Abstract
A previous 1H-NMR method allowed the quantification of ephedrine alkaloids; however, there were some disadvantages. The cyclized derivatives resulted from the impurities of diethyl ether were identified and benzene was selected as the better extraction solvent. The locations of ephedrine alkaloids were confirmed with 2D NMR. Therefore, a specific 1H-NMR method has been modified for the quantification of ephedrine alkaloids. Accordingly, twenty Ephedrae Herba samples could be classified into three classes: (I) E. sinica-like species; (II) E. intermedia-like species; (III) others (lower alkaloid contents). The results indicated that ephedrine and pseudoephedrine are the major alkaloids in Ephedra plants, but the concentrations vary greatly determined by the plant species and the collection locations.
1. Introduction
The genus of Ephedra belongs to the Ephedraceae family and contains more than 60 species which are widely distributed in the arid and semi-arid regions of Asia, Europe, Northern Africa (Sahara), Southwestern North America, and South America [1]. On the other hand, it is reported that there are 12 Ephedra species growing in China [2,3]. According to the Chinese Pharmacopeia, Ephedrae Herba is derived mainly from the aerial parts of E. sinica, E. equisetina, and E. intermedia [4,5,6]. Ephedrae Herba has been used in traditional Chinese medicine (TCM) for a long time as a diaphoretic, stimulant, and antiasthmatic. In addition, it could be used to treat bronchitis, acute nephritic edema, cough, and asthma, to induce perspiration, and to reduce fever [5,6].
The medicinal properties of Ephedra species could be classified based on the contents of ephedrine alkaloids, such as methylephedrine (ME) (1), ephedrine (EP) (2), norephedrine (NE) (3), norpseudoephedrine (NP) (4), methylpseudoephedrine (MP) (5), and pseudoephedrine (PE) (6) (structures as shown in Figure 1) [5,6]. It was reported that total ephedrine alkaloids found in the aerial parts of Ephedra species range from 0.02 to 3.4% [5]. The highest alkaloid content is found in E. equisetina (2.7 ± 0.6%), followed by E. intermedia (1.5 ± 0.7%) and E. sinica (1.4 ± 0.6%) [4]. E. sinica and E. equisetina mainly consist of l-ephedrine, whereas E. intermedia mainly contains d-pseudoephedrine [4]. In addition, the diverse geographical origins of the plant materials result in the total content of the main active alkaloids differing substantially from species to species [2,4].
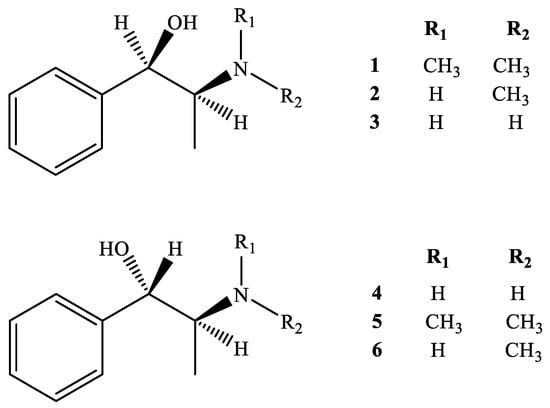
Figure 1.
Chemical structures of 1–6.
The pharmacological and toxicological effects of Ephedrae Herba depend on the individual ephedrine alkaloid type, its enantiomeric form, and receptor binding characteristics. Ephedrine (EP) is a sympathomimetic agonist at both the α- and β-adrenergic receptors, leading to an increased cardiac rate and contractility, peripheral vasoconstriction, bronchodilation, and central nervous system (CNS) stimulation [1,5]. The effects of vasoconstriction and bronchodilation explain the traditional use of Ephedra as a nasal decongestant and an anti-asthmatic [5]. However, EP enhances the release of catecholamines, thus triggering side effects on the cardiovascular system [1,7]. In recent years, dietary supplements containing Ephedrae Herba have been sold extensively for the treatment of obesity or for increasing performance in body building. These usages may be due to the CNS stimulation and thermogenic properties of EP [5]. Although EP does suppress appetite, the main mechanism for promoting weight loss appears to be by increasing the metabolic rate of adipose tissue [5]. Pseudoephedrine (PE) acts similarly, but with fewer CNS effects. Therefore, PE may be relatively safe for the treatment of non-chronic nasal congestion [1,8]. According to the Food and Drug Administration (FDA) assessment conducted in December 2003, dietary supplements that contain ephedrine alkaloids represent an unacceptable health risk. Consequently, the FDA banned usage of ephedrine alkaloids (regardless of their botanical origin) in dietary supplements [5,7]. The International Olympic Committee listed ephedrine and related compounds as stimulants in athletic sports in 2003. However, it is known that Ephedrae Herba products are often used for the treatment of colds; the International Athletic Committee has adopted a quantitative limitation for ephedrine alkaloids in urine. An athlete is regarded as “positive” for pseudoephedrine and norephedrine at 25 μg/mL, for ephedrine at 10 μg/mL, and for norpseudoephedrine at 5 μg/mL [9].
The regulated botanical origins of Chinese crude drug Mahuang (Ephedrae Herba) have been rapidly depleted because of habitat destruction and overharvesting [10]. Other Ephedra species that are not in the official pharmacopoeia, such as E. gerardiana, E. likiangensis, E. przewalskii, and E. minuta, are also used as Mahuang [11]. However, their reputation is not as good as that of the Ephedra species listed in the pharmacopoeia of China, and their ephedrine contents are usually lower according to the reports [11]. Therefore, determining the contents of ephedrine alkaloids in different Ephedra species is important to assess the quality of the crude drug and safe medication. Numerous analytical methods, such as thin-layer chromatography (TLC) [12], capillary electrophoresis (CE) [13], high-performance liquid chromatography (HPLC) [14,15,16,17,18,19,20,21,22,23], gas chromatography (GC) [9], and GC-mass spectrometry (GC-MS) [24,25], have been applied to the quantitative analysis of Ephedra alkaloids. Regarding the HPLC methods, the reversed-phase separation of basic analytes such as ephedrine alkaloids often results in broad and tailing bands that are caused by acidic sites on the column packing. The GC method has successfully separated and identified all six alkaloids. However, this method requires complex cleanup procedures and precolumn derivatization before analysis, leading to time-consuming protocols [26]. Hence, a simple, sensitive, accurate, and rapid method for the simultaneous identification and determination of Ephedra alkaloids for the quality control of Ephedra raw materials and commercial pharmaceutical prescriptions is urgently required.
High-resolution nuclear magnetic resonance (NMR) spectroscopy is regarded as a potent tool for the quality control of phytochemical preparations [27,28,29,30,31], clinical diagnosis [32], and monitoring of treatment [32]. Compared to classic analytical methods, the quantitative NMR (qNMR) offers several advantages. It allows for a rapid method implementation and simultaneous quantification of several metabolites without the standard compounds and any sample pretreatment steps. It is noninvasive, rapid, and does not require any sample pre-clean steps. In addition, it is not necessary to prepare the calibration curves based on standard compounds, and it monitors the constituents presented in herbal preparations simultaneously in a single analysis. In 2003, Kim et al. used qNMR for the quantitative analysis of four ephedrine analogues from Ephedra species including EP, PE, ME, and MP without any pre-cleaning steps [33]. Using this method, the contents of the ephedrine alkaloids can be analyzed within much shorter timeframes than the conventional chromatographic methods; however, in Kim et al., the determination was limited to four of the six Ephedra alkaloids. In 2021, Hung et. al. described a 1H-NMR spectroscopic method for the quantitative analysis of six ephedrine alkaloid derivatives in Ephedra species and related commercial traditional Chinese medicine prescriptions [34]. However, there were still some disadvantages associated with this method. First, some unusual derivatives were observed due to the utilization of ether. Second, the locations of some ephedrine alkaloid signals were confusing with 1H-NMR only. Therefore, in the present study, this method is further modified and utilized for the quantitative analysis of Ephedrae Herba samples collected from different regions. Hopefully, the developed method could be applied as the standard for the quantification of ephedrine alkaloids in the near future.
2. Results
2.1. Identification of the Cyclization Products
In our previous report, diethyl ether was selected for extraction of the ephedrine alkaloids; however, it would result in the cyclization products (Figure 2). In addition to the common ephedrine (2) and norephedrine (3), there were three characterized compounds with proton signals observed at δH 5.14 (7), 4.99 (8), 4.53 (9) ppm in the E. sinica extract (EP01), and these cyclization products could also be detected in the extracts of E. intermedia (EP03) and E. equisetina (EP02) (Figures S1 and S2). These products may be the result of the chemical reactions among the ephedrine alkaloids and the impurities of diethyl ether. The chemical structures of these cyclized ephedrine alkaloids were further determined by spectrometric and spectroscopic analyses.
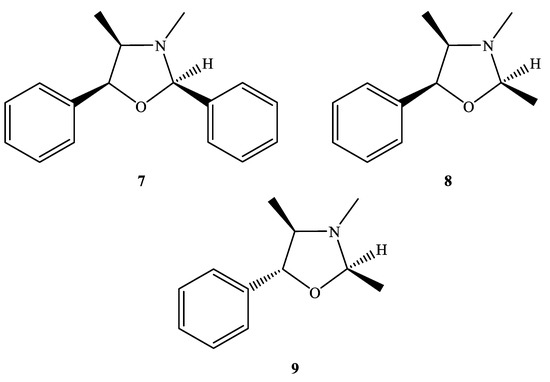
Figure 2.
Chemical structures of 7–9.
The molecular formula of compound 7 was deduced to be C17H19NO according to its ESI-TOF-MS analytical data (m/z 254.1548 for [M+H]+, calcd. for C17H20NO, 254.1545, error = 1.23 ppm) (Figure S3). Through the 1H-NMR, 13C-NMR, HMBC (Figures S4–S6), and HSQC-TOCSY (Figure 3) analyses, the structure of 7 was established to be (2R,4R,5S)-3,4-dimethyl-2,5-diphenyloxazolidine by comparison of its spectral characteristics with the literature data [35]. In addition, ephedrine (2) [36] and norephedrine (3) [37] could be confirmed with similar protocols. The molecular formula of compounds 8 and 9 were determined as C12H17NO, deduced from its ESI-TOF-MS analytical data (m/z 192.1380 for [M+H]+, calcd. for C12H18NO, 192.1388, error = −4.55 ppm) (Figure S7). Their structures were elucidated according to the spectroscopic analytical data (Figures S7–S13), and the HSQC-TOCSY spectra are shown in Figure 4 and Figure 5. In the total correlation spectroscopy (TOCSY) experiment, correlations between all protons within a given spin system were detected. Correlations are observed between remote protons as long as there are couplings between every intervening hydrogens [38]. It is very useful to identify protons of amino acids and sugars, including large molecules, such as peptides, proteins, and polysaccharides [38]. However, one of the disadvantages of TOCSY is the overlap between peaks close to one another. Heteronuclear single quantum coherence (HSQC)-TOCSY solves the major problem through resolution of crosspeaks into the 13C dimension and allows for easier characterization [39,40]. In the present case, it is very difficult to assign a chemical structure to each cyclized ephedrine alkaloid in crude extracts. After HSQC-TOCSY analysis, the fragments of rings from cyclized ephedrine alkaloids could be provided (Figure 4 and Figure 5), and the planar structures were supported by the heteronuclear multiple bond correlation (HMBC) spectrum (Figures S10 and S13). These structures could be further verified through their characteristic carbon signals (Figures S9 and S13). Therefore, 8 and 9 were identified as (2R,4R,5S)-2,3,4-trimethyl-5-phenyloxazolidine [35] and (2R,4R,5R)-2,3,4-trimethyl-5-phenyloxazolidine [35] (Figure 2), respectively, by comparison of their spectral data with those reported in the literature. The complete list of proton and carbon signals of 7–9 are provided in Table 1.
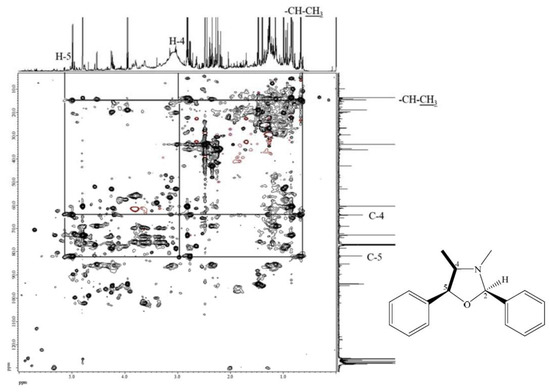
Figure 3.
HSQC-TOCSY of compound 7.
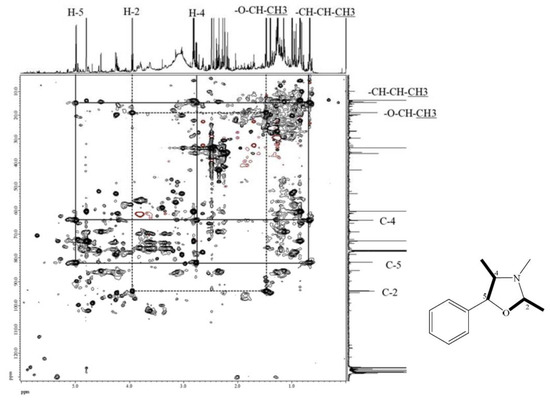
Figure 4.
HSQC-TOCSY of compound 8.
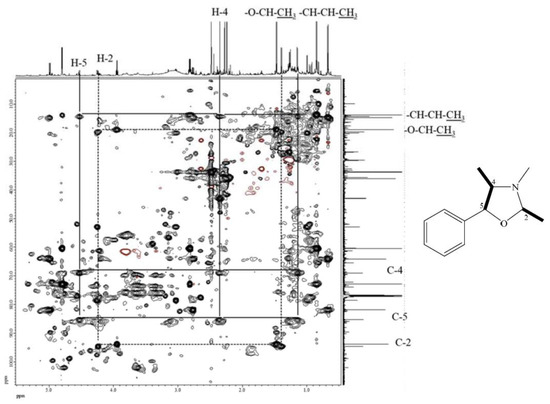
Figure 5.
HSQC-TOCSY of compound 9.

Table 1.
The key 1H-, and 13C-NMR signals of compounds 7–9.
Chloroform was also used for extraction, but it led to more impurities in the extracts (Figure S14). It produced more complex signals in the observed region for H-1 of ephedrine alkaloids at δ 4.0–5.0 ppm. Therefore, in the present study, benzene is selected as the extraction medium since few interferences at δ 4.0–5.0 ppm were observed and the resulting extracts were more pure (Figure S15).
2.2. Quantification of the Ephedrine Alkaloids
In our previous report [34] for the quantitative analysis of the six Ephedra alkaloids, i.e., methylephedrine (ME) (1), ephedrine (EP) (2), norephedrine (NE) (3), norpseudoephedrine (NP) (4), pseudoephedrine (PE) (5), and methylpseudoephedrine (MP) (6), H-1 was selected as a target signal in the 1H-NMR spectra because these signals of the ephedrine alkaloids have separate resonances and do not overlap with other signals from the extracts. Kim et al. recorded the 1H-NMR spectrum of four standards (ME, EP, MP, and PE) and analyzed their contents in Ephedra samples according to the chemical shifts in the standard spectrum [33]. However, some peaks would shift a little and even exchange their locations with neighbor alkaloids in different samples due to the interference from other constituents in the mixture. Thus, it is not easy to identify or quantify these alkaloids in these samples with 1H-NMR only. To ensure a correct NMR signal identification for each alkaloid, 1H-, HSQC-TOCSY and HMBC experiments were conducted to determine their positions (Figures S16–S23). Traditionally, TOCSY is very useful to identify protons of polymers, but often the overlapped peaks render distinction challenging [33]. To solve the problem, 2D-TOCSY is often utilized, for example, the 13C dimension of HSQC render distinction easier and more accurate (Figures S17, S19, S21 and S23). After the HSQC-TOCSY analysis of EP01, the H-1 of each alkaloid was determined and the quantification of corresponding ephedrine alkaloids was conducted.
Considering of the solubility of ephedrine alkaloids, CDCl3 was used as the solvent to ensure that all the extracts could be dissolved. The H-1 proton signal of each ephedrine alkaloid observed in the range of δ 4.0–5.0 ppm (doublet) was quite well separated from the others and did not overlap with other signals from the extract in the 1H-NMR spectrum (Figure 6). According to the literature reports and our 2D NMR analysis, the target protons (H-1) of EP and PE in the present study were resonating at δ 4.71 (d, J = 3.6 Hz) and 4.12 (d, J = 8.4 Hz), respectively. The H-1 proton signals of ME and NP are also well separated from each other and observed at δ 4.90 (d, J = 4.0 Hz) and 4.18 (d, J = 6.8 Hz), respectively. For two further alkaloids, namely NE and MP, the H-1 signals should be resonating close to δ 4.48 and 4.14; however, these peaks were not observed in this sample. Therefore, these data suggest that the H-1 signal is suitable for use as a target peak for quantification. Our results also highlight mistakes in the previous publication [33]. Although the identification of six alkaloids in Ephedrae Herba samples were unambiguous with the assisatance of 2D NMR experiments, routine determinations in other samples were achieved simply by comparison of chemical shift and coupling constants of H-1 signals of these alkaloids.
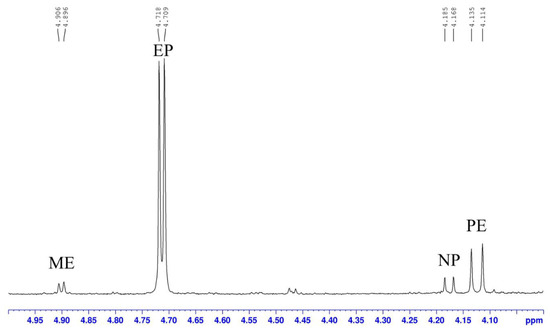
Figure 6.
The H-1 signals of ephedrine alkaloids in the expanded 1H-NMR of EP01 (recorded in CDCl3).
Twenty samples of Ephedrae Herba plants were collected from different locations in Taiwan and China. In total, eleven species of Ephedrae Herba were sampled among the commonly used medicinal plant materials in traditional Chinese medicines (Table 2). Powdered Ephedrae Herba was extracted according to the protocols described in the experimental section; the resulting weights of samples are listed in Table S1. A suitable internal standard should preferably be a stable compound with a signal in a non-crowded region of the 1H-NMR spectrum. Therefore, anthracene with a signal at δ 8.26 and the integral values remaining constant throughout 48 h was selected and added into the herbal extract samples. The 1H NMR spectra of these samples are shown in Figures S24–S43. In the case of the qNMR analysis, the amount of each alkaloid was calculated by the relative ratio of the intensity of H-1 signal to the known amount of anthracene with the Equation (1) (See Section 4.5) and the results of the ME, EP, NE, NP, and PE contents in these samples are shown in Table 3. The average recovery of EP was about 95% (in triplicate), and the LOD for EP under the present experimental parameter was 0.05 mg/mL.

Table 2.
Twenty Ephedrae Herba samples.

Table 3.
The contents of ephedrine alkaloids of different Ephedra plants.
3. Discussion
Various impurities including formaldehyde, acetaldehyde, and propionaldehyde were observed in diethyl ether even with the analytical grade chemicals [41]. It was reported that ephedrine alkaloids would be degraded under reaction with aliphatic and aromatic aldehydes [41,42,43,44,45,46]. Therefore, ephedrine derivatives were transformed into oxazolidines after extraction by diethyl ether. These cyclized products were determined by MS and NMR analysis (Figure 3, Figure 4, Figure 5 and Figures S3–S13). In the present research, benzene is selected as the extraction solvent since the extracted alkaloids were more pure than those extracted by diethyl ether (Figure S15). Although the contents of total ephedrine alkaloids are lower compared with the previous report [34], our recovery tests showed promising results for the present method. For example, in E. sinica, the content of ephedrine was reported as 8.49 mg/g, but in this study it was only 4.33 mg/g. Other Ephedra alkaloids also display the same tendency (Table 3). In the present qNMR results, total alkaloids contents were lower than those determined by HPLC [22]. It may be due to differences inherent to the plant species and collection locations. Nevertheless, the relative contents of Ephedra alkaloids could still be determined for comparison among different Ephedra species; the lower yields are not so relevant. Extraction with diethyl ether would definitely result in the cyllization products despite the higher extraction efficiency. Therefore, benzene was used for further extraction of other Ephedra plants and the obtained extracts were quantified by NMR analysis. Furthermore, no ephedrine alkaloids were observed in the residual water layers, since almost all alkaloid molecules were extracted from the organic layer under the present extraction conditions.
In total, twenty Ephedrae Herba samples were collected from different locations in Taiwan and China. After extraction following the present protocols, the obtained extracts were analyzed by the 1H-NMR spectroscopic method. Quantification results are presented in Table 3. The results indicate that ephedrine (EP) (2) and pseudoephedrine (PE) (6) are the main alkaloids in these Ephedra plants, but the concentrations of the six ephedrine alkaloids vary greatly with plant species and collection locations. Among the examined samples, eight showed total alkaloids content higher than 3 mg/g,; all of these were found to belong to E. sinica, E. equisetina, E. intermedia, E. monosperma, and E. saxatilis. The alkaloid contents of E. glauca and E. gerardiana are somewhat lower, ranging from 1 to 3 mg/g of dried plant material weight. Moreover, some samples did not contain these alkaloids, such as E. lepidosperma, E. minuta, E. przewalskii, and E. regeliana.
EP (2) was the main component in the tested samples derived from E. sinica, E. equisetina, E. gerardiana, and E. saxatilis. In contrast, in E. intermedia, E. glauca, E. przewalskii, and E. monosperma, the content of PE (6) was higher than that of EP (2). According to the Chinese pharmacopeia, Ephedrae Herba (Mahuang) is derived mainly from the aerial parts of E. sinica, E. equisetina, and E. intermedia [5]. Unfortunately, in China, the resources on which these three species depend have been rapidly depleted due to habitat destruction and overharvesting [10]. Based on the present analytical results, EP- and PE-rich species such as E. monosperma, E. saxatilis, E. glauca, and E. gerardiana could also be used as alternatives to Mahuang, even though they are not compiled in the official pharmacopoeia. According to our experimental quantification results, Ephedrae Herba samples could be classified into three classes: (I) E. sinica-like species (EP > PE), including samples EP01, EP02, EP04, EP11, EP17; (II) E. intermedia-like species (PE > EP), including samples EP03, EP08, EP10, EP12, EP16, EP18; and (III) others (lower alkaloid contents). Among the samples of Class I, EP01 contained the highest EP levels (4.33 mg/g), and EP11 presented the lowest (1.55 mg/g). Among the samples of Class II, PE was the richest in EP16 (4.41 mg/g); on the contrary, EP12 showed the lowest levels of PE (1.99 mg/g). Moreover, samples EP05, EP06, EP07, EP09, EP13, EP14, EP15, EP19, and EP20 (Class III) displayed very low alkaloid contents, with some alkaloids not detected by the present method. Kajimura et al. reported that alkaloid content was low in the early stages of stem growth [47]. Therefore, the present results may be attributed to the complex environment factors, mainly the growth periods. Furthermore, the previous report also indicates that E. przewalskii and E. lepidosperma contained few alkaloids [2]. The present results indicate that EP06 (E. intermedia), EP07, EP09 (E. przewalskii), EP13 (E. lepidosperma), EP14 (E. minuta), EP15 (E. regeliana), EP19, and EP20 did not show a significant presence of ephedrine alkaloids. Therefore, they were not suitable for use as an alternative to Mahuang.
Among the Class II samples, EP10 and EP18 were peculiar, since they were authenticated as E. equisetina and E. sinica, respectively. Based on the chemical and analytical results, they were classifed as E. intermedia-like species, since they exhibited higher levels of PE than EP. These results indicate that the two Ephedrae Herba samples may have been identified incorrectly. Based on these quantification data, the present analytical method could be considered as a feasible tool for quality control of commercial Mahuang products.
4. Materials and Methods
4.1. General
All the chemicals, unless specifically indicated otherwise, were purchased from Merck KGaA (Darmstadt, Germany). Anthracene (97.0%) and CDCl3 (99.9%) were obtained from Sigma-Aldrich (St. Louis, MO, USA). With respect to analyses, 2D NMR were recorded on the Jeol ECZ600R/S1 600 MHz (Jeol, Tokyo, Japan) and Agilent DD2 600 MHz (Agilent, Santa Clara, CA, USA), and quantitative 1H-NMR analyses were performed on the Bruker AV-400 400 MHz (Bruker, Billerica, MA, USA) NMR spectrometers with tetramethylsilane as the internal standard. Chemical shifts are reported in parts per million (ppm, δ). High resolution electrospray ionization mass spectrometry (HR-ESI-MS) were examined on a JEOL JMS-700 spectrometer (Jeol, Tokyo, Japan) that the experimental data were afforded in the positive-ion mode.
4.2. Plant Material and Authentication
Ephedrae Herba plants were collected in various regions and identified as shown in Table 2. The voucher specimen was deposited in the herbarium of the School of Pharmacy, National Cheng Kung University, Tainan, Taiwan.
4.3. 2D Analysis of the Ephedrine Alkaloids and Their Cyclization Derivatives
The powdered Ephedrae Herba samples were extracted following our previously reported protocols [34]. The resulted cyclization derivatives were observed in their 1H-NMR spectra (Figures S1 and S2). The molecular formula of cyclization derivatives were determined by HR-ESI-MS analytical data. 1H-, 13C-, HSQC-TOCSY, HMBC NMR analyses of the ephedrine alkaloids and cyclization products were recorded on the Jeol ECZ600R/S1 600 MHz NMR spectrometer with the Royal 5 mm probe.
4.4. Sample Extraction for qNMR Analysis
The extraction of alkaloids was performed according to the reported method [34] with minor modifications. Powdered Ephedrae Herba samples (~2 g) were immersed in 50 mL 0.5% HCl aqueous solution and extracted by ultrasonicator at 60 °C. After 30 min, the infusions were filtered through a suction filter. Each sample was extracted four times, and the pH of the combined solution was adjusted to 10.0 by Na2CO3; the suspension was successively obtained after centrifugation at 3000 rpm for 10 min. NaCl was added to the suspension until saturation and the suspension was then centrifuged at 3000 rpm for a further 10 min. The yielded upper layer was partitioned with benzene five times, and the combined organic solution was concentrated in vacuum to produce the Ephedrae Herba extract (Table S1).
4.5. qNMR Analysis of Ephedrine Alkaloids
Ephedrae Herba extracts and internal standard (anthracene) were dissolved in 0.6 mL CDCl3 and analyzed by Bruker AV-400 NMR spectrometer. One hundred scans were recorded in FID resolution, 0.39 Hz/point; spectrum digital resolution, 0.26 Hz/point; zero filling, 16,384; no digital filtering (apodization); spectra width, 6394 Hz; a 90 pulse was used to obtain the maximum sensitivity; relaxation delay, 20 s; acquisition time, 2.56 s. For quantitative analysis, the peak area of H-1 signals of these compounds (Table 4) were utilized since they were well separated in the region of δ 4.0–5.0 ppm, and the start and end points of the integration of each peak were selected manually. The alkaloid contents were calculated as followed.
A: Peak area of H-1 signal for each alkaloid (1H)
B: Peak area of anthracene (δH 8.36, 2H)
C: The concentration of anthracene (M)
D: The weight of herba extract
M: Molecular weight of each alkaloids

Table 4.
1H NMR signal of H-1 for the ephedrine alkaloids (δ in ppm).
Table 4.
1H NMR signal of H-1 for the ephedrine alkaloids (δ in ppm).
| Compound | H-1 |
|---|---|
| methylephedrine (ME) (1) | 4.96 |
| ephedrine (EP) (2) | 4.76 |
| norephedrine (NE) (3) | 4.52 |
| norpseudoephedrine (NP) (4) | 4.24 |
| methylpseudoephedrine (MP) (5) | 4.19 |
| pseudoephedrine (PE) (6) | 4.17 |
4.6. Recovery, Limit of Quantification (LOQ), and Limit of Detection (LOD) of Ephedrine
Recovery tests were selected to determine the accuracy of the method, in which three different concentrations of ephedrine (0.5, 1.0, 2.0 mg, respectively) were added to the sample and the recovery percentages were calculated using the measured contents divided by the contents of added standards and original sample obtained by 1H NMR analysis. A blank recovery sample was prepared and analyzed for the comparison. LOQ and LOD for ephedrine under the present NMR analytical conditions (100 scans) were determined at the signal-to-noise ratios of 10 and 3, respectively.
4.7. Characterization of Ephedrine Alkaloids by 2D NMR Analysis
The Ephedrae Herba extract was redissolved in CDCl3 and subjected to Agilent DD2 600 MHz for 2D analysis. For HMBC analysis, 64 scans were performed; the number of increments was 200. For HSQC-TOCSY examination, each experiment was scanned for 84 times; the number of increments was 128; mixing time was 80 ms. JEOL Delta V5 software was used for data processing.
5. Conclusions
In the present study, a modified 1H-NMR method is implemented successfully for the determination of six ephedrine alkaloids (1–6) in 20 samples of Ephedrae Herba collected from different regions across Taiwan and China. Benzene is selected as the better solvent for extraction of ephedrine alkaloids due to fewer interferences and no cyclization products. Furthermore, the locations of ephedrine alkaloid signals are unambiguously determined by 2D NMR analysis. The present method retains all the advantages of qNMR, including the rapid and simultaneous quantification of several targets, without the standard compounds and any sample pretreatment steps.
According to our experimental quantification results, the examined Ephedrae Herba samples could be divided into three classes: (I) E. sinica-like species (EP > PE), (II) E. intermedia-like species (PE > EP) and (III) others (lower alkaloid contents). The results showed that the main alkaloids in these Ephedra plants are ephedrine (EP) (2) and pseudoephedrine (PE) (6). Are the species containing no/low Ephedra alkaloids (Class III) botanically related? More experiments will need to be conducted to address it in the future. The concentrations of these six ephedrine alkaloids vary greatly depending on the plant species and collection location. These experimental data could provide the information related to the applicability of various Ephedra plants, in which the materials with low alkaloid contents are not suitable alternatives to Ephedrae Herba.
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms241411272/s1.
Author Contributions
Conceptualization, P.-C.K. and T.-S.W.; methodology, P.-C.K. and T.-S.W.; investigation, Y.-C.L., C.-H.W., T.H.L., G.-F.C. and M.-L.Y.; resources, Q.Y., L.H. and H.S.; data curation, Y.-C.L., T.H.L., G.-F.C., M.-L.Y., S.-H.L., H.-Y.H., Y.-H.W., Q.Y., L.H. and H.S.; writing—original draft preparation, P.-C.K. and Y.-C.L.; writing—review and editing, P.-C.K. and T.-S.W. All authors have read and agreed to the published version of the manuscript.
Funding
Authors wish to thank Kaiser Pharmaceutical Co. Ltd., Tainan, Taiwan for the financial support of the present research.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Original data can be obtained from corresponding author upon request.
Acknowledgments
Thanks are given to Rezwave Technology Inc., Taiwan for supporting Bruker TopSpin version 3.5 software. Authors also wish to thank Chia-An Tsai, Kaiser Pharmaceutical Co. Ltd., for his kind technical support.
Conflicts of Interest
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
1H-NMR: proton nuclear magnetic resonance; qNMR: quantitative nuclear magnetic resonance spectroscopy; 2D NMR: two-dimensions NMR; TCM: traditional Chinese medicine; ME: methylephedrine; EP: ephedrine; NE: norephedrine; NP: norpseudoephedrine; MP: methylpseudoephedrine; PE: pseudoephedrine; CNS: central nervous system; FDA: Food and Drug Administration; TLC: thin-layer chromatography; CE: capillary electrophoresis; HPLC: high-performance liquid chromatography; GC: gas-chromatography; GC-MS: GC-mass spectrometry; SDS: sodium dodecyl sulfate; HR-ESI-MS: High resolution electrospray ionization mass spectrometry; TOCSY: total correlation spectroscopy; HSQC: heteronuclear single quantum coherence; HMBC: heteronuclear multiple bond correlation.
References
- González-Juárez, D.E.; Escobedo-Moratilla, A.; Flores, J.; Hidalgo-Figueroa, S.; Martínez-Tagüeña, N.; Morales-Jiménez, J.; Muñiz-Ramírez, A.; Pastor-Palacios, G.; Pérez-Miranda, S.; Ramírez-Hernández, A.; et al. A review of the Ephedra genus: Distribution, ecology, ethnobotany, phytochemistry and pharmacological properties. Molecules 2020, 25, 3283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.S.; Tian, Z.; Lou, Z.C. Quality evaluation of twelve species of Chinese Ephedra (Ma huang). Yaoxue Xuebao 1989, 24, 865–871, [In Chinese, English abstract]. [Google Scholar]
- Zha, L.H.; Su, Z.G.; Zhang, G.Z.; Ouyang, F. Application and research on Ephedra resource. Zhiwuxue Tongbao 2002, 19, 396–405, [In Chinese, English abstract]. [Google Scholar]
- Hong, H.; Chen, H.-B.; Yang, D.-H.; Shang, M.-Y.; Wang, X.; Cai, S.-Q.; Mikage, M. Comparison of contents of five ephedrine alkaloids in three official origins of Ephedra herb in China by high-performance liquid chromatography. J. Nat. Med. 2011, 65, 623–628. [Google Scholar] [CrossRef]
- Abourashed, E.A.; El-Alfy, A.T.; Khan, I.A.; Walker, L. Ephedra in perspective-a current review. Phytother. Res. 2003, 17, 703–712. [Google Scholar] [CrossRef]
- Zhang, B.M.; Wang, Z.B.; Xin, P.; Wang, Q.H.; Bu, H.; Kuang, H.X. Phytochemistry and pharmacology of genus Ephedra. Chin. J. Nat. Med. 2018, 16, 811–828. [Google Scholar] [CrossRef]
- Andraws, R.; Chawla, P.; Brown, D.L. Cardiovascular effects of Ephedra alkaloids: A comprehensive review. Prog. Cardiovasc. Dis. 2005, 47, 217–225. [Google Scholar] [CrossRef]
- Sa-Ih, N.; Reakkamnuan, C.; Samerphob, N.; Cheaha, D.; Niyomdecha, S.; Kumarnsit, E. Local field potential power spectra and locomotor activity following treatment with pseudoephedrine in mice. Acta Neurobiol. Exp. 2020, 80, 19–31. [Google Scholar] [CrossRef]
- Van Eenoo, P.; Delbeke, F.T.; Roels, K.; De Backer, P. Simultaneous quantitation of ephedrines in urine by gas chromatography-nitrogen-phosphorus detection for doping control purposes. J. Chromatogr. B 2001, 760, 255–261. [Google Scholar] [CrossRef]
- Mikage, M.; Takahashi, A.; Chen, H.B.; Li, Q.S. Studies of Ephedra plants in Asia. Part 1. On the resources of Ephedra plants in China. Nat. Med. 2003, 57, 202–208. [Google Scholar]
- Long, C.; Kakiuchi, N.; Takahashi, A.; Komatsu, K.; Cai, S.; Mikage, M. Phylogenetic analysis of the DNA sequence of the non-coding region of nuclear ribosomal DNA and chloroplast of Ephedra plants in China. Planta Med. 2004, 70, 1080–1084. [Google Scholar] [CrossRef] [PubMed]
- Suedee, R.; Songkram, C.; Petmoreekul, A.; Sangkunakup, S.; Sankasa, S.; Kongyarit, N. Direct enantioseparation of adrenergic drugs via thin-layer chromatography using molecularly imprinted polymers. J. Pharm. Biomed. Anal. 1999, 19, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Mateus-Avois, L.; Mangin, P.; Saugy, M. Development and validation of a capillary zone electrophoresis method for the determination of ephedrine and related compounds in urine without extraction. J. Chromatogr. B 2003, 791, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Okamura, N.; Miki, H.; Harada, T.; Yamashita, S.; Masaoka, Y.; Nakamoto, Y.; Tsuguma, M.; Yoshitomi, H.; Yagi, A. Simultaneous determination of ephedrine, pseudoephedrine, norephedrine and methylephedrine in Kampo medicines by high-performance liquid chromatography. J. Pharm. Biomed. Anal. 1999, 20, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Li, H.X.; Ding, M.Y.; Lv, K.; Yu, J.Y. Simultaneous separation and determination of ephedrine alkaloids and tetramethylpyrazine in Ephedra sinica Stapf. by HPLC. J. Liq. Chromatogr. Relat. Technol. 2002, 25, 313–320. [Google Scholar] [CrossRef]
- Chan, K.H.; Pan, R.N.; Hsu, M.C. Simultaneous quantification of six ephedrines in a Mahwang preparation and in urine by high-performance liquid chromatography. Biomed. Chromatogr. 2005, 19, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Gurley, B.J.; Wang, P.; Gardner, S.F. Ephedrine-type alkaloid content of nutritional supplements containing Ephedra sinica (Ma-huang) as determined by high performance liquid chromatography. J. Pharm. Sci. 1998, 87, 1547–1553. [Google Scholar] [CrossRef] [PubMed]
- Imaz, C.; Carreras, D.; Navajas, R.; Rodriguez, C.; Rodriguez, A.; Maynar, J.; Cortes, R. Determination of ephedrines in urine by high-performance liquid chromatography. J. Chromatogr. 1993, 631, 201–205. [Google Scholar] [CrossRef]
- Roman, M.C. Determination of ephedrine alkaloids in botanicals and dietary supplements by HPLC-UV: Collaborative study. J. AOAC Int. 2004, 87, 1–14. [Google Scholar] [CrossRef]
- Hurlbut, J.A.; Carr, J.R.; Singleton, E.R.; Faul, K.C.; Madson, M.R.; Storey, J.M.; Thomas, T.L. Solid-phase extraction cleanup and liquid chromatography with ultraviolet detection of ephedrine alkaloids in herbal products. J. AOAC Int. 1998, 81, 1121–1127. [Google Scholar] [CrossRef]
- Ganzera, M.; Lanser, C.; Stuppner, H. Simultaneous determination of Ephedra sinica and Citrus aurantium var. amara alkaloids by ion-pair chromatography. Talanta 2005, 66, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Sheu, S.J.; Huang, M.H. Determination of Ephedra alkaloids by high-performance liquid chromatography. Chromatographia 2001, 54, 117–119. [Google Scholar] [CrossRef]
- Schaneberg, B.T.; Khan, I.A. Quantitative and qualitative HPLC analysis of thermogenic weight loss products. Pharmazie 2004, 59, 819–823. [Google Scholar] [PubMed]
- Gentili, S.; Torresi, A.; Marsili, R.; Chiarotti, M.; Macchia, T. Simultaneous detection of amphetamine-like drugs with headspace solid-phase microextraction and gas chromatography–mass spectrometry. J. Chromatogr. B 2002, 780, 183–192. [Google Scholar] [CrossRef]
- El-Haj, B.M.; Al-Amri, A.M.; Hassan, M.H.; Ali, H.S.; Bin Khadem, R.K. The use of cyclohexanone as a “derivatizing” reagent for the GC-MS detection of amphetamines and ephedrines in seizures and the urine. Forensic Sci. Int. 2003, 135, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.F.; Zhou, T.H.; Zhang, J.S.; Lou, Z.C. Analysis of alkaloids in Chinese Ephedra species by gas chromatographic methods. Phytochem. Anal. 1991, 2, 116–119. [Google Scholar] [CrossRef]
- Choi, Y.H.; Choi, H.-K.; Hazekamp, A.; Bermejo, P.; Schilder, Y.; Erkelens, C.; Verpoorte, R. Quantitative analysis of bilobalide and ginkgolides from Ginkgo biloba leaves and Ginkgo products using (1)H-NMR. Chem. Pharm. Bull. 2003, 51, 158–161. [Google Scholar] [CrossRef]
- Frédérich, M.; Choi, Y.H.; Verpoorte, R. Quantitative analysis of strychnine and brucine in Strychnos nux-vomica using 1H-NMR. Planta Med. 2003, 69, 1169–1171. [Google Scholar]
- Li, C.Y.; Lin, C.H.; Wu, C.C.; Lee, K.H.; Wu, T.S. Efficient 1H nuclear magnetic resonance method for improved quality control analyses of Ginkgo constituents. J. Agric. Food Chem. 2004, 52, 3721–3725. [Google Scholar] [CrossRef]
- Pauli, G.F.; Jaki, B.U.; Lankin, D.C. Quantitative 1H NMR: development and potential of a method for natural products analysis. J. Nat. Prod. 2005, 68, 133–149. [Google Scholar] [CrossRef]
- Li, C.Y.; Lin, C.H.; Wu, T.S. Quantitative analysis of camptothecin derivatives in Nothapodytes foetida using 1H-NMR method. Chem. Pharm. Bull. 2005, 53, 347–349. [Google Scholar] [CrossRef]
- Lindon, J.C.; Nicholson, J.K.; Everett, J.R. NMR spectroscopy of biofluids. Annu. Rep. NMR Spectrosc. 1999, 38, 1–88. [Google Scholar]
- Kim, H.K.; Choi, Y.H.; Chang, W.T.; Verpoorte, R. Quantitative analysis of ephedrine analogues from Ephedra species using 1H-NMR. Chem. Pharm. Bull. 2003, 51, 1382–1385. [Google Scholar] [CrossRef] [PubMed]
- Hung, H.-Y.; Lin, S.-M.; Li, C.-Y.; Lam, S.-H.; Chan, Y.-Y.; Liou, M.-J.; Shieh, P.-C.; Chen, F.-A.; Kuo, P.-C.; Wu, T.-S. A rapid and feasible 1H-NMR quantification method of ephedrine alkaloids in Ephedra herbal preparations. Molecules 2021, 26, 1599. [Google Scholar] [CrossRef]
- Contreras, R.; Santiesteban, F.; Paz-Sandoval, M.A.; Wrackmeyer, B. N-borane adducts of oxazolidines derived from ephedrine and pseudoephedrine. Study of stereochemistry by nuclear magnetic resonance. Tetrahedron 1984, 40, 3829–3838. [Google Scholar] [CrossRef]
- Fujita, M.; Hiyama, T. Erythro-directive reduction of α-substituted alkanones by means of hydrosilanes in acidic media. J. Org. Chem. 1988, 53, 5415–5421. [Google Scholar] [CrossRef]
- Rossi, S.; Porta, R.; Brenna, D.; Puglisi, A.; Benaglia, M. Stereoselective catalytic synthesis of active pharmaceutical ingredients in homemade 3D-printed mesoreactors. Angew. Chem. Int. Ed. 2017, 56, 4290–4294. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J.; Bryce, D.L. Relayed coherence transfer: TOCSY. In Spectrometric Identification of Organic Compounds, 8th ed.; Wiley: Hoboken, NJ, USA, 2014; pp. 254–259. [Google Scholar]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J.; Bryce, D.L. HMQC-TOCSY. In Spectrometric Identification of Organic Compounds, 8th ed.; Wiley: Hoboken, NJ, USA, 2014; p. 259. [Google Scholar]
- Kelly-Hunt, A.E.; Mehan, A.; Brooks, S.; Leanca, M.A.; McKay, J.E.D.; Mahamed, N.; Lambert, D.; Dempster, N.M.; Allen, R.J.; Evans, A.R.; et al. Synthesis and analytical characterization of purpurogallin: A pharmacologically active constituent of oak galls. J. Chem. Educ. 2022, 99, 983–993. [Google Scholar] [CrossRef]
- Beckett, A.H.; Jones, G.R. Hollingsbee DA. Degradation of (–)-ephedrine in solution and during extraction with diethyl ether. J. Pharm. Pharmacol. 1978, 30, 15–19. [Google Scholar] [CrossRef]
- Neelakantan, L. Asymmetric synthesis. II. Synthesis and absolute configuration of oxazolidines derived from (–)-ephedrine and aromatic aldehydes. J. Org. Chem. 1971, 36, 2256–2260. [Google Scholar] [CrossRef]
- Nishiyama, T.; Nishikawa, T.; Yamada, F. A stereochemical investigation of 2-methyl- and 2,5-dimethyl-3-phenyl-1,3-oxazolidines using NMR. J. Heterocycl. Chem. 1989, 26, 1687–1690. [Google Scholar] [CrossRef]
- Walker, R.B.; Wood, D.M.; Akmal, M.M.; Sharks, E. Effects of oxazolidines derived from (–) ephedrine in the rat. Gen. Pharmacol. 1992, 23, 729–732. [Google Scholar] [CrossRef] [PubMed]
- Agami, C.; Rizk, T. Role of solvent on the diastereoselectivity of oxazolidine formation from (–)-ephedrine. J. Chem. Soc. Chem. Commun. 1983, 1485–1486. [Google Scholar] [CrossRef]
- Lewis, R.J.; Huffine, E.F.; Chaturvedi, A.K.; Canfield, D.V.; Mattson, J. Formation of an interfering substance, 3,4-dimethyl-5-phenyl-1,3-oxazolidine, during a pseudoephedrine urinalysis. J. Forensic Sci. 2000, 45, 898–901. [Google Scholar] [CrossRef]
- Kajimura, K.; Iwamoto, Y.; Yamasaki, K.; Sakagami, Y.; Yokoyama, H.; Yoneda, K. Variation of growth and contents of ephedrine type alkaloids in Ephedra distachya. Nat. Med. 1994, 48, 122–125. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).