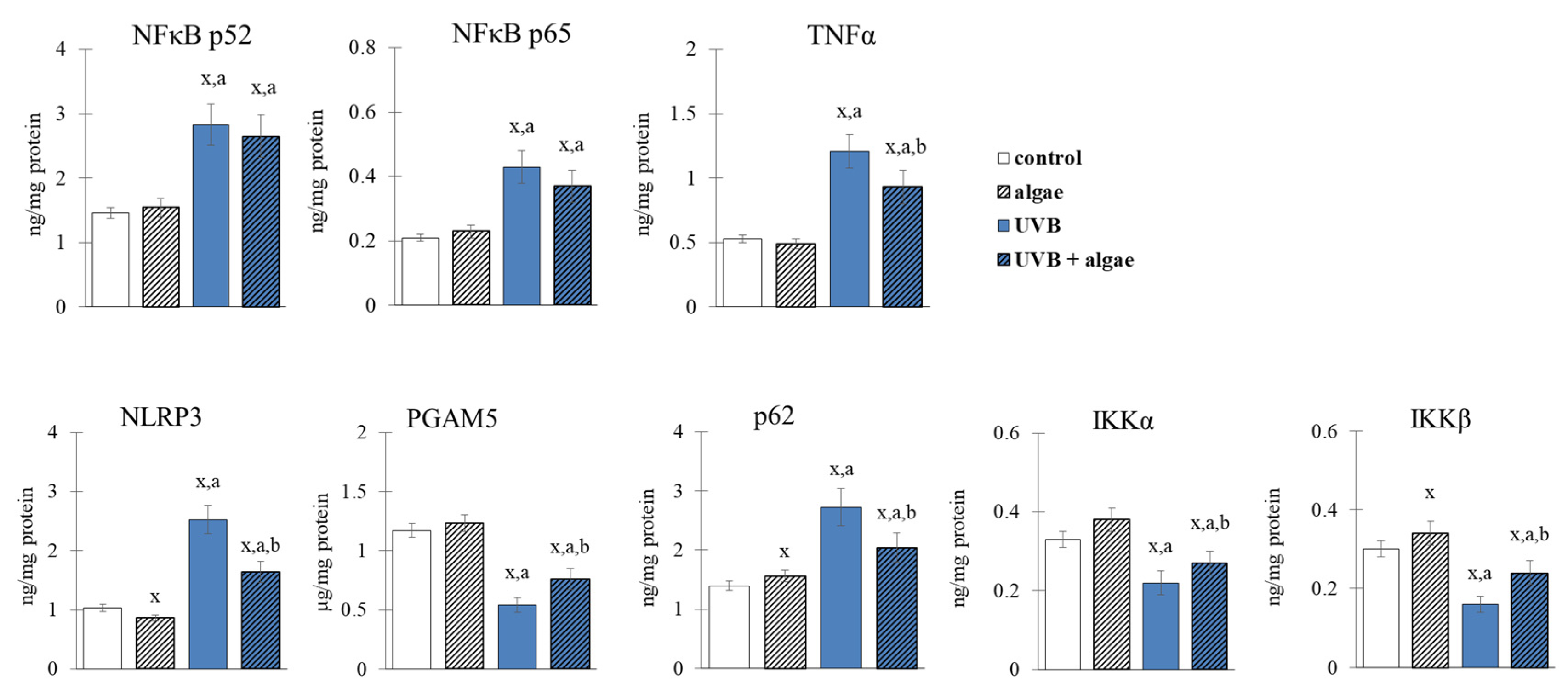
2.4. Anti-Inflammatory Effect of Lipid the Extract
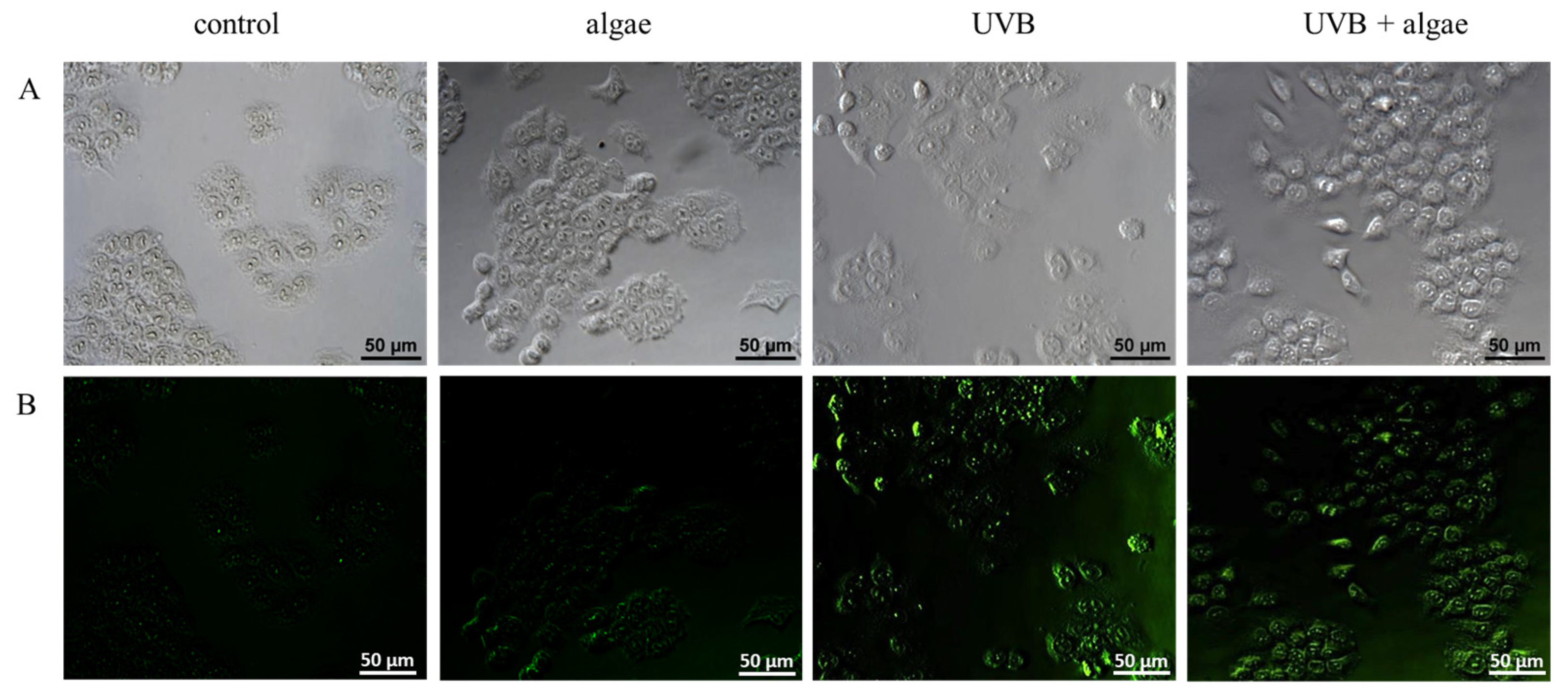
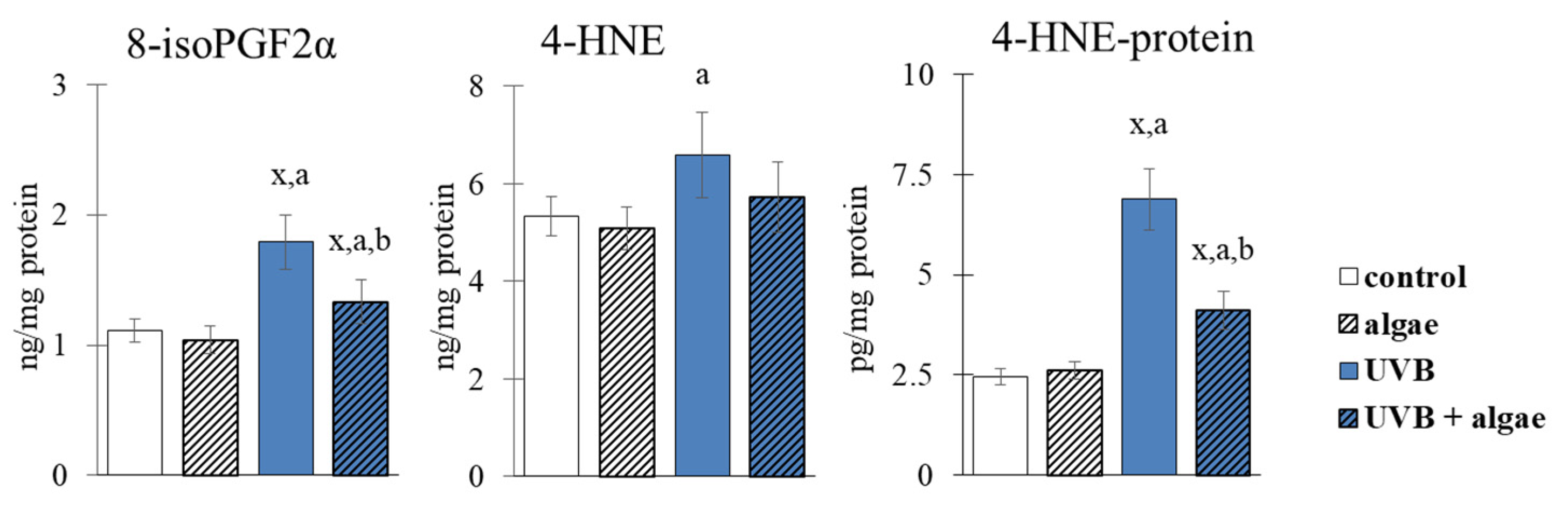
2.5. Lipid Peroxidation in Keratinocytes
3. Discussion
Future Perspectives and Limitations
4. Materials and Methods
4.1. Microalgal Material
4.2. Microalgae Lipid Extracts
4.3. Cell Culture
4.4. Transmembrane Transporters Activities
4.5. ROS Generation
4.6. Pro-Oxidant Enzymes Activities
4.7. Antioxidant Enzymes Activity and Low-Molecular Antioxidants Levels
4.8. Proteins Levels
4.9. Protein Localization
4.10. Quantification of Lipid Peroxidation Products
4.11. Protein-4-HNE Adducts Determination
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dąbrowska, A.K.; Spano, F.; Derler, S.; Adlhart, C.; Spencer, N.D.; Rossi, R.M. The relationship between skin function barrier properties and body-dependent factors. Skin Res. Technol. 2018, 24, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Khan, H.; Bahadar, R.; Riaz, A.; Asad, M.H.H. The impact of airborne pollution and exposure to solar ultraviolet radiation on skin: Mechanistic and physiological insight. Environ. Sci. Pollut. Res. 2020, 27, 28730–28736. [Google Scholar] [CrossRef] [PubMed]
- De Gruijl, F.R. Photocarcinogenesis: UVA vs. UVB. Methods Enzymol. 2000, 319, 359–366. [Google Scholar] [PubMed]
- Gu, Y.; Han, J.; Jiang, C.; Zhang, Y. Biomarkers oxidative stress and autophagy in skin aging. Ageing Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef] [PubMed]
- Ikehata, H.; Yamamoto, M. Roles of the KEAP1-NRF2 system in mammalian skin exposed to UV radiation. Toxicol. Appl. Pharmacol. 2018, 360, 69–77. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Svobodova, A.; Walterova, D.; Vostalova, J. Ultraviolet light induced alteration to the skin. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc. Czech Repub. 2006, 150, 25–38. [Google Scholar] [CrossRef]
- Berman, B.; Cockerell, C.J.; Zografos, P. Pathobiology of actinic keratosis: Ultraviolet-dependent keratinocyte proliferation. J. Am. Acad. Dermatol. 2013, 68, S10–S19. [Google Scholar] [CrossRef]
- Yardman-Frank, J.M.; Fisher, D.E. Skin pigmentation and its control: From ultraviolet radiation to stem cells. Exp. Dermatol. 2021, 30, 560–571. [Google Scholar] [CrossRef]
- Nakai, K.; Tsuruta, D. What are reactive oxygen species free radicals and oxidative stress in skin diseases? Int. J. Mol. Sci. 2021, 22, 10799. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef] [PubMed]
- García-Sánchez, A.; Miranda-Díaz, A.G.; Cardona-Muñoz, E.G. The Role of Oxidative Stress in Physiopathology and Pharmacological Treatment with Pro- And Antioxidant Properties in Chronic Diseases. Oxid. Med. Cell Longev. 2020, 2020, 2082145. [Google Scholar] [CrossRef] [PubMed]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L. Seaweeds as source of bioactive substances and skin care therapy-Cosmeceuticals algotheraphy and thalassotherapy. Cosmetics 2018, 5, 68. [Google Scholar] [CrossRef]
- Berthon, J.Y.; Nachat-Kappes, R.; Bey, M.; Cadoret, J.P.; Renimel, I.; Filaire, E. Marine algae as attractive source to skin care. Free Radic Res. 2017, 51, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Choo, W.T.; Teoh, M.L.; Phang, S.M.; Convey, P.; Yap, W.H.; Goh, B.H.; Beardall, J. Microalgae as Potential Anti-Inflammatory Natural Product Against Human Inflammatory Skin Diseases. Front. Pharmacol. 2020, 11, 1086. [Google Scholar] [CrossRef]
- Wang, H.M.D.; Li, X.C.; Lee, D.J.; Chang, J.S. Potential biomedical applications of marine algae. Bioresour. Technol. 2017, 244, 1407–1415. [Google Scholar] [CrossRef]
- Zhong, D.; Du, Z.; Zhou, M. Algae: A natural active material for biomedical applications. VIEW 2021, 2, 20200189. [Google Scholar] [CrossRef]
- Mourelle, M.; Gómez, C.; Legido, J. The Potential Use of Marine Microalgae and Cyanobacteria in Cosmetics and Thalassotherapy. Cosmetics 2017, 4, 46. [Google Scholar] [CrossRef]
- Adarme-Vega, T.C.; Lim, D.K.Y.; Timmins, M.; Vernen, F.; Li, Y.; Schenk, P.M. Microalgal biofactories: A promising approach towards sustainable omega-3 fatty acid production. Microb. Cell Fact. 2012, 11, 96. [Google Scholar] [CrossRef]
- Russo, B.; Brembilla, N.C.; Chizzolini, C. Interplay between keratinocytes and fibroblasts: A systematic review providing a new angle for understanding skin fibrotic disorders. Front. Immunol. 2020, 11, 648. [Google Scholar] [CrossRef]
- Conde, T.A.; Zabetakis, I.; Tsoupras, A.; Medina, I.; Costa, M.; Silva, J.; Neves, B.; Domingues, P.; Domingues, M.R. Microalgal lipid extracts have potential to modulate the inflammatory response: A critical review. Int. J. Mol. Sci. 2021, 22, 9825. [Google Scholar] [CrossRef] [PubMed]
- Banskota, A.H.; Stefanova, R.; Sperker, S.; Lall, S.P.; Craigie, J.S.; Hafting, J.T.; Critchley, A.T. Polar lipids from the marine macroalga Palmaria palmata inhibit lipopolysaccharide-induced nitric oxide production in RAW264,7 macrophage cells. Phytochemistry 2014, 101, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Robertson, R.C.; Guihéneuf, F.; Bahar, B.; Schmid, M.; Stengel, D.B.; Fitzgerald, G.F.; Ross, R.P.; Stanton, C. The anti-inflammatory effect of algae-derived lipid extracts on lipopolysaccharide (LPS)-stimulated human THP-1 macrophages. Mar. Drugs 2015, 13, 5402–5424. [Google Scholar] [CrossRef] [PubMed]
- Suda, S.; Atsumi, M.; Miyashita, H. Taxonomic characterization of a marine Nannochloropsis species, N. oceanica sp. nov. (Eustigmatophyceae). Phycologia 2002, 41, 273–279. [Google Scholar] [CrossRef]
- Rodolfi, L.; Chini Zittelli, G.; Bassi, N.; Padovani, G.; Biondi, N.; Bonini, G.; Tredici, M.R. Microalgae for oil: Strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol. Bioeng. 2009, 102, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Cabrita, A.R.; Guilherme-Fernandes, J.; Valente, I.M.; Almeida, A.; Lima, S.A.; Fonseca, A.J.; Maia, M.R. Nutritional Composition and Untargeted Metabolomics Reveal the Potential of Tetradesmus obliquus, Chlorella vulgaris and Nannochloropsis oceanica as Valuable Nutrient Sources for Dogs. Animals 2022, 12, 2643. [Google Scholar] [CrossRef]
- Ljubic, A.; Jacobsen, C.; Holdt, S.L.; Jakobsen, J. Microalgae Nannochloropsis oceanica as a future new natural source of vitamin D3. Food Chem. 2020, 320, 126627. [Google Scholar] [CrossRef]
- Vítor, A.C.M.; Godinho, M.; Francisco, A.E.; Silva, J.; Almeida, J.; Fialho, L.; Soldado, D.; Jerónimo, E.; Scollan, N.D.; Huws, S.A.; et al. Nannochloropsis oceanica microalga feeding increases long-chain omega-3 polyunsaturated fatty acids in lamb meat. Meat Sci. 2023, 197, 109053. [Google Scholar] [CrossRef]
- Du Preez, R.; Majzoub, M.E.; Thomas, T.; Panchal, S.K.; Brown, L. Nannochloropsis oceanica as a microalgal food intervention in diet-induced metabolic syndrome in rats. Nutrients 2021, 13, 3991. [Google Scholar] [CrossRef]
- Conde, T.; Lopes, D.; Łuczaj, W.; Neves, B.; Pinto, B.; Maurício, T.; Domingues, P.; Skrzydlewska, E.; Domingues, M.R. Algal lipids as modulators of skin disease: A critical review. Metabolites 2022, 12, 96. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Jung, J.H.; Kim, J.Y.; Heo, J.; Cho, D.H.; Kim, H.S.; Sungkwan, A.; In-Sook, A.; Bae, S. The protective effect of violaxanthin from Nannochloropsis oceanica against ultraviolet B-induced damage in normal human dermal fibroblasts. Photochem. Photobiol. 2019, 95, 595–604. [Google Scholar] [CrossRef]
- Martínez-Ruiz, M.; Martínez-González, C.A.; Kim, D.H.; Santiesteban-Romero, B.; Reyes-Pardo, H.; Villaseñor-Zepeda, K.R.; Meléndez-Sánchez, E.R.; Ramírez-Gamboa, D.; Díaz-Zamorano, A.L.; Sosa-Hernández, J.E.; et al. Microalgae Bioactive Compounds to Topical Applications Products—A Review. Molecules 2022, 27, 3512. [Google Scholar] [CrossRef] [PubMed]
- Gegotek, A.; Biernacki, M.; Ambrozewicz, E.; Surazyński, A.; Wroński, A.; Skrzydlewska, E. The cross-talk between electrophiles antioxidant defence and the endocannabinoid system in fibroblasts and keratinocytes after UVA and UVB irradiation. J. Dermatol. Sci. 2016, 81, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Atalay, S.; Gęgotek, A.; Wroński, A.; Domigues, P.; Skrzydlewska, E. Therapeutic application of cannabidiol on UVA and UVB irradiated rat skin. A proteomic study. J. Pharm. Biomed. Anal. 2021, 192, 113656. [Google Scholar] [CrossRef]
- Gęgotek, A.; Ambrożewicz, E.; Jastrząb, A.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Rutin and ascorbic acid cooperation in antioxidant and antiapoptotic effect on human skin keratinocytes and fibroblasts exposed to UVA and UVB radiation. Arch. Dermatol. Res. 2019, 311, 203–219. [Google Scholar] [CrossRef]
- Jastrząb, A.; Gęgotek, A.; Skrzydlewska, E. Cannabidiol Regulates the Expression of Keratinocyte Proteins Involved in the Inflammation Process through Transcriptional Regulation. Cells 2019, 8, 827. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Łuczaj, W.; Gęgotek, A.; Skrzydlewska, E. Antioxidants and HNE in redox homeostasis. Free Radic. Biol. Med. 2017, 111, 87–101. [Google Scholar] [CrossRef]
- Galano, J.M.; Mas, E.; Barden, A.; Mori, T.A.; Signorini, C.; De Felice, C.; Barrett, A.; Opere, C.; Pinot, E.; Schwedhelm, E.; et al. Isoprostanes and neuroprostanes: Total synthesis biological activity and biomarkers of oxidative stress in humans. Prostaglandins Lipid Mediat. 2013, 107, 95–102. [Google Scholar] [CrossRef]
- Gęgotek, A.; Domingues, P.; Skrzydlewska, E. Proteins involved in the antioxidant and inflammatory response in rutin-treated human skin fibroblasts exposed to UVA or UVB irradiation. J. Dermatol. Sci. 2018, 90, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Photoaging: UV radiation-induced inflammation and immunosuppression accelerate the aging process in the skin. Inflamm. Res. 2022, 71, 817–831. [Google Scholar] [CrossRef] [PubMed]
- Couto, D.; Conde, T.; Melo, T.; Neves, B.; Costa, M.; Cunha, P.; Guerra, I.; Correia, N.; Silva, J.T.; Pereira, H.; et al. Effects of outdoor and indoor cultivation on the polar lipid composition and antioxidant activity of Nannochloropsis oceanica and Nannochloropsis limnetica: A lipidomics perspective. Algal Res. 2022, 64, 102718. [Google Scholar] [CrossRef]
- Coderch, L.; López, O.; de la Maza, A.; Parra, J.L. Ceramides and skin function. Am. J. Clin. Dermatol. 2003, 4, 107–129. [Google Scholar] [CrossRef] [PubMed]
- Holleran, W.M.; Takagi, Y.; Uchida, Y. Epidermal sphingolipids: Metabolism, function, and roles in skin disorders. FEBS Lett. 2006, 580, 5456–5466. [Google Scholar] [CrossRef]
- Gęgotek, A.; Skrzydlewska, E. The Role of ABC Transporters in Skin Cells Exposed to UV Radiation. Int. J. Mol. Sci. 2022, 24, 115. [Google Scholar] [CrossRef]
- Atalay, S.; Gęgotek, A.; Skrzydlewska, E. Protective effects of cannabidiol on the membrane proteome of UVB-irradiated keratinocytes. Antioxidants 2021, 10, 402. [Google Scholar] [CrossRef]
- Choi, E.; Yi, Y.S.; Lee, J.; Park, S.H.; Kim, S.; Hossain, M.A.; Jang, S.G.; Choi, Y.I.; Park, K.J.; Kim, D.S.; et al. Anti-Apoptotic and Anti-Inflammatory Activities of Edible Fresh Water Algae Prasiola japonica in UVB-Irradiated Skin Keratinocytes. Am. J. Chin. Med. 2019, 47, 1853–1868. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, E.; Melo, T.; Reis, M.; Domingues, P.; Calado, R.; Abreu, M.H.; Domingues, M.R. Polar lipids composition antioxidant and anti-inflammatory activities of the atlantic red seaweed Grateloupia turuturu. Mar. Drugs 2021, 19, 414. [Google Scholar] [CrossRef]
- Han, E.J.; Kim, H.S.; Jung, K.; Asanka Sanjeewa, K.K.; Iresha Nadeeka Madushani Herath, K.H.; Lee, W.W.; Jee, Y.; Jeon, Y.J.; Lee, J.; Kim, T.; et al. Sargassum horneri ethanol extract ameliorates TNF-α/IFN-γ-induced inflammation in human keratinocytes and TPA-induced ear edema in mice. Food Biosci. 2021, 39, 100831. [Google Scholar] [CrossRef]
- Jayawardena, T.U.; Sanjeewa, K.K.A.; Wang, L.; Kim, W.S.; Lee, T.K.; Kim, Y.T.; Jeon, Y.J. Alginic Acid from Padina boryana Abate Particulate Matter-Induced Inflammatory Responses in Keratinocytes and Dermal Fibroblasts. Molecules 2020, 25, 5746. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Kim, K.; Heo, J.; Cho, D.H.; Kim, H.S.; Han, S.H.; Ahn, K.J.; An, I.S.; An, S.; Bae, S. Protective effect of Arthrospira platensis extracts against ultraviolet B-induced cellular senescence through inhibition of DNA damage and matrix metalloproteinase-1 expression in human dermal fibroblasts. J. Photochem. Photobiol. B Biol. 2017, 173, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Leitner, P.D.; Jakschitz, T.; Gstir, R.; Stuppner, S.; Perkams, S.; Kruus, M.; Trockenbacher, A.; Griesbeck, C.; Bonn, G.K.; Huber, L.A.; et al. Anti-Inflammatory Extract from Soil Algae Chromochloris zofingiensis Targeting TNFR/NF-κB Signaling at Different Levels. Cells 2022, 11, 1407. [Google Scholar] [CrossRef]
- Zheng, J.; Madduma Hewage, S.R.K.; Piao, M.J.; Kang, K.A.; Han, X.; Kang, H.K.; Yoo, E.S.; Koh, Y.S.; Lee, N.H.; Ko, C.S.; et al. Photoprotective effect of carpomitra costata extract against ultraviolet B-induced oxidative damage in human keratinocytes. J. Environ. Pathol. Toxicol. Oncol. 2016, 35, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; An, S.; Kim, K.B.; Heo, J.; Cho, D.H.; Oh, H.M.; Kim, H.S.; Bae, S. Extract of ettlia sp, YC001 exerts photoprotective effects against UVB irradiation in normal human dermal fibroblasts. J. Microbiol. Biotechnol. 2016, 26, 775–783. [Google Scholar] [CrossRef]
- Schlotterbeck, J.; Kolb, A.; Lämmerhofer, M. Free fatty acid profiling in marine algae extract by LC-MS/MS and isolation as well as quantification of the ω-3 fatty acid hexadeca-471013-tetraenoic acid. J. Sep. Sci. 2018, 41, 4286–4295. [Google Scholar] [CrossRef] [PubMed]
- Goiris, K.; Muylaert, K.; Fraeye, I.; Foubert, I.; De Brabanter, J.; De Cooman, L. Antioxidant potential of microalgae in relation to their phenolic and carotenoid content. J. Appl. Phycol. 2012, 24, 1477–1486. [Google Scholar] [CrossRef]
- Conde, T.A.; Couto, D.; Melo, T.; Costa, M.; Silva, J.; Domingues, M.R.; Domingues, P. Polar lipidomic profile shows Chlorococcum amblystomatis as a promising source of value-added lipids. Sci. Rep. 2021, 11, 4355. [Google Scholar] [CrossRef]
- Regueiras, A.; Huguet, Á.; Conde, T.; Couto, D.; Domingues, P.; Domingues, M.R.; Costa, A.M.; Silva, J.L.; da Vasconcelos, V.; Urbatzka, R. Potential Anti-Obesity Anti-Steatosis and Anti-Inflammatory Properties of Extracts from the Microalgae Chlorella vulgaris and Chlorococcum amblystomatis under Different Growth Conditions. Mar. Drugs 2021, 20, 9. [Google Scholar] [CrossRef]
- Bergé, J.P.; Debiton, E.; Dumay, J.; Durand, P.; Barthomeuf, C. In vitro anti-inflammatory and anti-proliferative activity of sulfolipids from the red alga Porphyridium cruentum. J. Agric. Food Chem. 2002, 50, 6227–6232. [Google Scholar] [CrossRef]
- Gao, Q.M.; Yu, K.; Xia, Y.; Shine, M.B.; Wang, C.; Navarre, D.; Kachroo, A.; Kachroo, P. Mono-and digalactosyldiacylglycerol lipids function nonredundantly to regulate systemic acquired resistance in plants. Cell Rep. 2014, 9, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- Landon, R.; Gueguen, V.; Petite, H.; Letourneur, D.; Pavon-Djavid, G.; Anagnostou, F. Impact of Astaxanthin on Diabetes Pathogenesis and Chronic Complications. Mar. Drugs 2020, 18, 357. [Google Scholar] [CrossRef] [PubMed]
- Jastrząb, A.; Skrzydlewska, E. Thioredoxin-dependent system. Application of inhibitors. J. Enzyme Inhib. Med. Chem. 2021, 36, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Łuczaj, W.; Dobrzyńska, I.; Wroński, A.; Domingues, M.R.; Domingues, P.; Skrzydlewska, E. Cannabidiol-Mediated Changes to the Phospholipid Profile of UVB-Irradiated Keratinocytes from Psoriatic Patients. Int. J. Mol. Sci. 2020, 21, 6592. [Google Scholar] [CrossRef] [PubMed]
- Jastrząb, A.; Jarocka-Karpowicz, I.; Markowska, A.; Wroński, A.; Gȩgotek, A.; Skrzydlewska, E. Antioxidant and Anti-inflammatory Effect of Cannabidiol Contributes to the Decreased Lipid Peroxidation of Keratinocytes of Rat Skin Exposed to UV Radiation. Oxid. Med. Cell Longev. 2021, 2021, 6647222. [Google Scholar] [CrossRef]
- Conde, T.A.; Neves, B.F.; Couto, D.; Melo, T.; Neves, B.; Costa, M.; Silva, J.; Domingues, P.; Domingues, M.R. Microalgae as sustainable bio-factories of healthy lipids: Evaluating fatty acid content and antioxidant activity. Mar. Drugs 2021, 19, 357. [Google Scholar] [CrossRef]
- Kim, M.S.; Oh, G.H.; Kim, M.J.; Hwang, J.K. Fucosterol Inhibits Matrix Metalloproteinase Expression and Promotes Type-1 Procollagen Production in UVB-induced HaCaT Cells. Photochem. Photobiol. 2013, 89, 911–918. [Google Scholar] [CrossRef]
- Huang, Y.; Li, W.; Su, Z.; Yuan Kong, A.N.T. The complexity of the Nrf2 pathway: Beyond the antioxidant response. J. Nutr. Biochem. 2015, 26, 1401–1413. [Google Scholar] [CrossRef]
- Atalay Ekiner, S.; Gęgotek, A.; Skrzydlewska, E. The molecular activity of cannabidiol in the regulation of Nrf2 system interacting with NF-κB pathway under oxidative stress. Redox Biol. 2022, 57, 102489. [Google Scholar] [CrossRef]
- Gęgotek, A.; Skrzydlewska, E. The role of transcription factor Nrf2 in skin cells metabolism. Arch. Dermatol. Res. 2015, 307, 385–396. [Google Scholar] [CrossRef]
- Correia-Sá, I.B.; Carvalho, C.M.; Serrão, P.V.; Loureiro, A.I.; Fernandes-Lopes, C.; Marques, M.; Vieira-Coelho, M.A. A new role for anandamide: Defective link between the systemic and skin endocannabinoid systems in hypertrophic human wound healing. Sci. Rep. 2020, 10, 11134. [Google Scholar] [CrossRef] [PubMed]
- Kohandel, Z.; Farkhondeh, T.; Aschner, M.; Samarghandian, S. Nrf2 a molecular therapeutic target for Astaxanthin. Biomed. Pharmacother. 2021, 137, 111374. [Google Scholar] [CrossRef]
- Inoue, Y.; Shimazawa, M.; Nagano, R.; Kuse, Y.; Takahashi, K.; Tsuruma, K.; Hayashi, M.; Ishibashi, T.; Maoka, T.; Hara, H. Astaxanthin analogs adonixanthin and lycopene activate Nrf2 to prevent light-induced photoreceptor degeneration. J. Pharmacol. Sci. 2017, 134, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Martín, M.A.; Ramos, S.; Granado-Serrano, A.B.; Rodríguez-Ramiro, I.; Trujillo, M.; Bravo, L.; Goya, L. Hydroxytyrosol induces antioxidant/detoxificant enzymes and Nrf2 translocation via extracellular regulated kinases and phosphatidylinositol-3-kinase/protein kinase B pathways in HepG2 cells. Mol. Nutr. Food Res. 2010, 54, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhang, X.S.; Wang, H.D.; Zhang, X.; Yu, Q.; Li, W.; Zhou, M.L.; Wang, X.L. Astaxanthin Activates Nuclear Factor Erythroid-Related Factor 2 and the Antioxidant Responsive Element (Nrf2-ARE) Pathway in the Brain after Subarachnoid Hemorrhage in Rats and Attenuates Early Brain Injury. Mar. Drugs 2014, 12, 6125–6141. [Google Scholar] [CrossRef]
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Kostov, R.V.; Canning, P. Keap1 the cysteine-based mammalian intracellular sensor for electrophiles and oxidants. Arch. Biochem. Biophys. 2017, 617, 84–93. [Google Scholar] [CrossRef]
- Stȩpkowski, T.M.; Kruszewski, M.K. Molecular cross-talk between the NRF2/KEAP1 signaling pathway autophagy and apoptosis. Free Radic. Biol. Med. 2011, 50, 1186–1195. [Google Scholar] [CrossRef]
- Abad, M.; Bedoya, L.; Bermejo, P. Natural Marine Anti-inflammatory Products. Mini-Rev. Med. Chem. 2008, 8, 740–754. [Google Scholar] [CrossRef]
- Öllinger, R.; Wang, H.; Yamashita, K.; Wegiel, B.; Thomas, M.; Margreiter, R.; Bach, F.H. Therapeutic applications of bilirubin and biliverdin in transplantation. Antioxid. Redox Signal. 2007, 9, 2175–2185. [Google Scholar] [CrossRef]
- Gao, W.; Guo, L.; Yang, Y.; Wang, Y.; Xia, S.; Gong, H.; Zhang, B.K.; Yan, M. Dissecting the crosstalk between Nrf2 and NF-κB response pathways in drug-induced toxicity. Front. Cell Dev. Biol. 2021, 9, 809952. [Google Scholar] [CrossRef]
- Hasegawa, T.; Nakashima, M.; Suzuki, Y. Nuclear DNA damage-triggered NLRP3 inflammasome activation promotes UVB-induced inflammatory responses in human keratinocytes. Biochem. Biophys. Res. Commun. 2016, 477, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Bashir, M.M.; Sharma, M.R.; Werth, V.P. TNF-α production in the skin. Arch. Dermatol. Res. 2009, 301, 87–91. [Google Scholar] [CrossRef]
- Zarkovic, N.; Biernacki, M.; Wójcik, P.; Jarocka-Karpowicz, I.; Orehovec, B.; Baršić, B.; Tarle, M.; Kmet, M.; Lukšić, I.; Skrzydlewska, E. Alterations of the Antioxidant and Inflammatory Response of the Peripheral Blood Granulocytes to SARS-CoV-2 Infection in the Deceased COVID-19 Patients. Immunol. Microbiol. 2022. [Google Scholar] [CrossRef]
- Velichkova, M.; Hasson, T. Keap1 Regulates the Oxidation-Sensitive Shuttling of Nrf2 into and out of the Nucleus via a Crm1-Dependent Nuclear Export Mechanism. Mol. Cell Biol. 2005, 25, 4501–4513. [Google Scholar] [CrossRef] [PubMed]
- Barros, A.; Pereira, H.; Campos JMarques, A.; Varela, J.; Silva, J. Heterotrophy as a tool to overcome the long and costly autotrophic scale-up process for large scale production of microalgae. Sci. Rep. 2019, 9, 13935. [Google Scholar] [CrossRef]
- Lees, M.; Sloanestanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Fotakis, G.; Timbrell, J.A. In vitro cytotoxicity assays: Comparison of LDH neutral red MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol. Lett. 2006, 160, 171–177. [Google Scholar] [CrossRef]
- Bradford, M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Dikalov, S.; Griendling, K.K.; Harrison, D.G. Measurement of reactive oxygen species in cardiovascular studies. Hypertension 2007, 49, 717–727. [Google Scholar] [CrossRef]
- Griendling, K.K.; Minieri, C.A.; Ollerenshaw, J.D.; Alexander, R.W. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ. Res. 1994, 74, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Prajda, N.; Weber, G. Malignant transformation-linked imbalance: Decreased xanthine oxidase activity in hepatomas. FEBS Lett. 1975, 59, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Sykes, J. A preliminary study of the superoxide dismutase content of some human tumors. Cancer Res. 1978, 38, 2759–2762. [Google Scholar]
- Geller, B.L.; Winge, D.R. A method for distinguishing CuZn- and Mn-containing superoxide dismutases. Anal. Biochem. 1983, 128, 86–92. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in Vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar] [PubMed]
- Mize, C.E.; Langdon, R.G. Hepatic Glutathione Reductase. J. Biol. Chem. 1962, 237, 1589–1595. [Google Scholar] [CrossRef]
- Maeso, N.; García-Martínez, D.; Rupérez, F.J.; Cifuentes, A.; Barbas, C. Capillary electrophoresis of glutathione to monitor oxidative stress and response to antioxidant treatments in an animal model. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2005, 822, 61–69. [Google Scholar] [CrossRef]
- Holmgren, A.; Lu, J. Thioredoxin and thioredoxin reductase: Current research with special reference to human disease. Biochem. Biophys. Res. Commun. 2010, 396, 120–124. [Google Scholar] [CrossRef]
- Butler, J.E. Enzyme-linked immunosorbent assay. J Immunoass. 2000, 21, 165–209. [Google Scholar] [CrossRef]
- Tsikas, D.; Rothmann, S.; Schneider, J.Y.; Gutzki, F.M.; Beckmann, B.; Frölich, J.C. Simultaneous GC-MS/MS measurement of malondialdehyde and 4-hydroxy-2-nonenal in human plasma: Effects of long-term L-arginine administration. Anal. Biochem. 2017, 524, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Coolen, S.A.; van Buuren, B.; Duchateau, G.; Upritchard, J.; Verhagen, H. Kinetics of biomarkers: Biological and technical validity of isoprostanes in plasma. Amino Acids 2005, 29, 429–436. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stasiewicz, A.; Conde, T.; Gęgotek, A.; Domingues, M.R.; Domingues, P.; Skrzydlewska, E. Prevention of UVB Induced Metabolic Changes in Epidermal Cells by Lipid Extract from Microalgae Nannochloropsis oceanica. Int. J. Mol. Sci. 2023, 24, 11302. https://doi.org/10.3390/ijms241411302
Stasiewicz A, Conde T, Gęgotek A, Domingues MR, Domingues P, Skrzydlewska E. Prevention of UVB Induced Metabolic Changes in Epidermal Cells by Lipid Extract from Microalgae Nannochloropsis oceanica. International Journal of Molecular Sciences. 2023; 24(14):11302. https://doi.org/10.3390/ijms241411302
Chicago/Turabian StyleStasiewicz, Anna, Tiago Conde, Agnieszka Gęgotek, Maria Rosário Domingues, Pedro Domingues, and Elżbieta Skrzydlewska. 2023. "Prevention of UVB Induced Metabolic Changes in Epidermal Cells by Lipid Extract from Microalgae Nannochloropsis oceanica" International Journal of Molecular Sciences 24, no. 14: 11302. https://doi.org/10.3390/ijms241411302
APA StyleStasiewicz, A., Conde, T., Gęgotek, A., Domingues, M. R., Domingues, P., & Skrzydlewska, E. (2023). Prevention of UVB Induced Metabolic Changes in Epidermal Cells by Lipid Extract from Microalgae Nannochloropsis oceanica. International Journal of Molecular Sciences, 24(14), 11302. https://doi.org/10.3390/ijms241411302