Antiproliferative, Antioxidant, Chemopreventive and Antiangiogenic Potential of Chromatographic Fractions from Anemonia sulcata with and without Its Symbiont Symbiodinium in Colorectal Cancer Therapy
Abstract
1. Introduction
2. Results
2.1. Antiproliferative Activity in CRC Cultured Cells
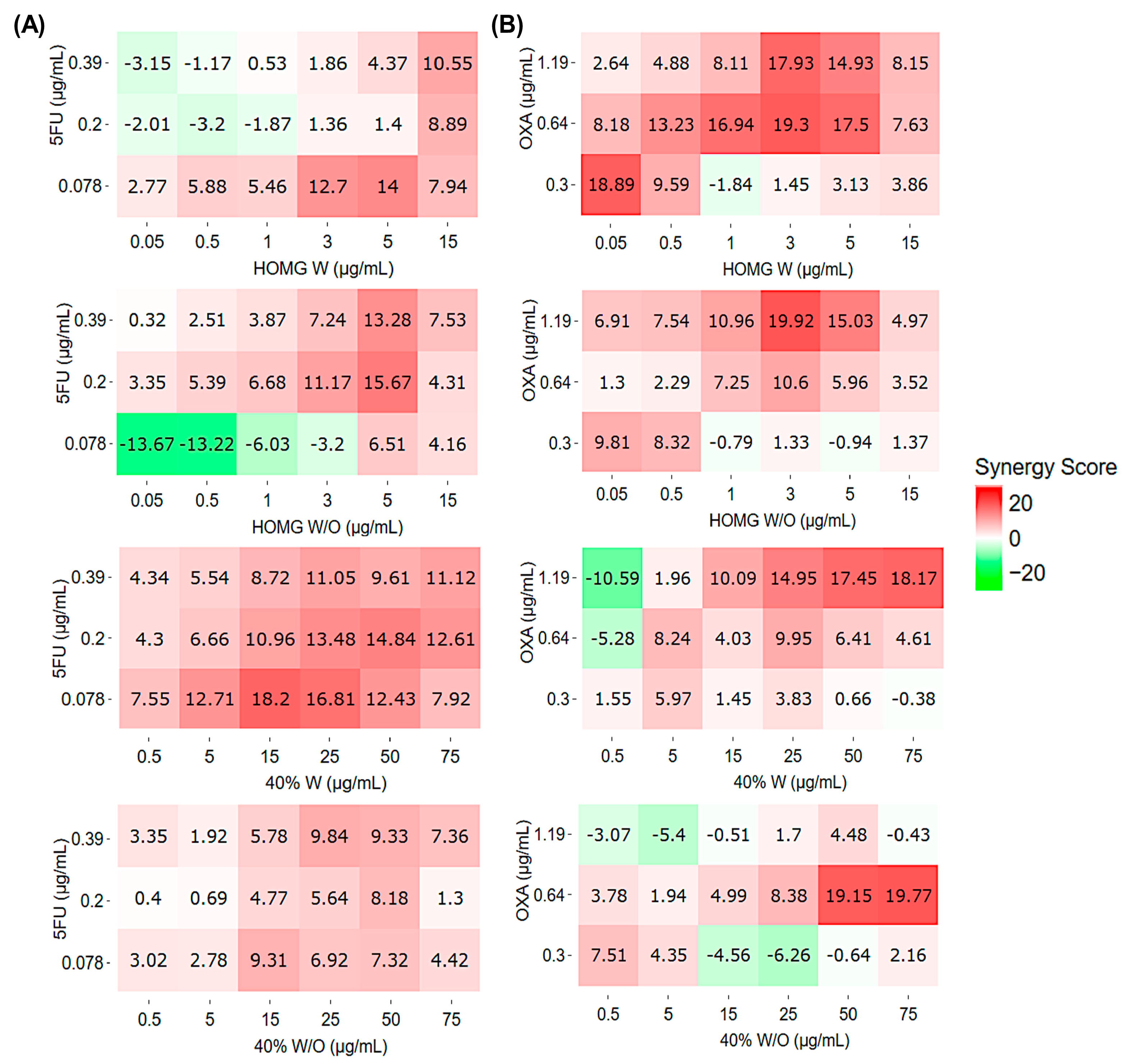
2.2. Synergistic Effect Analysis
2.3. HCT116-Tumorspheres Viability Assay
2.4. Protein Expression of Caspases 8 and 9, and PARP1
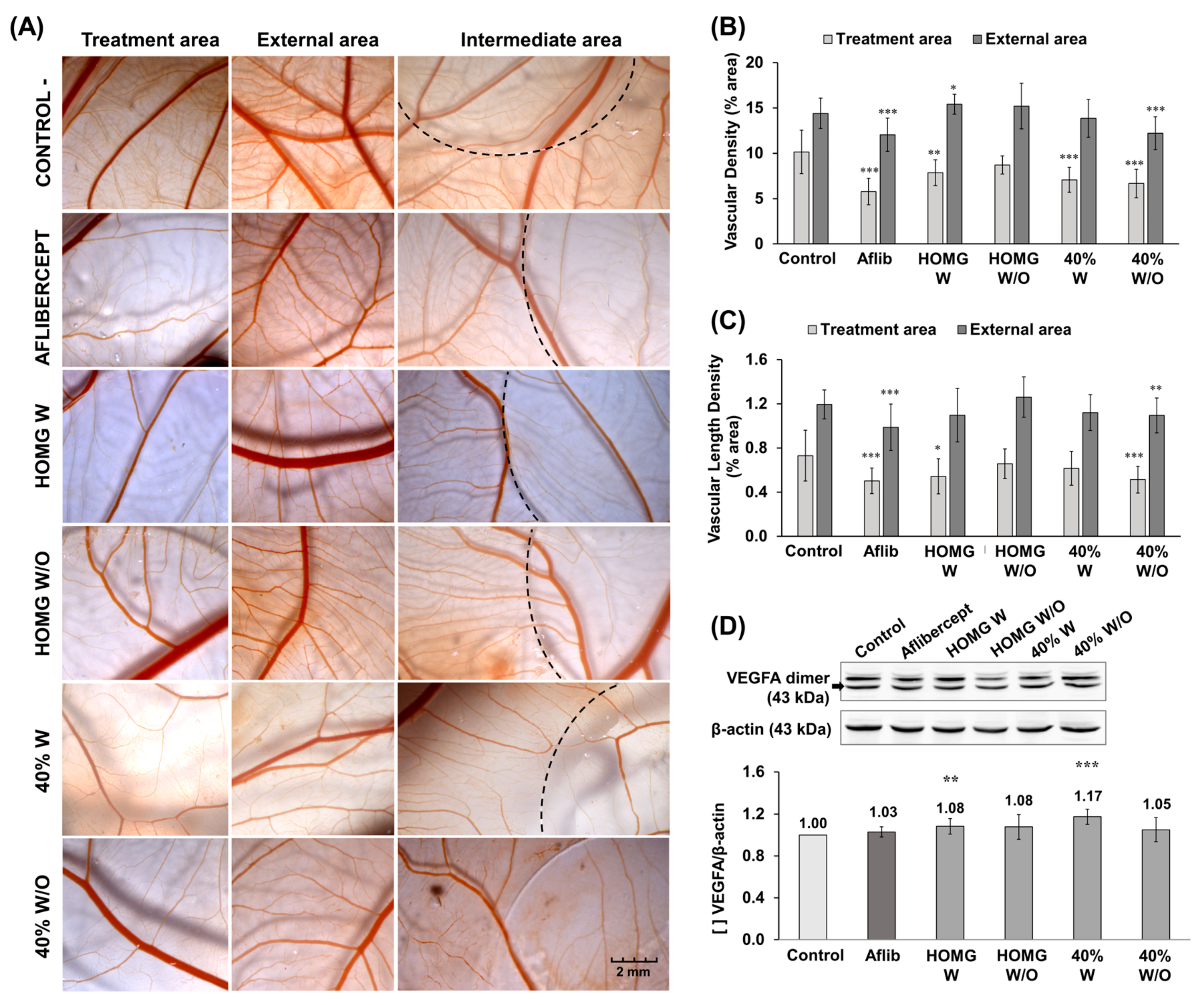
2.5. Antiangiogenic Activity
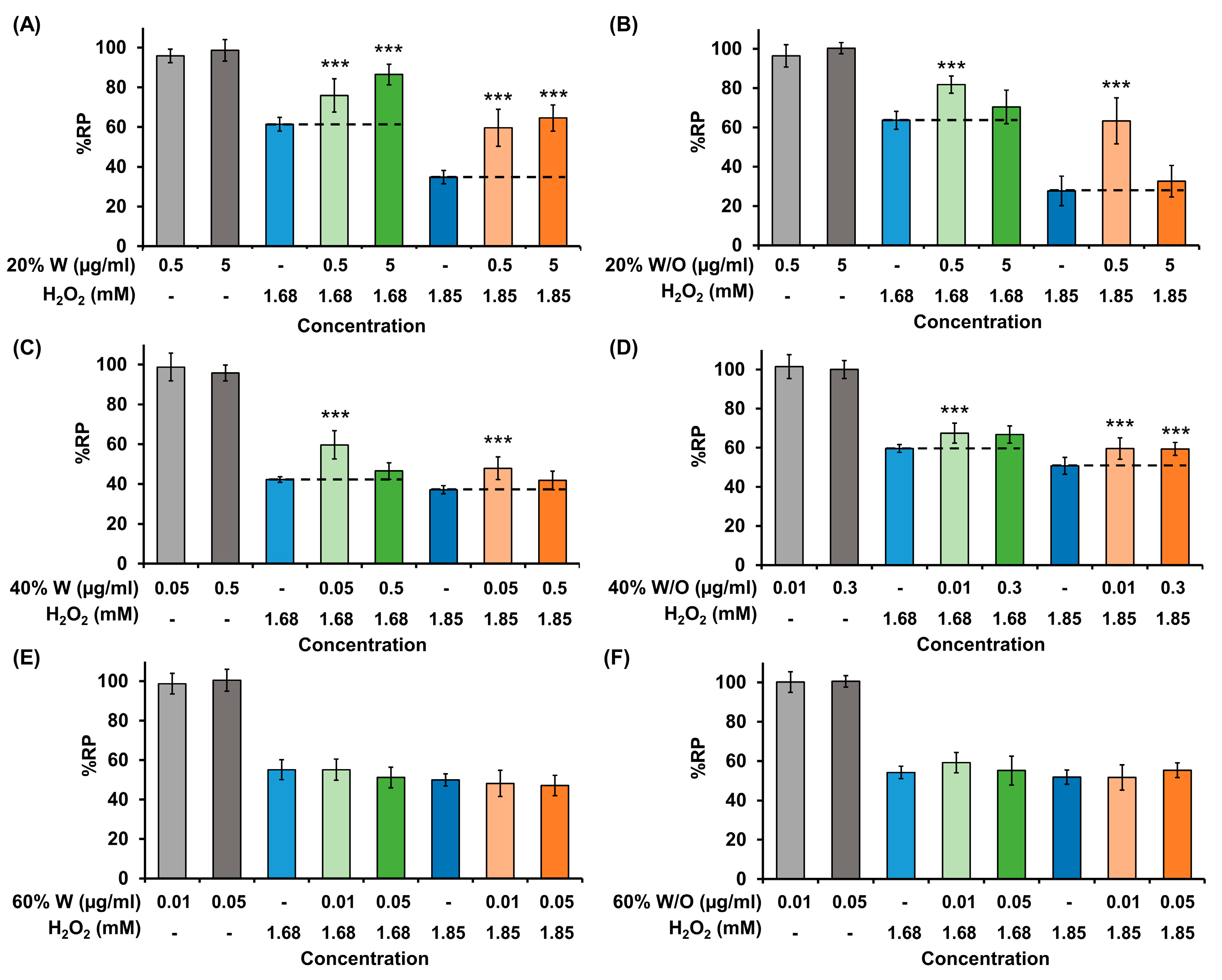
2.6. In Vitro Antioxidant Activity
2.7. Determination of Antioxidant Enzyme Induction Capacity
2.8. Determination of Detoxifying Enzyme Induction Capacity
2.9. Chemical Characterization of Active Fractions
2.9.1. Composition Analysis of the Active Fractions by HPLC-MS
2.9.2. Protein Identification by LC-MS/MS
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture
4.3. Animal Collection and Crude Homogenate Preparation
4.4. Chromatographic Fractionation of Crude Homogenate from A. sulcata
4.5. Cell Viability Assay
4.6. Synergistic Effect Analysis
4.7. Sphere Formation, Characterization, and Viability Assays
4.7.1. Sphere Formation Assay
4.7.2. Real Time Quantitative PCR (qPCR)
4.7.3. HCT116-Tumorspheres Viability Assay
4.8. Angiogenesis Assay—Chick Chorioallantoic Membrane (CAM) Assay
4.9. Western Blot Analysis (VEGFA, Caspase 8 and 9, PARP1 Expression)
4.10. Determination of In Vitro Antioxidant Activity
4.11. Determination of Antioxidant and Detoxifying Enzymes Induction Capacity
4.11.1. Treatment and Purification of the Cytosolic Fraction
4.11.2. Antioxidant Enzyme Assays
4.11.3. Detoxifying Enzyme Assays
4.12. Chemical characterization of Active Fractions
4.12.1. HPLC-MS Analysis
4.12.2. Protein Identification by LC-MS/MS (nLC-IT)
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haque, N.; Parveen, S.; Tang, T.; Wei, J.; Huang, Z. Marine Natural Products in Clinical Use. Mar. Drugs 2022, 20, 528. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, P.C.; Wilke, D.V.; Branco, P.C.; Bauermeister, A.; Rezende-Teixeira, P.; Gaudêncio, S.P.; Costa-Lotufo, L.V. Enriching Cancer Pharmacology with Drugs of Marine Origin. Br. J. Pharmacol. 2020, 177, 3–27. [Google Scholar] [CrossRef] [PubMed]
- Ilhan, H.A.; Pulat, Ç.Ç. Cytotoxic and Antitumor Compounds from Marine Invertebrates. In Encyclopedia of Marine Biotechnology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; pp. 2529–2584. ISBN 978-1-119-14380-2. [Google Scholar]
- Leal, M.C.; Puga, J.; Serôdio, J.; Gomes, N.C.M.; Calado, R. Trends in the Discovery of New Marine Natural Products from Invertebrates over the Last Two Decades—Where and What Are We Bioprospecting? PLoS ONE 2012, 7, e30580. [Google Scholar] [CrossRef]
- Casado-Amezúa, P.; Terrón-Sigler, A.; Pinzón, J.H.; Furla, P.; Forcioli, D.; Allemand, D.; Ribes, M.; Coma, R. General Ecological Aspects of Anthozoan-Symbiodinium Interactions in the Mediterranean Sea. In The Cnidaria, Past, Present and Future: The World of Medusa and Her Sisters; Goffredo, S., Dubinsky, Z., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 375–386. ISBN 978-3-319-31305-4. [Google Scholar]
- Revel, J.; Massi, L.; Mehiri, M.; Boutoute, M.; Mayzaud, P.; Capron, L.; Sabourault, C. Differential Distribution of Lipids in Epidermis, Gastrodermis and Hosted Symbiodinium in the Sea Anemone Anemonia Viridis. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2016, 191, 140–151. [Google Scholar] [CrossRef]
- Bosmans, F.; Tytgat, J. Sea Anemone Venom as a Source of Insecticidal Peptides Acting on Voltage-Gated Na+ Channels. Toxicon 2007, 49, 550–560. [Google Scholar] [CrossRef]
- Silva, T.C.; de Andrade, P.B.; Paiva-Martins, F.; Valentão, P.; Pereira, D.M. In Vitro Anti-Inflammatory and Cytotoxic Effects of Aqueous Extracts from the Edible Sea Anemones Anemonia Sulcata and Actinia Equina. Int. J. Mol. Sci. 2017, 18, 653. [Google Scholar] [CrossRef]
- Piccialli, I.; Tedeschi, V.; Boscia, F.; Ciccone, R.; Casamassa, A.; de Rosa, V.; Grieco, P.; Secondo, A.; Pannaccione, A. The Anemonia Sulcata Toxin BDS-I Protects Astrocytes Exposed to Aβ1-42 Oligomers by Restoring [Ca2+]i Transients and ER Ca2+ Signaling. Toxins 2020, 13, 20. [Google Scholar] [CrossRef]
- Cabeza, L.; Peña, M.; Martínez, R.; Mesas, C.; Galisteo, M.; Perazzoli, G.; Prados, J.; Porres, J.M.; Melguizo, C. Anemonia Sulcata and Its Symbiont Symbiodinium as a Source of Anti-Tumor and Anti-Oxidant Compounds for Colon Cancer Therapy: A Preliminary In Vitro Study. Biology 2021, 10, 134. [Google Scholar] [CrossRef]
- Bulati, M.; Longo, A.; Masullo, T.; Vlah, S.; Bennici, C.; Bonura, A.; Salamone, M.; Tagliavia, M.; Nicosia, A.; Mazzola, S.; et al. Partially Purified Extracts of Sea Anemone Anemonia Viridis Affect the Growth and Viability of Selected Tumour Cell Lines. BioMed Res. Int. 2016, 2016, 3849897. [Google Scholar] [CrossRef]
- Loret, E.P.; Luis, J.; Nuccio, C.; Villard, C.; Mansuelle, P.; Lebrun, R.; Villard, P.H. A Low Molecular Weight Protein from the Sea Anemone Anemonia Viridis with an Anti-Angiogenic Activity. Mar. Drugs 2018, 16, 134. [Google Scholar] [CrossRef]
- Merle, P.-L.; Sabourault, C.; Richier, S.; Allemand, D.; Furla, P. Catalase Characterization and Implication in Bleaching of a Symbiotic Sea Anemone. Free Radic. Biol. Med. 2007, 42, 236–246. [Google Scholar] [CrossRef]
- Pey, A.; Zamoum, T.; Christen, R.; Merle, P.-L.; Furla, P. Characterization of Glutathione Peroxidase Diversity in the Symbiotic Sea Anemone Anemonia Viridis. Biochimie 2017, 132, 94–101. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; Van Krieken, J.H.; Aderka, D.; Aranda Aguilar, E.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO Consensus Guidelines for the Management of Patients with Metastatic Colorectal Cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef]
- Weng, J.; Li, S.; Zhu, Z.; Liu, Q.; Zhang, R.; Yang, Y.; Li, X. Exploring Immunotherapy in Colorectal Cancer. J. Hematol. Oncol. 2022, 15, 95. [Google Scholar] [CrossRef]
- Marzagalli, M.; Fontana, F.; Raimondi, M.; Limonta, P. Cancer Stem Cells—Key Players in Tumor Relapse. Cancers 2021, 13, 376. [Google Scholar] [CrossRef]
- Salehi, B.; Selamoglu, Z.; Mileski, S.K.; Pezzani, R.; Redaelli, M.; Cho, W.C.; Kobarfard, F.; Rajabi, S.; Martorell, M.; Kumar, P.; et al. Liposomal Cytarabine as Cancer Therapy: From Chemistry to Medicine. Biomolecules 2019, 9, 773. [Google Scholar] [CrossRef]
- Tanni, K.A.; Truong, C.B.; Johnson, B.S.; Qian, J. Comparative Effectiveness and Safety of Eribulin in Advanced or Metastatic Breast Cancer: A Systematic Review and Meta-Analysis. Crit. Rev. Oncol. Hematol. 2021, 163, 103375. [Google Scholar] [CrossRef]
- Wang, J.; Wang, P.; Zeng, Z.; Lin, C.; Lin, Y.; Cao, D.; Ma, W.; Xu, W.; Xiang, Q.; Luo, L.; et al. Trabectedin in Cancers: Mechanisms and Clinical Applications. Curr. Pharm. Des. 2022, 28, 1949–1965. [Google Scholar] [CrossRef]
- Fares Amer, N.; Luzzatto Knaan, T. Natural Products of Marine Origin for the Treatment of Colorectal and Pancreatic Cancers: Mechanisms and Potential. Int. J. Mol. Sci. 2022, 23, 8048. [Google Scholar] [CrossRef]
- Han, N.; Li, J.; Li, X. Natural Marine Products: Anti-Colorectal Cancer In Vitro and In Vivo. Mar. Drugs 2022, 20, 349. [Google Scholar] [CrossRef] [PubMed]
- Manmuan, S.; Manmuan, P. Fucoxanthin Enhances 5-FU Chemotherapeutic Efficacy in Colorectal Cancer Cells by Affecting MMP-9 Invasive Proteins. J. Appl. Pharm. Sci. 2019, 9, 7–14. [Google Scholar] [CrossRef]
- Ramos, A.A.; Almeida, T.; Lima, B.; Rocha, E. Cytotoxic Activity of the Seaweed Compound Fucosterol, Alone and in Combination with 5-Fluorouracil, in Colon Cells Using 2D and 3D Culturing. J. Toxicol. Environ. Health Part A 2019, 82, 537–549. [Google Scholar] [CrossRef]
- Lopes-Costa, E.; Abreu, M.; Gargiulo, D.; Rocha, E.; Ramos, A.A. Anticancer Effects of Seaweed Compounds Fucoxanthin and Phloroglucinol, Alone and in Combination with 5-Fluorouracil in Colon Cells. J. Toxicol. Environ. Health Part A 2017, 80, 776–787. [Google Scholar] [CrossRef]
- Tsai, H.-L.; Tai, C.-J.; Huang, C.-W.; Chang, F.-R.; Wang, J.-Y. Efficacy of Low-Molecular-Weight Fucoidan as a Supplemental Therapy in Metastatic Colorectal Cancer Patients: A Double-Blind Randomized Controlled Trial. Mar. Drugs 2017, 15, 122. [Google Scholar] [CrossRef]
- Gbolahan, O.B.; O’Neil, B.H.; McRee, A.J.; Sanoff, H.K.; Fallon, J.K.; Smith, P.C.; Ivanova, A.; Moore, D.T.; Dumond, J.; Asher, G.N. A Phase I Evaluation of the Effect of Curcumin on Dose-limiting Toxicity and Pharmacokinetics of Irinotecan in Participants with Solid Tumors. Clin. Transl. Sci. 2022, 15, 1304–1315. [Google Scholar] [CrossRef]
- Howells, L.M.; Iwuji, C.O.O.; Irving, G.R.B.; Barber, S.; Walter, H.; Sidat, Z.; Griffin-Teall, N.; Singh, R.; Foreman, N.; Patel, S.R.; et al. Curcumin Combined with FOLFOX Chemotherapy Is Safe and Tolerable in Patients with Metastatic Colorectal Cancer in a Randomized Phase IIa Trial. J. Nutr. 2019, 149, 1133–1139. [Google Scholar] [CrossRef]
- Xu, R.; Wu, J.; Zhang, X.; Zou, X.; Li, C.; Wang, H.; Yuan, M.; Chen, M.; Sun, Q.; Liu, S. Modified Bu-Zhong-Yi-Qi Decoction Synergies with 5 Fluorouracile to Inhibits Gastric Cancer Progress via PD-1/PD- L1-Dependent T Cell Immunization. Pharmacol. Res. 2020, 152, 104623. [Google Scholar] [CrossRef]
- Bai, Y.; Xiong, Y.; Zhang, Y.-Y.; Cheng, L.; Liu, H.; Xu, K.; Wu, Y.-Y.; Field, J.; Wang, X.-D.; Zhou, L.-M. Tangeretin Synergizes with 5-Fluorouracil to Induce Autophagy through MicroRNA-21 in Colorectal Cancer Cells. Am. J. Chin. Med. 2022, 50, 1681–1701. [Google Scholar] [CrossRef]
- Tian, S.; Su, R.; Wu, K.; Zhou, X.; Vadgama, J.V.; Wu, Y. Diaporine Potentiates the Anticancer Effects of Oxaliplatin and Doxorubicin on Liver Cancer Cells. J. Pers. Med. 2022, 12, 1318. [Google Scholar] [CrossRef]
- Genovese, S.; Epifano, F.; Preziuso, F.; Slater, J.; Nangia-Makker, P.; Majumdar, A.P.N.; Fiorito, S. Gercumin Synergizes the Action of 5-Fluorouracil and Oxaliplatin against Chemoresistant Human Cancer Colon Cells. Biochem. Biophys. Res. Commun. 2020, 522, 95–99. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Miao, Z.-H. Marine-Derived Angiogenesis Inhibitors for Cancer Therapy. Mar. Drugs 2013, 11, 903–933. [Google Scholar] [CrossRef]
- Elez, E.; Gomez-Roca, C.; Soto Matos-Pita, A.; Argiles, G.; Valentin, T.; Coronado, C.; Iglesias, J.; Macarulla, T.; Betrian, S.; Fudio, S.; et al. First-in-Human Phase I Study of the Microtubule Inhibitor Plocabulin in Patients with Advanced Solid Tumors. Investig. New Drugs 2019, 37, 674–683. [Google Scholar] [CrossRef]
- Aspeslagh, S.; Awada, A.; Matos-Pita, S.A.; Aftimos, P.; Bahleda, R.; Varga, A.; Soria, J.-C. Phase I Dose-Escalation Study of Plitidepsin in Combination with Bevacizumab in Patients with Refractory Solid Tumors. Anticancer Drugs 2016, 27, 1021–1027. [Google Scholar] [CrossRef]
- Twelves, C.; Hoekman, K.; Bowman, A.; Vermorken, J.B.; Anthoney, A.; Smyth, J.; van Kesteren, C.; Beijnen, J.H.; Uiters, J.; Wanders, J.; et al. Phase I and Pharmacokinetic Study of Yondelis (Ecteinascidin-743; ET-743) Administered as an Infusion over 1 h or 3 h Every 21 Days in Patients with Solid Tumours. Eur. J. Cancer 2003, 39, 1842–1851. [Google Scholar] [CrossRef]
- Vilar, E.; Grünwald, V.; Schöffski, P.; Singer, H.; Salazar, R.; Iglesias, J.L.; Casado, E.; Cullell-Young, M.; Baselga, J.; Tabernero, J. A Phase I Dose-Escalating Study of ES-285, a Marine Sphingolipid-Derived Compound, with Repeat Dose Administration in Patients with Advanced Solid Tumors. Investig. New Drugs 2012, 30, 299–305. [Google Scholar] [CrossRef]
- Sahlberg, S.H.; Spiegelberg, D.; Glimelius, B.; Stenerlöw, B.; Nestor, M. Evaluation of Cancer Stem Cell Markers CD133, CD44, CD24: Association with AKT Isoforms and Radiation Resistance in Colon Cancer Cells. PLoS ONE 2014, 9, e94621. [Google Scholar] [CrossRef]
- Takeda, K.; Mizushima, T.; Yokoyama, Y.; Hirose, H.; Wu, X.; Qian, Y.; Ikehata, K.; Miyoshi, N.; Takahashi, H.; Haraguchi, N.; et al. Sox2 Is Associated with Cancer Stem-like Properties in Colorectal Cancer. Sci. Rep. 2018, 8, 17639. [Google Scholar] [CrossRef]
- Roudi, R.; Barodabi, M.; Madjd, Z.; Roviello, G.; Corona, S.P.; Panahi, M. Expression Patterns and Clinical Significance of the Potential Cancer Stem Cell Markers OCT4 and NANOG in Colorectal Cancer Patients. Mol. Cell. Oncol. 2020, 7, 1788366. [Google Scholar] [CrossRef]
- Terasaki, M.; Maeda, H.; Miyashita, K.; Tanaka, T.; Miyamoto, S.; Mutoh, M. A Marine Bio-Functional Lipid, Fucoxanthinol, Attenuates Human Colorectal Cancer Stem-like Cell Tumorigenicity and Sphere Formation. J. Clin. Biochem. Nutr. 2017, 61, 25–32. [Google Scholar] [CrossRef]
- Terasaki, M.; Matsumoto, N.; Hashimoto, R.; Endo, T.; Maeda, H.; Hamada, J.; Osada, K.; Miyashita, K.; Mutoh, M. Fucoxanthin Administration Delays Occurrence of Tumors in Xenograft Mice by Colonospheres, with an Anti-Tumor Predictor of Glycine. J. Clin. Biochem. Nutr. 2019, 64, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.; Silva, J.; Afonso, M.B.; Guedes, R.A.; Guedes, R.C.; Alvariño, R.; Pinteus, S.; Gaspar, H.; Goettert, M.I.; Alfonso, A.; et al. Disclosing the Antitumour Potential of the Marine Bromoditerpene Sphaerococcenol A on Distinct Cancer Cellular Models. Biomed. Pharmacother. 2022, 149, 112886. [Google Scholar] [CrossRef] [PubMed]
- Vizetto-Duarte, C.; Castelo-Branco, P.; Custódio, L. Marine Natural Products as a Promising Source of Therapeutic Compounds to Target Cancer Stem Cells. Curr. Med. Chem. 2021, 28, 4343–4355. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Xie, H.; Shen, W.; Shao, L.; Zeng, L.; Huang, X.; Zhu, Q.; Zhai, X.; Li, K.; Qiu, Z.; et al. The Synergism of Natural Compounds and Conventional Therapeutics against Colorectal Cancer Progression and Metastasis. Front. Biosci.-Landmark 2022, 27, 263. [Google Scholar] [CrossRef]
- Jeong, S.; Kim, D.Y.; Kang, S.H.; Yun, H.K.; Kim, J.L.; Kim, B.R.; Park, S.H.; Na, Y.J.; Jo, M.J.; Jeong, Y.A.; et al. Docosahexaenoic Acid Enhances Oxaliplatin-Induced Autophagic Cell Death via the ER Stress/Sesn2 Pathway in Colorectal Cancer. Cancers 2019, 11, 982. [Google Scholar] [CrossRef]
- Nagata, S. Apoptosis and Clearance of Apoptotic Cells. Annu. Rev. Immunol. 2018, 36, 489–517. [Google Scholar] [CrossRef]
- Mashimo, M.; Onishi, M.; Uno, A.; Tanimichi, A.; Nobeyama, A.; Mori, M.; Yamada, S.; Negi, S.; Bu, X.; Kato, J.; et al. The 89-KDa PARP1 Cleavage Fragment Serves as a Cytoplasmic PAR Carrier to Induce AIF-Mediated Apoptosis. J. Biol. Chem. 2021, 296, 100046. [Google Scholar] [CrossRef]
- Chaitanya, G.V.; Alexander, J.S.; Babu, P.P. PARP-1 Cleavage Fragments: Signatures of Cell-Death Proteases in Neurodegeneration. Cell Commun. Signal. 2010, 8, 31. [Google Scholar] [CrossRef]
- Zhang, Q.-T.; Liu, Z.-D.; Wang, Z.; Wang, T.; Wang, N.; Wang, N.; Zhang, B.; Zhao, Y.-F. Recent Advances in Small Peptides of Marine Origin in Cancer Therapy. Mar. Drugs 2021, 19, 115. [Google Scholar] [CrossRef]
- Ding, G.-F.; Huang, F.-F.; Yang, Z.-S.; Yu, D.; Yang, Y.-F. Anticancer Activity of an Oligopeptide Isolated from Hydrolysates of Sepia Ink. Chin. J. Nat. Med. 2011, 9, 151–155. [Google Scholar] [CrossRef]
- Quah, Y.; Mohd Ismail, N.I.; Ooi, J.L.S.; Affendi, Y.A.; Abd Manan, F.; Teh, L.-K.; Wong, F.-C.; Chai, T.-T. Purification and Identification of Novel Cytotoxic Oligopeptides from Soft Coral Sarcophyton Glaucum. J. Zhejiang Univ. Sci. B 2019, 20, 59–70. [Google Scholar] [CrossRef]
- Ahn, E.H.; Schroeder, J.J. Induction of Apoptosis by Sphingosine, Sphinganine, and C2-Ceramide in Human Colon Cancer Cells, but Not by C2-Dihydroceramide. Anticancer Res. 2010, 30, 2881–2884. [Google Scholar]
- D’Ambra, I.; Lauritano, C. A Review of Toxins from Cnidaria. Mar. Drugs 2020, 18, 507. [Google Scholar] [CrossRef]
- Brackenbury, W.J. Voltage-Gated Sodium Channels and Metastatic Disease. Channels 2012, 6, 352–361. [Google Scholar] [CrossRef]
- House, C.D.; Vaske, C.J.; Schwartz, A.M.; Obias, V.; Frank, B.; Luu, T.; Sarvazyan, N.; Irby, R.; Strausberg, R.L.; Hales, T.G.; et al. Voltage-Gated Na+ Channel SCN5A Is a Key Regulator of a Gene Transcriptional Network That Controls Colon Cancer Invasion. Cancer Res. 2010, 70, 6957–6967. [Google Scholar] [CrossRef]
- Lopez-Charcas, O.; Poisson, L.; Benouna, O.; Lemoine, R.; Chadet, S.; Pétereau, A.; Lahlou, W.; Guyétant, S.; Ouaissi, M.; Pukkanasut, P.; et al. Voltage-Gated Sodium Channel NaV1.5 Controls NHE-1-Dependent Invasive Properties in Colon Cancer Cells. Cancers 2022, 15, 46. [Google Scholar] [CrossRef]
- Angus, M.; Ruben, P. Voltage Gated Sodium Channels in Cancer and Their Potential Mechanisms of Action. Channels 2019, 13, 400–409. [Google Scholar] [CrossRef]
- Mao, W.; Zhang, J.; Körner, H.; Jiang, Y.; Ying, S. The Emerging Role of Voltage-Gated Sodium Channels in Tumor Biology. Front. Oncol. 2019, 9, 124. [Google Scholar]
- Zúñiga, L.; Cayo, A.; González, W.; Vilos, C.; Zúñiga, R. Potassium Channels as a Target for Cancer Therapy: Current Perspectives. OncoTargets Ther. 2022, 15, 783–797. [Google Scholar] [CrossRef]
- Angi, B.; Muccioli, S.; Szabò, I.; Leanza, L. A Meta-Analysis Study to Infer Voltage-Gated K+ Channels Prognostic Value in Different Cancer Types. Antioxidants 2023, 12, 573. [Google Scholar] [CrossRef]
- Castañeda, O.; Harvey, A.L. Discovery and Characterization of Cnidarian Peptide Toxins That Affect Neuronal Potassium Ion Channels. Toxicon Off. J. Int. Soc. Toxinol. 2009, 54, 1119–1124. [Google Scholar] [CrossRef]
- Song, M.S.; Park, S.M.; Park, J.S.; Byun, J.H.; Jin, H.J.; Seo, S.H.; Ryu, P.D.; Lee, S.Y. Kv3.1 and Kv3.4, Are Involved in Cancer Cell Migration and Invasion. Int. J. Mol. Sci. 2018, 19, 1061. [Google Scholar] [CrossRef] [PubMed]
- Ousingsawat, J.; Spitzner, M.; Puntheeranurak, S.; Terracciano, L.; Tornillo, L.; Bubendorf, L.; Kunzelmann, K.; Schreiber, R. Expression of Voltage-Gated Potassium Channels in Human and Mouse Colonic Carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2007, 13, 824–831. [Google Scholar] [CrossRef]
- Spitzner, M.; Ousingsawat, J.; Scheidt, K.; Kunzelmann, K.; Schreiber, R. Voltage-Gated K+ Channels Support Proliferation of Colonic Carcinoma Cells. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2007, 21, 35–44. [Google Scholar] [CrossRef]
- Pons-Cursach, R.; Casanovas, O. Mechanisms of Anti-Angiogenic Therapy. In Tumor Angiogenesis: A Key Target for Cancer Therapy; Marmé, D., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–25. ISBN 978-3-319-31215-6. [Google Scholar]
- Nieberler, M.; Reuning, U.; Reichart, F.; Notni, J.; Wester, H.-J.; Schwaiger, M.; Weinmüller, M.; Räder, A.; Steiger, K.; Kessler, H. Exploring the Role of RGD-Recognizing Integrins in Cancer. Cancers 2017, 9, 116. [Google Scholar] [CrossRef]
- Gothai, S.; Muniandy, K.; Gnanaraj, C.; Ibrahim, I.A.A.; Shahzad, N.; Al-Ghamdi, S.S.; Ayoub, N.; Veeraraghavan, V.P.; Kumar, S.S.; Esa, N.M.; et al. Pharmacological Insights into Antioxidants against Colorectal Cancer: A Detailed Review of the Possible Mechanisms. Biomed. Pharmacother. 2018, 107, 1514–1522. [Google Scholar] [CrossRef]
- Tarrant, A.M.; Reitzel, A.M.; Kwok, C.K.; Jenny, M.J. Activation of the Cnidarian Oxidative Stress Response by Ultraviolet Radiation, Polycyclic Aromatic Hydrocarbons and Crude Oil. J. Exp. Biol. 2014, 217, 1444–1453. [Google Scholar] [CrossRef]
- Dyshlovoy, S.A. Recent Updates on Marine Cancer-Preventive Compounds. Mar. Drugs 2021, 19, 558. [Google Scholar] [CrossRef]
- Nelson, M.M.; Phleger, C.F.; Mooney, B.D.; Nichols, P.D. Lipids of Gelatinous Antarctic Zooplankton: Cnidaria and Ctenophora. Lipids 2000, 35, 551–559. [Google Scholar] [CrossRef]
- Zhukova, N.V.; Titlyanov, E.A. Fatty Acid Variations in Symbiotic Dinoflagellates from Okinawan Corals. Phytochemistry 2003, 62, 191–195. [Google Scholar] [CrossRef]
- da Costa, E.; Amaro, H.M.; Melo, T.; Guedes, A.C.; Domingues, M.R. Screening for Polar Lipids, Antioxidant, and Anti-Inflammatory Activities of Gloeothece Sp. Lipid Extracts Pursuing New Phytochemicals from Cyanobacteria. J. Appl. Phycol. 2020, 32, 3015–3030. [Google Scholar] [CrossRef]
- da Costa, E.; Melo, T.; Reis, M.; Domingues, P.; Calado, R.; Abreu, M.H.; Domingues, M.R. Polar Lipids Composition, Antioxidant and Anti-Inflammatory Activities of the Atlantic Red Seaweed Grateloupia Turuturu. Mar. Drugs 2021, 19, 414. [Google Scholar] [CrossRef]
- Lee, S.; Lee, Y.S.; Jung, S.H.; Kang, S.S.; Shin, K.H. Anti-Oxidant Activities of Fucosterol from the Marine Algae Pelvetia Siliquosa. Arch. Pharm. Res. 2003, 26, 719–722. [Google Scholar] [CrossRef]
- Treignier, C.; Grover, R.; Ferrier-Pagés, C.; Tolosa, I. Effect of Light and Feeding on the Fatty Acid and Sterol Composition of Zooxanthellae and Host Tissue Isolated from the Scleractinian Coral Turbinaria Reniformis. Limnol. Oceanogr. 2008, 53, 2702–2710. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, W.; Aldahdooh, J.; Malyutina, A.; Shadbahr, T.; Tanoli, Z.; Pessia, A.; Tang, J. SynergyFinder Plus: Toward Better Interpretation and Annotation of Drug Combination Screening Datasets. Genom. Proteom. Bioinform. 2022, 20, 587–596. [Google Scholar] [CrossRef]
- Feng, Y.; Dai, X.; Li, X.; Wang, H.; Liu, J.; Zhang, J.; Du, Y.; Xia, L. EGF Signalling Pathway Regulates Colon Cancer Stem Cell Proliferation and Apoptosis. Cell Prolif. 2012, 45, 413–419. [Google Scholar] [CrossRef]
- Fuel, M.; Mesas, C.; Martínez, R.; Ortiz, R.; Quiñonero, F.; Prados, J.; Porres, J.M.; Melguizo, C. Antioxidant and Antiproliferative Potential of Ethanolic Extracts from Moringa Oleifera, Tropaeolum Tuberosum and Annona Cherimola in Colorrectal Cancer Cells. Biomed. Pharmacother. 2021, 143, 112248. [Google Scholar] [CrossRef]
- Martínez, R.; García-Beltrán, A.; Kapravelou, G.; Mesas, C.; Cabeza, L.; Perazzoli, G.; Guarnizo, P.; Rodríguez-López, A.; Andrés Vallejo, R.; Galisteo, M.; et al. In Vivo Nutritional Assessment of the Microalga Nannochloropsis Gaditana and Evaluation of the Antioxidant and Antiproliferative Capacity of Its Functional Extracts. Mar. Drugs 2022, 20, 318. [Google Scholar] [CrossRef]
- Ukeda, H.; Maeda, S.; Ishii, T.; Sawamura, M. Spectrophotometric Assay for Superoxide Dismutase Based on Tetrazolium Salt 3′-{1-[(Phenylamino)-Carbonyl]-3,4-Tetrazolium}-Bis(4-Methoxy-6-Nitro)Benzenesulfonic Acid Hydrate Reduction by Xanthine–Xanthine Oxidase. Anal. Biochem. 1997, 251, 206–209. [Google Scholar] [CrossRef]
- Cohen, G.; Kim, M.; Ogwu, V. A Modified Catalase Assay Suitable for a Plate Reader and for the Analysis of Brain Cell Cultures. J. Neurosci. Methods 1996, 67, 53–56. [Google Scholar]
- Lawrence, R.A.; Sunde, R.A.; Schwartz, G.L.; Hoekstra, W.G. Glutathione Peroxidase Activity in Rat Lens and Other Tissues in Relation to Dietary Selenium Intake. Exp. Eye Res. 1974, 18, 563–569. [Google Scholar] [CrossRef] [PubMed]



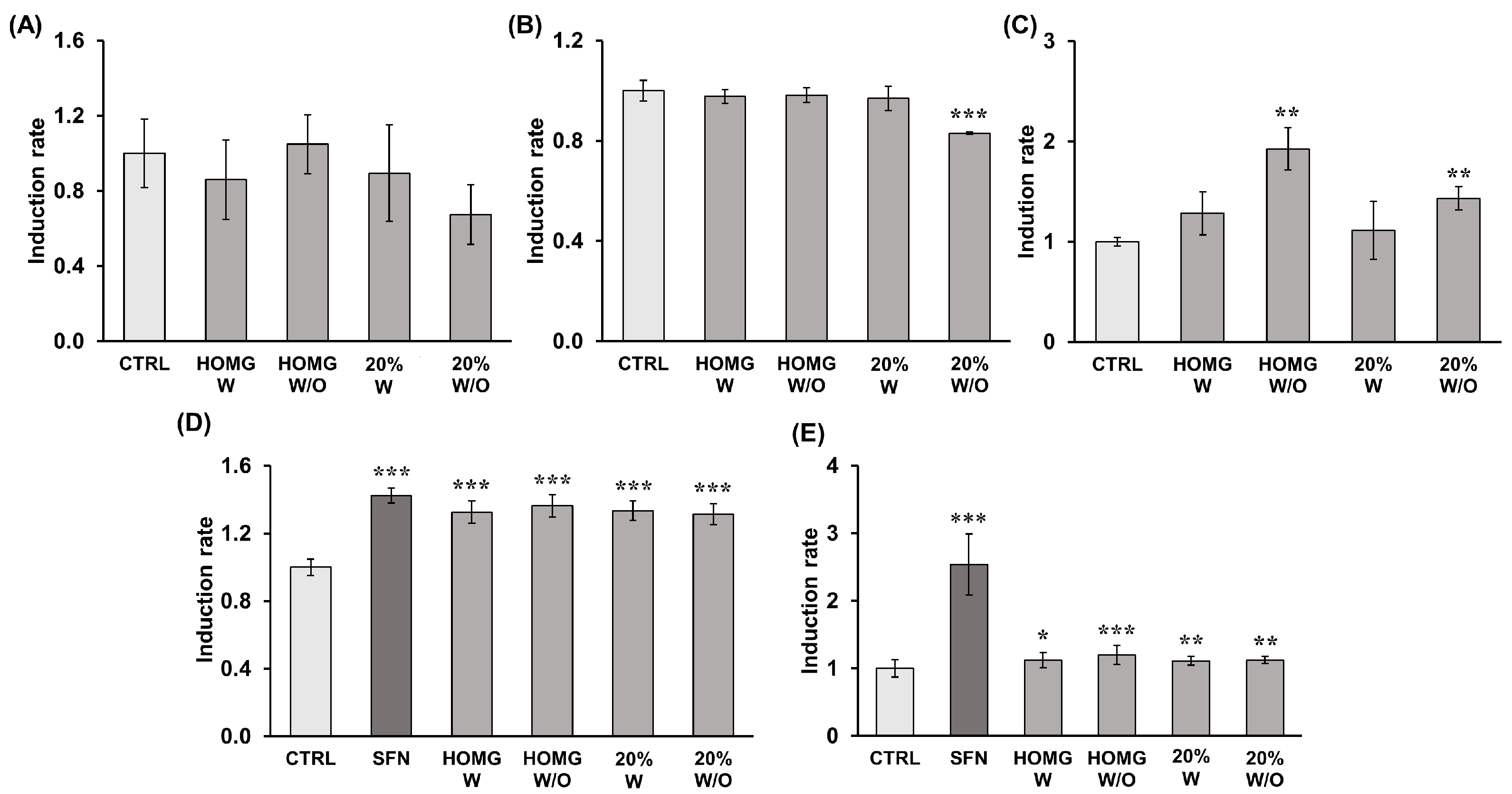

| Antioxidant Activity | ||||||
|---|---|---|---|---|---|---|
| * CAT Activity | * SOD Activity | * GPX Activity | ||||
| UA/mg protein | Induction Rate | UA/mg protein | Induction Rate | nmol NADPH/min/mg protein | Induction Rate | |
| Control | 1.39 ± 0.25 | 1.00 ± 0.18 | 3.43 ± 0.14 | 1.00 ± 0.04 | 4.68 ± 0.19 | 1.00 ± 0.04 |
| Homg W | 1.19 ± 0.29 | 0.86 ± 0.21 | 3.36 ± 0.09 | 0.98 ± 0.03 | 6.00 ± 1.00 | 1.28 ± 0.21 |
| Homg W/O | 1.45 ± 0.22 | 1.05 ± 0.16 | 3.37 ± 0.10 | 0.98 ± 0.03 | 9.01 ± 0.99 | 1.93 ± 0.21 |
| 20% W | 1.24 ± 0.36 | 0.89 ± 0.26 | 3.33 ± 0.16 | 0.97 ± 0.05 | 5.21 ± 1.35 | 1.11 ± 0.29 |
| 20% W/O | 0.93 ± 0.22 | 0.67 ± 0.16 | 2.85 ± 0.01 | 0.83 ± 0.00 | 6.70 ± 0.54 | 1.43 ± 0.12 |
| Detoxifying Capacity | ||||||
| * GST activity | * QR activity | |||||
| UA/mg protein | Induction Rate | UA/mg protein | Induction Rate | |||
| Control | 89.65 ± 4.40 | 1 ± 0.049 | 967.58 ± 122.52 | 1.00 ± 0.126 | ||
| Sulforaphane | 127.63 ± 3.88 | 1.42 ± 0.043 | 2456.88 ± 435.17 | 2.54 ± 0.450 | ||
| Homg W | 118.92 ± 6.04 | 1.33 ± 0.067 | 1082.25 ± 108.94 | 1.12 ± 0.113 | ||
| Homg W/O | 122.24 ± 5.95 | 1.36 ± 0.066 | 1162.42 ± 136.91 | 1.20 ± 0.141 | ||
| 20% W | 119.70 ± 5.13 | 1.34 ± 0.057 | 1075.27 ± 61.97 | 1.11 ± 0.064 | ||
| 20% W/O | 117.78 ± 5.61 | 1.31 ± 0.063 | 1087.00 ± 51.83 | 1.12 ± 0.054 | ||
| Family | UniProt Accession Number | Protein | Organism | Mass (Da) | Score | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Recommended Name | Short Name | Alternative Name | 20% W | 20% W/O | 40% W | 40% W/O | ||||
| Sea anemone sodium channel (Nav) inhibitory toxin family. Type I subfamily | P0DL49 | Delta-actitoxin-Avd1c 1 | Delta-AITX-Avd1c 1 | A. viridis toxin 2 (Av2; Avt2) Neurotoxin 2 Toxin 2c1; Toxin 2c4; Toxin Av2-1 | A. viridis | 8999 | 21 ± 0 | - | - | 265 ± 38 |
| B1NWT7 | Delta-actitoxin-Avd1c 4 | Delta-AITX-Avd1c 4 | Toxin 2c2 Toxin 2c3 | A. viridis | 8870 | - | - | - | 238 | |
| P01529 | Delta-actitoxin-Avd1d | Delta-AITX-Avd1d | Neurotoxin 5 ATX-V; As5; Toxin V | A. sulcata | 5222 | - | - | 1017 ± 210 | 278 | |
| P0DL52 | Delta-actitoxin-Avd1e 1 | Delta-AITX-Avd1e 1 | Neurotoxin 2; Av2 Toxin 2-4; Toxin 2c5 | A. viridis | 9013 | - | - | 1253 ± 179 | 264 ± 50 | |
| P0DMZ1 | Delta-actitoxin-Avd1h | Delta-AITX-Avd1h | Neurotoxin 1-1 | A. viridis | 9116 | - | - | 83 ± 24 | - | |
| Sea anemone sodium channel (Nav) inhibitory toxin family | A0A0S1M162 | Sodium channel toxin protein, partial | - | - | A. sulcata | 9234 | - | - | 291 ± 71 | 278 ± 27 |
| Sea anemone type 1 potassium channel (Kv1) toxin family. Type 1b subfamily | Q9TWG1 | Kappa-actitoxin-Avd6a | Kappa-AITX-Avd6a | Kaliseptine (AsKS) | A. sulcata | 4180 | 26 ± 5 | 53 ± 9 | - | - |
| P0DN00 | U-actitoxin-Avd9a | U-AITX-Avd9a | Potassium channel toxin Avtx-6 | A. viridis | 9383 | - | - | - | 23 | |
| Sea anemone type 1 potassium channel (Kv1) toxin family | A0A0S1M179 | Type I potassium channel toxin protein | - | - | A. sulcata | 7957 | - | - | 73 ± 28 | 65 ± 40 |
| Venom Kunitz-type family. Sea anemone type 2 potassium channel (Kv2) toxin subfamily | Q9TWG0 | KappaPI-actitoxin-Avd3b | KappaPI-AITX-Avd3b | Kunitz-type serine protease inhibitor Kalicludine-1 (AsKC1) | A. sulcata | 7028 | 639 ± 51 | 1248 ± 48 | 784 ± 33 | 997 ± 166 |
| Q9TWF9 | KappaPI-actitoxin-Avd3c | KappaPI-AITX-Avd3c | Kalicludine-2 (AsKC2) | A. sulcata | 7115 | 545 ± 59 | 1009 ± 68 | 725 | 700 | |
| Q9TWF8 | KappaPI-actitoxin-Avd3d | KappaPI-AITX-Avd3d | Kalicludine-3 (AsKC3) | A. sulcata | 7075 | 440 ± 91 | 418 ± 3 | 524 ± 26 | 478 ± 89 | |
| P0DN07 | U-actitoxin-Avd3g | U-AITX-Avd3g | AsKC4 | A. viridis | 9709 | 294 ± 38 | 415 ± 32 | 386 ± 51 | 414 ± 77 | |
| P0DN13 | U-actitoxin-Avd3l | U-AITX-Avd3l | AsKC9 | A. viridis | 9813 | 278 ± 12 | 435 ± 36 | 443 ± 65 | 438 ± 106 | |
| P0DN15 | U-actitoxin-Avd3n | U-AITX-Avd3n | AsKC11 | A. viridis | 10,398 | - | - | 307 | - | |
| P0DN16 | U-actitoxin-Avd3o | U-AITX-Avd3o | AsKC12 | A. viridis | 10,415 | - | - | 310 ± 48 | - | |
| Sea anemone type 3 (BDS) potassium channel (Kv3) toxin family | P0DMX7 | Kappa-actitoxin-Avd4c | Kappa-AITX-Avd4c | Blood depressing substance 3 (BDS-3) | A. viridis | 8895 | 486 | - | 1778 ± 312 | 2116 |
| P0DMX8 | Kappa-actitoxin-Avd4d | Kappa-AITX-Avd4d | Blood depressing substance 4 (BDS-4) | A. viridis | 8939 | 200 | - | 1963 ± 408 | 2164 ± 165 | |
| P0DMX9 | Kappa-actitoxin-Avd4e | Kappa-AITX-Avd4e | Blood depressing substance 5 (BDS-5) | A. viridis | 8827 | 215 | 621 ± 28 | - | 1843 ± 135 | |
| P0DMY1 | Kappa-actitoxin-Avd4g | Kappa-AITX-Avd4g | Blood depressing substance 7 (BDS-7) | A. viridis | 8807 | - | - | 840 ± 101 | - | |
| P0DMY4 | Kappa-actitoxin-Avd4j | Kappa-AITX-Avd4j | Blood depressing substance 10 (BDS-10) | A. viridis | 9492 | - | - | 482 ± 44 | 625 | |
| P0DMY7 | Kappa-actitoxin-Avd4m | Kappa-AITX-Avd4m | Blood depressing substance 13 (BDS-13) | A. viridis | 8845 | 121 ± 41 | 20 | 727 ± 46 | 810 ± 19 | |
| A0A0S1M190 | Type III potassium channel toxin protein, partial | - | - | A. sulcata | 8923 | - | - | 1767 ± 311 | 1947 ± 202 | |
| A0A0S1M170 | Type III potassium channel toxin protein, partial | - | - | A. sulcata | 9520 | - | - | 481 ± 44 | 662 ± 34 | |
| A0A0S1M169 | Type III potassium channel toxin protein, partial | - | - | A. sulcata | 9195 | - | - | 200 ± 28 | 1058 ± 118 | |
| A0A0S1M1A6 | Type III potassium channel toxin protein | - | - | A. sulcata | 9539 | - | - | 214 ± 3 | 466 ± 170 | |
| A0A0S1M174 | Type III potassium channel toxin protein, partial | - | - | A. sulcata | 9436 | - | - | 66 ± 17 | 50 ± 14 | |
| A0A0S1M194 | Type III potassium channel toxin protein | - | - | A. sulcata | 7882 | - | - | - | 67 ± 25 | |
| A0A0S1M195 | Type III potassium channel toxin protein | - | - | A. sulcata | 8327 | - | - | - | 36 | |
| A0A0S1M185 | Type III potassium channel toxin protein | - | - | A. sulcata | 8284 | - | - | 26 | - | |
| Non-classical Kazal-type elastase inhibitor | P16895 | PI-actitoxin-Avd5a | PI-AITX-Avd5a | Non-classical Kazal-type elastase inhibitor | A. sulcata | 5472 | - | - | 60 | 27 |
| Sea anemone 8 toxin family | P0DMZ3 | U-actitoxin-Avd8a | U-AITX-Avd8a | Avtx-1 | A. viridis | 9128 | - | - | 521 ± 46 | 530 ± 46 |
| P0DMZ6 | U-actitoxin-Avd8d | U-AITX-Avd8d | Avtx-4 | A. viridis | 9527 | 47 ± 24 | 50 ± 12 | 155 ± 35 | 174 ± 6 | |
| P0DMZ7 | U-actitoxin-Avd8e | U-AITX-Avd8e | Avtx-5 | A. viridis | 9874 | - | - | 37 ± 3 | 85 | |
| Sea anemone structural class 9a family | P0DMZ8 | U-actitoxin-Avd13a/b Cleaved into 2 chains: Avd13a and Avd13b | U-AITX-Avd13a/b | Peptide toxin AV-1 | A. viridis | 18,716 | 71 | 195 ± 38 | - | 149 ± 77 |
| Small cysteine-rich protein (SCRiP) family | P0DL61 | Small cysteine-rich protein (Fragment) | Avir_SCRiP; SCRiP | - | A. viridis | 7717 | 35 ± 5 | - | 21 | 71 ± 2 |
| Family | UniProt Accession Number | Protein | Organism | Mass (Da) | Score | |||||
| Recommended name | Short name | Alternative name | 20% W | 20% W/O | 40% W | 40% W/O | ||||
| Antioxidant enzymes | Q8I807 | Copper/zinc superoxide dismutase (CuZnSODb) | A. viridis | 15,950 | 131 ± 35 | 108 ± 6 | 924 ± 117 | 526 ± 9 | ||
| A0EJ86 | Catalase | A. viridis | 57,729 | 30 ± 5 | 112 ± 29 | 483 ± 105 | 580 ± 164 | |||
| A0A1D8RF20 | Glutathione peroxidase | A. viridis | 28,042 | - | 31 ± 7 | 163 ± 69 | 203 ± 75 | |||
| A0A1D8RF24 | Glutathione peroxidase | A. viridis | 18,957 | - | - | 42 ± 23 | 26 | |||
| Q9GPI6 | Green fluorescent protein as(S)FP499—Chain A | A. sulcata | 25,401 | 390 ± 78 | 1224 ± 50 | 3282 ± 242 | 2547 ± 141 | |||
| Q9GZ28 | GFP-like non-fluorescent chromoprotein FP595—Cleaved into 2 chains: Chain 1 and chain 2 | A. sulcata | 26,301 | 333 ± 99 | 1018 ± 44 | 2340 ± 498 | 2213 ± 210 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peña, M.; Mesas, C.; Perazzoli, G.; Martínez, R.; Porres, J.M.; Doello, K.; Prados, J.; Melguizo, C.; Cabeza, L. Antiproliferative, Antioxidant, Chemopreventive and Antiangiogenic Potential of Chromatographic Fractions from Anemonia sulcata with and without Its Symbiont Symbiodinium in Colorectal Cancer Therapy. Int. J. Mol. Sci. 2023, 24, 11249. https://doi.org/10.3390/ijms241411249
Peña M, Mesas C, Perazzoli G, Martínez R, Porres JM, Doello K, Prados J, Melguizo C, Cabeza L. Antiproliferative, Antioxidant, Chemopreventive and Antiangiogenic Potential of Chromatographic Fractions from Anemonia sulcata with and without Its Symbiont Symbiodinium in Colorectal Cancer Therapy. International Journal of Molecular Sciences. 2023; 24(14):11249. https://doi.org/10.3390/ijms241411249
Chicago/Turabian StylePeña, Mercedes, Cristina Mesas, Gloria Perazzoli, Rosario Martínez, Jesús M. Porres, Kevin Doello, Jose Prados, Consolación Melguizo, and Laura Cabeza. 2023. "Antiproliferative, Antioxidant, Chemopreventive and Antiangiogenic Potential of Chromatographic Fractions from Anemonia sulcata with and without Its Symbiont Symbiodinium in Colorectal Cancer Therapy" International Journal of Molecular Sciences 24, no. 14: 11249. https://doi.org/10.3390/ijms241411249
APA StylePeña, M., Mesas, C., Perazzoli, G., Martínez, R., Porres, J. M., Doello, K., Prados, J., Melguizo, C., & Cabeza, L. (2023). Antiproliferative, Antioxidant, Chemopreventive and Antiangiogenic Potential of Chromatographic Fractions from Anemonia sulcata with and without Its Symbiont Symbiodinium in Colorectal Cancer Therapy. International Journal of Molecular Sciences, 24(14), 11249. https://doi.org/10.3390/ijms241411249







