Abstract
Nitric oxide (NO) is a key signaling molecule in almost all organisms and is active in a variety of physiological and pathological processes. Our understanding of the peculiarities and functions of this simple gas has increased considerably by extending studies to non-mammal vertebrates and invertebrates. In this review, we report the nitric oxide synthase (Nos) genes so far characterized in chordates and provide an extensive, detailed, and comparative analysis of the function of NO in the aquatic chordates tunicates, cephalochordates, teleost fishes, and amphibians. This comprehensive set of data adds new elements to our understanding of Nos evolution, from the single gene commonly found in invertebrates to the three genes present in vertebrates.
1. Introduction
Thanks to its peculiar features, nitric oxide (NO) has attracted a good deal of interest in recent decades. NO is a short-lived, fast-diffusing signal molecule acting in almost all organisms, including bacteria, plants, fungi, and metazoa [1,2,3]. Dissolved NO diffuses readily across plasmatic membrane and interacts with DNA, lipids, and proteins of neighboring cells [2].
NO importance in human physiology and pathology, recognized by the Nobel Prize award in 1998, has increased significantly through the discovery that this simple gas is intimately involved in regulating all aspects of human lives. Nowadays, it is clear that NO is involved in a large variety of vertebrates’ physiological processes, including proliferation, differentiation, apoptosis, macrophage activity, neurotransmission, cardiovascular homeostasis, and several pathological conditions, such as stroke, diabetes, and cancer [4]. Parallel studies performed in invertebrate animals have widened our knowledge of the various roles played by NO in different species (for a review, see [5]), revealing the ubiquitous occurrence and biological functions of this compound [3].
Unlikely, different methodologies with different levels of reliability are used to detect Nos in the different organisms so far investigated. Immunochemistry assays that do not have species-specific antibodies or an indirect NADPH-diaphorase method are generally less specific than the detection of mRNA. Consequently, in some cases, the results may not be congruent and therefore cannot always be generalized.
The majority of chordates, a monophyletic clade [6,7], live in aquatic environments, ranging from oceans to inland waters to estuaries, and are therefore adapted to a wide range of physicochemical conditions [8]. In this review, we report the function of NO in aquatic chordates, including tunicates, cephalochordates, teleost fishes, and amphibians, and highlight the conserved NO roles. This review mainly deals with the Nos-dependent NO production and function as a major component of NO homeostasis, also providing information on Nos genes found in chordates to obtain further insights into Nos evolution.
2. NO Homeostasis
NO is one of the main actors in the cellular redox landscape of living organisms, and its homeostasis is of paramount importance for its functional roles at all levels in an organism, from the molecular to the whole body (Figure 1). As a signaling molecule, NO typically exerts its physiological functions by (i) reversibly binding to hemoproteins, forming an iron-nitrosyl complex; (ii) S-nitrosylation of proteins (thiol residues); and (iii) reacting with amines (N-nitroso compounds) [9]. Thioldisulfide exchange reactions modulate protein structure and function and maintain cellular redox homeostasis [10]. The soluble guanylate cyclase (sGC), an almost ubiquitous hemoprotein, is a primary target of NO. The binding with NO via its ferrous heme activates by 100- to 200-fold the sGC enzyme [11]. Interestingly, among small diatomic signaling molecules, NO binds five-coordinate ferrous heme species, unlike O2 and CO, which bind to six-coordinate species [3]. NO can also bind to non-heme iron and copper ions [3]. The latter can be important in modulating cytochrome c oxidase and mitochondrial respiration [12,13,14].
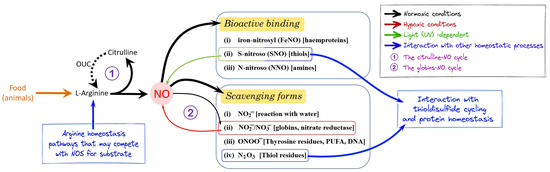
Figure 1.
Schematic resuming of all major transformations putatively involved in nitric oxide (NO) homeostasis. Under normoxic conditions, NO is synthesized by Nos enzymes from L-arginine. In organisms possessing the ornithine–urea cycle (OUC), the co-product of the reaction, L-citrulline, is reconverted to L-arginine (the citrulline–NO cycle). NO signaling may occur by (i) forming an iron-nitrosyl complex with hemoproteins; (ii) promoting protein post-translational modifications via S-nitrosylation, forming S-nitroso compounds with thiol residues (SNO); and (iii) forming N-nitroso (NNO) compounds. NO can be (i) rapidly converted to nitrite with dissolved O2 by NO scavenging reactions or can be (ii) inactivated to nitrate by oxygenated heme proteins. Excess NO (iii) can react with superoxide to form peroxynitrite (ONOO−), which in turn can interact with lipids, DNA, and proteins to give nitrated derivatives or (iv) react with oxygen to form N2O3, which interacts with thiol groups to give nitrosylated proteins. When de novo production via Nos is compromised under low O2, NO can be produced by nitrite reduction in heme proteins (the globin–NO cycle), possibly associated with nitrate conversion to nitrite by xanthine oxidoreductase. The interactions with arginine and protein homeostasis may be significant in determining the balance between NO formation and scavenging.
NO needs to be produced in the proximity of the site of action being very reactive, and its diffusion gradient spans relatively short distances due to a series of scavenging reactions (Figure 1) [15]. In the presence of oxygen, NO may (i) rapidly react with water forming nitrite (NO2−); (ii) be inactivated to nitrate (NO3−) by oxygenated globins (as hemoglobin, myoglobin, and neuroglobin); (iii) generate peroxynitrite (ONOO−) or dinitrogen trioxide (N2O3), which in turn may form reactive nitrogen species and affect the cellular redox state. All reactive nitrogen species produced in these scavenging processes can contribute to protein, lipid, and DNA nitration [16], protein S-nitrosylation [9], and S-glutathionylation [17], thus affecting protein homeostasis [10,18].
Under normoxic conditions, NO is mainly produced by nitric oxide synthase (Nos), an enzyme that requires molecular oxygen and several cofactors, synthesizing NO and citrulline from L-arginine [19]. Citrulline can be recycled to L-arginine via the ornithine–urea cycle (OUC), a process mainly studied in vertebrates, although OUC enzymes appear to be widely present among prokaryotes and eukaryotes [20,21]. Nos can produce nanomolar to micromolar concentrations of NO, under constitutive or induced activity, respectively.
Under low oxygen environmental or tissue conditions, Nos-dependent NO production is insufficient, and alternative mechanisms are involved. In particular, globins can act as nitrite reductases, generating NO [15]. Indeed, NO3− (via a putative nitrate reductase activity, as described, for example, in mammals and fish [22,23]) and NO2− serve as a bioavailable reservoir of NO in blood and tissues (globin–NO cycling) [15]. NO can also be generated from NO2, NO3−, and S-nitrosothiols in a decomposition process mediated by UV light that has been proposed to modulate blood perfusion in the skin [24].
The regulation of NO production and scavenging is essential for the proper NO function as a signaling molecule. The NO profile in a cell or tissue is defined by its concentration, persistence, and spatial orientation, and is determined by both physical and chemical processes. Under normoxic conditions, the activity and rate of NO production are primarily determined by controlling Nos transcription and translation, and by post-transcriptional and post-translational modifications [25,26], as well as by Nos cofactors or substrate availability, while Nos-independent pathways are mainly regulated by changes in the oxygen tension [10]. Additionally, Nos activity can be modulated by hydrogen sulfide (H2S), a relatively poorly studied signaling molecule [27].
From the above, it is clear that the multitude of NO derivative species (NOx, see Table 1) produced in living cells have varying significance, depending on the diversity of the target reactions that these species can undergo in the complex biological environment (Figure 1) [27]. This complexity is at the base of the double-edged sword behavior of NO in biological systems. Its freely diffusing capacity, sufficiently long lifetime, and very high affinity with ferrous heme make NO a highly efficient signaling molecule. On the other hand, NO can be noxious as a source of strong oxidizing agents that can damage plasmatic membranes, proteins, and DNA [16].

Table 1.
The oxidized or reduced derivatives of NO in biological systems, forming the complex of NOx (i.e., the pool of NO and its oxidized or reduced derivative species that can be found in living cells) [27,28].
The interaction with arginine homeostasis is critical in regulating NO production. The role of arginase in modulating Nos has been particularly investigated [29,30]. This interaction between Nos and arginase is more complex than simple substrate competition [31], depending on the complexity of cellular and whole-body arginine fluxes [30]. Arginine, which is semi-essential or conditionally essential in vertebrates, is the substrate for several enzymatic pathways leading to the biosynthesis of urea, ornithine, agmantine, creatine, citrulline, and glutamate [32]. Arginine is implicated in collagen synthesis and in the modulation of immuno-responses [33,34]. The complex interaction between arginine and NO homeostasis is epitomized by the so-called “arginine paradox”. In mammals, endothelial NO synthesis can be regulated by modulating extracellular arginine concentration, despite the fact that the intracellular arginine concentration usually exceeds the Km for arginine of eNOS. No definitive explanation for this paradox is yet available [33].
The overproduction of NO is at the base of a wide range of physio-pathological alterations, including neurodegenerative diseases, inflammation, and ischemia, mainly via peroxynitrite excess, protein S-nitrosylation, and nitration (SNOs) [35,36,37,38,39]
Apart from reduced or altered enzymatic expression or functionality, NO under-production can result from Nos uncoupling, in which the enzyme produces superoxide at the expense of NO, due to deficiency of the cofactor tetrahydrobiopterin or the substrate L-arginine, as well as Nos S-glutathionylation. Nos uncoupling strongly contributes to oxidative stress [40,41].
3. Nos Evolution in Deuterostomes
The Nos gene family has experienced loss and duplication events in several animal lineages, although the gene structure remains nearly unchanged [42].
In mammals, three Nos genes are reported, encoding for three distinct proteins often improperly defined as isoforms. Two of these genes, the neuronal (nNos) and the endothelial Nos (eNos), are constitutively expressed mainly in neurons and endothelium, respectively, whereas the third gene, the inducible Nos (iNos), is expressed in macrophages upon stressful stimuli. However, the different Nos genes are not exclusively expressed in a single cell type, e.g., neurons, endothelium, and macrophages, as initially believed. Moreover, the inducible Nos may act constitutively in some cells (see for details: [42,43]). Therefore, the above-mentioned classical nomenclature appears nowadays inadequate and, to avoid confusion, here we adopt the Nos1, Nos2, and Nos3 gene nomenclature, which reflects the chronology of their identification (Table 2).

Table 2.
The table reports the multiple acronyms of Nos genes and proteins present in the literature. The selected nomenclature for duplicate Nos takes into account the gene or genome duplication events. * Nomenclature adopted in this manuscript.
The corresponding proteins have different properties and localization. Synthetically, Nos1 is calcium-dependent and constitutively expressed in neurons and several other tissues, including muscles. Nos2 is calcium-independent and is inducible in macrophages but is also present in other tissues such as cardiac myocytes, glial cells, vascular smooth muscle cells, hepatocytes, chondrocytes, and keratinocytes. Nos3 is calcium-dependent and constitutively expressed in the endothelium but is also present in the myocardium, brain, and other tissues. These discrepancies are linked to structural differences: Nos1 and Nos3 possess an inhibitory loop sequence, responsible for their calcium-dependency, which is absent in Nos2; Nos1 has a PDZ domain, while Nos3 shows myristoylation and palmitoylation motifs [42,44].
The three distinct Nos present in deuterostomes probably originated from an ancestral Nos that structurally resembles the Nos1 for the presence of PDZ domain. Multiple Nos gene copies have been found in invertebrate deuterostomes, including sea urchin, Amphioxus, and early branching vertebrates like lamprey, resulting from lineage-specific gene duplication events (Table 3) [42,45]. On the contrary, a single Nos copy has been identified so far in the ascidian sea squirt (Table 3) [46].

Table 3.
NOS gene repertoire in deuterostomes. Table reports exhaustive information on Nos genes available in deuterostomes, with a special focus on chordates. The number of Nos orthologs per each species and the corresponding bibliographic references are shown. The symbol # indicates that Ciona robusta was previously named Ciona intestinalis, and * indicates that a third Nos gene should be expected in those species, although one of the orthologs is still missing, probably for genomic quality issues.
Whole-genome duplication events (WGD) [56,57] and the consequent increase in genomes complexity have expanded the number of paralogs and their functional specialization in vertebrates: Nos1, Nos2, and Nos3 (Figure 2). This gene repertoire is conserved in Sarcopterygians and early diverging Actinopterygians, as polypterids, acipenserids, bowfins, and gars, but shows a highly diversified landscape in teleosts. Teleosts underwent the teleost genome duplication (TGD) that produced, generally, an amplification of the gene repertoire (Figure 2) [58]. The loss of Nos3 has been detected in all the analyzed teleosts, except for elephant fish, which still possess the complete Nos repertoire [54]. The evolutionary history of Nos2 reflects a more complex scenario with multiple independent lineage-specific duplications, as found in cavefish, catfish, or northern pike, and 4R tetraploidization events occurred in cyprinids and salmonids (Cs4R and Ss4R, respectively) [54]. Despite all this, Nos2 has been lost at the stem of Neoteleosts. The Nos1 gene is instead ubiquitously present, as a single gene copy, in gnathostomes, except for duplicates deriving from 4R events [54].
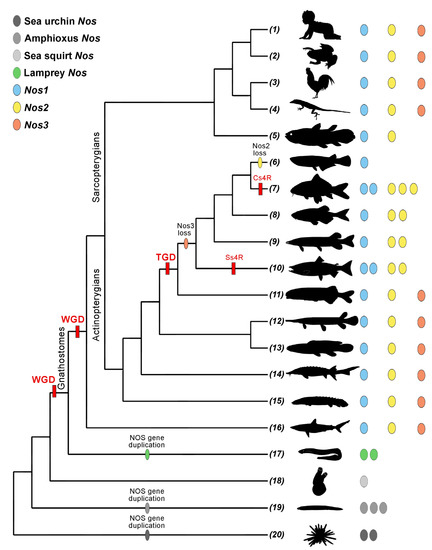
Figure 2.
Nos repertoire in deuterostomes. Silhouettes are from PhyloPic (PhyloPic—free silhouette images of life forms) and represent the following: (1) Human (Homo sapiens); (2) Frog (Xenopus laevis and Xenopus tropicalis); (3) Chicken (Gallus gallus); (4) Lizard (Anolis carolinensis); (5) Coelacanth (Latimeria chalumnae); (6) Medaka (Oryzias latipes); (7) Carp (Carassius auratus, Cyprinus carpio, and Sinocyclocheilus anshuiensis); (8) Zebrafish (Danio rerio); (9) Northern pike (Esox lucius); (10) Salmon (Oncorhynchus mykiss and Salmo salar); (11) Elephant fish (Paramormyrops kingsleyae); (12) Spotted gar (Lepisosteus oculatus); (13) Bowfin (Amia calva); (14) Sturgeon (Acipenser ruthenus); (15) Bichir (Polypterus senegalus); (16) Shark (Scyliorhinus torazame); (17) Lamprey (Lethenteron camtschaticum, Petromyzon marinus); (18) Sea squirt (Ciona robusta and Ciona savignyi); (19) Amphioxus (Asymmetron lucayanum, Branchiostoma belcheri, Branchiostoma floridae, and Branchiostoma lanceolatum); (20) Sea urchin (Hemicentrotus pulcherrimus and Strongylocentrotus purpuratus). Abbreviations: Cs4R, carp-specific genome duplication; Ss4R, salmonid-specific genome duplication; TGD, teleost genome duplication; VGD, vertebrate genome duplication.
It remains to be demonstrated if multiple Nos duplicates are involved in specific biological pathways (sub-functionalization) and whether a compensatory mechanism exists to functionally compensate the lost genes.
4. NO Function in Cephalochordates
In 2011, Andreakis and collaborators described three different Nos genes in the Amphioxus Branchiostoma floridae’s genome, called NosA, NosB, and NosC, which were not orthologues of the three human Nos genes based on phylogenetic analysis. The analysis of gene and protein structures allowed the identification of NosA and NosC as neuronal-like types, thanks to the presence of the N-terminal PDZ domain and of the inhibitory loop sequence. NosB misses both those domains, suggesting that it could represent an inducible-like Nos, similarly to the human Nos2 [42]. Nos genes have also been found in other cephalochordate species, such as Branchiostoma lanceolatum, Branchiostoma belcheri, and Asymmetron lucayanum, and thanks to a dedicated phylogenetic analysis using more cephalochordate species, it was confirmed that they originated from two cephalochordate-specific gene duplications [45]. The three Nos in Amphioxus show a complementary expression pattern, from early embryogenesis to adulthood, indicating a putative NO role in multiple processes spanning the entire Amphioxus’ life. NO deriving from NosB was hypothesized to have an important role during gastrulation, since NosB in B. lanceolatum was expressed in between endoderm and ectoderm at early and middle gastrula [45]. Later during embryogenesis, particularly during neurulation, NO from NosC was found to have a key role in the antero-posterior patterning of the pharyngeal region. For the normal development of the pharynx, NO cooperates with another important and ancient signaling molecule, Retinoic Acid (RA) [49]. During the successive developmental phases, both in B. lanceolatum and B. floridae larvae, Nos expression was observed in the intestine and the club-shaped gland [45,59]. The club-shaped gland is involved in the production of mucus for capturing food particles entering the mouth, and this suggests a role of NO in the feeding behavior that is a conserved feature in other animals [60,61,62,63,64,65,66]. The neuronal expression of NosC, detected from neurula to larva stages, is a strong indication of the NO’s Involvement in neurogenesis and neurotransmission [45,49]. NosC is the only Amphioxus Nos gene for which a putative enhancer, conserved among B. lanceolatum, B. floridae, and B. belcheri, has been identified using a transphyletic approach [52]. Such an enhancer was active in an in vivo reporter assay in the CNS of ascidian embryos and recapitulated the endogenous Nos expression, indicating conservation of the Nos regulatory machinery among invertebrate chordates [52]. The neuronal role of NO is important in the adult as well, where Nos and NO were identified in the cerebral vesicle and neurons of the neural cord [50,67]. In the adult B. belcheri, apart from a role in the nervous system, NO is implicated in the maturation of germinal cells, both egg and sperm, and in the immune response [50,51,67,68]. In particular, Nos transcripts were detected in macrophages in the lymphoid cavities of metapleural folds and of the branchial coelom as well as in the gut and the endostyle, which are known to be immune-system-associated organs [50,51,67]. At the time of the experiments, the above-mentioned authors ignored the existence of three different Nos genes in Amphioxus, and based on expression profiles shown in B. lanceolatum, it is plausible that they detected NosA, which is, in fact, exclusively expressed in adulthood [45].
5. NO Function in Tunicates
In tunicates, NO is involved in various processes from developmental to adult stages. Nos expression during the various phases of embryonic development has been analyzed by real-time PCR experiments in two ascidian species: Ciona robusta and Herdmania momus. Unfortunately, no data are present in the literature about Nos or NO localization in tunicate early stages of development, although a progressive increase in Nos gene expression is evident from neurulation onwards [46,69]. Most studies are centered on Nos expression and NO function during larval development and metamorphosis.
Metamorphosis represents a key developmental event in which there is a radical transition from a larval to a juvenile/adult body plan and the need to control two overlapping developmental programs simultaneously: larval and juvenile development [70]. All metamorphoses involve the differentiation of juvenile/adult structures, the degeneration of larval structures, metamorphic competence, and a habitat change [70]. In tunicates, these changes are entirely different in each of the classes. Whereas ascidians undergo a dramatic metamorphosis, members of the Appendicularia retain a larval body plan in the adult, and Thaliacea exhibit a direct development [71]. It has been shown that in C. robusta, NO exerts a fundamental role for the correct larval structural organization. Interestingly, dynamic expression patterns of Nos genes and proteins as well as NO signals in the nervous system, tail muscle, and epidermis seem to correlate with the different phases of metamorphosis induction and progression [5]. As larval development proceeds, a progressive shift of Nos expression and NO signal along the antero-posterior axis of the larva is observed that is intimately connected to the different phases of induction of larval settlement and tail regression. At the early and middle larva stages, Nos expression and NO localization in the anterior palps and CNS are necessary for settlement induction. Later on, at the late larva stage, Nos expression and NO signal along the tail nervous system, muscles, and notochord control change in the swimming behavior and induction of tail resorption [5,46].
Most of our knowledge of the involvement of NO in ascidian metamorphosis comes from the pharmacological treatments of larvae with NO donors or inhibitors of Nos or guanylyl cyclase. The modulation in NO levels in the ascidians C. robusta, Boltenia villosa, Cnemidocarpa finmarkiensis, and H. momus affect the frequency of metamorphosis, resulting in tail retraction and resorption that characterize the juvenile morphology [46,69,72,73,74]. These results are further supported by Nos gene and protein expression and NO localization in the tail epidermis and tail tip at all regression stages and NO diffusion in all tail tissues, including notochord and muscle cells in C. robusta [5,46] and C. finmarkiensis [73].
The detection of NOS, by the indirect histochemical method of NADPH-diaphorase activity, in specific regions of adult Styela rustica and Molgula citrina has suggested the involvement of NO in several functions, including in the processing of the sensory information, regulation of reproduction, immune response, regulation of respiratory and digestive functions, muscle and intestinal tones, and epithelial cell functioning, and the synthesis of secretory products [75].
Of particular interest is the involvement of NO in the immune function in some tunicates. Among the different types of blood cells of Styela plicata, the lymphocyte-like cells produce most of the NO, as revealed by NADPH-diaphorase and immunostaining with antibodies against inducible Nos [76]. Also, hemocytes of Phallusia nigra contain NO, detected using the cell-permeant 4-Amino-5-Methylamino-2′,7′-Difluorofluorescein Diacetate (DAF-FM-DA), which becomes fluorescent after reaction with NO [77]. No conclusive data were obtained by functional experiments in which NO production was investigated in hemocytes under inflammatory conditions after treatment with lipopolysaccharide (LPS) or zymosan A [76,77].
6. NO Function in Teleosts
Teleost species account for about half of all vertebrate species known today and have diversified into virtually every aquatic habitat on the planet. Salinity, temperature, and O2 availability have been the main drivers of fish evolution. The long evolutionary history and the wide variety of aquatic environmental conditions explain the high diversity in this group of animals.
As in mammals, in fish, NO is involved in a large number of physiological processes, including development, cardiovascular homeostasis, neurotransmission and neuromodulation, and immune defense. The signaling role of NO in fish has often been studied from the perspective of its involvement in animal responses and adaptation to environmental stress, i.e., any environmental condition or change that challenges the homeostatic and allostatic processes of animals. The ability of animals to cope with stress-dependent challenges represents a fundamental mechanism for maintaining organism homeostasis. NO is involved, for example, in the teleost response to osmotic stress [78], hypoxia [79], and thermal stress [80].
It should be noted that a large body of information on the role of NO in teleosts comes from a limited number of model species, mainly cyprinids and salmonids, the winner being the zebrafish embryo. This situation limits the possibility of thoroughly evaluating the functional role of NO in this group of organisms characterized by a large diversity. As reported in Figure 2, due to a complex evolutionary process, Nos1 is present in all species of Gnatostomata so far examined, whereas Nos3 is lost in Clupeocephala, and Nos2 is duplicated in several species, but lost in Neoteleostei [54]. Therefore, apparently, only one gene, Nos1, accounts for all enzymatic NO synthesis and the wide range of NO roles in Neoteleostei.
6.1. Role of NO in Fish Reproduction and Development
As in mammals, in teleosts, NO is critical in both oocyte maturation and egg activation and maturation upon fertilization [15]. Nos plays a role in oocyte maturation [81], although a fine-tuning of NO appears necessary, as both too low and too high NO levels have negative consequences. The mechanism of such a fine-tuning process has yet to be clarified.
NO is involved in the calcium rise necessary for egg activation upon fertilization [82]. Interestingly, maternal zebrafish transcript reported no detection of the Nos enzymes in eggs and embryos until 6 h post fertilization [83]. Hence, it is unclear which source of NO is involved in egg activation. Globin X, which is expressed at high levels in the eggs at fertilization, has been proposed to play this role [15], given its efficient nitrite reductase activity [84].
The availability of fish larval models for studying the development of vertebrates, mainly zebrafish (Danio rerio) and medaka (Oryzias latipes), has also offered the opportunity to gain significant insights into the role played by NO in fish development.
In the developing zebrafish, the strongest NO-positive sites in 5-day post fertilization larvae are the notochord, bulbous arteriosus, and cranial bones [85]. Nos inhibitors did not completely abrogate the NO signal, suggesting that there are additional Nos-independent sources of NO in the developing embryo [85]. NO is involved in the control of vasculature during development [86,87,88] and stimulates angiogenesis and hematopoiesis [87,89]. The canonical NO signaling (NO activating sGC and catalyzing the synthesis of cGMP) targets several proteins, including Bone Morphogenetic Protein-4 (Bmp4), which is involved in determining the positioning of the heart during embryonic development [90].
NO plays a key role in the development and plasticity of the CNS during both embryonic and post-embryonic stages of life [91]. Studies performed in different species on the developing nervous system have implicated NO in neural differentiation, pathfinding, and synapse formation [91]. NO is involved in motor axon development and branching [92], and in the cranial neural crest patterning, differentiation and convergence during craniofacial morphogenesis [93]. It has been suggested that NO and/or the cGMP generated by soluble GC play a role in neuronal signaling and neuronal development in the medaka fish embryonic retina [94].
6.2. Role of NO in Teleost Cardiorespiratory Function, Osmoregulation, and Feeding
NO acts as a paracrine and endocrine modulator in the gills, heart, blood vessels, kidney, and intestine, playing a crucial role in highly integrated processes such as respiratory gas distribution, osmoregulation, and gut function.
Despite the absence of Nos3, there is plenty of evidence for a role of Nos-derived NO in the homeostasis of the cardiovascular system. Nos3 immunoreactivity (i.e., using anti-mammalian eNos, often indicated as eNos-like) has been found in the heart and endothelial cells of several species [53,95], and the role of NO in controlling cardiac function is well established [9,80,96]. The presence of Nos3-like and Nos2 in the different districts of the fish heart has been described (as reviewed in [97]), with evidence for an NO role as an autocrine and paracrine molecule modulating its functionality. Nos1 immunoreactive signals have also been detected in the goldfish heart’s intracardiac nervous system (ICNS) as part of the complex neuronal set necessary for fine-tuning cardiac function [98].
NO donors (e.g., SNP) and Nos inhibitors (e.g., L-NAME) affect vascular resistance, confirming its putative vasodilatory function in fish [86,99,100]. The occurrence of perivascular nitrergic neurons, with Nos1 as a likely primary source of NO, innervating the vasculature of some teleost species, is also described [53,101].
Few studies have also addressed the occurrence and role of the classical NO-sGC-cGMP pathway in the fish’s cardiorespiratory system. The occurrence of GC-activating protein (GCAP) genes has been demonstrated in pufferfish (Fugu rubripes), zebrafish, and medaka [90,102,103,104]. As in rats, mice, and humans, the genes encoding the sGCa1 and b1 isoforms are closely located in medaka, accounting for their transcriptional coordination [105]. Indirect evidence of sGC-dependent cardiac modulation by NO has been reported in adult goldfish (Carassius auratus) [106] and zebrafish larvae [107,108].
In the teleost gill vascular district, NO-dependent vasoconstriction, rather than vasodilation, has been described. For example, NO-cGMP signaling has been reported in the freshwater eel branchial vasculature [109], with NO-dependent vasoconstriction. Gill Nos1 immunoreactivity has been reported in killifish (Fundulus heteroclitus) [110], catfish (Heteropneustes fossilis) [111], Gadus morhus [112], African bonytongue (Heterotis niloticus), [113], and Antarctic fish (Trematomus bernacchii and Chionodraco hamatus) [114].
NO has been involved in controlling ion transport in gill and kidney epithelia in several species [110,115,116]. The NO-cGMP system is likely involved in the regulation of Na+/K+-ATPase (the leading actor of osmoregulation in fish) in the kidney of brown trout (Salmo trutta) [115]. Nos1 has also been localized in the rainbow trout kidney [117]. NO also plays a role in the regulation of NaCl transport in the intestines of marine and air-breathing fish [118,119]. Nos1, which is highly expressed in the middle intestine of rainbow trout (Oncorhynchus mykiss) [120], is upregulated during sea water acclimation [121].
The respiratory rhythm of zebrafish is affected by manipulating the NO system [122]. In larvae, NO donors increase ventilation, while the same manipulation inhibits adult ventilation [122]. NO and Nos have also been involved in the complex cardiorespiratory and osmoregulatory adjustments during aestivation in lungfish (Protopterus sp.) [123].
NO generally has an inhibitory effect on intestinal smooth muscle cells, and NOS has been found in gut neurons of several teleosts [120,124,125,126]. A nitrergic inhibitory tone has been shown in Atlantic cod and zebrafish intestine [127,128], but not in rainbow trout stomach [129]. The enteric neurons and nitrergic inhibitory tonus develop before the onset of exogenous feeding in zebrafish [128] and Atlantic cod [127]. The zebrafish mutant lessen, characterized by a reduced number of proliferating ENS precursor cells and a delayed and disturbed intestinal motility, presents a significantly lower number of nitrergic neurons than the wild type [130]. In Anguilla anguilla, parasitized by the worm Contracaecum rudolphii, Bosi et al. [131] discovered an increase in the density of nerve fibers harboring endogenous Nos1.
6.3. Role of NO in the Immune System of Fish
The teleost immune system comprises the most basic immune organs, immune cells, and molecules among vertebrates. The teleost innate immunity faces pathogen infection and initiates the adaptive immune response, which, compared with that in higher vertebrates, shows some limitations, such as a limited antibody library, slow proliferation, and maturation of lymphocytes and longer memory time [132].
Teleosts have monocytes/macrophages and neutrophils. These cells are considered professional phagocytes that recognize a wide range of particles or antigens and have a strong and efficient endocytosis ability [132]. Classically, activated macrophages express high levels of inducible NO synthase (Nos2). It has been reported that macrophages express Nos2 when stimulated by IFNγ, TNFα, or bacterial MAMPs [133]. Simultaneous production of superoxide and NO intermediates can form peroxynitrite, which additionally serves potent antiparasitic/antimicrobial functions [134,135].
The involvement of Nos2 in the response to infection has been identified and characterized in various teleost species. Fish macrophages’ diversity and plasticity are similar to that found in mammals [136]. Fish macrophages produce NO [137,138]. Nos2 can be detected at a high level when the pathogen Enteromyxum scophthalmi infects turbot [139]. Common carp macrophages stimulated by LPS or protozoan blood flagellate Trypanoplasma borreli produce a large amount of Nos2, as shown by RT-PCR [140]. Nos2 is also highly expressed in zebrafish, although not upregulated following Frascinella infection [141]. Nos2 expression in gills has also been reported in rainbow trout infected with Gram-positive pathogens [142]. These studies suggest that the production of NO by teleost macrophages can be a significant component of the teleost immune response to pathogens.
Mammalian macrophages not only play a role in initiating inflammation but also in the resolution phase of inflammation (macrophage polarization, with metabolically distinct pro-inflammatory, M1, and anti-inflammatory, M2, phenotypes) [136]. Macrophage polarization has also been reported in common carp Cyprinus carpio carpio [143,144], where the M1 macrophages are characterized by high NO production and low arginase activity, while the M2 macrophages are characterized by high arginase activity, so exploiting the Nos–arginase competition for substrates [144].
6.4. Role of NO in the Teleost Nervous System, and Control of Locomotion and Behavior
Nos-derived NO is an important neurotransmitter in the CNS that is involved in the regulation of most systemic functions, as well as learning, sensory processing, and other forms of neural plasticity [145].
In ray-finned fish, early expression of Nos1 mRNA in the CNS has been shown [55,91,104,146]. In adult teleosts, peripheral organs contain Nos1, as in mammals [147,148]. The co-expression of Nos1 with serotonin and tyrosine hydroxylase-immunoreactive fibers in the skin and gills of the giant mudskipper, Periophthalmodon schlosseri, likely related to the amphibious lifestyle of this species, was found by Zaccone et al. [149].
Interestingly, NO signaling is involved in several aspects of fish behavior. Using zebrafish knockouts for the Nos1 gene, Penglee et al. [150] reported high dopamine levels and a low inter-individual distance (high shoal cohesion) in shoaling juveniles of zebrafish related to the brain’s low levels of NO. Moreover, zebrafish Nos1 knockouts are less aggressive and display anxiety-like behavior [151]. Brain Nos1 is among the genes associated with aggressiveness in female zebrafish [152]. Recently, fish oxytocin (also called isotocin) has been involved in the regulation of social fear contagion in zebrafish [153]. Since oxytocin can stimulate NO production [154], we can indirectly speculate on NO involvement in social fear in fish.
Although the distribution of Nos1-expressing cells seems to be conserved in most teleosts, with a high degree of detection in the telencephalon and diencephalon and reduced expression in more caudal brain regions [55,155], diversity of function can be observed. For example, in the African cichlid fish Astatotilapia burtoni, Nos1-expressing cells were found in the inner nuclear layer and from the olfactory bulbs to the hindbrain, including within specific nuclei involved in decision-making, sensory processing, neuroendocrine regulation, and the expression of social behaviors [156]. These authors demonstrated that NO signaling might play diverse roles depending on sex and male dominance, likely mediating the sensory perception required for structuring male social hierarchies or male courtship in this species characterized by territoriality, male hierarchy, and maternal mouthbrooding. Using RT-PCR, they reported the occurrence of Nos1-expressing cells in the retina [156], in agreement with the hypothesis of a significant role of NO signaling in modulating retinal light response [157], but not in the olfactory epithelia. Using immunocytochemistry, Nos1 has also been detected in the olfactory receptor neurons of tilapia, Oreochromis mossambicus [158] and in the retina of goldfish and catfish [159].
NO is among the major players involved in the neuromodulation of the locomotor central pattern generating (CPG) networks both in vertebrates [160] and invertebrates [161]. During animal development, the requirements of locomotor control systems may undergo significant changes at the molecular (receptors, ion channels, pumps, and transporters in individual neurons), cellular (changes in existing neurons or formation of new neurons), or systemic (new neuronal networks) levels in relation to the emergence of new or modified neuromuscular structures and behaviors [162]. In zebrafish, Nos1 mRNA expression is detected at 19 h post fertilization (hpf) in the forebrain, and Nos-positive neurons increase in the brainstem [91]. However, despite the fact that no evidence of NO directly modulating the swimming CPG has been reported so far, the zebrafish swimming system as a whole is profoundly modulated by NO signaling, which plays a role in regulating muscle innervation and neuromuscular transmission [92,163].
7. NO Function in Amphibia
In Amphibia, there is evidence of the role of NO as a signaling molecule in several functional processes. What is particularly studied is its role in Anura’s metamorphosis.
Nos immunoreactivity was found in the enteric nervous system of axolotl, Ambystoma mexicanum (Urodela) [164]. The NO/cGMP signaling pathway was functional, and its components were widely distributed throughout specific cell types in the outer and inner salamander retina [165].
Xenopus tropicalis possesses a Nos3 gene, which is orthologous of mammalian Nos3 (as determined using gene synteny and phylogenetic analyses). Nos3 mRNA expression was highest in lung and skeletal muscle and lowest in the liver, gut, kidney, heart, and brain. Nos immunoreactivity was observed in the proximal tubule of the kidney and endocardium of the heart but not in the endothelium of blood vessels. Thus, X. tropicalis has a Nos3 gene that appears not to be expressed in the vascular endothelium [166].
A putative role of Nos2 and Nos3 has been reported in the larval, pre-metamorphic, and adult phases of the newt Triturus italicus (Amphibia, Urodela), using indirect immunofluorescence techniques [167]. In X. laevis, NO activates eggs parthenogenesis [168] and is involved in the development of the mouth and neural crest [169].
NO and Metamorphosis in Anura
The metamorphosis in amphibians consists of the transformation of the larva to a miniature adult replicate, with a change in the lifestyle from aquatic to terrestrial or semi-terrestrial [170]. Metamorphosis is guided by the hormone thyroxine, and it is nearly unnoticeable in Apoda and Urodela, but dramatic in Anura [170]. Anuran larvae (tadpoles) are strikingly different from the adult. Their metamorphosis requires comprehensive structural and physiological reorganization, involving substantial histological and physiological changes: absorption of the extensive tadpole tail and development of limbs and complex multicellular skin glands, rearrangement of the visual system, gut alterations as the animal changes from a herbivore to a carnivore, and gill resorption as gas exchange occurs in newly developed lungs as the amphibian adapts from an aquatic to a terrestrial environment [170].
This change in the anuran body plan is associated with profound changes in the behavioral-locomotor system, with a gradual switch from swimming, in which trust is generated by undulatory oscillations of the trunk and tail, to the limb-based propulsion of adults. This is associated with the degeneration of the Mauthner neurons (a pair of neural cells involved in controlling the escape response of tadpoles) and the rearrangement of the visual system from a lateral position (due to panoramic vision, an adaptation to the herbivorous prey lifestyle of tadpoles) to a frontal position that is appropriate for the predatory lifestyle of adults [162].
All these changes require critical but gradual modifications in the central and peripheral nervous pathways involved in controlling locomotory and visual functions [171] that are consistent with the behavioral requirements for continuous locomotor capability throughout metamorphosis.
NO appears to be deeply involved in the anuran metamorphosis process, particularly the dramatic change in locomotion modality and related behavioral patterns. The role of NO in reconfiguring the sensory and locomotor neural networks during metamorphosis has been proposed in Rana esculenta by identifying Nos immunoreactivity in the root ganglia and dorsal horn [172]. In X. laevis, the distribution of Nos1 and Nos2 was found to be stage-specific, and the Nos1 gene expression was up-regulated by thyroxin treatment [173].
NO is one of the three neuromodulators, together with dopamine and serotonin, that potently influence CPG outputs in the development of X. laevis tadpole locomotion [162]. Their roles switch from predominantly inhibitory early in development to mainly excitatory at the later stages. Nos-positive neurons appear very early in the Xenopus brainstem. Until stage 42, NO exerts an inhibitory role on tadpole swimming via the presynaptic facilitation of inhibitory transmitter release [162]. At this stage, the tadpole lives on yolk and swims only when stimulated by potential predators, remaining otherwise motionless. Successively, Nos-positive neuron populations expand through the brain and spinal cord, and the NO role switches from inhibitory to facilitatory, although the mechanism involved is unclear [162]. This switch is associated with a dramatic change in locomotor behavior in Xenopus tadpoles as they become free-swimming and active filter feeders.
Another dramatic change in amphibian physiology during metamorphosis is in the respiratory system, from gill-based aquatic respiration to lung-based air respiration. NO is also involved in this process. Early in development, NO provides a tonic inhibitory input to gill and lung burst activity, but as development progresses, NO provides an excitatory input to lung ventilation [174]. Likely, NO provides a tonic input to the respiratory CPG during development, and this changing role reflects the modulatory influence of NO on inhibitory or excitatory modulators or neurotransmitters involved in the generation of respiratory rhythm [174].
8. Conservation of NO Functions in Aquatic Chordates
The analysis of NO’s multiple functions in aquatic chordates revealed a series of conserved roles both during embryonic development and in adult organism life. This gaseous molecule provides regulation and fine-tuning of a wide range of physiological processes (Figure 3).
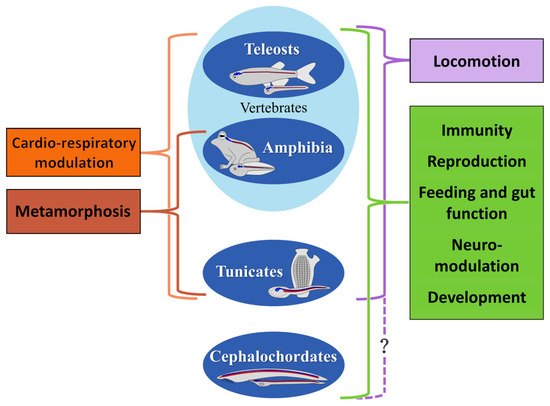
Figure 3.
Comparison of the major physiological functions of NO in aquatic chordates. The NO roles in immunity, reproduction, feeding and gut function, neuro-modulation, and development are common to all chordates (green). The role in locomotion is conserved among vertebrates and tunicates (lilac), but no data are available in cephalochordates (question mark). NO function in metamorphosis is common to all chordates with an indirect development (light brown). Cardio-respiratory modulation by NO is reported in vertebrates and tunicates (orange).
A common NO function among different chordate species is in the maturation of eggs and sperm. Similarly to teleosts, and also in cephalochordates, NO is involved in the maturation of germinal cells [15,50,51,67,68,81]. An analogue function in tunicates could be suggested by the presence of NADPH-diaphorase in adult gonads (Figure 3) [75].
During development, NO plays crucial roles in the definition of head structures in all chordate species investigated so far. The Nos1 loss-of-function induces severe defects in the anterior structures in vertebrate Xenopus and zebrafish [169]. Similarly, endogenous NO reduction in amphioxus during development prevents the correct development of the mouth and pharyngeal structures [45,49]
In ascidians, the larval palps represent the most anterior sensory organs where Nos is expressed, further confirming the conservation of this messenger activity in the development of the anterior sensory regions among chordates (Figure 3). In tunicates, these anterior structures assure larval settlement. Interestingly, NO has a conserved function in the regulation of larval settlement in all metazoans undergoing metamorphosis [175]. As reported in other chordates, NO’s prominent role in the larval settlement is the modulation of the CPG neural circuits for the swimming behavior of tunicate larvae [5] and for fish locomotion [162,163]. As pointed out in Figure 3 (question mark), this role has not yet been investigated in cephalochordates. Nevertheless, since this role is present in the other chordates and in non-chordate invertebrates [176,177], it is most likely also conserved in amphioxus.
The ascidian larval settlement is followed by metamorphosis induction, where NO’s neuromodulatory role is also highly conserved (Figure 3). An interesting parallel can be observed comparing NO activities in tail retraction in chordates with an indirect development [5,46,178].
A relatively well-defined and conserved role of NO is the modulatory activity of the chordate immune system (Figure 3). In adult cephalochordates and tunicates, NO is present in immune-system-associated organs and cells [50,51,67,76,77]. Accordingly, Nos2 activation in the immune response to infection is observed in various teleost species [139,140].
In the adult stage of aquatic vertebrates, NO is likely involved in all the main physiological processes, such as sensory information processing, cardiorespiratory function, feeding and digestion, neuroendocrine regulation, and behavior [156]. It is not easy to detail the parallelism among tunicates, teleosts, and amphibians. However, the data on tunicate NADPH localization in the adult endostyle and in the nerve filaments innervating the muscles of the heart, the digestive system, and the gill sac suggest the occurrence of these NO functions already in the invertebrate chordates (Figure 3) [75]. These roles have considerably specialized following the development of the more complex lifestyles of vertebrates.
In conclusion, our comparative analyses of Nos expression and NO function in aquatic chordates evidenced a high degree of conservation from non-vertebrate chordates to vertebrates from development to the adult stage. Nevertheless, further functional studies will be necessary for non-vertebrate chordates to confirm this hypothesis, sometimes inferred only from common data on expression or behavior.
Author Contributions
Conceptualization, A.L. and A.P.; supervision, A.L., S.D., C.A. and A.P.; writing—review and editing, A.L., G.A., F.C., S.D., C.A. and A.P. All authors have read and agreed to the published version of the manuscript.
Funding
G.A. and F.C. were supported by MUR CIR_0029 Human Capital.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data is available upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hansen, M.N.; Jensen, F.B. Nitric Oxide Metabolites in Goldfish under Normoxic and Hypoxic Conditions. J. Exp. Biol. 2010, 213, 3593–3602. [Google Scholar] [CrossRef] [PubMed]
- Moroz, L.L.; Romanova, D.Y.; Nikitin, M.A.; Sohn, D.; Kohn, A.B.; Neveu, E.; Varoqueaux, F.; Fasshauer, D. The Diversification and Lineage-Specific Expansion of Nitric Oxide Signaling in Placozoa: Insights in the Evolution of Gaseous Transmission. Sci. Rep. 2020, 10, 13020. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, M.; Giordano, D.; Verde, C.; Poole, R.K. The Evolution of Nitric Oxide Function: From Reactivity in the Prebiotic Earth to Examples of Biological Roles and Therapeutic Applications. Antioxidants 2022, 11, 1222. [Google Scholar] [CrossRef] [PubMed]
- Hirst, D.G.; Robson, T. Nitric Oxide Physiology and Pathology. Methods Mol. Biol. 2011, 704, 1–13. [Google Scholar] [CrossRef]
- Locascio, A.; Vassalli, Q.A.; Castellano, I.; Palumbo, A. Novel Insights on Nitric Oxide Synthase and NO Signaling in Ascidian Metamorphosis. Int. J. Mol. Sci. 2022, 23, 3505. [Google Scholar] [CrossRef]
- Annona, G.; Holland, N.D.; D’Aniello, S. Evolution of the Notochord. Evodevo 2015, 6, 30. [Google Scholar] [CrossRef]
- Gupta, R.S. Molecular Signatures That Are Distinctive Characteristics of the Vertebrates and Chordates and Supporting a Grouping of Vertebrates with the Tunicates. Mol. Phylogenet. Evol. 2016, 94, 383–391. [Google Scholar] [CrossRef]
- McNab, B.K. The Physiological Ecology of Vertebrates: A View from Energetics; Comstock Publishing Associates, Cornell University Press: Ithaca, NY, USA, 2002; 576p. [Google Scholar]
- Gattuso, A.; Garofalo, F.; Cerra, M.C.; Imbrogno, S. Hypoxia Tolerance in Teleosts: Implications of Cardiac Nitrosative Signals. Front. Physiol. 2018, 9, 366. [Google Scholar] [CrossRef]
- Thomas, D.D.; Heinecke, J.L.; Ridnour, L.A.; Cheng, R.Y.; Kesarwala, A.H.; Switzer, C.H.; McVicar, D.W.; Roberts, D.D.; Glynn, S.; Fukuto, J.M.; et al. Signaling and Stress: The Redox Landscape in NOS2 Biology. Free Radic. Biol. Med. 2015, 87, 204–225. [Google Scholar] [CrossRef]
- Potter, L.R. Guanylyl Cyclase Structure, Function and Regulation. Cell Signal. 2011, 23, 1921–1926. [Google Scholar] [CrossRef]
- Mason, M.G.; Nicholls, P.; Wilson, M.T.; Cooper, C.E. Nitric Oxide Inhibition of Respiration Involves Both Competitive (Heme) and Noncompetitive (Copper) Binding to Cytochrome c Oxidase. Proc. Natl. Acad. Sci. USA 2006, 103, 708–713. [Google Scholar] [CrossRef]
- Sarti, P.; Forte, E.; Mastronicola, D.; Giuffrè, A.; Arese, M. Cytochrome c Oxidase and Nitric Oxide in Action: Molecular Mechanisms and Pathophysiological Implications. Biochim. Biophys. Acta 2012, 1817, 610–619. [Google Scholar] [CrossRef]
- Poderoso, J.J.; Helfenberger, K.; Poderoso, C. The Effect of Nitric Oxide on Mitochondrial Respiration. Nitric Oxide 2019, 88, 61–72. [Google Scholar] [CrossRef]
- Rochon, E.R.; Corti, P. Globins and Nitric Oxide Homeostasis in Fish Embryonic Development. Mar. Genomics 2020, 49, 100721. [Google Scholar] [CrossRef]
- Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. The Nitration of Proteins, Lipids and DNA by Peroxynitrite Derivatives-Chemistry Involved and Biological Relevance. Stresses 2022, 2, 53–64. [Google Scholar] [CrossRef]
- Xiong, Y.; Uys, J.D.; Tew, K.D.; Townsend, D.M. S-Glutathionylation: From Molecular Mechanisms to Health Outcomes. Antioxid. Redox Signal. 2011, 15, 233. [Google Scholar] [CrossRef]
- Radzinski, M.; Oppenheim, T.; Metanis, N.; Reichmann, D. The Cys Sense: Thiol Redox Switches Mediate Life Cycles of Cellular Proteins. Biomolecules 2021, 11, 469. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric Oxide Synthases: Regulation and Function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef]
- Allen, A.E.; Dupont, C.L.; Oborník, M.; Horák, A.; Nunes-Nesi, A.; McCrow, J.P.; Zheng, H.; Johnson, D.A.; Hu, H.; Fernie, A.R.; et al. Evolution and Metabolic Significance of the Urea Cycle in Photosynthetic Diatoms. Nature 2011, 473, 203–207. [Google Scholar] [CrossRef]
- Song, X.; Teng, L.; Zhao, X. Re-Examining the Origin and Functions of the Urea Cycle. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Jansson, E.Å.; Huang, L.; Malkey, R.; Govoni, M.; Nihlén, C.; Olsson, A.; Stensdotter, M.; Petersson, J.; Holm, L.; Weitzberg, E.; et al. A Mammalian Functional Nitrate Reductase That Regulates Nitrite and Nitric Oxide Homeostasis. Nat. Chem. Biol. 2008, 4, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.N.; Lundberg, J.O.; Filice, M.; Fago, A.; Christensen, N.M.G.; Jensen, F.B. The Roles of Tissue Nitrate Reductase Activity and Myoglobin in Securing Nitric Oxide Availability in Deeply Hypoxic Crucian Carp. J. Exp. Biol. 2016, 219, 3875–3883. [Google Scholar] [CrossRef] [PubMed]
- Pelegrino, M.T.; Paganotti, A.; Seabra, A.B.; Weller, R.B. Photochemistry of Nitric Oxide and S-Nitrosothiols in Human Skin. Histochem. Cell Biol. 2020, 153, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Casper, I.; Nowag, S.; Koch, K.; Hubrich, T.; Bollmann, F.; Henke, J.; Schmitz, K.; Kleinert, H.; Pautz, A. Post-Transcriptional Regulation of the Human Inducible Nitric Oxide Synthase (INOS) Expression by the Cytosolic Poly (A)-Binding Protein (PABP). Nitric Oxide 2013, 33, 6–17. [Google Scholar] [CrossRef]
- Pautz, A.; Li, H.; Kleinert, H. Regulation of NOS Expression in Vascular Diseases. Front. Biosci. Landmark 2021, 26, 85–101. [Google Scholar] [CrossRef]
- Lehnert, N.; Scheidt, W.R. Preface for the Inorganic Chemistry Forum: The Coordination Chemistry of Nitric Oxide and Its Significance for Metabolism, Signaling, and Toxicity in Biology. Inorg. Chem. 2010, 49, 6223–6225. [Google Scholar] [CrossRef]
- Hill, B.G.; Dranka, B.P.; Bailey, S.M.; Lancaster, J.R.; Darley-Usmar, V.M. What Part of NO Don’t You Understand? Some Answers to the Cardinal Questions in Nitric Oxide Biology. J. Biol. Chem. 2010, 285, 19699–19704. [Google Scholar] [CrossRef]
- Caldwell, R.B.; Toque, H.A.; Narayanan, S.P.; Caldwell, R.W. Arginase: An Old Enzyme with New Tricks. Trends Pharmacol. Sci. 2015, 36, 395–405. [Google Scholar] [CrossRef]
- Caldwell, R.W.; Rodriguez, P.C.; Toque, H.A.; Priya Narayanan, S.; Caldwell, R.B. Arginase: A Multifaceted Enzyme Important in Health and Disease. Physiol. Rev. 2018, 98, 641–665. [Google Scholar] [CrossRef]
- Momma, T.Y.; Ottaviani, J.I. There Is No Direct Competition between Arginase and Nitric Oxide Synthase for the Common Substrate L-Arginine. Nitric Oxide 2022, 129, 16–24. [Google Scholar] [CrossRef]
- Morris, S.M. Enzymes of Arginine Metabolism. J. Nutr. 2004, 134, 2743S–2747S. [Google Scholar] [CrossRef]
- Gambardella, J.; Khondkar, W.; Morelli, M.B.; Wang, X.; Santulli, G.; Trimarco, V. Arginine and Endothelial Function. Biomedicines 2020, 8, 277. [Google Scholar] [CrossRef]
- Martí i Líndez, A.-A.; Reith, W. Arginine-Dependent Immune Responses. Cell. Mol. Life Sci. 2021, 78, 5303–5324. [Google Scholar] [CrossRef]
- Premont, R.T.; Reynolds, J.D.; Zhang, R.; Stamler, J.S. Role of Nitric Oxide Carried by Hemoglobin in Cardiovascular Physiology. Circ. Res. 2020, 126, 129–158. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Ghasemi, A. Role of Nitric Oxide in Insulin Secretion and Glucose Metabolism. Trends Endocrinol. Metab. 2020, 31, 118–130. [Google Scholar] [CrossRef]
- Bladowski, M.; Gawrys, J.; Gajecki, D.; Szahidewicz-Krupska, E.; Sawicz-Bladowska, A.; Doroszko, A. Role of the Platelets and Nitric Oxide Biotransformation in Ischemic Stroke: A Translative Review from Bench to Bedside. Oxid. Med. Cell. Longev. 2020, 2020, 2979260. [Google Scholar] [CrossRef]
- Tewari, D.; Sah, A.N.; Bawari, S.; Nabavi, S.F.; Dehpour, A.R.; Shirooie, S.; Braidy, N.; Fiebich, B.L.; Vacca, R.A.; Nabavi, S.M. Role of Nitric Oxide in Neurodegeneration: Function, Regulation, and Inhibition. Curr. Neuropharmacol. 2020, 19, 114–126. [Google Scholar] [CrossRef]
- Fukuto, J.M.; Perez-Ternero, C.; Zarenkiewicz, J.; Lin, J.; Hobbs, A.J.; Toscano, J.P. Hydropersulfides (RSSH) and Nitric Oxide (NO) Signaling: Possible Effects on S-Nitrosothiols (RS-NO). Antioxidants 2022, 11, 169. [Google Scholar] [CrossRef]
- Daiber, A.; Kröller-Schön, S.; Oelze, M.; Hahad, O.; Li, H.; Schulz, R.; Steven, S.; Münzel, T. Oxidative Stress and Inflammation Contribute to Traffic Noise-Induced Vascular and Cerebral Dysfunction via Uncoupling of Nitric Oxide Synthases. Redox Biol. 2020, 34, 101506. [Google Scholar] [CrossRef]
- Park, L.; Hochrainer, K.; Hattori, Y.; Ahn, S.J.; Anfray, A.; Wang, G.; Uekawa, K.; Seo, J.; Palfini, V.; Blanco, I.; et al. Tau Induces PSD95–Neuronal NOS Uncoupling and Neurovascular Dysfunction Independent of Neurodegeneration. Nat. Neurosci. 2020, 23, 1079–1089. [Google Scholar] [CrossRef]
- Andreakis, N.; D’Aniello, S.; Albalat, R.; Patti, F.P.; Garcia-Fernandez, J.; Procaccini, G.; Sordino, P.; Palumbo, A. Evolution of the Nitric Oxide Synthase Family in Metazoans. Mol. Biol. Evol. 2011, 28, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Godtman, R.A.; Hallsberg, L.; Löf-Öhlin, Z.; Peeker, R.; Delbro, D. Constitutive Expression of Inducible Nitric Oxide Synthase in Healthy Rat Urothelium? Med. J. Swed. AB 2021, 55, 493–497. [Google Scholar] [CrossRef]
- Stuehr, D.J.; Haque, M.M. Nitric Oxide Synthase Enzymology in the 20 Years after the Nobel Prize. Br. J. Pharmacol. 2019, 176, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Annona, G.; Caccavale, F.; Pascual-Anaya, J.; Kuratani, S.; De Luca, P.; Palumbo, A.; D’Aniello, S. Nitric Oxide Regulates Mouth Development in Amphioxus. Sci. Rep. 2017, 7, 8432. [Google Scholar] [CrossRef]
- Comes, S.; Locascio, A.; Silvestre, F.; d’Ischia, M.; Russo, G.L.; Tosti, E.; Branno, M.; Palumbo, A. Regulatory Roles of Nitric Oxide during Larval Development and Metamorphosis in Ciona Intestinalis. Dev. Biol. 2007, 306, 772–784. [Google Scholar] [CrossRef]
- Yaguchi, J.; Yaguchi, S. Sea Urchin Larvae Utilize Light for Regulating the Pyloric Opening. BMC Biol. 2021, 19, 64. [Google Scholar] [CrossRef]
- Yaguchi, J.; Yaguchi, S. Evolution of Nitric Oxide Regulation of Gut Function. Proc. Natl. Acad. Sci. USA 2019, 116, 5607–5612. [Google Scholar] [CrossRef]
- Caccavale, F.; Annona, G.; Subirana, L.; Escriva, H.; Bertrand, S.; D’Aniello, S. Crosstalk between Nitric Oxide and Retinoic Acid Pathways Is Essential for Amphioxus Pharynx Development. Elife 2021, 10, e58295. [Google Scholar] [CrossRef]
- Chen, D.; Lin, Y.; Zhang, H. Characterization and Expression of Two Amphioxus DDAH Genes Originating from an Amphioxus-Specific Gene Duplication. Gene 2008, 410, 75–81. [Google Scholar] [CrossRef]
- Lin, Y.S.; Chen, D.Y.; Zhang, W.; Cai, Z.P.; Chen, Z.K.; Zhang, N.; Mao, B.Y.; Zhang, H.W. Characterization of the Immune Defense Related Tissues, Cells, and Genes in Amphioxus. Sci. China Life Sci. 2011, 54, 999–1004. [Google Scholar] [CrossRef]
- Caccavale, F.; Coppola, U.; Vassalli, Q.A.; La Vecchia, C.; Palumbo, A.; D’Aniello, E.; Locascio, A.; Ristoratore, F.; D’Aniello, S. Transphyletic Conservation of Nitric Oxide Synthase Regulation in Cephalochordates and Tunicates. Dev. Genes Evol. 2020, 230, 329–338. [Google Scholar] [CrossRef]
- Donald, J.A.; Forgan, L.G.; Cameron, M.S. The Evolution of Nitric Oxide Signalling in Vertebrate Blood Vessels. J. Comp. Physiol. B 2015, 185, 153–171. [Google Scholar] [CrossRef]
- Annona, G.; Sato, I.; Pascual-Anaya, J.; Osca, D.; Braasch, I.; Voss, R.; Stundl, J.; Soukup, V.; Ferrara, A.; Fontenot, Q.; et al. Evolution of the Nitric Oxide Synthase Family in Vertebrates and Novel Insights in Gill Development. Proc. R. Soc. B Biol. Sci. 2022, 289, 667. [Google Scholar] [CrossRef]
- Annona, G.; Ferran, J.L.; De Luca, P.; Conte, I.; Postlethwait, J.H.; D’Aniello, S. Expression Pattern of Nos1 in the Developing Nervous System of Ray-Finned Fish. Genes 2022, 13, 918. [Google Scholar] [CrossRef]
- Dehal, P.; Boore, J.L. Two Rounds of Whole Genome Duplication in the Ancestral Vertebrate. PLoS Biol. 2005, 3, e314. [Google Scholar] [CrossRef]
- Nakatani, Y.; Shingate, P.; Ravi, V.; Pillai, N.E.; Prasad, A.; McLysaght, A.; Venkatesh, B. Reconstruction of Proto-Vertebrate, Proto-Cyclostome and Proto-Gnathostome Genomes Provides New Insights into Early Vertebrate Evolution. Nat. Commun. 2021, 12, 4489. [Google Scholar] [CrossRef]
- Pasquier, J.; Braasch, I.; Batzel, P.; Cabau, C.; Montfort, J.; Nguyen, T.; Jouanno, E.; Berthelot, C.; Klopp, C.; Journot, L.; et al. Evolution of Gene Expression after Whole-Genome Duplication: New Insights from the Spotted Gar Genome. J. Exp. Zool. B Mol. Dev. Evol. 2017, 328, 709–721. [Google Scholar] [CrossRef]
- Godoy, L.; Gonzàlez-Duarte, R.; Albalat, R. S-Nitrosogluthathione Reductase Activity of Amphioxus ADH3: Insights into the Nitric Oxide Metabolism. Int. J. Biol. Sci. 2006, 2, 117. [Google Scholar] [CrossRef]
- Colasanti, M.; Venturini, G.; Merante, A.; Musci, G.; Lauro, G.M. Nitric Oxide Involvement in Hydra Vulgaris Very Primitive Olfactory-Like System. J. Neurosci. 1997, 17, 493–499. [Google Scholar] [CrossRef]
- Colasanti, M.; Venturini, G. Nitric Oxide in Invertebrates. Mol. Neurobiol. 1998, 17, 157–174. [Google Scholar] [CrossRef]
- Moroz, L.L.; Norekian, T.P.; Pirtle, T.J.; Robertson, K.J.; Satterlie, R.A. Distribution of NADPH-Diaphorase Reactivity and Effects of Nitric Oxide on Feeding and Locomotory Circuitry in the Pteropod Mollusc, Clione Limacina. J. Comp. Neurol. 2000, 427, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Sadamoto, H.; Ogawa, H.; Kitamura, Y.; Oka, K.; Tanishita, K.; Ito, E. Nitric Oxide Generation around Buccal Ganglia Accompanying Feeding Behavior in the Pond Snail, Lymnaea Stagnalis. Neurosci. Res. 2000, 38, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.I.; Tachibana, T.; Hasebe, Y.; Masuda, N.; Ueda, H. Peripheral or Central Administration of Nitric Oxide Synthase Inhibitor Affects Feeding Behavior in Chicks. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 148, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Susswein, A.J.; Chiel, H.J. Nitric Oxide as a Regulator of Behavior: New Ideas from Aplysia Feeding. Prog. Neurobiol. 2012, 97, 304–317. [Google Scholar] [CrossRef]
- Hazut, N.; Rapps, K.; Kristt, D.A.; Susswein, A.J.; Weller, A. Nitric Oxide and L-Arginine Regulate Feeding in Satiated Rats. Appetite 2019, 132, 44–54. [Google Scholar] [CrossRef]
- Zhang, S.; Li, L.; Li, H.; Guo, H. Histochemical Localization of Constitutive Nitric Oxide Synthases in Amphioxus Branchiostoma Belcheri Tsingtauense. J. Mar. Biol. Assoc. UK 2002, 82, 1041–1042. [Google Scholar] [CrossRef]
- Yongjun, W.; Shicui, Z. Localization of Nitric Oxide Synthase in the Developing Gonads of Amphioxus Branchiostoma Belcheri Tsingtauense. Acta Oceanol. Sin. 2005, 24, 120–126. [Google Scholar]
- Ueda, N.; Degnan, S.M. Nitric Oxide Acts as a Positive Regulator to Induce Metamorphosis of the Ascidian Herdmania Momus. PLoS ONE 2013, 8, e72797. [Google Scholar] [CrossRef]
- Heyland, A.; Moroz, L.L. Signaling Mechanisms Underlying Metamorphic Transitions in Animals. Integr. Comp. Biol. 2006, 46, 743–759. [Google Scholar] [CrossRef]
- Paris, M.; Laudet, V. The History of a Developmental Stage: Metamorphosis in Chordates. Genesis 2008, 46, 657–672. [Google Scholar] [CrossRef]
- Castellano, I.; Ercolesi, E.; Palumbo, A. Nitric Oxide Affects ERK Signaling through Down-Regulation of MAP Kinase Phosphatase Levels during Larval Development of the Ascidian Ciona Intestinalis. PLoS ONE 2014, 9, e102907. [Google Scholar] [CrossRef]
- Bishop, C.D.; Bates, W.R.; Brandhorst, B.P. Regulation of Metamorphosis in Ascidians Involves NO/CGMP Signaling and HSP90. J. Exp. Zool. 2001, 289, 374–384. [Google Scholar] [CrossRef]
- Ercolesi, E.; Tedeschi, G.; Fiore, G.; Negri, A.; Maffioli, E.; D’Ischia, M.; Palumbo, A. Protein Nitration as Footprint of Oxidative Stress-Related Nitric Oxide Signaling Pathways in Developing Ciona Intestinalis. Nitric Oxide 2012, 27, 18–24. [Google Scholar] [CrossRef]
- Zaitseva, O.V.; Romanov, V.N.; Markosova, T.G. Distribution of NADPH-Diaphorase Activity in Organs and Tissues of Adult Ascidians. Dokl. Biol. Sci. 2012, 444, 180–183. [Google Scholar] [CrossRef]
- de Barros, C.M.; De Carvalho, D.R.; Andrade, L.R.; Pavão, M.S.G.; Allodi, S. Nitric Oxide Production by Hemocytes of the Ascidian Styela Plicata. Cell Tissue Res. 2009, 338, 117–128. [Google Scholar] [CrossRef]
- de Barros, C.M.; de Abreu Mello, A.; Allodi, S. Norepinephrine Depresses the Nitric Oxide Production in the Ascidian Hemocytes. J. Invertebr. Pathol. 2012, 111, 182–185. [Google Scholar] [CrossRef]
- Cioni, C.; Angiulli, E.; Toni, M. Nitric Oxide and the Neuroendocrine Control of the Osmotic Stress Response in Teleosts. Int. J. Mol. Sci. 2019, 20, 489. [Google Scholar] [CrossRef]
- Peter, M.C.S.; Gayathry, R.; Peter, V.S. Inducible Nitric Oxide Synthase/Nitric Oxide System as a Biomarker for Stress and Ease Response in Fish: Implication on Na+ Homeostasis During Hypoxia. Front. Physiol. 2022, 13, 795. [Google Scholar] [CrossRef]
- Filice, M.; Imbrogno, S.; Gattuso, A.; Cerra, M.C. Hypoxic and Thermal Stress: Many Ways Leading to the NOS/NO System in the Fish Heart. Antioxidants 2021, 10, 1401. [Google Scholar] [CrossRef]
- Li, J.; Zhou, W.; Wang, Y.; Niu, C. The Dual Role of CGMP in Oocyte Maturation of Zebrafish. Biochem. Biophys. Res. Commun. 2018, 499, 998–1003. [Google Scholar] [CrossRef]
- Stricker, S.A. Comparative Biology of Calcium Signaling during Fertilization and Egg Activation in Animals. Dev. Biol. 1999, 211, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; Van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript Assembly and Quantification by RNA-Seq Reveals Unannotated Transcripts and Isoform Switching during Cell Differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Corti, P.; Xue, J.; Tejero, J.; Wajih, N.; Sun, M.; Stolz, D.B.; Tsang, M.; Kim-Shapiro, D.B.; Gladwin, M.T. Globin X Is a Six-Coordinate Globin That Reduces Nitrite to Nitric Oxide in Fish Red Blood Cells. Proc. Natl. Acad. Sci. USA 2016, 113, 8538–8543. [Google Scholar] [CrossRef]
- Lepiller, S.; Laurens, V.; Bouchot, A.; Herbomel, P.; Solary, E.; Chluba, J. Imaging of Nitric Oxide in a Living Vertebrate Using a Diaminofluorescein Probe. Free Radic. Biol. Med. 2007, 43, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, R.; Schwerte, T.; Pelster, B. Nitric Oxide and Vascular Reactivity in Developing Zebrafish, Danio Rerio. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 279, R2200–R2207. [Google Scholar] [CrossRef]
- Pelster, B.; Grillitsch, S.; Schwerte, T. NO as a Mediator during the Early Development of the Cardiovascular System in the Zebrafish. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2005, 142, 215–220. [Google Scholar] [CrossRef]
- North, T.E.; Goessling, W.; Peeters, M.; Li, P.; Ceol, C.; Lord, A.M.; Weber, G.J.; Harris, J.; Cutting, C.C.; Huang, P.; et al. Hematopoietic Stem Cell Development Is Dependent on Blood Flow. Cell 2009, 137, 736–748. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, P.; Wei, Y.; Gao, Y.; Patient, R.; Liu, F. A Blood Flow–Dependent Klf2a-NO Signaling Cascade Is Required for Stabilization of Hematopoietic Stem Cell Programming in Zebrafish Embryos. Blood 2011, 118, 4102–4110. [Google Scholar] [CrossRef]
- Veerkamp, J.; Rudolph, F.; Cseresnyes, Z.; Priller, F.; Otten, C.; Renz, M.; Schaefer, L.; Abdelilah-Seyfried, S. Unilateral Dampening of Bmp Activity by Nodal Generates Cardiac Left-Right Asymmetry. Dev. Cell 2013, 24, 660–667. [Google Scholar] [CrossRef]
- Holmqvist, B.; Ellingsen, B.; Forsell, J.; Zhdanova, I.; Alm, P. The Early Ontogeny of Neuronal Nitric Oxide Synthase Systems in the Zebrafish. J. Exp. Biol. 2004, 207, 923–935. [Google Scholar] [CrossRef]
- Bradley, S.; Tossell, K.; Lockley, R.; McDearmid, J.R. Nitric Oxide Synthase Regulates Morphogenesis of Zebrafish Spinal Cord Motoneurons. J. Neurosci. 2010, 30, 16818. [Google Scholar] [CrossRef]
- Kong, Y.; Grimaldi, M.; Curtin, E.; Dougherty, M.; Kaufman, C.; White, R.M.; Zon, L.I.; Liao, E.C. Neural Crest Development and Craniofacial Morphogenesis Is Coordinated by Nitric Oxide and Histone Acetylation. Chem. Biol. 2014, 21, 488–501. [Google Scholar] [CrossRef]
- Harumi, T.; Watanabe, T.; Yamamoto, T.; Tanabe, Y.; Suzuki, N. Expression of Membrane-Bound and Soluble Guanylyl Cyclase MRNAs in Embryonic and Adult Retina of the Medaka Fish Oryzias Latipes. Zool. Sci. 2003, 20, 133–140. [Google Scholar] [CrossRef]
- Giordano, D.; Verde, C.; Corti, P. Nitric Oxide Production and Regulation in the Teleost Cardiovascular System. Antioxidants 2022, 11, 957. [Google Scholar] [CrossRef]
- Imbrogno, S.; Verri, T.; Filice, M.; Barca, A.; Schiavone, R.; Gattuso, A.; Cerra, M.C. Shaping the Cardiac Response to Hypoxia: NO and Its Partners in Teleost Fish. Curr. Res. Physiol. 2022, 5, 193–202. [Google Scholar] [CrossRef]
- Imbrogno, S.; Tota, B.; Gattuso, A. The Evolutionary Functions of Cardiac NOS/NO in Vertebrates Tracked by Fish and Amphibian Paradigms. Nitric Oxide 2011, 25, 1–10. [Google Scholar] [CrossRef]
- Newton, C.M.; Stoyek, M.R.; Croll, R.P.; Smith, F.M. Regional Innervation of the Heart in the Goldfish, Carassius Auratus: A Confocal Microscopy Study. J. Comp. Neurol. 2014, 522, 456–478. [Google Scholar] [CrossRef]
- Agnisola, C. Role of Nitric Oxide in the Control of Coronary Resistance in Teleosts. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2005, 142, 178–187. [Google Scholar] [CrossRef]
- Agnisola, C.; Pellegrino, D. Role of Nitric Oxide in Vascular Regulation in Fish. Adv Exp Biol 2007, 1, 293–310. [Google Scholar] [CrossRef]
- Olson, K.R.; Donald, J.A. Nervous Control of Circulation—The Role of Gasotransmitters, NO, CO, and H2S. Acta Histochem. 2009, 111, 244–256. [Google Scholar] [CrossRef]
- Imanishi, Y.; Yang, L.; Sokal, I.; Filipek, S.; Palczewski, K.; Baehr, W. Diversity of Guanylate Cyclase-Activating Proteins (GCAPs) in Teleost Fish: Characterization of Three Novel GCAPs (GCAP4, GCAP5, GCAP7) from Zebrafish (Danio Rerio) and Prediction of Eight GCAPs (GCAP1-8) in Pufferfish (Fugu rubripes). J. Mol. Evol. 2004, 59, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, L.R.; Ahuja, G.; Ivandic, I.; Syed, A.S.; Marioni, J.C.; Korsching, S.I.; Logan, D.W. Molecular and Neuronal Homology between the Olfactory Systems of Zebrafish and Mouse. Sci. Rep. 2015, 5, 11487. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Yao, Y.; Harumi, T.; Suzuki, N. Localization of the Nitric Oxide/CGMP Signaling Pathway-Related Genes and Influences of Morpholino Knock-down of Soluble Guanylyl Cyclase on Medaka Fish Embryogenesis. Zool. Sci. 2003, 20, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Mikami, T.; Kusakabe, T.; Suzuki, N. Tandem Organization of Medaka Fish Soluble Guanylyl Cyclase A1 and Β1 Subunit Genes. J. Biol. Chem. 1999, 274, 18567–18573. [Google Scholar] [CrossRef]
- Imbrogno, S.; Capria, C.; Tota, B.; Jensen, F.B. Nitric Oxide Improves the Hemodynamic Performance of the Hypoxic Goldfish (Carassius auratus) Heart. Nitric Oxide 2014, 42, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Vishnolia, K.K.; Rakovic, A.; Hoene, C.; Tarhbalouti, K.; Aherrahrou, Z.; Erdmann, J. SGC Activity and Regulation of Blood Flow in a Zebrafish Model System. Front. Physiol. 2021, 12, 217. [Google Scholar] [CrossRef]
- Xiyuan, Z.; Fink, R.H.A.; Mosqueira, M. NO-SGC Pathway Modulates Ca2+ Release and Muscle Contraction in Zebrafish Skeletal Muscle. Front. Physiol. 2017, 8, 607. [Google Scholar] [CrossRef]
- Pellegrino, D.; Sprovieri, E.; Mazza, R.; Randall, D.J.; Tota, B. Nitric Oxide-CGMP-Mediated Vasoconstriction and Effects of Acetylcholine in the Branchial Circulation of the Eel. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2002, 132, 447–457. [Google Scholar] [CrossRef]
- Hyndman, K.A.; Choe, K.P.; Havird, J.C.; Rose, R.E.; Piermarini, P.M.; Evans, D.H. Neuronal Nitric Oxide Synthase in the Gill of the Killifish, Fundulus Heteroclitus. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2006, 144, 510–519. [Google Scholar] [CrossRef]
- Mauceri, A.; Fasulo, S.; Ainis, L.; Licata, A.; Rita Lauriano, E.; Martfnez, A.; Mayer, B.; Zaccone, G. Neuronal Nitric Oxide Synthase (NNOS) Expression in the Epithelial Neuroendocrine Cell System and Nerve Fibers in the Gill of the Catfish, Heteropneustes Fossilis. Acta Histochem. 1999, 101, 437–448. [Google Scholar] [CrossRef]
- Gibbins, I.L.; Olsson, C.; Holmgren, S. Distribution of Neurons Reactive for NADPH-Diaphorase in the Branchial Nerves of a Teleost Fish, Gadus Morhua. Neurosci. Lett. 1995, 193, 113–116. [Google Scholar] [CrossRef]
- Zaccone, G.; Capillo, G.; Aragona, M.; Alesci, A.; Cupello, C.; Lauriano, E.R.; Guerrera, M.C.; Kuciel, M.; Zuwala, K.; Germana, A.; et al. Gill Structure and Neurochemical Markers in the African Bonytongue (Heterotis niloticus): A Preliminary Study. Acta Histochem. 2022, 124, 151954. [Google Scholar] [CrossRef]
- Garofalo, F.; Santovito, G.; Amelio, D. Morpho-Functional Effects of Heat Stress on the Gills of Antarctic T. bernacchii and C. hamatus. Mar. Pollut. Bull. 2019, 141, 194–204. [Google Scholar] [CrossRef]
- Tipsmark, C.K. Regulation of Na+/K+-ATPase Activity by Nitric Oxide in the Kidney and Gill of the Brown Trout (Salmo trutta). J. Exp. Biol. 2003, 206, 1503–1510. [Google Scholar] [CrossRef]
- Ebbesson, L.O.E. Nitric Oxide Synthase in the Gill of Atlantic Salmon: Colocalization with and Inhibition of Na+,K+-ATPase. J. Exp. Biol. 2005, 208, 1011–1017. [Google Scholar] [CrossRef]
- Jiménez, A.; Esteban, F.J.; Sánchez-López, A.M.; Pedrosa, J.A.; Del Moral, M.L.; Hernández, R.; Blanco, S.; Barroso, J.B.; Rodrigo, J.; Peinado, M.A. Immunohistochemical Localisation of Neuronal Nitric Oxide Synthase in the Rainbow Trout Kidney. J. Chem. Neuroanat. 2001, 21, 289–294. [Google Scholar] [CrossRef]
- Trischitta, F.; Pidalà, P.; Faggio, C. Nitric Oxide Modulates Ionic Transport in the Isolated Intestine of the Eel, Anguilla Anguilla. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 148, 368–373. [Google Scholar] [CrossRef]
- Peter, V.S. Nitric Oxide Rectifies Acid-Base Disturbance and Modifies Thyroid Hormone Activity during Net Confinement of Air-Breathing Fish (Anabas testudineus Bloch). Gen. Comp. Endocrinol. 2013, 181, 115–121. [Google Scholar] [CrossRef]
- Gerber, L.; Madsen, S.S.; Jensen, F.B. Cortisol Regulates Nitric Oxide Synthase in Freshwater and Seawater Acclimated Rainbow Trout, Oncorhynchus mykiss. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2017, 204, 1–8. [Google Scholar] [CrossRef]
- Gerber, L.; Jensen, F.B.; Madsen, S.S. Dynamic Changes in Nitric Oxide Synthase Expression Are Involved in Seawater Acclimation of Rainbow Trout Oncorhynchus mykiss. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 314, R552–R562. [Google Scholar] [CrossRef]
- Porteus, C.S.; Pollack, J.; Tzaneva, V.; Kwong, R.W.M.; Kumai, Y.; Abdallah, S.J.; Zaccone, G.; Lauriano, E.R.; Milsom, W.K.; Perry, S.F. A Role for Nitric Oxide in the Control of Breathing in Zebrafish (Danio rerio). J. Exp. Biol. 2015, 218, 3746–3753. [Google Scholar] [CrossRef] [PubMed]
- Tota, B.; Amelio, D.; Cerra, M.C.; Garofalo, F. The Morphological and Functional Significance of the NOS/NO System in the Respiratory, Osmoregulatory, and Contractile Organs of the African Lungfish. Acta Histochem 2018, 120, 654–666. [Google Scholar] [CrossRef] [PubMed]
- Olsson, C.; Holmgren, S. The Control of Gut Motility. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2001, 128, 479–501. [Google Scholar] [CrossRef]
- Olsson, C.; Holmgren, S. Autonomic Control of Gut Motility: A Comparative View. Auton. Neurosci. 2011, 165, 80–101. [Google Scholar] [CrossRef]
- Ceccotti, C.; Giaroni, C.; Bistoletti, M.; Viola, M.; Crema, F.; Terova, G. Neurochemical Characterization of Myenteric Neurons in the Juvenile Gilthead Sea Bream (Sparus aurata) Intestine. PLoS ONE 2018, 13, e0201760. [Google Scholar] [CrossRef]
- Olsson, C.; Holmgren, S. Pacap and Nitric Oxide Inhibit Contractions in the Proximal Intestine of the Atlantic Cod, Gadus Morhua. J. Exp. Biol. 2000, 203, 575–583. [Google Scholar] [CrossRef]
- Holmberg, A.; Olsson, C.; Holmgren, S. The Effects of Endogenous and Exogenous Nitric Oxide on Gut Motility in Zebrafish Danio Rerio Embryos and Larvae. J. Exp. Biol. 2006, 209, 2472–2479. [Google Scholar] [CrossRef]
- Olsson, C.; Aldman, G.; Larsson, A.; Holmgren, S. Cholecystokinin Affects Gastric Emptying and Stomach Motility in the Rainbow Trout Oncorhynchus Mykiss. J. Exp. Biol. 1999, 202, 161–170. [Google Scholar] [CrossRef]
- Uyttebroek, L.; Shepherd, I.T.; Vanden Berghe, P.; Hubens, G.; Timmermans, J.P.; Van Nassauw, L. The Zebrafish Mutant Lessen: An Experimental Model for Congenital Enteric Neuropathies. Neurogastroenterol. Motil. 2016, 28, 345–357. [Google Scholar] [CrossRef]
- Bosi, G.; Maynard, B.J.; Pironi, F.; Dezfuli, B.S. Parasites and the Neuroendocrine Control of Fish Intestinal Function: An Ancient Struggle between Pathogens and Host. Parasitology 2022, 149, 1842–1861. [Google Scholar] [CrossRef]
- Zhu, W.; Su, J. Immune Functions of Phagocytic Blood Cells in Teleost. Rev. Aquac. 2022, 14, 630–646. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the Full Spectrum of Macrophage Activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Denicola, A.; Rubbo, H.; Rodriguez, D.; Radi, R. Peroxynitrite-Mediated Cytotoxicity to Trypanosoma Cruzi. Arch. Biochem. Biophys. 1993, 304, 279–286. [Google Scholar] [CrossRef]
- Henard, C.A.; Vázquez-Torres, A. Nitric Oxide and Salmonella Pathogenesis. Front. Microbiol. 2011, 2, 84. [Google Scholar] [CrossRef]
- Wiegertjes, G.F.; Wentzel, A.S.; Spaink, H.P.; Elks, P.M.; Fink, I.R. Polarization of Immune Responses in Fish: The ‘Macrophages First’ Point of View. Mol. Immunol. 2016, 69, 146–156. [Google Scholar] [CrossRef]
- Neumann, N.F.; Fagan, D.; Belosevic, M. Macrophage Activating Factor(s) Secreted by Mitogen Stimulated Goldfish Kidney Leukocytes Synergize with Bacterial Lipopolysaccharide to Induce Nitric Oxide Production in Teleost Macrophages. Dev. Comp. Immunol. 1995, 19, 473–482. [Google Scholar] [CrossRef]
- Buentello, J.A.; Gatlin, D.M. Nitric Oxide Production in Activated Macrophages from Channel Catfish (Ictalurus Punctatus): Influence of Dietary Arginine and Culture Media. Aquaculture 1999, 179, 513–521. [Google Scholar] [CrossRef]
- Losada, A.P.; Bermúdez, R.; Faílde, L.D.; Quiroga, M.I. Quantitative and Qualitative Evaluation of INOS Expression in Turbot (Psetta maxima) Infected with Enteromyxum scophthalmi. Fish. Shellfish Immunol. 2012, 32, 243–248. [Google Scholar] [CrossRef]
- Saeij, J.P.J.; Stet, R.J.M.; Groeneveld, A.; Verburg-Van Kemenade, L.B.M.; Van Muiswinkel, W.B.; Wiegertjes, G.F. Molecular and Functional Characterization of a Fish Inducible-Type Nitric Oxide Synthase. Immunogenetics 2000, 51, 339–346. [Google Scholar] [CrossRef]
- Vojtech, L.N.; Sanders, G.E.; Conway, C.; Ostland, V.; Hansen, J.D. Host Immune Response and Acute Disease in a Zebrafish Model of Francisella Pathogenesis. Infect. Immun. 2009, 77, 914–925. [Google Scholar] [CrossRef]
- Campos-Perez, J.J.; Ward, M.; Grabowski, P.S.; Ellis, A.E.; Secombes, C.J. The Gills Are an Important Site of INOS Expression in Rainbow Trout Oncorhynchus Mykiss after Challenge with the Gram-Positive Pathogen Renibacterium salmoninarum. Immunology 2000, 99, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Forlenza, M.; Fink, I.R.; Raes, G.; Wiegertjes, G.F. Heterogeneity of Macrophage Activation in Fish. Dev. Comp. Immunol. 2011, 35, 1246–1255. [Google Scholar] [CrossRef] [PubMed]
- Wentzel, A.S.; Janssen, J.J.E.; de Boer, V.C.J.; van Veen, W.G.; Forlenza, M.; Wiegertjes, G.F. Fish Macrophages Show Distinct Metabolic Signatures Upon Polarization. Front. Immunol. 2020, 11, 152. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Mancuso, C.; Calvani, M.; Rizzarelli, E.; Butterfield, D.A.; Giuffrida Stella, A.M. Nitric Oxide in the Central Nervous System: Neuroprotection versus Neurotoxicity. Nat. Rev. Neurosci. 2007, 8, 766–775. [Google Scholar] [CrossRef]
- Øyan, A.M.; Nilsen, F.; Goksøyr, A.; Holmqvist, B. Partial cloning of constitutive and inducible nitric oxide synthases and detailed neuronal expression of NOS mRNA in the cerebellum and optic tectum of adult Atlantic salmon (Salmo salar). Mol. Brain Res. 2000, 78, 38–49. [Google Scholar] [CrossRef]
- Zhou, L.; Bai, R.; Tian, J.; Liu, X.; Lu, D.; Zhu, P.; Liu, Y.; Zeng, L.; Luo, W.; Zhang, Y.; et al. Bioinformatic comparisons and tissue expression of the neuronal nitric oxide synthase (nNOS) gene from the red drum (Sciaenops ocellatus). Fish Shellfish. Immunol. 2009, 27, 577–584. [Google Scholar] [CrossRef]
- Brüning, G.; Hattwig, K.; Mayer, B. Nitric Oxide Synthase in the Peripheral Nervous System of the Goldfish, Carassius Auratus. Cell Tissue Res. 1996, 284, 87–98. [Google Scholar] [CrossRef]
- Zaccone, G.; Lauriano, E.R.; Kuciel, M.; Capillo, G.; Pergolizzi, S.; Alesci, A.; Ishimatsu, A.; Ip, Y.K.; Icardo, J.M. Identification and Distribution of Neuronal Nitric Oxide Synthase and Neurochemical Markers in the Neuroepithelial Cells of the Gill and the Skin in the Giant Mudskipper, Periophthalmodon schlosseri. Zoology 2017, 125, 41–52. [Google Scholar] [CrossRef]
- Penglee, R.; Gao, L.; Huang, Y.; Liu, L.; Nimitkul, S.; Bao, B. The Role of Nitric Oxide and Neuronal Nitric Oxide Synthase in Zebrafish (Danio rerio) Shoaling. Aquac. Fish 2021, 6, 565–573. [Google Scholar] [CrossRef]
- Carreño Gutiérrez, H.; O’Leary, A.; Freudenberg, F.; Fedele, G.; Wilkinson, R.; Markham, E.; van Eeden, F.; Reif, A.; Norton, W.H.J. Nitric Oxide Interacts with Monoamine Oxidase to Modulate Aggression and Anxiety-like Behaviour. Eur. Neuropsychopharmacol. 2020, 30, 30–43. [Google Scholar] [CrossRef]
- Filby, A.L.; Paull, G.C.; Hickmore, T.F.A.; Tyler, C.R. Unravelling the Neurophysiological Basis of Aggression in a Fish Model. BMC Genom. 2010, 11, 498. [Google Scholar] [CrossRef]
- Akinrinade, I.; Kareklas, K.; Teles, M.C.; Reis, T.K.; Gliksberg, M.; Petri, G.; Levkowitz, G.; Oliveira, R.F. Evolutionarily Conserved Role of Oxytocin in Social Fear Contagion in Zebrafish. Science 2023, 379, 1232–1237. [Google Scholar] [CrossRef]
- Rettori, V.; Canteros, G.; Renoso, R.; Gimeno, M.; McCann, S.M. Oxytocin Stimulates the Release of Luteinizing Hormone-Releasing Hormone from Medial Basal Hypothalamic Explants by Releasing Nitric Oxide. Proc. Natl. Acad. Sci. USA 1997, 94, 2741–2744. [Google Scholar] [CrossRef]
- Bordieri, L.; Bonaccorsi Di Patti, M.C.; Miele, R.; Cioni, C. Partial Cloning of Neuronal Nitric Oxide Synthase (NNOS) CDNA and Regional Distribution of NNOS MRNA in the Central Nervous System of the Nile Tilapia Oreochromis Niloticus. Mol. Brain Res. 2005, 142, 123–133. [Google Scholar] [CrossRef]
- Mobley, R.B.; Ray, E.J.; Maruska, K.P. Expression and Localization of Neuronal Nitric Oxide Synthase in the Brain and Sensory Tissues of the African Cichlid Fish Astatotilapia burtoni. J. Comp. Neurol. 2022, 530, 2901–2917. [Google Scholar] [CrossRef]
- Vielma, A.H.; Retamal, M.A.; Schmachtenberg, O. Nitric Oxide Signaling in the Retina: What Have We Learned in Two Decades? Brain Res. 2012, 1430, 112–125. [Google Scholar] [CrossRef]
- Singru, P.S.; Sakharkar, A.J.; Subhedar, N. Neuronal Nitric Oxide Synthase in the Olfactory System of an Adult Teleost Fish Oreochromis mossambicus. Brain Res. 2003, 977, 157–168. [Google Scholar] [CrossRef]
- Liepe, B.A.; Stone, C.; Koistinaho, J.; Copenhagen, D.R. Nitric Oxide Synthase in Muller Cells and Neurons of Salamander and Fish Retina. J. Neurosci. 1994, 14, 7641–7654. [Google Scholar] [CrossRef]
- Miles, G.B.; Sillar, K.T. Neuromodulation of Vertebrate Locomotor Control Networks. Physiology 2011, 26, 393–411. [Google Scholar] [CrossRef]
- Pirtle, T.J.; Satterlie, R.A. Cyclic Guanosine Monophosphate Modulates Locomotor Acceleration Induced by Nitric Oxide but Not Serotonin in Clione limacina Central Pattern Generator Swim Interneurons. Integr. Org. Biol. 2021, 3, obaa045. [Google Scholar] [CrossRef]
- Hachoumi, L.; Sillar, K.T. Developmental Stage-Dependent Switching in the Neuromodulation of Vertebrate Locomotor Central Pattern Generator Networks. Dev. Neurobiol. 2020, 80, 42–57. [Google Scholar] [CrossRef] [PubMed]
- Jay, M.; Bradley, S.; McDearmid, J.R. Effects of Nitric Oxide on Neuromuscular Properties of Developing Zebrafish Embryos. PLoS ONE 2014, 9, e86930. [Google Scholar] [CrossRef]
- Maake, C.; Kloas, W.; Szendefi, M.; Reinecke, M. Neurohormonal Peptides, Serotonin, and Nitric Oxide Synthase in the Enteric Nervous System and Endocrine Cells of the Gastrointestinal Tract of Neotenic and Thyroid Hormone-Treated Axolotls (Ambystoma mexicanum). Cell Tissue Res. 1999, 297, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Blom, J.J.; Blute, T.A.; Eldred, W.D. Functional Localization of the Nitric Oxide/CGMP Pathway in the Salamander Retina. Vis. Neurosci. 2009, 26, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Trajanovska, S.; Donald, J.A. Endothelial Nitric Oxide Synthase in the Amphibian, Xenopus Tropicalis. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2011, 158, 274–281. [Google Scholar] [CrossRef]
- Brunelli, E.; Perrotta, I.; Talarico, E.; Tripepi, S. Localization of Two Nitric Oxide Synthase Isoforms, ENOS and INOS, in the Skin of Triturus Italicus (Amphibia, Urodela) during Development. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2005, 142, 249–255. [Google Scholar] [CrossRef]
- Jeseta, M.; Marin, M.; Tichovska, H.; Melicharova, P.; Cailliau-Maggio, K.; Martoriati, A.; Lescuyer-Rousseau, A.; Beaujois, R.; Petr, J.; Sedmikova, M.; et al. Nitric Oxide-Donor SNAP Induces Xenopus Eggs Activation. PLoS ONE 2012, 7, e41509. [Google Scholar] [CrossRef]
- Jacox, L.; Sindelka, R.; Chen, J.; Rothman, A.; Dickinson, A.; Sive, H. The Extreme Anterior Domain Is an Essential Craniofacial Organizer Acting through Kinin-Kallikrein Signaling. Cell Rep. 2014, 8, 596–609. [Google Scholar] [CrossRef]
- Vitt, L.J.; Caldwell, J.P. Herpetology: An Introductory Biology of Amphibians and Reptiles, 4th ed.; Academic Press: Cambridge, MA, USA, 2013; 757p. [Google Scholar] [CrossRef]
- Sillar, K.T.; Combes, D.; Ramanathan, S.; Molinari, M.; Simmers, J. Neuromodulation and Developmental Plasticity in the Locomotor System of Anuran Amphibians during Metamorphosis. Brain Res. Rev. 2008, 57, 94–102. [Google Scholar] [CrossRef]
- Cristino, L.; Florenzano, F.; Bentivoglio, M.; Guglielmotti, V. Nitric Oxide Synthase Expression and Cell Changes in Dorsal Root Ganglia and Spinal Dorsal Horn of Developing and Adult Rana Esculenta Indicate a Role of Nitric Oxide in Limb Metamorphosis. J. Comp. Neurol. 2004, 472, 423–436. [Google Scholar] [CrossRef]
- Johnson, J.; Manzo, W.; Gardner, E.; Menon, J. Reactive Oxygen Species and Anti-Oxidant Defenses in Tail of Tadpoles, Xenopus Laevis. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2013, 158, 101–108. [Google Scholar] [CrossRef]
- Hedrick, M.S.; Chen, A.K.; Jessop, K.L. Nitric Oxide Changes Its Role as a Modulator of Respiratory Motor Activity during Development in the Bullfrog (Rana catesbeiana). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2005, 142, 231–240. [Google Scholar] [CrossRef]
- Ueda, N.; Richards, G.S.; Degnan, B.M.; Kranz, A.; Adamska, M.; Croll, R.P.; Degnan, S.M. An Ancient Role for Nitric Oxide in Regulating the Animal Pelagobenthic Life Cycle: Evidence from a Marine Sponge. Sci. Rep. 2016, 6, 37546. [Google Scholar] [CrossRef]
- Moroz, L.L.; Meech, R.W.; Sweedler, J.V.; Mackie, G.O. Nitric Oxide Regulates Swimming in the Jellyfish Aglantha Digitale. J. Comp. Neurol. 2004, 471, 26–36. [Google Scholar] [CrossRef]
- Newcomb, J.M.; Watson, W.H. Modulation of Swimming in the Gastropod Melibe leonina by Nitric Oxide. J. Exp. Biol. 2002, 205, 397–403. [Google Scholar] [CrossRef]
- Mahapatra, C.; Achary, A.S.; Patra, D. Protein S-Nitrosylation: Nitric Oxide Signalling during Anuran Tail Regression. Acta Histochem. 2022, 124, 151899. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).