Targeting Protein Aggregates with Natural Products: An Optional Strategy for Neurodegenerative Diseases
Abstract
1. Introduction
2. Study of Natural Products on Neurodegenerative Diseases
3. Natural Products Reduce Amyloid-β Aggregates and Toxicity
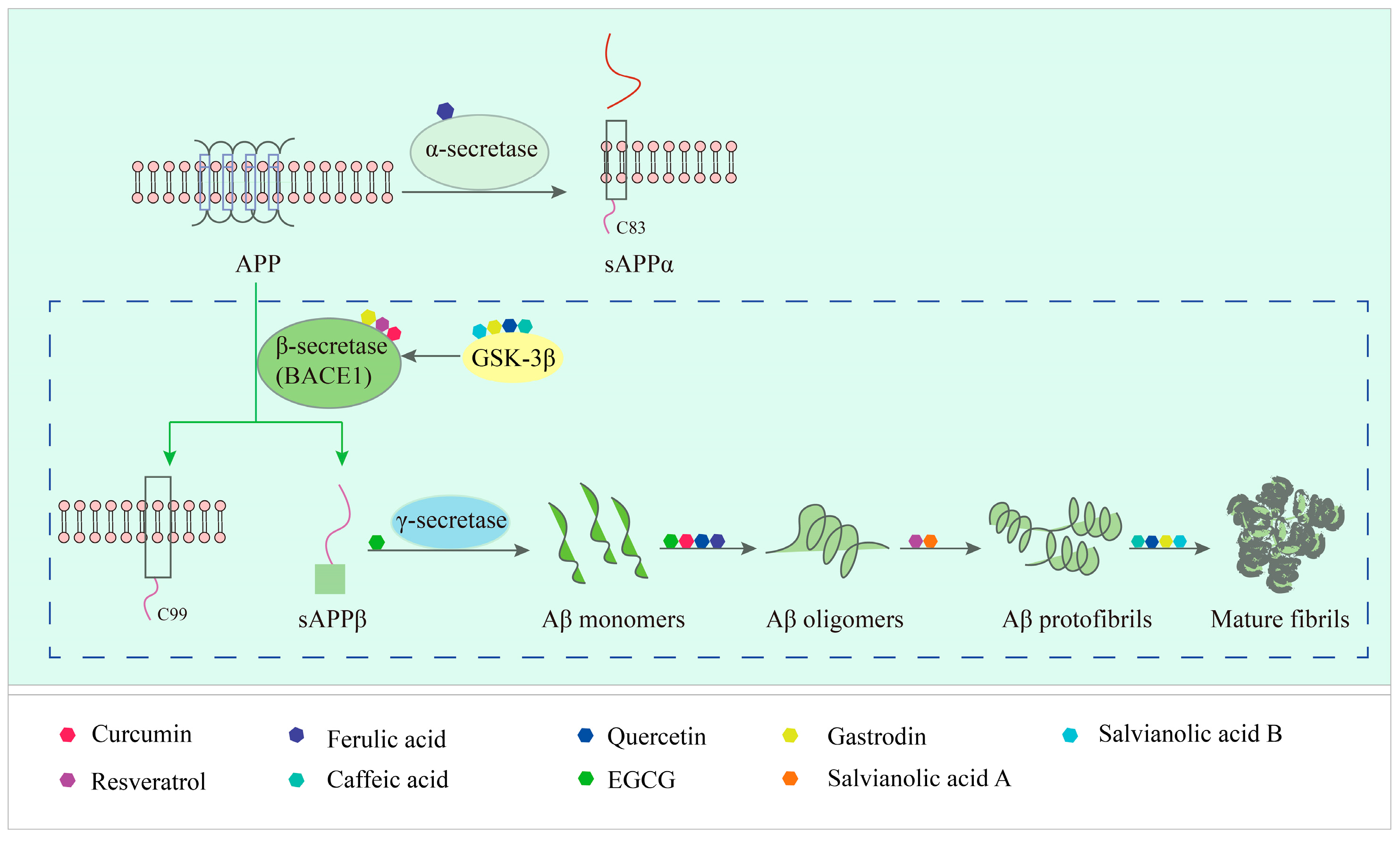
3.1. The Process of Amyloid-β Protein Formation
3.2. Specific Description of Natural Products Targeting Amyloid-β Action
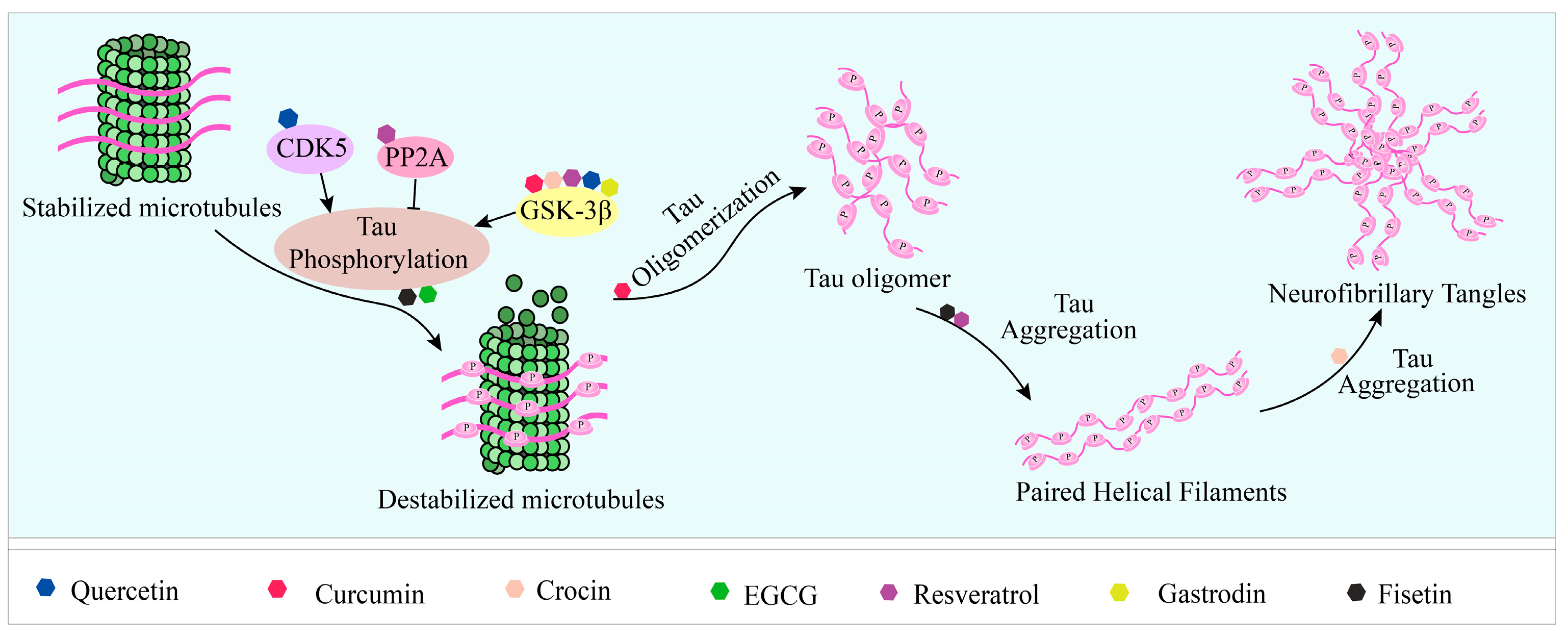
4. Natural Products Reduce Tau Aggregation by Affecting Aggregate Formation, Disaggregation, and Key Enzyme Activity
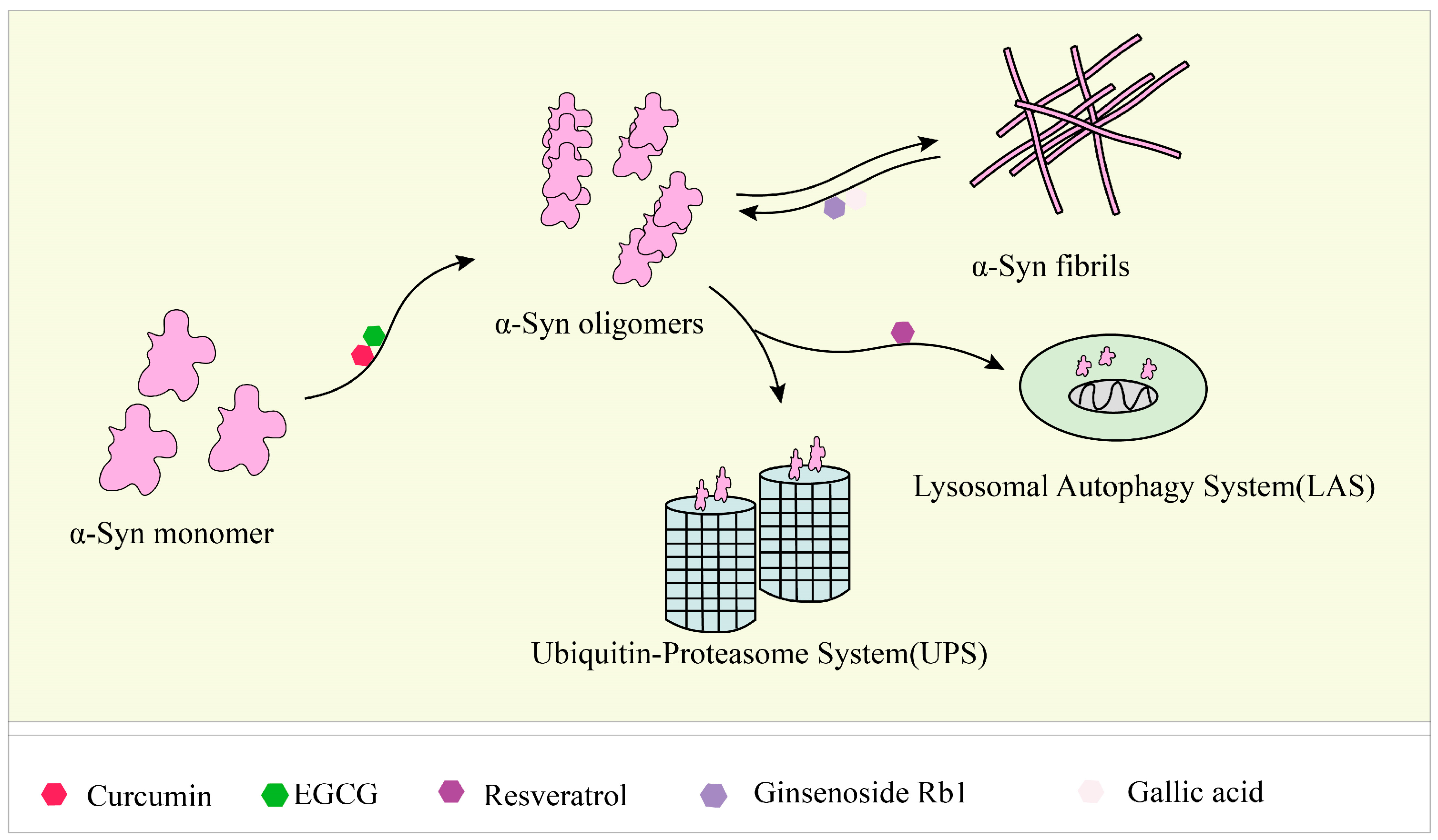
5. Natural Products Inhibit, Degrade, and Remodel α-Syn Fibrils to Reduce Accumulation and Toxicity
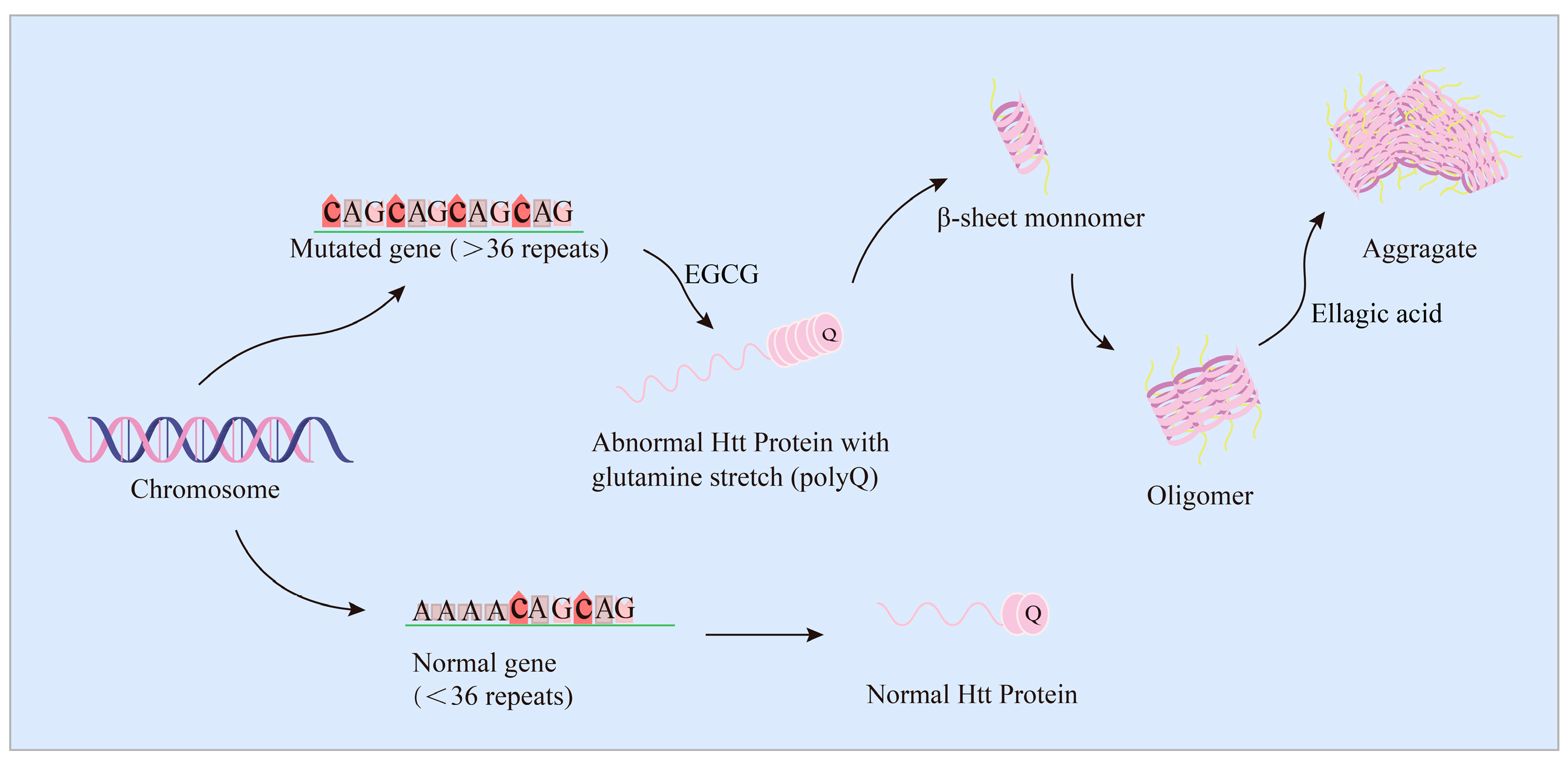
6. EGCG and Ellagic Acid Dose-Dependently Inhibit Htt Protein Aggregates and Increase Cell Viability
7. EGCG Directly Targets the Structural Domain of the FUS Protein to Inhibit Aggregate Formation
8. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, N.; Tan, M.A.; An, S.S.A. Phytosterols: Potential metabolic modulators in neurodegenerative diseases. Int. J. Mol. Sci. 2021, 22, 12255. [Google Scholar] [CrossRef]
- Gauthier, S.; Rosa-Neto, P.; Morais, J.A.; Webster, C. World Alzheimer Report 2021 Journey through the Diagnosis of Dementia; Alzheimer’s Disease International: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Dorsey, E.R.; Sherer, T.; Okun, M.S.; Bloem, B.R. The Emerging Evidence of the Parkinson Pandemic. J. Parkinsons. Dis. 2018, 8, S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Cerri, S.; Mus, L.; Blandini, F. Parkinson’s Disease in Women and Men: What’s the Difference? J. Parkinsons. Dis. 2019, 9, 501–515. [Google Scholar] [CrossRef]
- Pringsheim, T.; Wiltshire, K.; Day, L.; Dykeman, J.; Steeves, T.; Jette, N. The incidence and prevalence of Huntington’s disease: A systematic review and meta-analysis. Mov. Disord. 2012, 27, 1083–1091. [Google Scholar] [CrossRef]
- Caron, N.S.; Dorsey, E.R.; Hayden, M.R. Therapeutic approaches to Huntington disease: From the bench to the clinic. Nat. Rev. Drug Discov. 2018, 17, 729–750. [Google Scholar] [CrossRef] [PubMed]
- Mathis, S.; Goizet, C.; Soulages, A.; Vallat, J.M.; Masson, G.L. Genetics of amyotrophic lateral sclerosis: A review. J. Neurol. Sci. 2019, 399, 217–226. [Google Scholar] [PubMed]
- Hassan, S.S.U.; Samanta, S.; Dash, R.; Karpinski, T.M.; Habibi, E.; Sadiq, A.; Ahmadi, A.; Bunagu, S. The neuroprotective effects of fisetin, a natural flavonoid in neurodegenerative diseases: Focus on the role of oxidative stress. Front. Pharmacol. 2022, 13, 1015835. [Google Scholar]
- Glenner, G.G.; Wong, C.W. Alzheimer’s disease: Initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 1984, 120, 885–890. [Google Scholar] [CrossRef]
- Grundke-Iqbal, I.; Iqbal, K.; Quinlan, M.; Tung, Y.C.; Zaidi, M.S.; Wisniewski, H.M. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J. Biol. Chem. 1986, 261, 6084–6089. [Google Scholar] [CrossRef]
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. Alpha-synuclein in Lewy bodies. Nature 1997, 388, 839–840. [Google Scholar] [CrossRef]
- Polymeropoulos, M.H.; Lavedan, C.; Leroy, E.; Ide, S.E.; Dehejia, A.; Dutra, A.; Pike, B.; Root, H.; Rubenstein, J.; Boyer, R.; et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Sci. N. Y. 1997, 276, 2045–2047. [Google Scholar] [CrossRef]
- Bates, G. Huntingtin aggregation and toxicity in Huntington’s disease. Lancet 2003, 361, 1642–1644. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.P.; Brown, R.H., Jr.; Cleveland, D.W. Decoding ALS: From genes to mechanism. Nature 2016, 539, 197–206. [Google Scholar] [CrossRef]
- Salem, M.A.; Perez de Souza, L.; Serag, A.; Fernie, A.R.; Farag, M.A.; Ezzat, S.M.; Alseekh, S. Metabolomics in the context of plant natural products research: From sample preparation to metabolite analysis. Metabolites 2020, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Mohd Sairazi, N.S.; Sirajudeen, K.N.S. Natural products and their bioactive compounds: Neuroprotective potentials against neurodegenerative diseases. Evid. Based Complement. Alternat. Med. 2020, 2020, 6565396. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Song, J.; Kong, L.; Yuan, T.; Li, W.; Zhang, W.; Hou, B.; Lu, Y.; Du, G. The strategies and techniques of drug discovery from natural products. Pharmacol. Therapeut. 2020, 216, 107686. [Google Scholar] [CrossRef] [PubMed]
- Essa, M.M.; Vijayan, R.K.; Castellano-Gonzalez, G.; Memon, M.A.; Braidy, N.; Guillemin, G.J. Neuroprotective effect of natural products against Alzheimer’s disease. Neurochem. Res. 2012, 37, 1829–1842. [Google Scholar] [CrossRef]
- Chen, X.; Drew, J.; Berney, W.; Lei, W. Neuroprotective natural products for Alzheimer’s disease. Cells 2021, 10, 1309. [Google Scholar] [CrossRef]
- Wang, Z.; He, C.; Shi, J.S. Natural products for the treatment of neurodegenerative diseases. Curr. Med. Chem. 2020, 27, 5790–5828. [Google Scholar] [CrossRef]
- Taylor, E.; Kim, Y.; Zhang, K.; Chau, L.; Nguyen, B.C.; Rayalam, S.; Wang, X. Antiaging Mechanism of natural compounds: Effects on autophagy and oxidative stress. Molecules 2022, 27, 4396. [Google Scholar] [CrossRef]
- Scrivo, A.; Bourdenx, M.; Pampliega, O.; Cuervo, A.M. Selective autophagy as a potential therapeutic target for neurodegenerative disorders. Lancet Neurol. 2018, 17, 802–815. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Lin, Q.; Liang, Y. Plant-derived antioxidants protect the nervous system from aging by inhibiting oxidative stress. Front. Aging Neurosci. 2020, 12, 209. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Ahn, N.H.; Choi, S.B.; Kwon, Y.; Yang, S.H. Natural products targeting amyloid beta in Alzheimer’s disease. Int. J. Mol. Sci. 2021, 22, 2341. [Google Scholar] [CrossRef] [PubMed]
- Witter, S.; Samoson, A.; Vilu, R.; Witter, R. Screening of nutraceuticals and plant extracts for inhibition of amyloid-β fibrillation. J. Alzheimers Dis. 2020, 73, 1003–1012. [Google Scholar] [CrossRef]
- Noori, T.; Dehpour, A.R.; Sureda, A.; Sobarzo-Sanchez, E.; Shirooie, S. Role of natural products for the treatment of Alzheimer’s disease. Eur. J. Pharmacol. 2021, 898, 173974. [Google Scholar] [CrossRef]
- Rocha-Gonzalez, H.I.; Ambriz-Tututi, M.; Granados-Soto, V. Resveratrol: A natural compound with pharmacological potential in neurodegenerative diseases. CNS Neurosci. Ther. 2008, 14, 234–247. [Google Scholar] [CrossRef]
- Rao, T.; Tan, Z.; Peng, J.; Guo, Y.; Chen, Y.; Zhou, H.; Ouyang, D. The pharmacogenetics of natural products: A pharmacokinetic and pharmacodynamic perspective. Pharmacol. Res. 2019, 146, 104283. [Google Scholar] [CrossRef]
- Rodrigues, T.; Reker, D.; Schneider, P.; Schneider, G. Counting on natural products for drug design. Nat. Chem. 2016, 8, 531–541. [Google Scholar] [CrossRef]
- Al-Rahbi, B.; Zakaria, R.; Othman, Z.; Hassan, A.; Ahmad, A.H. Protective effects of tualang honey against oxidative stress and anxiety-like behaviour in stressed ovariectomized rats. Internat. Schol. Res. Not. 2014, 2014, 521065. [Google Scholar] [CrossRef]
- Ryu, S.; Koo, S.; Ha, K.-T.; Kim, S. Neuroprotective effect of Korea red ginseng extract on 1-methyl-4-phenyl-pyridinium-induced apoptosis in PC12 cells. Anim. Cells Syst. 2016, 20, 363–368. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, L. Resveratrol suppresses abeta-induced microglial activation through the TXNIP/TRX/NLRP3 signaling pathway. DNA Cell Biol. 2019, 38, 874–879. [Google Scholar] [CrossRef]
- Yang, X.; Qiang, X.; Li, Y.; Luo, L.; Xu, R.; Zheng, Y.; Cao, Z.; Tan, Z.; Deng, Y. Pyridoxine-resveratrol hybrids Mannich base derivatives as novel dual inhibitors of AChE and MAO-B with antioxidant and metal-chelating properties for the treatment of Alzheimer’s disease. Bioorg. Chem. 2017, 71, 305–314. [Google Scholar] [CrossRef]
- Carrizzo, A.; Forte, M.; Damato, A.; Trimarco, V.; Salzano, F.; Bartolo, M.; Maciag, A.; Puca, A.A.; Vecchione, C. Antioxidant effects of resveratrol in cardiovascular, cerebral and metabolic diseases. Food Chem. Toxicol. 2013, 61, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Wang, X.B.; Kong, L.Y. Design, synthesis and biological evaluation of imine resveratrol derivatives as multi-targeted agents against Alzheimer’s disease. Eur. J. Med. Chem. 2014, 71, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Gomes, B.A.Q.; Silva, J.P.B.; Romeiro, C.F.R.; Dos Santos, S.M.; Rodrigues, C.A.; Goncalves, P.R.; Sakai, J.T.; Mendes, P.F.S.; Varela, E.L.P.; Monteiro, M.C. Neuroprotective mechanisms of resveratrol in Alzheimer’s disease: Role of SIRT1. Oxid. Med. Cell. Longev. 2018, 2018, 8152373. [Google Scholar] [CrossRef] [PubMed]
- Zwolak, I. Epigallocatechin gallate for management of heavy metal-induced oxidative stress: Mechanisms of action, efficacy, and concerns. Int. J. Mol. Sci. 2021, 22, 4027. [Google Scholar] [CrossRef]
- Saeed, A.A.; Genové, G.; Li, T.; Lütjohann, D.; Olin, M.; Mast, N.; Pikuleva, I.A.; Crick, P.; Wang, Y.; Griffiths, W.; et al. Effects of a disrupted blood-brain barrier on cholesterol homeostasis in the brain. J. Biol. Chem. 2014, 289, 23712–23722. [Google Scholar] [CrossRef]
- Andrade, S.; Nunes, D.; Dabur, M.; Ramalho, M.J.; Pereira, M.C.; Loureiro, J.A. Therapeutic potential of natural compounds in neurodegenerative diseases: Insights from clinical trials. Pharmaceutics 2023, 15, 212. [Google Scholar] [CrossRef]
- Turner, R.S.; Thomas, R.G.; Craft, S.; van Dyck, C.H.; Mintzer, J.; Reynolds, B.A.; Brewer, J.B.; Rissman, R.A.; Raman, R.; Aisen, P.S. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology 2015, 85, 1383–1391. [Google Scholar] [CrossRef]
- Zia, A.; Farkhondeh, T.; Pourbagher-Shahri, A.M.; Samarghandian, S. The role of curcumin in aging and senescence: Molecular mechanisms. Biomed. Pharmacother. 2021, 134, 111119. [Google Scholar] [CrossRef]
- Jabczyk, M.; Nowak, J.; Hudzik, B.; Zubelewicz-Szkodzińska, B. Curcumin and its potential impact on microbiota. Nutrients 2021, 13, 2004. [Google Scholar] [CrossRef] [PubMed]
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X. A review of curcumin and its derivatives as anticancer agents. Int. J. Mol. Sci. 2019, 20, 1033. [Google Scholar] [CrossRef]
- Han, Y.; Chen, R.; Lin, Q.; Liu, Y.; Ge, W.; Cao, H.; Li, J. Curcumin improves memory deficits by inhibiting HMGB1-RAGE/TLR4-NF-kappaB signalling pathway in APPswe/PS1dE9 transgenic mice hippocampus. J. Cell. Mol. Med. 2021, 25, 8947–8956. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Zhang, Y.; Yang, J.; Liu, X.; Wang, Y.; Xu, B.; Jia, J. Curcumin alleviates beta amyloid-induced neurotoxicity in HT22 Cells via upregulating SOD2. J. Mol. Neurosci. 2019, 67, 540–549. [Google Scholar] [CrossRef]
- Fu, Z.; Aucoin, D.; Ahmed, M.; Ziliox, M.; Van Nostrand, W.E.; Smith, S.O. Capping of abeta42 oligomers by small molecule inhibitors. Biochemistry. 2014, 53, 7893–7903. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.N.; Chiu, S.W.; Benoit, J.; Chew, L.Y.; Mu, Y. The effect of curcumin on the stability of abeta dimers. J. Phys. Chem. B. 2012, 116, 7428–7435. [Google Scholar] [CrossRef]
- Thapa, A.; Vernon, B.C.; De la Pena, K.; Soliz, G.; Moreno, H.A.; Lopez, G.P.; Chi, E.Y. Membrane-mediated neuroprotection by curcumin from amyloid-beta-peptide-induced toxicity. Langmuir. 2013, 29, 11713–11723. [Google Scholar] [CrossRef]
- Caesar, I.; Jonson, M.; Nilsson, K.P.; Thor, S.; Hammarstrom, P. Curcumin promotes A-beta fibrillation and reduces neurotoxicity in transgenic Drosophila. PLoS ONE 2012, 7, e31424. [Google Scholar] [CrossRef]
- Chainoglou, E.; Hadjipavlou-Litina, D. Curcumin in health and diseases: Alzheimer’s disease and curcumin analogues, derivatives, and hybrids. Int. J. Mol. Sci. 2020, 21, 1975. [Google Scholar] [CrossRef]
- Rane, J.S.; Bhaumik, P.; Panda, D. Curcumin Inhibits Tau Aggregation and Disintegrates Preformed Tau Filaments in vitro. J. Alzheimers. Dis. 2017, 60, 999–1014. [Google Scholar] [CrossRef]
- Ahmad, B.; Lapidus, L.J. Curcumin prevents aggregation in α-synuclein by increasing reconfiguration rate. J. Biol. Chem. 2012, 287, 9193–9199. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yu, Y.; Li, X.; Ross, C.A.; Smith, W.W. Curcumin protects against A53T alpha-synuclein-induced toxicity in a PC12 inducible cell model for Parkinsonism. Pharmacol. Res. 2011, 63, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Melgar, A.; Izquierdo-Ramirez, P.J.; Grinan-Ferre, C.; Pallas, M.; Martin, M.; Albasanz, J.L. Neuroprotective effects of resveratrol by modifying cholesterol metabolism and abeta processing in SAMP8 Mice. Int. J. Mol. Sci. 2022, 23, 7580. [Google Scholar] [CrossRef]
- Rahman, M.H.; Akter, R.; Bhattacharya, T.; Abdel-Daim, M.M.; Alkahtani, S.; Arafah, M.W.; Al-Johani, N.S.; Alhoshani, N.M.; Alkeraishan, N.; Alhenaky, A.; et al. Resveratrol and neuroprotection: Impact and its therapeutic potential in Alzheimer’s disease. Front. Pharmacol. 2020, 11, 619024. [Google Scholar] [CrossRef] [PubMed]
- Al-Edresi, S.; Alsalahat, I.; Freeman, S.; Aojula, H.; Penny, J. Resveratrol-mediated cleavage of amyloid beta(1-42) peptide: Potential relevance to Alzheimer’s disease. Neurobiol. Aging. 2020, 94, 24–33. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, G.W.; Liang, Z.M.; Sheng, S.Y.; Shi, Y.S.; Peng, L.; Wang, Y.P.; Wang, F.; Zhang, X.M. Resveratrol improves cognition and decreases amyloid plaque formation in Tg6799 mice. Mol. Med. Rep. 2019, 19, 3783–3790. [Google Scholar] [CrossRef]
- Sun, X.Y.; Dong, Q.X.; Zhu, J.; Sun, X.; Zhang, L.F.; Qiu, M.; Yu, X.L.; Liu, R.T. Resveratrol rescues tau-induced cognitive deficits and neuropathology in a mouse model of tauopathy. Curr. Alzheimer Res. 2019, 16, 710–722. [Google Scholar] [CrossRef]
- Guo, Y.J.; Dong, S.Y.; Cui, X.X.; Feng, Y.; Liu, T.; Yin, M.; Kuo, S.H.; Tan, E.K.; Zhao, W.J.; Wu, Y.C. Resveratrol alleviates MPTP-induced motor impairments and pathological changes by autophagic degradation of α-synuclein via SIRT1-deacetylated LC3. Mol. Nutr. Food. Res. 2016, 60, 2161–2175. [Google Scholar] [CrossRef]
- Ono, K.; Hirohata, M.; Yamada, M. Ferulic acid destabilizes preformed beta-amyloid fibrils in vitro. Biochem. Biophys. Res. Commun. 2005, 336, 444–449. [Google Scholar] [CrossRef]
- Salamanova, E.; Atanasova, M.; Dimitrov, I.; Doytchinova, I. Effects of curcumin and ferulic acid on the folding of amyloid-beta peptide. Molecules 2021, 26, 2815. [Google Scholar] [CrossRef]
- Arai, T.; Ohno, A.; Mori, K.; Kuwata, H.; Mizuno, M.; Imai, K.; Hara, S.; Shibanuma, M.; Kurihara, M.; Miyata, N.; et al. Inhibition of amyloid fibril formation and cytotoxicity by caffeic acid-conjugated amyloid-beta C-terminal peptides. Bioorg. Med. Chem. Lett. 2016, 26, 5468–5471. [Google Scholar] [CrossRef]
- Alghamdi, A.; Birch, D.J.S.; Vyshemirsky, V.; Rolinski, O.J. Impact of the Flavonoid Quercetin on beta-amyloid aggregation revealed by intrinsic fluorescence. J. Phys. Chem. B 2022, 126, 7229–7237. [Google Scholar] [CrossRef]
- Godoy, J.A.; Lindsay, C.B.; Quintanilla, R.A.; Carvajal, F.J.; Cerpa, W.; Inestrosa, N.C. Quercetin exerts differential neuroprotective effects against H2O2 and abeta aggregates in hippocampal neurons: The role of mitochondria. Mol. Neurobiol. 2017, 54, 7116–7128. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Krishnakumar, V.G.; Morya, V.; Gupta, S.; Datta, B. Nanobiocatalyst facilitated aglycosidic quercetin as a potent inhibitor of tau protein aggregation. Int. J. Biol. Macromol. 2019, 138, 168–180. [Google Scholar] [CrossRef]
- Ehrnhoefer, D.E.; Bieschke, J.; Boeddrich, A.; Herbst, M.; Masino, L.; Lurz, R.; Engemann, S.; Pastore, A.; Wanker, E.E. EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Nat. Struct. Mol. Biol. 2008, 15, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Palhano, F.L.; Lee, J.; Grimster, N.P.; Kelly, J.W. Toward the molecular mechanism(s) by which EGCG treatment remodels mature amyloid fibrils. J. Am. Chem. Soc. 2013, 135, 7503–7510. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; VanSchouwen, B.; Jafari, N.; Ni, X.; Ortega, J.; Melacini, G. Molecular Mechanism for the (-)-Epigallocatechin Gallate-Induced Toxic to Nontoxic Remodeling of Aβ Oligomers. J. Am. Chem. Soc. 2017, 139, 13720–13734. [Google Scholar] [CrossRef]
- Bieschke, J.; Russ, J.; Friedrich, R.P.; Ehrnhoefer, D.E.; Wobst, H.; Neugebauer, K.; Wanker, E.E. EGCG remodels mature alpha-synuclein and amyloid-beta fibrils and reduces cellular toxicity. PNAS. 2010, 107, 7710–7715. [Google Scholar] [CrossRef]
- Teng, Y.; Zhao, J.; Ding, L.; Ding, Y.; Zhou, P. Complex of EGCG with Cu(II) suppresses amyloid aggregation and Cu(II)-Induced cytotoxicity of alpha-synuclein. Molecules 2019, 24, 2940. [Google Scholar] [CrossRef]
- Choi, J.S.; Braymer, J.J.; Nanga, R.P.; Ramamoorthy, A.; Lim, M.H. Design of small molecules that target metal-abeta species and regulate metal-induced abeta aggregation and neurotoxicity. Proc. Natl. Acad. Sci. USA 2010, 107, 21990–21995. [Google Scholar] [CrossRef]
- DeToma, A.S.; Krishnamoorthy, J.; Nam, Y.; Lee, H.J.; Brender, J.R.; Kochi, A.; Lee, D.; Onnis, V.; Congiu, C.; Manfredini, S.; et al. Synthetic flavonoids, aminoisoflavones: Interaction and reactivity with metal-free and metal-associated amyloid-beta species. Chem. Sci. 2014, 5, 4851–4862. [Google Scholar] [CrossRef] [PubMed]
- Gueroux, M.; Fleau, C.; Slozeck, M.; Laguerre, M.; Pianet, I. Epigallocatechin 3-gallate as an inhibitor of tau phosphorylation and aggregation: A molecular and structural insight. J. Prev. Alzheimers Dis. 2017, 4, 218–225. [Google Scholar] [PubMed]
- Tang, Y.; Huang, D.; Zhang, M.H.; Zhang, W.S.; Tang, Y.X.; Shi, Z.X.; Deng, L.; Zhou, D.H.; Lu, X.Y. Salvianolic Acid B inhibits abeta generation by modulating BACE1 activity in SH-SY5Y-APPsw cells. Nutrients 2016, 8, 333. [Google Scholar] [CrossRef] [PubMed]
- Jianwei Liu, Y.W. Jing Guo, Jinyan Sun, Qinfeng Sun, Salvianolic Acid B improves cognitive impairment by inhibiting neuroinflammation and decreasing Aβ level in porphyromonas gingivalis-infected mice. Aging 2020, 12, 10117–10128. [Google Scholar]
- Zeng, Y.-Q.; Gu, J.-H.; Chen, L.; Zhang, T.-T.; Zhou, X.-F. Gastrodin as a multi-target protective compound reverses learning memory deficits and AD-like pathology in APP/PS1 transgenic mice. J. Funct. Foods 2021, 77, 104324. [Google Scholar] [CrossRef]
- Tan, X.; Liang, Z.; Li, Y.; Zhi, Y.; Yi, L.; Bai, S.; Forest, K.H.; Nichols, R.A.; Dong, Y.; Li, Q.X. Isoorientin, a GSK-3beta inhibitor, rescues synaptic dysfunction, spatial memory deficits and attenuates pathological progression in APP/PS1 model mice. Behav. Brain Res. 2021, 398, 112968. [Google Scholar] [CrossRef]
- Habtemariam, S. Molecular pharmacology of rosmarinic and salvianolic acids: Potential seeds for Alzheimer’s and vascular dementia drugs. Int. J. Mol. Sci. 2018, 19, 458. [Google Scholar] [CrossRef]
- Cornejo, A.; Aguilar Sandoval, F.; Caballero, L.; Machuca, L.; Munoz, P.; Caballero, J.; Perry, G.; Ardiles, A.; Areche, C.; Melo, F. Rosmarinic acid prevents fibrillization and diminishes vibrational modes associated to beta sheet in tau protein linked to Alzheimer’s disease. J. Enzyme Inhib. Med. Chem. 2017, 32, 945–953. [Google Scholar] [CrossRef]
- Taguchi, R.; Hatayama, K.; Takahashi, T.; Hayashi, T.; Sato, Y.; Sato, D.; Ohta, K.; Nakano, H.; Seki, C.; Endo, Y.; et al. Structure-activity relations of rosmarinic acid derivatives for the amyloid beta aggregation inhibition and antioxidant properties. Eur. J. Med. Chem. 2017, 138, 1066–1075. [Google Scholar] [CrossRef]
- Sun, X.; Zhu, J.; Sun, X.Y.; Ji, M.; Yu, X.L.; Liu, R.T. Ellagic acid rescues motor and cognitive deficits in the R6/2 mouse model of Huntington’s disease by lowering mutant huntingtin protein. Food Funct. 2020, 11, 1334–1348. [Google Scholar] [CrossRef]
- Xiao, S.; Lu, Y.; Wu, Q.; Yang, J.; Chen, J.; Zhong, S.; Eliezer, D.; Tan, Q.; Wu, C. Fisetin inhibits tau aggregation by interacting with the protein and preventing the formation of beta-strands. Int. J. Biol. Macromol. 2021, 178, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Georgiadou, G.; Tarantilis, P.A.; Pitsikas, N. Effects of the active constituents of Crocus sativus L. crocins, in an animal model of obsessive-compulsive disorder. Neurosci. Lett. 2012, 528, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Ardah, M.T.; Paleologou, K.E.; Lv, G.; Menon, S.A.; Abul Khair, S.B.; Lu, J.H.; Safieh-Garabedian, B.; Al-Hayani, A.A.; Eliezer, D.; Li, M.; et al. Ginsenoside Rb1 inhibits fibrillation and toxicity of alpha-synuclein and disaggregates preformed fibrils. Neurobiol. Dis. 2015, 74, 89–101. [Google Scholar] [CrossRef]
- Liu, Y.; Carver, J.A.; Calabrese, A.N.; Pukala, T.L. Gallic acid interacts with alpha-synuclein to prevent the structural collapse necessary for its aggregation. Biochim. Biophys. Acta. 2014, 1844, 1481–1485. [Google Scholar] [CrossRef] [PubMed]
- Ardah, M.T.; Paleologou, K.E.; Lv, G.; Abul Khair, S.B.; Kazim, A.S.; Minhas, S.T.; Al-Tel, T.H.; Al-Hayani, A.A.; Haque, M.E.; Eliezer, D.; et al. Structure activity relationship of phenolic acid inhibitors of alpha-synuclein fibril formation and toxicity. Front. Aging. Neurosci. 2014, 6, 197. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, G.; Sancarlo, D.; Ruan, Q.; Yu, Z.; Panza, F.; Daniele, A.; Greco, A.; Seripa, D. Phytochemicals in the Treatment of Alzheimer’s Disease: A Systematic Review. Curr. Drug Targets 2017, 18, 1487–1498. [Google Scholar] [CrossRef]
- Thinakaran, G.; Koo, E.H. Amyloid precursor protein trafficking, processing, and function. J. Biol. Chem. 2008, 283, 29615–29619. [Google Scholar] [CrossRef]
- Ono, K.; Watanabe-Nakayama, T. Aggregation and structure of amyloid β-protein. Neurochem. Int. 2021, 151, 105208. [Google Scholar] [CrossRef]
- Calfio, C.; Gonzalez, A.; Singh, S.K.; Rojo, L.E.; Maccioni, R.B. The emerging Role of nutraceuticals and phytochemicals in the prevention and treatment of Alzheimer’s disease. J. Alzheimers Dis. 2020, 77, 33–51. [Google Scholar] [CrossRef]
- Simunkova, M.; Alwasel, S.H.; Alhazza, I.M.; Jomova, K.; Kollar, V.; Rusko, M.; Valko, M. Management of oxidative stress and other pathologies in Alzheimer’s disease. Arch. Toxicol. 2019, 93, 2491–2513. [Google Scholar] [CrossRef]
- Peng, Y.; Ao, M.; Dong, B.; Jiang, Y.; Yu, L.; Chen, Z.; Hu, C.; Xu, R. Anti-inflammatory effects of curcumin in the inflammatory diseases: Status, limitations and countermeasures. Drug Des. Dev. Ther. 2021, 15, 4503–4525. [Google Scholar] [CrossRef]
- Ly, P.T.; Wu, Y.; Zou, H.; Wang, R.; Zhou, W.; Kinoshita, A.; Zhang, M.; Yang, Y.; Cai, F.; Woodgett, J.; et al. Inhibition of GSK3β-mediated BACE1 expression reduces Alzheimer-associated phenotypes. J. Clin. Investig. 2013, 123, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Zheng, N.; Zhou, H.B.; Huang, J. Curcumin inhibits BACE1 expression through the interaction between ERbeta and NFkappaB signaling pathway in SH-SY5Y cells. Mol. Cell. Biochem. 2020, 463, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Liang, Y.; Liang, F.; Shen, N.; Shinozuka, K.; Yu, J.T.; Ran, C.; Quan, Q.; Tanzi, R.E.; Zhang, C. A Curcumin analog reduces levels of the Alzheimer’s disease-associated amyloid-beta protein by modulating abetaPP processing and autophagy. J. Alzheimers Dis. 2019, 72, 761–771. [Google Scholar] [CrossRef]
- Jakubowski, J.M.; Orr, A.A.; Le, D.A.; Tamamis, P. Interactions between curcumin derivatives and amyloid-beta fibrils: Insights from molecular dynamics simulations. J. Chem. Inf. Model. 2020, 60, 289–305. [Google Scholar] [CrossRef] [PubMed]
- Fidelis, E.M.; Savall, A.S.P.; da Luz Abreu, E.; Carvalho, F.; Teixeira, F.E.G.; Haas, S.E.; Bazanella Sampaio, T.; Pinton, S. Curcumin-loaded nanocapsules reverses the depressant-like behavior and oxidative stress induced by beta-amyloid in mice. Neuroscience 2019, 423, 122–130. [Google Scholar] [CrossRef]
- Ruan, Y.; Xiong, Y.; Fang, W.; Yu, Q.; Mai, Y.; Cao, Z.; Wang, K.; Lei, M.; Xu, J.; Liu, Y.; et al. Highly sensitive Curcumin-conjugated nanotheranostic platform for detecting amyloid-beta plaques by magnetic resonance imaging and reversing cognitive deficits of Alzheimer’s disease via NLRP3-inhibition. J. Nanobiotechnol. 2022, 20, 322. [Google Scholar] [CrossRef]
- Mathew, A.; Fukuda, T.; Nagaoka, Y.; Hasumura, T.; Morimoto, H.; Yoshida, Y.; Maekawa, T.; Venugopal, K.; Kumar, D.S. Curcumin loaded-PLGA nanoparticles conjugated with Tet-1 peptide for potential use in Alzheimer’s disease. PLoS ONE 2012, 7, e32616. [Google Scholar] [CrossRef]
- Wang, N.; Zhou, Y.; Zhao, L.; Wang, C.; Ma, W.; Ge, G.; Wang, Y.; Ullah, I.; Muhammad, F.; Alwayli, D.; et al. Ferulic acid delayed amyloid beta-induced pathological symptoms by autophagy pathway via a fasting-like effect in Caenorhabditis elegans. Food Chem. Toxicol. 2020, 146, 111808. [Google Scholar] [CrossRef]
- Wang, N.Y.; Li, J.N.; Liu, W.L.; Huang, Q.; Li, W.X.; Tan, Y.H.; Liu, F.; Song, Z.H.; Wang, M.Y.; Xie, N.; et al. Ferulic acid ameliorates Alzheimer’s disease-like pathology and repairs cognitive decline by preventing capillary hypofunction in APP/PS1 Mice. Neurotherapeutics 2021, 18, 1064–1080. [Google Scholar] [CrossRef]
- Kim, J.H.; Wang, Q.; Choi, J.M.; Lee, S.; Cho, E.J. Protective role of caffeic acid in an Aβ25-35-induced Alzheimer’s disease model. Nut. Res. Pract. 2015, 9, 5480. [Google Scholar] [CrossRef]
- Li, H.; Yu, X.; Li, C.; Ma, L.; Zhao, Z.; Guan, S.; Wang, L. Caffeic acid protects against Abeta toxicity and prolongs lifespan in Caenorhabditis elegans models. Food Funct. 2021, 12, 1219–1231. [Google Scholar] [CrossRef]
- Andrade, S.; Pereira, M.C.; Loureiro, J.A. Caffeic acid loaded into engineered lipid nanoparticles for Alzheimer’s disease therapy. Colloids Surf. B Biointerfaces 2023, 225, 113270. [Google Scholar] [CrossRef]
- Shimmyo, Y.; Kihara, T.; Akaike, A.; Niidome, T.; Sugimoto, H. Flavonols and flavones as BACE-1 inhibitors: Structure-activity relationship in cell-free, cell-based and in silico studies reveal novel pharmacophore features. Biochim. Biophys. Acta 2008, 1780, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Paris, D.; Mathura, V.; Ait-Ghezala, G.; Beaulieu-Abdelahad, D.; Patel, N.; Bachmeier, C.; Mullan, M. Flavonoids lower Alzheimer's Aβ production via an NFκB dependent mechanism. Bioinformation. 2011, 6, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Li, K.; Fu, T.; Wan, C.; Zhang, D.; Song, H.; Zhang, Y.; Liu, N.; Gan, Z.; Yuan, L. Quercetin ameliorates Aβ toxicity in Drosophila AD model by modulating cell cycle-related protein expression. Oncotarget. 2016, 7, 67716–67731. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, S.; Li, J.; Sun, Y.; Hasimu, H.; Liu, R.; Zhang, T. Quercetin protects human brain microvascular endothelial cells from fibrillar beta-amyloid1-40-induced toxicity. Acta. Pharm. Sin. B 2015, 5, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Tang, Y.; Zhan, C.; Wei, G. Green tea extract EGCG plays a dual role in abeta(42) protofibril disruption and membrane protection: A molecular dynamic study. Chem. Phys. Lipids 2021, 234, 105024. [Google Scholar] [CrossRef]
- Zhang, J.S.; Zhou, S.F.; Wang, Q.; Guo, J.N.; Liang, H.M.; Deng, J.B.; He, W.Y. Gastrodin suppresses BACE1 expression under oxidative stress condition via inhibition of the PKR/eIF2alpha pathway in Alzheimer’s disease. Neuroscience 2016, 325, 1–9. [Google Scholar] [CrossRef]
- Li, M.; Qian, S. Gastrodin protects neural progenitor cells against amyloid beta (1-42)-induced neurotoxicity and improves hippocampal neurogenesis in amyloid beta (1-42)-injected mice. J. Mol. Neurosci. 2016, 60, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, C.; Shen, W. Gastrodin alleviates memory deficits and reduces neuropathology in a mouse model of Alzheimer’s disease. Neuropathology 2014, 34, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zou, Y.; Xu, H.; Fan, L.; Guo, H.; Li, X.; Li, G.; Zhang, X.; Dong, M. Gastrodin protect primary cultured rat hippocampal neurons against amyloid-beta peptide-induced neurotoxicity via ERK1/2-Nrf2 pathway. Brain Res. 2012, 1482, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.Y.; Wang, L.; Ge, H.; Lu, X.L.; Pei, Z.; Gu, Q.; Xu, J. Salvianolic acid A, a polyphenolic derivative from Salvia miltiorrhiza bunge, as a multifunctional agent for the treatment of Alzheimer’s disease. Mol. Divers. 2013, 17, 515–524. [Google Scholar] [CrossRef]
- Durairajan, S.S.; Yuan, Q.; Xie, L.; Chan, W.S.; Kum, W.F.; Koo, I.; Liu, C.; Song, Y.; Huang, J.D.; Klein, W.L.; et al. Salvianolic acid B inhibits abeta fibril formation and disaggregates preformed fibrils and protects against abeta-induced cytotoxicty. Neuroche. Int. 2008, 52, 741–750. [Google Scholar] [CrossRef]
- Lin, Y.H.; Liu, A.H.; Wu, H.L.; Westenbroek, C.; Song, Q.L.; Yu, H.M.; Ter Horst, G.J.; Li, X.J. Salvianolic acid B, an antioxidant from salvia miltiorrhiza, prevents abeta(25-35)-induced reduction in BPRP in PC12 cells. Biochem. Biophys. Res. Commun. 2006, 348, 593–599. [Google Scholar] [CrossRef]
- He, Y.; Jia, K.; Li, L.; Wang, Q.; Zhang, S.; Du, J.; Du, H. Salvianolic acid B attenuates mitochondrial stress against abeta toxicity in primary cultured mouse neurons. Biochem. Biophys. Res. Commun. 2018, 498, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Paudel, P.; Seong, S.H.; Kim, J.A.; Jung, H.A.; Choi, J.S. Computational insights into beta-site amyloid precursor protein enzyme 1 (BACE1) inhibition by tanshinones and salvianolic acids from Salvia miltiorrhiza via molecular docking simulations. Comput. Biol. Chem. 2018, 74, 273–285. [Google Scholar] [CrossRef]
- Baek, S.; Park, S.; Shin, J.; Lee, J.-S.; Kim, H.Y.; Han, G.; Kim, Y. Investigation of memory-enhancing botanical mixture and their isolated compounds for inhibition of amyloid-β and tau aggregation. Appl. Biol. Chem. 2020, 63, 13765. [Google Scholar] [CrossRef]
- Jayatunga, D.P.W.; Hone, E.; Fernando, W.; Garg, M.L.; Verdile, G.; Martins, R.N. Mitoprotective effects of a synergistic nutraceutical combination: Basis for a prevention strategy against Alzheimer’s disease. Front. Aging Neurosci. 2021, 13, 781468. [Google Scholar] [CrossRef]
- Dourado, N.S.; Souza, C.D.S.; de Almeida, M.M.A.; Bispo da Silva, A.; Dos Santos, B.L.; Silva, V.D.A.; De Assis, A.M.; da Silva, J.S.; Souza, D.O.; Costa, M.F.D.; et al. Neuroimmunomodulatory and neuroprotective effects of the flavonoid apigenin in in vitro models of neuroinflammation associated with Alzheimer’s disease. Front. Aging Neurosci. 2020, 12, 119. [Google Scholar] [CrossRef]
- Nikbakht, F.; Khadem, Y.; Haghani, S.; Hoseininia, H.; Moein Sadat, A.; Heshemi, P.; Jamali, N. Protective role of apigenin against abeta 25-35 toxicity via inhibition of mitochondrial cytochrome crelease. Basic Clin. Neurosci. 2019, 10, 557–566. [Google Scholar]
- Siddique, Y.H.; Rahul; Ara, G.; Afzal, M.; Varshney, H.; Gaur, K.; Subhan, I.; Mantasha, I.; Shahid, M. Beneficial effects of apigenin on the transgenic Drosophila model of Alzheimer’s disease. Chem. Biol. Interact. 2022, 366, 110120. [Google Scholar] [CrossRef] [PubMed]
- Min, Z.; Tang, Y.; Hu, X.T.; Zhu, B.L.; Ma, Y.L.; Zha, J.S.; Deng, X.J.; Yan, Z.; Chen, G.J. Cosmosiin increases ADAM10 expression via mechanisms involving 5’UTR and PI3K signaling. Front. Mol. Neurosci. 2018, 11, 198. [Google Scholar] [CrossRef]
- Gu, L.; Cai, N.; Li, M.; Bi, D.; Yao, L.; Fang, W.; Wu, Y.; Hu, Z.; Liu, Q.; Lin, Z.; et al. Inhibitory effects of macelignan on tau phosphorylation and abeta aggregation in the cell model of Alzheimer’s disease. Front. Nutr. 2022, 9, 892558. [Google Scholar] [CrossRef] [PubMed]
- Hitl, M.; Kladar, N.; Gavarić, N.; Božin, B. Rosmarinic acid-human pharmacokinetics and health benefits. Planta Medica 2021, 87, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.; Hwang, B.R.; Lee, M.H.; Lee, S.; Cho, E.J. Perilla frutescens var. japonica and rosmarinic acid improve amyloid-beta25-35 induced impairment of cognition and memory function. Nutr. Res. Pract. 2016, 10, 274–281. [Google Scholar] [CrossRef]
- Mirza, F.J.; Amber, S.; Sumera; Hassan, D.; Ahmed, T.; Zahid, S. Rosmarinic acid and ursolic acid alleviate deficits in cognition, synaptic regulation and adult hippocampal neurogenesis in an abeta(1-42)-induced mouse model of Alzheimer’s disease. Phytomedicine 2021, 83, 153490. [Google Scholar] [CrossRef] [PubMed]
- Dani, M.; Brooks, D.J.; Edison, P. Tau imaging in neurodegenerative diseases. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1139–1150. [Google Scholar] [CrossRef] [PubMed]
- Giacobini, E.; Gold, G. Alzheimer disease therapy--moving from amyloid-β to tau. Nat. Rev. Neurol. 2013, 9, 677–686. [Google Scholar] [CrossRef]
- Spillantini, M.G.; Goedert, M. Tau pathology and neurodegeneration. Lancet Neurol. 2013, 12, 609–622. [Google Scholar] [CrossRef]
- Lee, H.G.; Perry, G.; Moreira, P.I.; Garrett, M.R.; Liu, Q.; Zhu, X.; Takeda, A.; Nunomura, A.; Smith, M.A. Tau phosphorylation in Alzheimer’s disease: Pathogen or protector? Trends Mol. Med. 2005, 11, 164–169. [Google Scholar] [CrossRef]
- Chabrier, M.A.; Cheng, D.; Castello, N.A.; Green, K.N.; LaFerla, F.M. Synergistic effects of amyloid-beta and wild-type human tau on dendritic spine loss in a floxed double transgenic model of Alzheimer’s disease. Neurobiol. Dis. 2014, 64, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, D.W.; Hwo, S.Y.; Kirschner, M.W. Physical and chemical properties of purified tau factor and the role of tau in microtubule assembly. J. Mol. Bio. 1977, 116, 227–247. [Google Scholar] [CrossRef]
- Ballatore, C.; Lee, V.M.; Trojanowski, J.Q. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat. Rev. Neurosci. 2007, 8, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Herrup, K.; Carrillo, M.C.; Schenk, D.; Cacace, A.; Desanti, S.; Fremeau, R.; Bhat, R.; Glicksman, M.; May, P.; Swerdlow, R.; et al. Beyond amyloid: Getting real about nonamyloid targets in Alzheimer’s disease. Alzheimers Dement. 2013, 9, 452–458.e1. [Google Scholar] [CrossRef] [PubMed]
- Stoothoff, W.H.; Johnson, G.V. Tau phosphorylation: Physiological and pathological consequences. Biochim. Biophys. Acta 2005, 1739, 280–297. [Google Scholar] [CrossRef]
- von Bergen, M.; Barghorn, S.; Biernat, J.; Mandelkow, E.M.; Mandelkow, E. Tau aggregation is driven by a transition from random coil to beta sheet structure. Biochim. Biophys. Acta 2005, 1739, 158–166. [Google Scholar] [CrossRef]
- Mietelska-Porowska, A.; Wasik, U.; Goras, M.; Filipek, A.; Niewiadomska, G. Tau protein modifications and interactions: Their role in function and dysfunction. Int. J. Mol. Sci. 2014, 15, 4671–4713. [Google Scholar] [CrossRef]
- Goedert, M.; Ghetti, B.; Spillantini, M.G. Tau gene mutations in frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17). Their relevance for understanding the neurogenerative process. Ann. N. Y. Acad. Sci. 2000, 920, 74–83. [Google Scholar] [CrossRef]
- Hutton, M. Molecular genetics of chromosome 17 tauopathies. Ann. N. Y. Acad. Sci. 2000, 920, 63–73. [Google Scholar] [CrossRef]
- Shafiei, S.S.; Guerrero-Munoz, M.J.; Castillo-Carranza, D.L. Tau oligomers: Cytotoxicity, propagation, and mitochondrial damage. Front. Aging Neurosci. 2017, 9, 83. [Google Scholar] [CrossRef]
- Congdon, E.E.; Sigurdsson, E.M. Tau-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 2018, 14, 399–415. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Choi, K.J.; Cho, S.J.; Yun, S.M.; Jeon, J.P.; Koh, Y.H.; Song, J.; Johnson, G.V.; Jo, C. Fisetin stimulates autophagic degradation of phosphorylated tau via the activation of TFEB and Nrf2 transcription factors. Sci. Rep. 2016, 6, 24933. [Google Scholar] [CrossRef]
- Ait-Bouziad, N.; Lv, G.; Mahul-Mellier, A.L.; Xiao, S.; Zorludemir, G.; Eliezer, D.; Walz, T.; Lashuel, H.A. Discovery and characterization of stable and toxic Tau/phospholipid oligomeric complexes. Nat. Commun. 2017, 8, 1678. [Google Scholar] [CrossRef]
- Ahmad, A.; Ali, T.; Park, H.Y.; Badshah, H.; Rehman, S.U.; Kim, M.O. Neuroprotective effect of fisetin against amyloid-beta-induced cognitive/synaptic dysfunction, neuroinflammation, and neurodegeneration in adult mice. Mol. Neurobiol. 2017, 54, 2269–2285. [Google Scholar] [CrossRef] [PubMed]
- Soeda, S.; Ochiai, T.; Paopong, L.; Tanaka, H.; Shoyama, Y.; Shimeno, H. Crocin suppresses tumor necrosis factor-alpha-induced cell death of neuronally differentiated PC-12 cells. Life Sci. 2001, 69, 2887–2898. [Google Scholar] [CrossRef]
- Ochiai, T.; Ohno, S.; Soeda, S.; Tanaka, H.; Shoyama, Y.; Shimeno, H. Crocin prevents the death of rat pheochromyctoma (PC-12) cells by its antioxidant effects stronger than those of alpha-tocopherol. Neurosci. Lett. 2004, 362, 61–64. [Google Scholar] [CrossRef]
- Inoue, E.; Shimizu, Y.; Masui, R.; Hayakawa, T.; Tsubonoya, T.; Hori, S.; Sudoh, K. Effects of saffron and its constituents, crocin-1, crocin-2, and crocetin on alpha-synuclein fibrils. J. Nat. Med. 2018, 72, 274–279. [Google Scholar] [CrossRef]
- Sugiura, M.; Shoyama, Y.; Saito, H.; Abe, K. The effects of ethanol and crocin on the induction of long-term potentiation in the CA1 region of rat hippocampal slices. Jap. J. Of. Pharmacol. 1995, 67, 395–397. [Google Scholar] [CrossRef] [PubMed]
- Karakani, A.M.; Riazi, G.; Ghaffari, S.M.; Ahmadian, S.; Mokhtari, F.; Firuzi, M.J.; Bathaie, S.Z. Inhibitory effect of corcin on aggregation of 1N/4R human tau protein in vitro. Iran. J. Basic Med. Sci. 2015, 18, 485–492. [Google Scholar]
- Rashedinia, M.; Lari, P.; Abnous, K.; Hosseinzadeh, H. Protective effect of crocin on acrolein-induced tau phosphorylation in the rat brain. Acta Neurobiol. Exp. 2015, 75, 208–219. [Google Scholar]
- Chalatsa, I.; Arvanitis, D.A.; Koulakiotis, N.S.; Giagini, A.; Skaltsounis, A.L.; Papadopoulou-Daifoti, Z.; Tsarbopoulos, A.; Sanoudou, D. The crocus sativus compounds trans-crocin 4 and trans-crocetin modulate the amyloidogenic pathway and tau misprocessing in Alzheimer disease neuronal cell culture models. Front. Neurosci. 2019, 13, 249. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.; Yokota, T.; Ashihara, H.; Lean, M.E.; Crozier, A. Plant foods and herbal sources of resveratrol. J. Agric. Food. Chem. 2002, 50, 3337–3340. [Google Scholar] [CrossRef]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef]
- Jeon, B.T.; Jeong, E.A.; Shin, H.J.; Lee, Y.; Lee, D.H.; Kim, H.J.; Kang, S.S.; Cho, G.J.; Choi, W.S.; Roh, G.S. Resveratrol attenuates obesity-associated peripheral and central inflammation and improves memory deficit in mice fed a high-fat diet. Diabetes 2012, 61, 1444–1454. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.X.; Shaikh, S.; Wang, J.Z.; Zaidi, T.; Grundke-Iqbal, I.; Iqbal, K. Phosphatase activity toward abnormally phosphorylated tau: Decrease in Alzheimer disease brain. J. Neurochem. 1995, 65, 732–773. [Google Scholar] [CrossRef]
- Gong, C.X.; Singh, T.J.; Grundke-Iqbal, I.; Iqbal, K. Phosphoprotein phosphatase activities in Alzheimer disease brain. J. Neurochem. 1993, 61, 921–927. [Google Scholar] [CrossRef]
- Vogelsberg-Ragaglia, V.; Schuck, T.; Trojanowski, J.Q.; Lee, V.M. PP2A mRNA expression is quantitatively decreased in Alzheimer’s disease hippocampus. Exp. Neurol. 2001, 168, 402–412. [Google Scholar] [CrossRef]
- Wang, Y.J.; Chen, G.H.; Hu, X.Y.; Lu, Y.P.; Zhou, J.N.; Liu, R.Y. The expression of calcium/calmodulin-dependent protein kinase II-alpha in the hippocampus of patients with Alzheimer’s disease and its links with AD-related pathology. Brain Res. 2005, 1031, 101–108. [Google Scholar] [CrossRef]
- Pei, J.J.; Braak, E.; Braak, H.; Grundke-Iqbal, I.; Iqbal, K.; Winblad, B.; Cowburn, R.F. Distribution of active glycogen synthase kinase 3beta (GSK-3beta) in brains staged for Alzheimer disease neurofibrillary changes. J. Neurotaph. Exp. Neur. 1999, 58, 1010–1019. [Google Scholar] [CrossRef]
- He, X.; Li, Z.; Rizak, J.D.; Wu, S.; Wang, Z.; He, R.; Su, M.; Qin, D.; Wang, J.; Hu, X. Resveratrol attenuates formaldehyde induced hyperphosphorylation of tau protein and cytotoxicity in N2a cells. Front. Neurosci. 2016, 10, 598. [Google Scholar] [CrossRef]
- Trockenbacher, A.; Suckow, V.; Foerster, J.; Winter, J.; Krauss, S.; Ropers, H.H.; Schneider, R.; Schweiger, S. MID1, mutated in Opitz syndrome, encodes an ubiquitin ligase that targets phosphatase 2A for degradation. Nat. Genet. 2001, 29, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Schweiger, S.; Matthes, F.; Posey, K.; Kickstein, E.; Weber, S.; Hettich, M.M.; Pfurtscheller, S.; Ehninger, D.; Schneider, R.; Krauß, S. Resveratrol induces dephosphorylation of Tau by interfering with the MID1-PP2A complex. Sci. Rep. 2017, 7, 13753. [Google Scholar] [CrossRef] [PubMed]
- Shati, A.A.; Alfaifi, M.Y. Trans-resveratrol Inhibits Tau phosphorylation in the brains of control and cadmium chloride-treated rats by activating PP2A and PI3K/Akt induced-inhibition of GSK3beta. Neurochem. Res. 2019, 44, 357–373. [Google Scholar] [CrossRef] [PubMed]
- Jhang, K.A.; Park, J.S.; Kim, H.S.; Chong, Y.H. Resveratrol ameliorates tau hyperphosphorylation at Ser396 Site and oxidative damage in rat hippocampal slices exposed to vanadate: Implication of ERK1/2 and GSK-3beta signaling cascades. J. Agric. Food Chem. 2017, 65, 9626–9634. [Google Scholar] [CrossRef]
- Means, J.C.; Lopez, A.A.; Koulen, P. Resveratrol protects optic nerve head astrocytes from oxidative stress-induced cell death by preventing caspase-3 activation, tau dephosphorylation at Ser(422) and formation of misfolded protein aggregates. Cell. Mol. Neurobiol. 2020, 40, 911–926. [Google Scholar] [CrossRef]
- Ishisaka, A.; Mukai, R.; Terao, J.; Shibata, N.; Kawai, Y. Specific localization of quercetin-3-O-glucuronide in human brain. Arch. Biochem. Biophys. 2014, 557, 11–17. [Google Scholar] [CrossRef]
- Chen, J.; Deng, X.; Liu, N.; Li, M.; Liu, B.; Fu, Q.; Qu, R.; Ma, S. Quercetin attenuates tau hyperphosphorylation and improves cognitive disorder via suppression of ER stress in a manner dependent on AMPK pathway. J. Funct. Foods 2016, 22, 463–476. [Google Scholar] [CrossRef]
- Kimura, T.; Ishiguro, K.; Hisanaga, S. Physiological and pathological phosphorylation of tau by Cdk5. Front. Mol. Neurosci. 2014, 7, 65. [Google Scholar] [CrossRef]
- Shen, X.Y.; Luo, T.; Li, S.; Ting, O.Y.; He, F.; Xu, J.; Wang, H.Q. Quercetin inhibits okadaic acid-induced tau protein hyperphosphorylation through the Ca2+-calpain-p25-CDK5 pathway in HT22 cells. Int. J. Mol. Med. 2018, 41, 1138–1146. [Google Scholar] [CrossRef]
- Huang, H.C.; Xu, K.; Jiang, Z.F. Curcumin-mediated neuroprotection against amyloid-beta-induced mitochondrial dysfunction involves the inhibition of GSK-3beta. J. Alzheimers Dis. 2012, 32, 981–996. [Google Scholar] [CrossRef]
- Huang, H.C.; Tang, D.; Xu, K.; Jiang, Z.F. Curcumin attenuates amyloid-beta-induced tau hyperphosphorylation in human neuroblastoma SH-SY5Y cells involving PTEN/Akt/GSK-3beta signaling pathway. J. Recept. Signal Transduct. Res. 2014, 34, 26–37. [Google Scholar] [CrossRef]
- Wobst, H.J.; Sharma, A.; Diamond, M.I.; Wanker, E.E.; Bieschke, J. The green tea polyphenol (-)-epigallocatechin gallate prevents the aggregation of tau protein into toxic oligomers at substoichiometric ratios. FEBS Lett. 2015, 589, 77–83. [Google Scholar] [CrossRef]
- Chesser, A.S.; Ganeshan, V.; Yang, J.; Johnson, G.V. Epigallocatechin-3-gallate enhances clearance of phosphorylated tau in primary neurons. Nutr. Neurosci. 2016, 19, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Gong, E.J.; Park, H.R.; Kim, M.E.; Piao, S.; Lee, E.; Jo, D.G.; Chung, H.Y.; Ha, N.C.; Mattson, M.P.; Lee, J. Morin attenuates tau hyperphosphorylation by inhibiting GSK3beta. Neurobiol. Dis. 2011, 44, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Qu, J.; Zhang, W.; Bai, M.; Zhou, Q.; Zhang, Z.; Li, Z.; Miao, J. Morin reverses neuropathological and cognitive impairments in APPswe/PS1dE9 mice by targeting multiple pathogenic mechanisms. Neuropharmacology 2016, 108, 1–13. [Google Scholar] [CrossRef] [PubMed]
- An, F.; Bai, Y.; Xuan, X.; Bian, M.; Zhang, G.; Wei, C. 1,8-Cineole ameliorates advanced glycation end products-induced Alzheimer’s disease-like pathology in vitro and in vivo. Molecules 2022, 27, 3913. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, A.; Aoyama, Y.; Nguyen, T.T.; Shin, E.J.; Kim, H.C.; Yamada, S.; Nakai, T.; Nagai, T.; Yokosuka, A.; Mimaki, Y.; et al. Nobiletin, a citrus flavonoid, ameliorates cognitive impairment, oxidative burden, and hyperphosphorylation of tau in senescence-accelerated mouse. Behav. Brain Res. 2013, 250, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.T.; Qian, Z.M.; He, X.; Gong, Q.; Wu, K.C.; Jiang, L.R.; Lu, L.N.; Zhu, Z.J.; Zhang, H.Y.; Yung, W.H.; et al. Reducing iron in the brain: A novel pharmacologic mechanism of huperzine A in the treatment of Alzheimer’s disease. Neurobiol. Aging 2014, 35, 1045–1054. [Google Scholar] [CrossRef]
- Laurent, C.; Eddarkaoui, S.; Derisbourg, M.; Leboucher, A.; Demeyer, D.; Carrier, S.; Schneider, M.; Hamdane, M.; Muller, C.E.; Buee, L.; et al. Beneficial effects of caffeine in a transgenic model of Alzheimer’s disease-like tau pathology. Neurobiol. Aging 2014, 35, 2079–2090. [Google Scholar] [CrossRef]
- Shasaltaneh, M.D.; Naghdi, N.; Ramezani, S.; Alizadeh, L.; Riazi, G.H. Protection of beta boswellic acid against streptozotocin-induced Alzheimer’s model by reduction of tau phosphorylation level and enhancement of reelin expression. Planta Med. 2022, 88, 367–379. [Google Scholar]
- Xiao, S.; Wu, Q.; Yao, X.; Zhang, J.; Zhong, W.; Zhao, J.; Liu, Q.; Zhang, M. Inhibitory effects of isobavachalcone on tau protein aggregation, tau phosphorylation, and oligomeric tau-induced apoptosis. ACS Chem. Neurosci. 2021, 12, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Gorantla, N.V.; Das, R.; Mulani, F.A.; Thulasiram, H.V.; Chinnathambi, S. Neem derivatives inhibits tau aggregation. J. Alzheimers Dis. Rep. 2019, 3, 169–178. [Google Scholar] [CrossRef]
- Khare, S.D.; Chinchilla, P.; Baum, J. Multifaceted interactions mediated by intrinsically disordered regions play key roles in alpha synuclein aggregation. Curr. Opin. Struc. Biol. 2023, 80, 102579. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.D.; Rueter, S.M.; Trojanowski, J.Q.; Lee, V.M. Synucleins are developmentally expressed, and alpha-synuclein regulates the size of the presynaptic vesicular pool in primary hippocampal neurons. J. Neurosci. 2000, 20, 3214–3220. [Google Scholar] [CrossRef]
- Uversky, V.N.; Lee, H.J.; Li, J.; Fink, A.L.; Lee, S.J. Stabilization of partially folded conformation during alpha-synuclein oligomerization in both purified and cytosolic preparations. J. Biol. Chem. 2001, 276, 43495–43498. [Google Scholar] [CrossRef] [PubMed]
- Paillusson, S.; Gomez-Suaga, P.; Stoica, R.; Little, D.; Gissen, P.; Devine, M.J.; Noble, W.; Hanger, D.P.; Miller, C.C.J. α-Synuclein binds to the ER-mitochondria tethering protein VAPB to disrupt Ca(2+) homeostasis and mitochondrial ATP production. Acta Neuropathol. 2017, 134, 129–149. [Google Scholar] [CrossRef]
- Lindström, V.; Gustafsson, G.; Sanders, L.H.; Howlett, E.H.; Sigvardson, J.; Kasrayan, A.; Ingelsson, M.; Bergström, J.; Erlandsson, A. Extensive uptake of α-synuclein oligomers in astrocytes results in sustained intracellular deposits and mitochondrial damage. Mol. Cell. Neurosci. 2017, 82, 143–156. [Google Scholar] [CrossRef]
- Uversky, V.N. Hot, Hotter, and Hottest Trends in α-Synuclein Research. Curr. Protein. Pept. Sci. 2015, 16, 682–687. [Google Scholar] [CrossRef]
- Büeler, H. Impaired mitochondrial dynamics and function in the pathogenesis of Parkinson’s disease. Exp. Neurol. 2009, 218, 235–246. [Google Scholar] [CrossRef]
- Niranjan, R. The role of inflammatory and oxidative stress mechanisms in the pathogenesis of Parkinson’s disease: Focus on astrocytes. Mol. Neurobiol. 2014, 49, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhu, M.; Rajamani, S.; Uversky, V.N.; Fink, A.L. Rifampicin inhibits alpha-synuclein fibrillation and disaggregates fibrils. Chem. Biol. 2004, 11, 1513–1521. [Google Scholar] [CrossRef]
- Goel, A.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin as “Curecumin”: From kitchen to clinic. Biochem. Pharmacol. 2008, 75, 787–809. [Google Scholar] [CrossRef] [PubMed]
- Cole, G.M.; Teter, B.; Frautschy, S.A. Neuroprotective effects of curcumin. Ad. Exp. Med. Biol. 2007, 595, 197–212. [Google Scholar]
- Lim, G.P.; Chu, T.; Yang, F.; Beech, W.; Frautschy, S.A.; Cole, G.M. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J. Neurosci. 2001, 21, 8370–8377. [Google Scholar] [CrossRef]
- Yang, F.; Lim, G.P.; Begum, A.N.; Ubeda, O.J.; Simmons, M.R.; Ambegaokar, S.S.; Chen, P.P.; Kayed, R.; Glabe, C.G.; Frautschy, S.A.; et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J. Biol. Chem. 2005, 280, 5892–5901. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Nehru, B. Curcumin affords neuroprotection and inhibits alpha-synuclein aggregation in lipopolysaccharide-induced Parkinson’s disease model. Inflammopharmacology 2018, 26, 349–360. [Google Scholar] [CrossRef]
- Ahsan, N.; Mishra, S.; Jain, M.K.; Surolia, A.; Gupta, S. Curcumin Pyrazole and its derivative (N-(3-Nitrophenylpyrazole) Curcumin inhibit aggregation, disrupt fibrils and modulate toxicity of Wild type and Mutant alpha-Synuclein. Sci. Rep. 2015, 5, 9862. [Google Scholar] [CrossRef]
- Singh, B.N.; Shankar, S.; Srivastava, R.K. Green tea catechin, epigallocatechin-3-gallate (EGCG): Mechanisms, perspectives and clinical applications. Biochem. Pharmacol. 2011, 82, 1807–1821. [Google Scholar] [CrossRef]
- Masuda, M.; Suzuki, N.; Taniguchi, S.; Oikawa, T.; Nonaka, T.; Iwatsubo, T.; Hisanaga, S.; Goedert, M.; Hasegawa, M. Small molecule inhibitors of alpha-synuclein filament assembly. Biochemistry 2006, 45, 6085–6094. [Google Scholar] [CrossRef]
- Lorenzen, N.; Nielsen, S.B.; Yoshimura, Y.; Vad, B.S.; Andersen, C.B.; Betzer, C.; Kaspersen, J.D.; Christiansen, G.; Pedersen, J.S.; Jensen, P.H.; et al. How epigallocatechin gallate can inhibit α-synuclein oligomer toxicity in vitro. J. Biol. Chem. 2014, 289, 21299–21310. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Hong, C.S.; Lee, S.; Yang, J.E.; Park, Y.I.; Lee, D.; Hyeon, T.; Jung, S.; Paik, S.R. Radiating amyloid fibril formation on the surface of lipid membranes through unit-assembly of oligomeric species of α-synuclein. PLoS ONE 2012, 7, e47580. [Google Scholar] [CrossRef]
- Yang, J.E.; Rhoo, K.Y.; Lee, S.; Lee, J.T.; Park, J.H.; Bhak, G.; Paik, S.R. EGCG-mediated protection of the membrane disruption and cytotoxicity caused by the ‘active oligomer’ of alpha-synuclein. Sci. Rep. 2017, 7, 17945. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhou, S.; Shi, D.; Bai, Q.; Liu, H.; Yao, X. Influence of EGCG on alpha-synuclein (alphaS) aggregation and identification of their possible binding mode: A computational study using molecular dynamics simulation. Chem. Biol. Drug Des. 2018, 91, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Tang, Y.; Wei, G. Epigallocatechin Gallate Destabilizes alpha-Synuclein Fibril by Disrupting the E46-K80 Salt-Bridge and Inter-protofibril Interface. ACS Chem. Neurosci. 2020, 11, 4351–4361. [Google Scholar] [CrossRef]
- Dehay, B.; Martinez-Vicente, M.; Caldwell, G.A.; Caldwell, K.A.; Yue, Z.; Cookson, M.R.; Klein, C.; Vila, M.; Bezard, E. Lysosomal impairment in Parkinson's disease. Movement. Disorders. 2013, 28, 725–732. [Google Scholar] [CrossRef]
- Bourdenx, M.; Bezard, E.; Dehay, B. Lysosomes and α-synuclein form a dangerous duet leading to neuronal cell death. Front. Neuroanat. 2014, 8, 83. [Google Scholar] [CrossRef]
- Gu, W.Z.; Chen, R.; Brandwein, S.; McAlpine, J.; Burres, N. Isolation, purification, and characterization of immunosuppressive compounds from tripterygium: Triptolide and tripdiolide. Int. J. Immunopharmaco. 1995, 17, 351–356. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, W.J.; Wang, X.M. Triptolide with potential medicinal value for diseases of the central nervous system. CNS Neurosci. Ther. 2013, 19, 76–82. [Google Scholar] [CrossRef]
- Hu, G.; Gong, X.; Wang, L.; Liu, M.; Liu, Y.; Fu, X.; Wang, W.; Zhang, T.; Wang, X. Triptolide promotes the clearance of alpha-synuclein by enhancing autophagy in neuronal cells. Mol. Neurobiol. 2017, 54, 2361–2372. [Google Scholar] [CrossRef]
- Eskandari, H.; Ghanadian, M.; Noleto-Dias, C.; Lomax, C.; Tawfike, A.; Christiansen, G.; Sutherland, D.S.; Ward, J.L.; Mohammad-Beigi, H.; Otzen, D.E. Inhibitors of alpha-synuclein fibrillation and oligomer toxicity in rosa damascena: The all-pervading powers of flavonoids and phenolic glycosides. ACS Chem. Neurosci. 2020, 11, 3161–3173. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Fernandez, M.; Hornedo-Ortega, R.; Cerezo, A.B.; Troncoso, A.M.; Garcia-Parrilla, M.C. Melatonin, protocatechuic acid and hydroxytyrosol effects on vitagenes system against alpha-synuclein toxicity. Food. Chem. Toxicol. 2019, 134, 110817. [Google Scholar] [CrossRef]
- Tabrizi, S.J.; Ghosh, R.; Leavitt, B.R. Huntingtin lowering strategies for disease modification in Huntington’s disease. Neuron 2019, 101, 801–819. [Google Scholar] [CrossRef] [PubMed]
- Caron, N.S.; Wright, G.E.B.; Hayden, M.R. Huntington Disease. In GeneReviews; Adam, M.P., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993–2023. [Google Scholar]
- Gil, J.M.; Rego, A.C. Mechanisms of neurodegeneration in Huntington’s disease. Eur. J. Neurosci. 2008, 27, 2803–2820. [Google Scholar] [CrossRef] [PubMed]
- Franco-Iborra, S.; Plaza-Zabala, A.; Montpeyo, M.; Sebastian, D.; Vila, M.; Martinez-Vicente, M. Mutant HTT (huntingtin) impairs mitophagy in a cellular model of Huntington disease. Autophagy 2021, 17, 672–689. [Google Scholar] [CrossRef]
- Landrum, E.; Wetzel, R. Biophysical underpinnings of the repeat length dependence of polyglutamine amyloid formation. J. Biol. Chem. 2014, 289, 10254–10260. [Google Scholar] [CrossRef]
- Legleiter, J.; Mitchell, E.; Lotz, G.P.; Sapp, E.; Ng, C.; DiFiglia, M.; Thompson, L.M.; Muchowski, P.J. Mutant huntingtin fragments form oligomers in a polyglutamine length-dependent manner in vitro and in vivo. J. Biol. Chem. 2010, 285, 14777–14790. [Google Scholar] [CrossRef]
- Lum, P.T.; Sekar, M.; Gan, S.H.; Bonam, S.R.; Shaikh, M.F. Protective effect of natural products against Huntington’s disease: An overview of scientific evidence and understanding their mechanism of action. ACS Chem. Neurosci. 2021, 12, 391–418. [Google Scholar] [CrossRef]
- Kim, A.; Lalonde, K.; Truesdell, A.; Gomes Welter, P.; Brocardo, P.S.; Rosenstock, T.R.; Gil-Mohapel, J. New avenues for the treatment of Huntington’s disease. Int. J. Mol. Sci. 2021, 22, 8363. [Google Scholar] [CrossRef]
- Gupta, M.; Sanjana; Singh, N.; Singh, B.; Alam, P. Role of natural products in alleviation of Huntington’s disease: An overview. S. Afr. J. Bot. 2022, 151, 263–276. [Google Scholar] [CrossRef]
- Ehrnhoefer, D.E.; Duennwald, M.; Markovic, P.; Wacker, J.L.; Engemann, S.; Roark, M.; Legleiter, J.; Marsh, J.L.; Thompson, L.M.; Lindquist, S.; et al. Green tea (-)-epigallocatechin-gallate modulates early events in huntingtin misfolding and reduces toxicity in Huntington’s disease models. Hum. Mol. Genet. 2006, 15, 2743–2751. [Google Scholar] [CrossRef] [PubMed]
- Varga, J.; Der, N.P.; Zsindely, N.; Bodai, L. Green tea infusion alleviates neurodegeneration induced by mutant Huntingtin in Drosophila. Nutr. Neurosci. 2020, 23, 183–189. [Google Scholar] [CrossRef]
- Beasley, M.; Stonebraker, A.R.; Hasan, I.; Kapp, K.L.; Liang, B.J.; Agarwal, G.; Groover, S.; Sedighi, F.; Legleiter, J. Lipid membranes influence the ability of small molecules to inhibit Huntingtin fibrillization. Biochemistry 2019, 58, 4361–4373. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Panuganti, V.; Jha, S.; Roy, I. Harmine acts as an indirect inhibitor of intracellular protein aggregation. ACS Omega 2020, 5, 5620–5628. [Google Scholar] [CrossRef]
- Portz, B.; Lee, B.L.; Shorter, J. FUS and TDP-43 phases in health and disease. Trends Biochem. Sci. 2021, 46, 550–563. [Google Scholar] [CrossRef]
- Ji, Y.; Li, F.; Qiao, Y. Modulating liquid-liquid phase separation of FUS: Mechanisms and strategies. J. Mater. Chem. B 2022, 10, 8616–8628. [Google Scholar] [CrossRef]
- Dormann, D.; Haass, C. Fused in sarcoma (FUS): An oncogene goes awry in neurodegeneration. Mol. Cell. Neurosci. 2013, 56, 475–486. [Google Scholar] [CrossRef]
- Reber, S.; Jutzi, D.; Lindsay, H.; Devoy, A.; Mechtersheimer, J.; Levone, B.R.; Domanski, M.; Bentmann, E.; Dormann, D.; Mühlemann, O.; et al. The phase separation-dependent FUS interactome reveals nuclear and cytoplasmic function of liquid-liquid phase separation. Nucleic Acids Res. 2021, 49, 7713–7731. [Google Scholar] [CrossRef] [PubMed]
- Carey, J.L.; Guo, L. Liquid-Liquid Phase Separation of TDP-43 and FUS in physiology and pathology of neurodegenerative diseases. Front. Mol. Biosci. 2022, 9, 826719. [Google Scholar] [CrossRef]
- Lenard, A.J.; Zhou, Q.; Madreiter-Sokolowski, C.; Bourgeois, B.; Habacher, H.; Khanna, Y.; Madl, T. EGCG promotes FUS condensate formation in a methylation-dependent manner. Cells 2022, 11, 592. [Google Scholar] [CrossRef]
- Hu, S.; Maiti, P.; Ma, Q.; Zuo, X.; Jones, M.R.; Cole, G.M.; Frautschy, S.A. Clinical development of curcumin in neurodegenerative disease. Expert. Rev. Neurother. 2015, 15, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Rigacci, S.; Stefani, M. Nutraceuticals and amyloid neurodegenerative diseases: A focus on natural phenols. Expert. Rev. Neurother. 2015, 15, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Arseneault, M.; Sanderson, T.; Murthy, V.; Ramassamy, C. Challenges for research on polyphenols from foods in Alzheimer’s disease: Bioavailability, metabolism, and cellular and molecular mechanisms. J. Agr. Food Chem. 2008, 56, 4855–4873. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Simon, J.E.; Wu, Q. A critical review on grape polyphenols for neuroprotection: Strategies to enhance bioefficacy. Crit. Rev. Food Sci. Nutr. 2020, 60, 597–625. [Google Scholar] [CrossRef]
- Bilia, A.R.; Piazzini, V.; Risaliti, L.; Vanti, G.; Casamonti, M.; Wang, M.; Bergonzi, M.C. Nanocarriers: A successful tool to increase solubility, stability and optimise bioefficacy of natural constituents. Curr. Med. Chem. 2019, 26, 4631–4656. [Google Scholar] [CrossRef] [PubMed]
- Renaud, J.; Martinoli, M.G. Considerations for the use of polyphenols as therapies in neurodegenerative diseases. Int. J. Mol. Sci. 2019, 20, 1883. [Google Scholar] [CrossRef] [PubMed]

| Compound | Chemical Structure Formula | Source | Target * | Effect on Aggregates | Reference |
|---|---|---|---|---|---|
| Curcumin | 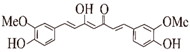 | Ginger | Aβ ②④⑤⑥ tau ①②⑥ α-Syn ①⑥ | (I) Inhibits formation (II) Disassembles aggregates | [46,47,48,49,50,51,52,53] |
| Resveratrol (RES) |  | Berries, grapes, and peanuts | Aβ ②⑥ tau ①②⑥ α-Syn ⑥ | (I) Inhibits formation (II) Disassembles aggregates | [54,55,56,57,58,59] |
| Ferulic acid (FA) | 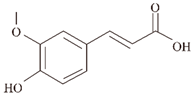 | Plants | Aβ ④⑥ | Inhibits formation | [60,61] |
| Caffeic acid (CA) | 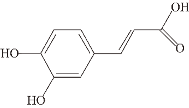 | Plants, fruits, wine, coffee, olive oil, and legumes | Aβ ②⑥ | Inhibits formation | [62] |
| Quercetin |  | Apples, berries, grapes, cherries, broccoli, and etc. | Aβ ②, tau ①②⑥ | (Ⅰ) Inhibits formation (Ⅱ) Disassembles aggregates | [63,64,65] |
| Epigallocatechin-3-gallocatechin (EGCG) |  | Green tea | Aβ ①, tau ①, α-Syn ①,Htt ①③, and FUS ① | (Ⅰ) Disassembles aggregates (Ⅱ) Inhibits formation (Ⅲ) Inhibits toxicity (Ⅳ) Remodels aggregates | [66,67,68,69,70,71,72,73] |
| Salvianolic acid A |  | Chinese herb Salvia miltiorrhiza | Aβ ② | (Ⅰ) Inhibits formation (Ⅱ) Disassembles aggregates | [74] |
| Salvianolic acid B | 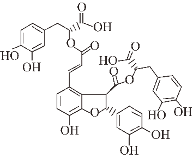 | Chinese herb Salvia miltiorrhiza | Aβ ② | (Ⅰ) Inhibits formation (Ⅱ) Disassembles aggregates | [75] |
| Gastrodin | 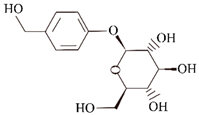 | Chinese traditional medicinal herbs tianma | Aβ ②⑥ | (Ⅰ) Inhibits formation (Ⅱ) Promotes clearance | [76] |
| Isoorientin | 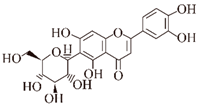 | Plants | Aβ ②⑥ | Inhibits formation | [77] |
| Rosmarinic acid |  | Plants | Aβ ⑥ | Disassembles aggregates | [78,79,80] |
| Ellagic acid | 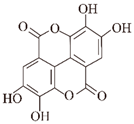 | Pomegranates, raspberries, strawberries, etc. | Htt ②⑥ | Inhibits formation | [81] |
| Fisetin |  | Fruits and vegetables | tau ①②⑥ | (Ⅰ) Inhibits formation (Ⅱ) Reduces insoluble protein | [82] |
| Crocin |  | Crocus sativus L. | tau ①②⑥ | Inhibits formation | [83] |
| Ginsenoside Rb1 | 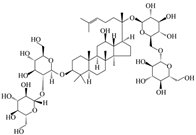 | Ginseng | α-Syn ① | (Ⅰ) Inhibits formation (Ⅱ) Disassembles aggregates | [84] |
| Gallic acid | 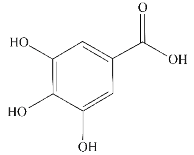 | Tea | α-Syn ① | (Ⅰ) Inhibits formation (Ⅱ) Disassembles aggregates | [85,86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, L.; Wang, Y.; Liu, S.; Liu, B.; Jin, X.; Cao, X. Targeting Protein Aggregates with Natural Products: An Optional Strategy for Neurodegenerative Diseases. Int. J. Mol. Sci. 2023, 24, 11275. https://doi.org/10.3390/ijms241411275
Xiang L, Wang Y, Liu S, Liu B, Jin X, Cao X. Targeting Protein Aggregates with Natural Products: An Optional Strategy for Neurodegenerative Diseases. International Journal of Molecular Sciences. 2023; 24(14):11275. https://doi.org/10.3390/ijms241411275
Chicago/Turabian StyleXiang, Lingzhi, Yanan Wang, Shenkui Liu, Beidong Liu, Xuejiao Jin, and Xiuling Cao. 2023. "Targeting Protein Aggregates with Natural Products: An Optional Strategy for Neurodegenerative Diseases" International Journal of Molecular Sciences 24, no. 14: 11275. https://doi.org/10.3390/ijms241411275
APA StyleXiang, L., Wang, Y., Liu, S., Liu, B., Jin, X., & Cao, X. (2023). Targeting Protein Aggregates with Natural Products: An Optional Strategy for Neurodegenerative Diseases. International Journal of Molecular Sciences, 24(14), 11275. https://doi.org/10.3390/ijms241411275







