Abstract
Hepatitis B infection caused by the hepatitis B virus is a life-threatening cause of liver fibrosis, cirrhosis, and hepatocellular carcinoma. Researchers have produced multiple in vivo models for hepatitis B virus (HBV) and, currently, there are no specific laboratory animal models available to study HBV pathogenesis or immune response; nonetheless, their limitations prevent them from being used to study HBV pathogenesis, immune response, or therapeutic methods because HBV can only infect humans and chimpanzees. The current study is the first of its kind to identify a suitable chemically induced liver cirrhosis/HCC model that parallels HBV pathophysiology. Initially, data from the peer-reviewed literature and the GeneCards database were compiled to identify the genes that HBV and seven drugs (acetaminophen, isoniazid, alcohol, D-galactosamine, lipopolysaccharide, thioacetamide, and rifampicin) regulate. Functional enrichment analysis was performed in the STRING server. The network HBV/Chemical, genes, and pathways were constructed by Cytoscape 3.6.1. About 1546 genes were modulated by HBV, of which 25.2% and 17.6% of the genes were common for alcohol and lipopolysaccharide-induced hepatitis. In accordance with the enrichment analysis, HBV activates the signaling pathways for apoptosis, cell cycle, PI3K-Akt, TNF, JAK-STAT, MAPK, chemokines, NF-kappa B, and TGF-beta. In addition, alcohol and lipopolysaccharide significantly activated these pathways more than other chemicals, with higher gene counts and lower FDR scores. In conclusion, alcohol-induced hepatitis could be a suitable model to study chronic HBV infection and lipopolysaccharide-induced hepatitis for an acute inflammatory response to HBV.
1. Introduction
Hepatitis B virus (HBV) is a member of the family Hepadnaviridae, possessing a 3.2 kb short genome with largely double-stranded DNA [1]. The human sodium taurocholate co-transporting polypeptide (NTCP) receptor and the viral envelope protein (HBsAg) interact in a remarkably species-specific manner to allow HBV to enter human hepatocytes [2]. Several liver diseases, including cirrhosis, hepatocellular carcinoma, and liver fibrosis, can develop in those with chronic HBV infection [2]. Despite significant advancements in the diagnosis, prevention, and treatment of chronic hepatitis B (CHB), over 296 million individuals worldwide still have the HBV infection and account for an estimated 820,000 deaths, mostly by cirrhosis and hepatocellular carcinoma (HCC) [3]. Injections of interferon and oral nucleoside analogs are used to treat persistent HBV infection [4]. Currently, HIV and HBV polymerase reverse transcriptase inhibitors are licensed treatments for HBV, and only 30–40% of individuals with chronic HBV (CHB) react to interferon therapy [4]. The World Health Organization (WHO) recommends entecavir and tenofovir for the treatment of CHB [3].
Several HBV cell culture-based systems, such as HepG2T14, HepG2.2.15, Q7 HBV-21, HepG2-4A5, and HepAD38, have been created and have been employed for cultivating the virus to conduct in vitro HBV inhibitor screening and investigate the control of viral replication [5]. In vivo models, however, have been and will continue to be essential for understanding the mechanisms underlying HBV pathogenesis, HBV-induced immune responses, and the testing of new antiviral therapeutic regimens [5]. Numerous in vivo models, such as those using chimpanzees, tupaiids, woodchucks, ducks, and woolly monkeys, have been produced since the “Australian antigen” was discovered. However, these animals are not routinely utilized as experimental hosts due to ethical and cost concerns [6]. Additionally, with the lack of small animal models that reproduce human-like HBV infections, it becomes extremely difficult to understand possible HBV disease mechanisms and develop efficient treatments [7]. Although an HBV mouse model may be suitable, this approach has several drawbacks. The 1.3-HBV transgenic mouse model, which has 1.3-HBV incorporated into the murine genome, is immune to HBV, does not cause liver damage, and does not produce cccDNA [8]. To maintain cells for six months, hydrodynamic injection (HDI)-based replication-competent HBV transgenic mice with HBV replicons, such as 1.2- or 1.3-HBV or HBV circle genomes, are hydrodynamically injected into mice. With the right vector, they can cause liver fibrosis and are expressed in 10–25% of murine hepatocytes post-inoculation. HBV genotype affects viral persistence [8]. Adeno-HBV transgenic mice were developed by injecting adenovirus vectors containing the HBV genome [9]. These mice become immunologically tolerant to HBV due to an altered T cell profile (an advantage for immunotolerant studies) and the absence of detectable cccDNA [10]. Apart from the above-mentioned models, various chemical-induced models (alcohol, acetaminophen, lipopolysaccharides, isoniazid, etc.) are also utilized to evaluate the hepatoprotective potential of compounds [11,12]. These chemically induced models alter multiple genes and pathways (PI3K-Akt, TNF, JAK-STAT, MAPK, Chemokine, NF-kappa B, TGF-beta signaling pathways, Apoptosis, and Cell cycle) and result in the development of hepatitis [13], which may be similar to that of HBV-induced hepatitis. Therefore, the goal of the current study was to combine gene set enrichment and network pharmacology analyses to pinpoint the chemical-induced hepatitis model that most closely resembles the pathophysiology of HBV; the current study’s workflow is presented (Figure 1).
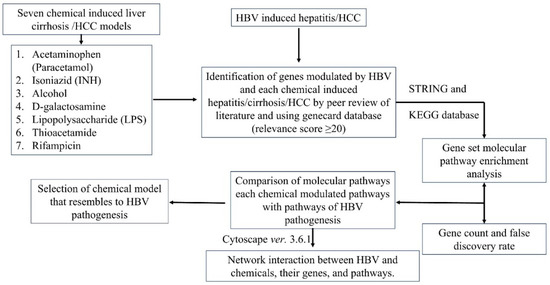
Figure 1.
Workflow of the current study.
2. Results
2.1. Identification of HBV-Associated and Chemically Induced Hepatitis Genes
Based on the literature review, 42 genes (from 36 articles) were obtained which were modulated in HBV-induced hepatitis. Similarly, from the GeneCards database, 1538 genes were obtained with a relevance score greater than 20. Among the 1538 genes, interferon-γ (IFN-γ) had the highest relevance score of 166.85, while KHK (Ketohexokinase) had the lowest relevance score of 20.00. Likewise, the genes obtained based upon the literature review were 66 genes for alcohol (from 65 articles), 38 genes for acetaminophen (from 11 articles), 30 genes for isoniazid (from 10 articles), 31 genes for D-galactosamine (from 14 articles), 44 genes for lipopolysaccharide (from 39 articles), 33 genes for rifampicin (from 26 articles), and 33 genes for thioacetamide (from 23 articles) were obtained and, similarly, were 407, 128,48, 52, 260, 24, 31 from the GeneCards database, respectively. From the list of genes for alcohol-induced hepatitis articles, alcohol dehydrogenase 1b (ADH1B), β-polypeptide had the highest relevance score of 98.78, and Serpin Family C member 1 (SERPINC1) had the lowest relevance score of 20.03. Interestingly TNF had the highest relevance scores of 75.06, 77.71, 69.39, 95.44, 51.13 for acetaminophen, isoniazid, D-galactosamine, lipopolysaccharide, and rifampicin-induced hepatitis, respectively, and for thioacetamide, IL6 had the highest relevance score of 55.21. UGT1A4, CYP2A6, CSF2, INRS, GSTM1, and BMP6 had the lowest relevance score of 20.08, 20.03, 20.47, 20.02, 20.0, and 20.02, respectively. The list of genes/protein molecules regulated by the HBV and each chemical-induced hepatitis which is obtained from a peer review of the literature (along with references [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226,227,228,229,230,231,232,233]) and the GeneCards database are summarized in Supplementary Tables S1–S8.
2.2. Analysis of Genes Involved in Hepatic Toxicity
In HBV-induced hepatitis, 2% (31) were common in both the literature review and the GeneCards database, while for acetaminophen, isoniazid, alcohol, D-galactosamine, lipopolysaccharide, thioacetamide, and rifampicin the common genes between the literature review and the GeneCards database were found to be 9.2% (14), 5.6% (4), 8.8% (33), 15.3% (11), 9% (25), 10.5% (6), and 5.6% (3), respectively. Figure 2 represents the common genes between the literature review and GeneCards.
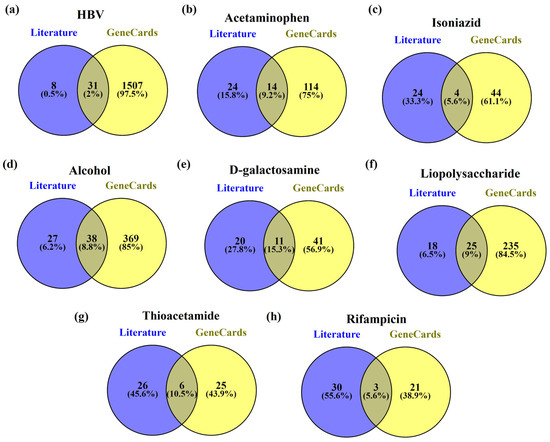
Figure 2.
The genes common in both the literature review and GeneCards for (a) HBV, (b) acetaminophen, (c) isoniazid, (d) alcohol, (e) D-galactosamine, (f) lipopolysaccharide, (g) thioacetamide, and (h) rifampicin.
Further, the common genes present in HBV and acetaminophen, isoniazid, alcohol, D-galactosamine, lipopolysaccharide, thioacetamide, and rifampicin-induced hepatitis were found to be 9% (140), 3.7% (57), 25.2% (399), 3.8% (59), 17.6% (273), 3.4% (52) and 3.2% (50) respectively. Among them, HBV genes with alcohol genes had the highest similarity i.e., 25.2% whereas lipopolysaccharides had the second highest similarity i.e., 17.6%. Figure 3 represents the common genes between HBV and chemical-induced hepatitis.
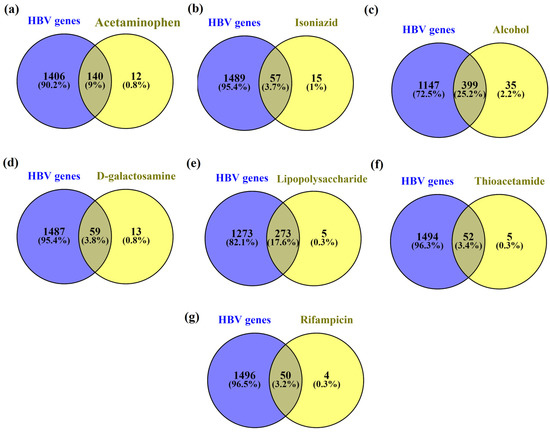
Figure 3.
Common genes between HBV (a) acetaminophen, (b) isoniazid, (c) alcohol, (d) D-galactosamine, (e) lipopolysaccharide, (f) thioacetamide, and (g) rifampicin.
2.3. Functional Enrichment Analysis to Assess the Hepatotoxicity
Initially, HBV, acetaminophen, isoniazid, alcohol, D-galactosamine, lipopolysaccharide, thioacetamide, and rifampicin were identified to modulate 1546, 152, 72, 434, 72, 278, 57, and 54 genes, respectively. The enrichment analysis of these individual sets of the gene revealed 217, 185, 184, 200, 185, 202, 167, and 172 molecular pathways, respectively. Supplementary Tables S9–S16 represent the molecular pathways modulated by HBV and chemicals.
In the HBV-induced hepatitis model, out of 217 pathways modulated, nine pathways—namely, PI3K-Akt, TNF, JAK-STAT, MAPK, Chemokine, NF-kappa B, TGF-beta signaling pathways, Apoptosis, and Cell cycle—were prioritized to compare with chemically-induced hepatitis, as these pathways were significantly associated with the progression of hepatocellular carcinoma induced by HBV (refer KEGG ID: hsa05161). Among them, the PI3K-Akt signaling pathway scored the lowest FDR of 1.39E−33 and the highest gene count of 57, whereas TNF, JAK-STAT, MAPK, Chemokine, NF-kappa B, TGF-beta signaling pathways, Apoptosis, and Cell cycle scored the lowest FDR of 1.56E−27, 1.38E−22, 5.4E−20, 7.77E−13, 6.49E−12, 7.26E−27, 4.34E−09, respectively, and gene counts of 34, 33, 38, 24, 18, 11, 35, and 16, respectively. Figure 4 represents the network of HBV-modulated genes and pathways.
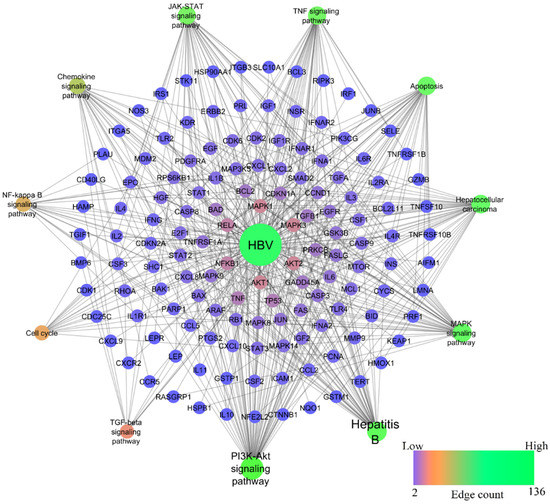
Figure 4.
Network representation of HBV-modulated genes and pathways.
Among chemical-induced, lipopolysaccharide-induced hepatitis had the highest similarity compared to HBV-induced, whereas alcohol-induced hepatitis was found to be the second highest similarity with HBV. Table 1 represents the pathways modulated by HBV and selected chemicals. Lipopolysaccharide was found to modulate 202 molecular pathways, in which the PI3K-Akt signaling pathway scored the lowest FDR of 2.46E−41 and the highest gene count of 58, whereas TNF, JAK-STAT, MAPK, Chemokine, NF-kappa B, TGF-β signaling pathways, apoptosis, and cell cycle scored the lowest FDR of 4.49E−46, 1.48E−35, 5.8E−30, 3.71E−21, 4.11E−38, 0.000000036, 2.35E−38, and 7.55E−08 and gene counts of 45, 41, 44, 30, 38, 12, 41, and 13, respectively. Figure 5 represents the network of lipopolysaccharide-modulated genes and pathways.

Table 1.
Functional enrichment analysis of genes modulated by HBV and selected chemicals.
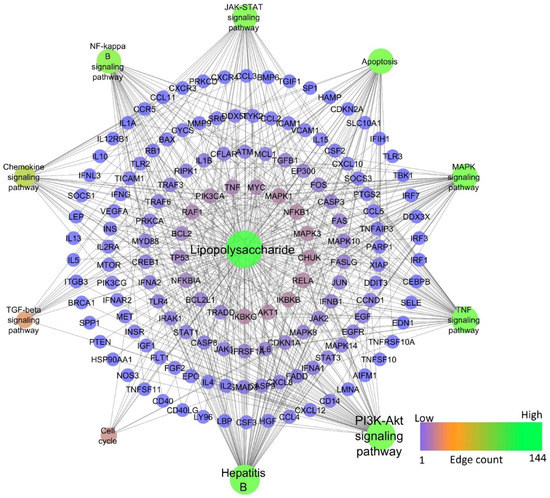
Figure 5.
Network representation of lipopolysaccharide-modulated genes and pathways.
Similar to HBV-induced hepatitis, alcohol-induced hepatitis shared the second-highest degree of similarity. Alcohol was found to modulate 200 molecular pathways, in which the PI3K-Akt signaling pathway scored the lowest FDR of 2.87E−21 and the highest gene count of 31, whereas TNF, JAK-STAT, MAPK, Chemokine, NF-kB, TGF-beta signaling pathways, Apoptosis, and Cell cycle scored the lowest FDR of 5.92E−23, 2.93E−14, 4.07E−16, 3.25E−10, 3.10E−11, 3.36E−05, 3.77E−24, 0.0063 and gene counts of 23, 18, 24, 15, 13, 7, 25, 5, respectively. Figure 6 represents the network of alcohol-modulated genes and pathways.
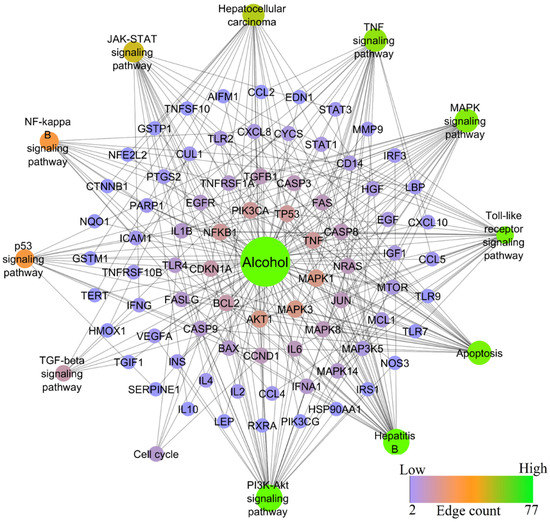
Figure 6.
Network representation of alcohol-modulated genes and pathways.
3. Discussion
Animal models are widely used to study the pathophysiology of chronic hepatitis B and to develop new drugs or treatment methods [234]. HBV can only infect humans and chimpanzees [235]. However, due to ethical and practical concerns, chimpanzees are not commonly utilized in HBV research [235]. Additionally, efforts have been conducted to spread HBV to smaller non-human primates. The tree shrew is the only rodent other than a primate that has been found to be susceptible to HBV infection, but the in vivo system still needs major improvement [7,236]. As a result of the absence of viral entry, cccDNA synthesis, and viral dissemination, mice with the HBV genome transfected, transduced, or transgenic can only support HBV replication, leaving the HBV life cycle unfinished. When human liver cells that maintain HBV infection are transplanted into immuno-deficient mice, the animals show apparent immunodeficiency, and their maintenance systems are very sophisticated [237]. As a result, the majority of gains in HBV research have been made utilizing mice models of HBV replication or infection, or models of HBV-related hepadnaviral infection [236,237,238].
In line with previous investigations, some of the drugs used to cause hepatitis in rats are known to cause pathophysiology that is comparable to the pathogenesis of HBV in people [239,240,241]. Therefore, the goal of the current study was to use gene set enrichment and network pharmacology analysis to find a chemically induced hepatitis model that is similar to HBV pathogenesis. The study determined that the pathogenesis of HBV is similar in the alcohol- and LPS-induced hepatitis models. About 42 and 1538 genes were first gathered for HBV from the literature and GeneCards, respectively, of which 31 genes (2%) were common. In the enrichment analysis, 1546 genes were involved in 217 molecular pathways, in which nine pathways—namely, PI3K-Akt, TNF, JAK-STAT, MAPK, Chemokine, NF-kappa B, TGF-beta signaling pathways, Apoptosis, and Cell cycle—were majorly associated with HBV infection (KEGG ID: hsa05161). These pathways were significantly targeted by both LPS and alcohol (Figure 7 and Table 1).
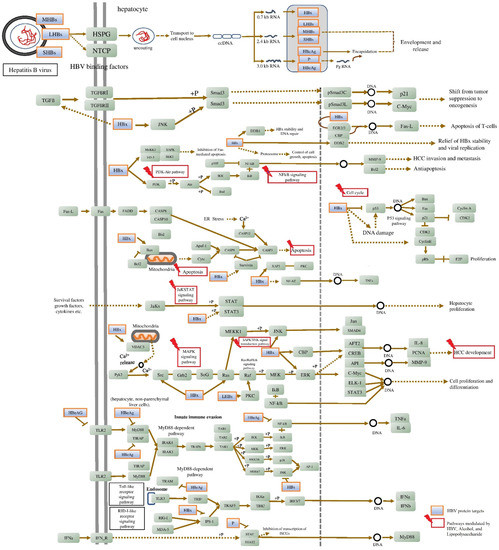
Figure 7.
Molecular pathways triggered by alcohol and LPS resembled the HBV pathogenesis. The figure information is retrieved from the KEGG database “Hepatitis B: hsa05161”.
The PI3K/Akt signaling pathway is associated with a variety of biological processes caused by enzymes, including glucose metabolism. Phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) and mitogen-activated protein kinase (MAPK) signaling are examples of signal transduction cascades that could be activated by HBV HBx, which is primarily present in the cytoplasm [242]. Alcohol inhibits the liver’s insulin signaling pathway, which leads to irregularities in the metabolism of glucose and lipids. This is one of the key factors contributing to the development of alcoholic liver disease (ALD) [243]. Alcohol was found to increase apoptotic expression and PI3K/Akt signaling while lowering hepatic perfusion, hence promoting cirrhosis [244]. Similarly, LPS is also reported to activate the PI3K/Akt and MAPKs [245]. In this study, HBV, alcohol, and LPS targeted the PI3K-Akt signaling pathway by modulating 57, 31, and 58 genes and the MAPK signaling pathway by 38, 24, and 44 genes, respectively. Among them, AKT1, AKT2, MAPK1, MAPK3, NFKB1, TNF, and BCL2 genes were identified as hub genes within the network. The activation of these signal pathways may contribute to liver cell malignant transformation.
TNF-α, one of the most important inflammatory cytokines, was first identified as an anti-tumor cytokine that resulted in tumor necrosis. Inflammation is fundamentally mediated by TNF-α, which also promotes the growth of cancers. Researchers have found that compared to healthy liver tissue, HCC expresses TNF-α at substantially higher levels [246]. TNF-α is a potent NF-kB signaling activator; during HBV infection, it increases HBx intracellular concentration by enhancing its stability and is essential for the onset and progression of HCC [247], whereas in alcohol-induced hepatitis, alcohol increases hepatocytes’ susceptibility to TNF-α-induced apoptosis. TNF-α levels were higher in both chronic drinkers and animal models fed alcohol over an extended period of time. In all kinds of liver cells, the NF-kB is a key regulator of cellular stress. In the cytoplasm of dormant cells, the family of NF-kB proteins, including RelA/p65, RelB, c-Rel, and p50, exist as dimers in a complex with inhibitory kB molecules [248]. Chronic alcohol use is thought to prime the liver by inducing basal and LPS-stimulated TNF-α and persistent NF-kB activation [249]. Hepatic macrophages’ expression of pro-inflammatory mediators is significantly regulated by NF-kB [249]. The activation of TLR4 by circulating LPS on liver macrophages, which results in NF-B activation and the generation of pro-inflammatory cytokines, is linked to chronic alcohol-mediated liver damage [250]. In this study, HBV, alcohol, and LPS targeted the TNF-α signaling pathway by modulating 34, 23, and 45 genes and the NF-kB signaling pathway by 18, 5, and 13 genes, respectively. The LPS has the lowest FDR score for TNF and NF-kB signaling pathways, i.e., 4.49E−46 and 4.11E−38, respectively. While for HBV and alcohol the FDR score for the TNF signaling pathway was 1.56E−27 and 5.92E−23, for the NF-kB signaling pathway it was 6.49E−12 and 3.10E−11, respectively. This indicates LPS possesses a significant effect on TNF and NF-kB signaling pathways. TGF-β a crucial cytokine that promotes fibrosis in a variety of chronic liver disorders and HCC. Overactivation of the TGF-β signaling pathway increases cell migration and invasion. The HBV HBx upregulates TGF-β on HCC progression by downregulating protein phosphatase magnesium-dependent 1A (PPM1a) [251]. Alcohol and LPS are also reported to increase the TGF-β and are prevalent in ALD. In this study, HBV, alcohol, and LPS targeted TGF-β signaling pathways by modulating 11, 7, and 12 genes, respectively.
Among the HBV proteins, HBx is the one that has been most commonly linked to the suppression of apoptosis and the stimulation of HCC development. Through the overexpression of PI3K and the stimulation of Akt phosphorylation, HBx stimulates the phosphatidylinositol-4,5-bisphosphate 3-kinase-protein kinase B (PI3K-Akt) pathway to suppress apoptosis. Drp-1 and Parkin are brought to the mitochondria by HBx to promote mitochondrial fission and mitophagy, which suppresses the intrinsic apoptotic pathway. Additionally, the activation of Akt inhibits BAD from moving to the mitochondria and apoptosis from occurring. HBx stimulates the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling by degrading IκB. HBV may reduce the activity of the kinase that activates JNK in the MAPK-JNK pathway [252]. Similarly, chronic alcohol use reduces the mitochondrial maximum oxygen uptake rate, which in turn makes hepatocytes more vulnerable to alcohol-induced hypoxia and liver damage [253]. Similar to LPS, which is a highly pro-inflammatory molecule, endothelial responses to LPS include the production of cytokines, adhesion molecules, and tissue factors, as well as apoptotic endothelial cell death [254]. Activation of the NF-B TLR4/PI3K/Akt/GSK-3, cytokine, and other signaling pathways is how LPS most commonly causes apoptosis. The 35, 25, and 41 genes in the network were respectively targeted by HBV, alcohol, and LPS in this investigation to induce apoptosis.
The JAK/STAT signaling system is crucial for several physiological processes, such as cell division, stem cell maintenance, differentiation, and immune/inflammatory response control. Additionally, it has been shown that JAK/STAT signaling controls gluconeogenesis and liver regeneration. Different cytokines and growth factors, including interleukins, interferons, and members of the EGF family also activate the JAK/STAT pathway by binding to their respective transmembrane receptors. The current study reports that the HBV, alcohol, and LPS modulate 33, 18, and 41 genes to modulate the JAK/STAT pathway. It is well known that chronic alcohol use and LPS decrease ILs and IFN-induced STAT1 activation, which in turn lowers NK cell function in the liver and speeds up the development of hepatic fibrosis. STATs activation via ILs and IFN is necessary for hepatic regeneration [250]. However, investigation has revealed that HBV HBx also controls cellular growth and death in addition to having a significant impact on the innate immune response and viral replication. HBX controls the activity of JAK1, JAK2, and TYK2. Cho et al. indicated that HBX may prevent TYK2 activation, lowering the expression of the IFN- receptor 1 (IFNAR1) and preventing signal transduction mediated by exogenous IFNs [255]. The HBX-mediated interaction of SH2 domain-containing 5 (SH2D5) with transketolase (TKT) may activate STAT3 to increase HCC cell proliferation, and HBx was also reported to drive SH2D5 expression in HCC cells. IL-6 is essential for STAT3 activation. As it is, HBX has been shown to increase IL-6 expression in hepatoma cells [255]. On the other hand, alcohol and LPS are also well reported to increase the level of IL-6 and IL-6-facilitated acute inflammatory response in the liver, causing the development of chronic liver injury [256,257]. The researchers also identified that the liver damage in IL-6 knockout mice after alcohol feeding may be due to STAT3-independent signaling of IL-6 in hepatocytes. Hence, this confirms that IL-6 mediated liver damage is due to STAT3 activation [258]. On looking into the overall outcome of the study, HBV and chemicals cause hepatocellular carcinoma (HCC) through a multifactorial process and molecular pathways. Animal models of chemically induced HCC resemble hepatocarcinogenesis of HBV and this research sheds light on the screening of novel anti-HBV and hepatoprotective molecules using alcohol and LPS as a chemical-induced hepatitis model.
4. Materials and Methods
4.1. Identification of HBV-Associated and Chemically Induced Hepatitis Genes
A peer review of the literature and GeneCards database were used to collect the information on genes that are modulated by HBV and the selected chemicals to produce hepatitis, namely, acetaminophen, isoniazid, alcohol, D-galactosamine, lipopolysaccharide, thioacetamide, and rifampicin were selected to compare with the HBV-induced hepatitis. In the GeneCards database, the genes with a relevance score ≥20 were considered for evaluation to obtain the most relevant data. Here, we set a relevance score ≥20 cut-off to avoid the large number of genes that cause errors during enrichment analysis. Further, Venny 2.1 [259] was utilized to identify the common genes between the literature and GeneCards with HBV and chemically-induced hepatitis. In addition, the Kyoto Encyclopedia of Genes and Genomes (KEGG) database accession number “hepatitis B: hsa05161” was utilized to collect the molecular pathway regulated in HBV infection.
4.2. Gene set Molecular Pathway Enrichment Analysis
The set of genes collected for HBV and chemical-induced hepatitis were submitted to the STRING database [260]. The set of gene-regulated molecular pathways was retrieved from the STRING database inbuilt KEGG pathway database [261]. Further, the obtained list of pathways of HBV-induced hepatitis was matched with pathways collected from “KEGG ID: hsa05161” and finalized with the matched pathways for HBV-modulated pathways for further analysis. In a similar manner, the list of pathways for chemically induced hepatitis and the HBV pathways were compared for similarity based on gene counts and false discovery rate (FDR) [262,263].
4.3. Network Construction
The network between HBV and chemicals with their targets (involved in hepatitis) and the regulated pathways were constructed using Cytoscape (https://cytoscape.org/ (accessed on 20 February 2023)) version 3.6.1 [264]. The constructed network was recognized as directed and inspected by translating node size and color to low values corresponding to small sizes and bright colors toward the edge count. In addition, the edge size and color were mapped to edge betweenness, with low values corresponding to small sizes and low values equating to bright colors [265,266].
5. Conclusions
The GeneCards database was utilized in the current investigation to collect genes affected by HBV and several substances thought to induce hepatitis. It also underwent a thorough peer review process. Out of seven chemically induced hepatitis cases, alcohol- and LPS-induced hepatitis were found to share similar molecular pathways with HBV-induced hepatitis, according to gene set enrichment and network pharmacology analysis. Apoptosis, Cell cycle, PI3K-Akt, TNF, JAK-STAT, MAPK, Chemokine, NF-kappa B, and TGF-β signaling pathways were the major pathways modulated by HBV, which were also targeted by alcohol and LPS with significant gene counts and FDR scores, since alcohol is used to investigate chronic hepatitis and LPS is used to examine acute hepatitis. In contrast to HBV-induced hepatitis in rodents, alcohol-induced chronic hepatitis may be the option to study chronic hepatitis in rodents.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms241311146/s1.
Author Contributions
Methodology, validation, formal analysis, investigation, data curation, and writing—original draft, writing—review and editing, V.S.P.; methodology, conceptualization, resources, writing—review and editing, supervision, project administration, and funding acquisition, D.R.H.; writing—original draft, data curation, G.H.S.; writing—review and editing, P.K.; data curation, writing—review and editing, S.S.G.; co-supervision, writing—review and editing, validation, and funding acquisition, S.R. and H.V.H.; co-supervision, writing—review and editing, investigation, validation, S.S.J.; All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Indian Council of Medical Research, Department of Health Research, New Delhi (ICMR grant no. ISRM/12(61)2019) and ICMR-National Institute of Traditional Medicine, Belagavi, India.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article (and/or) its Supplementary Materials.
Acknowledgments
The authors gratefully acknowledge the extramural funding of the Indian Council of Medical Research, Department of Health Research, New Delhi, and ICMR-National Institute of Traditional Medicine, Belagavi for providing resources and intramural funding. V.S.P. is thankful to the KLE Academy of Higher Education and Research, Belagavi, India, for supporting his Ph.D. studies.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liang, T.J. Hepatitis B: The virus and disease. Hepatology 2009, 49, S13–S21. [Google Scholar] [CrossRef] [PubMed]
- Niu, B.; Hann, H. Hepatitis B Virus—Related Hepatocellular Hepatitis Hepatocellular Carcinoma: Carcinoma: Carcinogenesis, Prevention, and Treatment. Updates Liver Cancer 2017, 13, 69–93. [Google Scholar] [CrossRef]
- Hepatitis B. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b (accessed on 6 March 2022).
- Stein, L.; Loomba, R. Drug Targets in Hepatitis B Virus Infection. Infect. Disord. Drug Targets 2012, 9, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Maya, S.; Ploss, A. Animal Models of Hepatitis B Virus Infection–Success, Challenges, and Future Directions. Viruses 2021, 13, 777. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, X.; Wu, M.; Ghildyal, R.; Yuan, Z. Animal Models for the Study of Hepatitis B Virus Pathobiology and Immunity: Past, Present, and Future. Front. Microbiol. 2021, 12, 715450. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.N.; Zhu, B.; Ai, L.; Yang, D.L.; Wang, B.J. Animal Models for the Study of Hepatitis B Virus Infection. Zool. Res. 2018, 39, 25. [Google Scholar] [CrossRef]
- Du, Y.; Broering, R.; Li, X.; Zhang, X.; Liu, J.; Yang, D.; Lu, M. In Vivo Mouse Models for Hepatitis B Virus Infection and Their Application. Front. Immunol. 2021, 12, 766534. [Google Scholar] [CrossRef]
- Ye, L.; Yu, H.; Li, C.; Hirsch, M.L.; Zhang, L.; Samulski, R.J.; Li, W.; Liu, Z. Adeno-Associated Virus Vector Mediated Delivery of the HBV Genome Induces Chronic Hepatitis B Virus Infection and Liver Fibrosis in Mice. PLoS ONE 2015, 10, e0130052. [Google Scholar] [CrossRef]
- Cheng, L.; Li, F.; Bility, M.T.; Murphy, C.M.; Su, L. Modeling Hepatitis B Virus Infection, Immunopathology and Therapy in Mice. Antivir. Res. 2015, 121, 1–8. [Google Scholar] [CrossRef]
- McGill, M.R.; Jaeschke, H. Animal Models of Drug-Induced Liver Injury. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1031. [Google Scholar] [CrossRef]
- Czekaj, P.; Król, M.; Limanówka, Ł.; Michalik, M.; Lorek, K.; Gramignoli, R. Assessment of Animal Experimental Models of Toxic Liver Injury in the Context of Their Potential Application as Preclinical Models for Cell Therapy. Eur. J. Pharmacol. 2019, 861, 172597. [Google Scholar] [CrossRef]
- Gu, X.; Manautou, J.E. Molecular Mechanisms Underlying Chemical Liver Injury. Expert Rev. Mol. Med. 2012, 14, e4. [Google Scholar] [CrossRef]
- Biermer, M.; Puro, R.; Schneider, R.J. Tumor necrosis factor alpha inhibition of hepatitis B virus replication involves disruption of capsid integrity through activation of NF-κB. J. Virol. 2003, 77, 4033–4042. [Google Scholar] [CrossRef]
- Bouchard, M.; Giannakopoulos, S.; Wang, E.H.; Tanese, N.; Schneider, R.J. Hepatitis B virus HBx protein activation of cyclin A–cyclin-dependent kinase 2 complexes and G1 transit via a Src kinase pathway. J. Virol. 2001, 175, 4247–4257. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.Y.-K.; Tse, A.P.-W.; Chiu, E.Y.-T.; Sze, K.M.-F.; Koh, H.-Y.; Ng, I.O.-L.; Wong, C.C.-L. Abstract 4520: Hepatitis B virus X protein regulates hypoxia-inducible factor-1alpha (HIF-1 alpha) and lysyl oxidase like 2 (LOXL2) pathway in hepatocellular carcinoma. Cancer Res. 2017, 77, 4520. [Google Scholar] [CrossRef]
- Cheng, P.; Li, Y.; Yang, L.; Wen, Y.; Shi, W.; Mao, Y.; Chen, P.; Lv, H.; Tang, Q.; Wei, Y. Hepatitis B virus X protein (HBx) induces G2/M arrest and apoptosis through sustained activation of cyclin B1-CDK1 kinase. Oncol. Rep. 2009, 22, 1101–1107. [Google Scholar]
- Durantel, D.; Zoulim, F. Interplay between hepatitis B virus and TLR2-mediated innate immune responses: Can restoration of TLR2 functions be a new therapeutic option? J. Hepatol. 2012, 57, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Huang, C.; Qin, Y.; McCombs, J.E.; Yuan, Q.; Harry, B.L.; Palmer, A.E.; Xia, N.-S.; Xue, D. Hepatitis B virus X protein targets Bcl-2 proteins to increase intracellular calcium, required for virus replication and cell death induction. Proc. Natl. Acad. Sci. USA 2012, 109, 18471–18476. [Google Scholar] [CrossRef]
- Guégan, J.P.; Frémin, C.; Baffet, G. The MAPK MEK1/2-ERK1/2 pathway and its implication in hepatocyte cell cycle control. Int. J. Hepatolog. 2012, 2012, 328372. [Google Scholar] [CrossRef]
- Hösel, M.; Quasdorff, M.; Ringelhan, M.; Kashkar, H.; Debey-Pascher, S.; Sprinzl, M.F.; Bockmann, J.-H.; Arzberger, S.; Webb, D.; von Olshausen, G.; et al. Hepatitis B Virus Activates Signal Transducer and Activator of Transcription 3 Supporting Hepatocyte Survival and Virus Replication. Cell. Mol. Gastroenterol. Hepatol. 2017, 4, 339–363. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-L.; Ren, T.-Y.; Cao, S.-W.; Zheng, S.-H.; Hu, X.-M.; Hu, Y.-W.; Lin, L.; Chen, J.; Zheng, L.; Wang, Q. HBx-related long non-coding RNA DBH-AS1 promotes cell proliferation and survival by activating MAPK signaling in hepatocellular carcinoma. Oncotarget 2015, 6, 33791–33804. [Google Scholar] [CrossRef]
- Huang, R.; Yang, C.-C.; Liu, Y.; Xia, J.; Su, R.; Xiong, Y.-L.; Wang, G.-Y.; Sun, Z.-H.; Yan, X.-M.; Lu, S.; et al. Association of serum gamma-glutamyl transferase with treatment outcome in chronic hepatitis B patients. World J. Gastroenterol. 2015, 21, 9957–9965. [Google Scholar] [CrossRef]
- Hyodo, N.; Nakamura, I.; Imawari, M. Hepatitis B core antigen stimulates interleukin-10 secretion by both T cells and monocytes from peripheral blood of patients with chronic hepatitis B virus infection. Clin. Exp. Immunol. 2004, 135, 462–466. [Google Scholar] [CrossRef]
- Iwamoto, M.; Saso, W.; Sugiyama, R.; Ishii, K.; Ohki, M.; Nagamori, S.; Suzuki, R.; Aizaki, H.; Ryo, A.; Yun, J.-H.; et al. Epidermal growth factor receptor is a host-entry cofactor triggering hepatitis B virus internalization. Proc. Natl. Acad. Sci. USA 2019, 116, 8487–8492. [Google Scholar] [CrossRef]
- Jiang, C.-Y.; Zeng, W.-Q.; Chen, Y.-X.; Dai, F.-H.; Jiang, P. Effect of HBV on the expression of SREBP in the hepatocyte of chronic hepatitis B patients combined with hepatic fatty change. Chin. J. Hepatol. 2011, 19, 608–613. [Google Scholar]
- Kim, D.H.; Kang, H.S.; Kim, K.-H. Roles of hepatocyte nuclear factors in hepatitis B virus infection. World J. Gastroenterol. 2016, 22, 7017–7029. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.; Chang, L.; Wu, L.; Yuan, Y.-F. IL-6 Plays a Crucial Role in HBV Infection. J. Clin. Transl. Hepatol. 2015, 3, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Sun, T.; Park, S.; Shen, G.; Liu, J. The role of hepatitis B virus X protein is related to its differential intracellular localization. Acta Biochim. Biophys. Sin. 2011, 43, 583–588. [Google Scholar] [CrossRef]
- Matsuura, K.; Isogawa, M.; Tanaka, Y. Host genetic variants influencing the clinical course of Hepatitis B virus infection. J. Med. Virol. 2015, 88, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Mehde, A.A.; Mehdi, W.A.; Jasim, A.M. Study Several Biochemical Parameters into Patient’s with Hepatitis B Virus. Glob. J. Med. Res. Dis. 2013, 13, 2249–4618. [Google Scholar]
- Nielsen, K.O.; Mirza, A.H.; Kaur, S.; Jacobsen, K.S.; Winther, T.N.; Glebe, D.; Pociot, F.; Hogh, B.; Størling, J. Hepatitis B virus suppresses the secretion of insulin-like growth factor binding protein 1 to facilitate anti-apoptotic IGF-1 effects in HepG2 cells. Exp. Cell. Res. 2018, 370, 399–408. [Google Scholar] [CrossRef]
- Park, U.S.; Park, S.K.; Lee, Y.I.; Park, J.G.; Lee, Y.I. Hepatitis B virus-X protein upregulates the expression of p21wafl/cipl and prolongs G1→S transition via a p53-independent pathway in human hepatoma cells. Oncogene 2000, 19, 3384–3394. [Google Scholar] [CrossRef]
- Qiao, L.; Wu, Q.; Lu, X.; Zhou, Y.; Fernández-Alvarez, A.; Ye, L.; Zhang, X.; Han, J.; Casado, M.; Liu, Q. SREBP-1a activation by HBx and the effect on hepatitis B virus enhancer II/core promoter. Biochem. Biophys. Res. Commun. 2013, 432, 643–649. [Google Scholar] [CrossRef][Green Version]
- Romporn, S.; Hirankarn, N.; Tangkijvanich, P.; Kimkong, I. Association of IFNAR2 and IL10RB genes in chronic hepatitis B virus infection. Tissue Antigens 2013, 82, 21–25. [Google Scholar] [CrossRef]
- Sepehri, Z.; Kiani, Z.; Alavian, S.M.; Arababadi, M.K.; Kennedy, D.H. The link between TLR7 signaling and hepatitis B virus infection. Life Sci. 2016, 158, 63–69. [Google Scholar] [CrossRef]
- Shahrakyvahed, A.; Sanchooli, J.; Sanadgol, N.; Arababadi, M.K.; Kennedy, D. TLR9: An important molecule in the fight against hepatitis B virus. Postgrad. Med. J. 2014, 90, 396–401. [Google Scholar] [CrossRef]
- Tan, G.; Song, H.; Xu, F.; Cheng, G. When Hepatitis B Virus Meets Interferons. Front. Microbiol. 2018, 9, 1611. [Google Scholar] [CrossRef]
- Tornesello, M.L.; Buonaguro, L.; Izzo, F.; Buonaguro, F.M. Molecular alterations in hepatocellular carcinoma associated with hepatitis B and hepatitis C infections. Oncotarget 2016, 7, 25087–25102. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, J.; Wang, Q.; Du, J.; Wang, B. The CDKN2A polymorphisms and the susceptibility of HBV-related gestational diabetes mellitus. J. Clin. Lab. Anal. 2018, 32, e22423. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hao, J.; Liu, X.; Wang, H.; Zeng, X.; Yang, J.; Li, L.; Kuang, X.; Zhang, T. The mechanism of apoliprotein A1 down-regulated by Hepatitis B virus. Lipids Heal. Dis. 2016, 15, 64. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, T.; Hu, D.; Weng, X.; Wang, X.; Chen, P.-J.; Luo, X.; Wang, H.; Ning, Q. Intracellular hepatitis B virus increases hepatic cholesterol deposition in alcoholic fatty liver via hepatitis B core protein. J. Lipid Res. 2018, 59, 58–68. [Google Scholar] [CrossRef]
- Wittkop, L.; Schwarz, A.; Cassany, A.; Grün-Bernhard, S.; Delaleau, M.; Rabe, B.; Cazenave, C.; Gerlich, W.; Glebe, D.; Kann, M. Inhibition of protein kinase C phosphorylation of hepatitis B virus capsids inhibits virion formation and causes intracellular capsid accumulation. Cell. Microbiol. 2010, 12, 962–975. [Google Scholar] [CrossRef]
- Xiang, K.; Wang, B. Role of the PI3K-AKT-mTOR pathway in hepatitis B virus infection and replication. Mol. Med. Rep. 2018, 17, 4713–4719. [Google Scholar] [CrossRef]
- Xiao, Q.; Fu, B.; Chen, P.; Liu, Z.Z.; Wang, W.; Ye, Q. Three polymorphisms of tumor necrosis factor-alpha and hepatitis B virus related hepatocellular carcinoma: A meta-analysis. Medicine 2016, 95, e5609. [Google Scholar] [CrossRef]
- Xie, Q.; Su, Y.; Dykema, K.; Johnson, J.; Koeman, J.; De Giorgi, V.; Huang, A.; Schlegel, R.; Essenburg, C.; Kang, L.; et al. Overexpression of HGF promotes HBV-induced hepatocellular carcinoma progression and is an effective indicator for Met-targeting therapy. Genes Cancer 2013, 4, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Peng, B.; Liu, Y.; Xu, G.; He, W.; Ren, B.; Jing, Z.; Sui, J.; Li, W. Viral Entry of Hepatitis B and D Viruses and Bile Salts Transportation Share Common Molecular Determinants on Sodium Taurocholate Cotransporting Polypeptide. J. Virol. 2014, 88, 3273–3284. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, X.; Liao, X.; Han, C.; Yu, T.; Qin, W.; Zhu, G.; Su, H.; Yu, L.; Liu, X.; et al. Aldehyde dehydrogenase 1 (ALDH1) isoform expression and potential clinical implications in hepatocellular carcinoma. PLoS ONE 2017, 12, e0182208. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Ye, L. Effects of hepatitis B virus X protein on the development of liver cancer. J. Lab. Clin. Med. 2006, 147, 58–66. [Google Scholar] [CrossRef]
- Contoreggi, C.; Chrousos, G.P.; Di Mascio, M. Chronic distress and the vulnerable host: A new target for HIV treatment and prevention? Neurobehav. HIV Med. 2016, 7, 53–75. [Google Scholar] [CrossRef] [PubMed]
- Brind, A.M.; Hurlstone, A.; Edrisinghe, D.; Gilmore, I.; Fisher, N.; Pirmohamed, M.; Fryer, A. The Role of Polymorphisms of Glutathione S-transferases Gstm1, M3, P1, T1 and A1 in Susceptibility to Alcoholic Liver Disease. Alcohol. Alcohol. 2004, 39, 478–483. [Google Scholar] [CrossRef]
- Affò, S.; Dominguez, M.; Lozano, J.J.; Sancho-Bru, P.; Rodrigo-Torres, D.; Morales-Ibanez, O.; Moreno, M.; Millán, C.; Loaeza-Del-Castillo, A.; Altamirano, J.; et al. Transcriptome analysis identifies TNF superfamily receptors as potential therapeutic targets in alcoholic hepatitis. Gut 2012, 62, 452–460. [Google Scholar] [CrossRef]
- Sid, B.; Verrax, J.; Calderon, P.B. Role of oxidative stress in the pathogenesis of alcohol-induced liver disease. Free Radic. Res. 2013, 47, 894–904. [Google Scholar] [CrossRef]
- Bailey, S.M.; Patel, V.B.; Young, T.A.; Asayama, K.; Cunningham, C.C. Chronic Ethanol Consumption Alters the Glutathione/Glutathione Peroxidase-1 System and Protein Oxidation Status in Rat Liver. Alcohol. Clin. Exp. Res. 2001, 25, 726–733. [Google Scholar] [CrossRef]
- Blednov, Y.A.; Benavidez, J.M.; Black, M.; Ferguson, L.B.; Schoenhard, G.L.; Goate, A.M.; Edenberg, H.J.; Wetherill, L.; Hesselbrock, V.; Foroud, T.; et al. Peroxisome Proliferator-Activated Receptors α and γ are Linked with Alcohol Consumption in Mice and Withdrawal and Dependence in Humans. Alcohol. Clin. Exp. Res. 2014, 39, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Blendis, L.; Dotan, I. Anti-TNF therapy for severe acute alcoholic hepatitis: What went wrong? Gastroenterology 2004, 127, 1637–1639. [Google Scholar] [CrossRef] [PubMed]
- I Cederbaum, A. Hepatoprotective effects of S-adenosyl-L-methionine against alcohol- and cytochrome P450 2E1-induced liver injury. World J. Gastroenterol. 2010, 16, 1366–1376. [Google Scholar] [CrossRef]
- Chen, L.; Wang, F.; Sun, X.; Zhou, J.; Gao, L.; Jiao, Y.; Hou, X.; Qin, C.; Zhao, J. Chronic ethanol feeding impairs AMPK and MEF2 expression and is associated with GLUT4 decrease in rat myocardium. Exp. Mol. Med. 2010, 42, 205–215. [Google Scholar] [CrossRef]
- Coccini, T.; Castoldi, A.F.; Gandini, C.; Randine, G.; Vittadini, G.; Baiardi, P.; Manzo, L. Platelet monoamine oxidase b activity as a state marker for alcoholism: Trend over time during withdrawal and influence of smoking and gender. Alcohol. Alcohol. 2002, 37, 566–572. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sayaf, K.; Gabbia, D.; Russo, F.P.; De Martin, S. The Role of Sex in Acute and Chronic Liver Damage. Int. J. Mol. Sci. 2022, 23, 10654. [Google Scholar] [CrossRef]
- Crews, F.T.; Walter, T.J.; Coleman, L.G.; Vetreno, R.P. Toll-like receptor signaling and stages of addiction. Psychopharmacology 2017, 234, 1483–1498. [Google Scholar] [CrossRef] [PubMed]
- de la Monte, S.; Derdak, Z.; Wands, J.R. Alcohol, insulin resistance and the liver-brain axis. J. Gastroenterol. Hepatol. 2012, 27, 33–41. [Google Scholar] [CrossRef]
- De Minicis, S.; A Brenner, D. Oxidative stress in alcoholic liver disease: Role of NADPH oxidase complex. J. Gastroenterol. Hepatol. 2008, 23, S98–S103. [Google Scholar] [CrossRef]
- Albano, E. Oxidative mechanisms in the pathogenesis of alcoholic liver disease. Mol. Asp. Med. 2008, 29, 9–16. [Google Scholar] [CrossRef]
- Fortier, M.; Cadoux, M.; Boussetta, N.; Pham, S.; Donné, R.; Couty, J.-P.; Desdouets, C.; Celton-Morizur, S. Hepatospecific ablation of p38α MAPK governs liver regeneration through modulation of inflammatory response to CCl4-induced acute injury. Sci. Rep. 2019, 9, 14614. [Google Scholar] [CrossRef]
- Ge, Y.; Belcher, S.M.; Pierce, D.R.; Light, K.E. Altered expression of Bcl2, Bad and Bax mRNA occurs in the rat cerebellum within hours after ethanol exposure on postnatal day 4 but not on postnatal day 9. Mol. Brain Res. 2004, 129, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Heit, C.; Dong, H.; Chen, Y.; Thompson, D.C.; Deitrich, R.A.; Vasiliou, V.K. The Role of CYP2E1 in Alcohol Metabolism and Sensitivity in the Central Nervous System; Springer: Berlin/Heidelberg, Germany, 2013; pp. 235–247. [Google Scholar] [CrossRef]
- Hill, D.B.; D’Souza, N.B.; Lee, E.Y.; Burikhanov, R.; Deaciuc, I.V.; de Villiers, W.J.S. A Role for Interleukin-10 in Alcohol-Induced Liver Sensitization to Bacterial Lipopolysaccharide. Alcohol. Clin. Exp. Res. 2002, 26, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Kim, W.-H.; Tian, Z.; Jaruga, B.; Ishac, E.; Shen, X.; Gao, B. Elevated interleukin-6 during ethanol consumption acts as a potential endogenous protective cytokine against ethanol-induced apoptosis in the liver: Involvement of induction of Bcl-2 and Bcl-xL proteins. Oncogene 2002, 21, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, N.; Wang, L.; Mukhopadhyay, P.; Park, O.; Jeong, W.I.; Lafdil, F.; Osei–Hyiaman, D.; Moh, A.; Fu, X.Y.; Pacher, P.; et al. Cell Type–Dependent Pro- and Anti-Inflammatory Role of Signal Transducer and Activator of Transcription 3 in Alcoholic Liver Injury. Gastroenterology 2008, 134, 1148–1158. [Google Scholar] [CrossRef]
- Cao, H.; Xi, S.; He, W.; Ma, X.; Liu, L.; Xu, J.; Zhang, K.; Li, Y.; Jin, L. The effects of Gentiana dahurica Fisch on alcoholic liver disease revealed by RNA sequencing. J. Ethnopharmacol. 2020, 279, 113422. [Google Scholar] [CrossRef]
- Jairam, S.; Edenberg, H.J. Single-Nucleotide Polymorphisms Interact to Affect ADH7 Transcription. Alcohol. Clin. Exp. Res. 2014, 38, 921–929. [Google Scholar] [CrossRef]
- Jarvelainen, H.A.; Oinonen, T.; Lindros, K.O. Alcohol-Induced Expression of the CD14 Endotoxin Receptor Protein in Rat Kupffer Cells. Alcohol. Clin. Exp. Res. 1997, 21, 1547–1551. [Google Scholar] [CrossRef]
- Jogunoori, W.; Mishra, L. Role TGF-Β Alcohol-Induc. Liver Dis. 2018, 1032, 93–104. [Google Scholar] [CrossRef]
- Khodja, Y.; Samuels, M.E. Ethanol-mediated upregulation of APOA1 gene expression in HepG2 cells is independent of de novo lipid biosynthesis. Lipids Health Dis. 2020, 19, 144. [Google Scholar] [CrossRef]
- Kirpich, I.; Ghare, S.; Zhang, J.; Gobejishvili, L.; Kharebava, G.; Barve, S.J.; Barker, D.; Moghe, A.; McClain, C.J.; Barve, S. Binge Alcohol-Induced Microvesicular Liver Steatosis and Injury are Associated with Down-Regulation of Hepatic Hdac1, 7, 9, 10, 11 and Up-Regulation of Hdac3. Alcohol. Clin. Exp. Res. 2012, 36, 1578–1586. [Google Scholar] [CrossRef]
- Köken, T.; Gürsoy, F.; Kahraman, A. Long-term Alcohol Consumption Increases Pro-Matrix Metalloproteinase-9 Levels via Oxidative Stress. J. Med. Toxicol. 2010, 6, 126–130. [Google Scholar] [CrossRef]
- Lecomte, E.; Herbeth, B.; Paille, F.; Steinmetz, J.; Artur, Y.; Siest, G. Changes in serum apolipoprotein and lipoprotein profile induced by chronic alcohol consumption and withdrawal: Determinant effect on heart disease? Clin. Chem. 1996, 42, 1666–1675. [Google Scholar] [CrossRef] [PubMed]
- Leite, L.N.; Lacchini, R.; Carnio, E.C.; Queiroz, R.H.; Tanus-Santos, J.E.; de Oliveira, A.M.; Tirapelli, C.R. Ethanol Consumption Increases Endothelin-1 Expression and Reactivity in the Rat Cavernosal Smooth Muscle. Alcohol Alcohol. 2013, 48, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Hu, M.; Rogers, C.Q.; Shen, Z.; You, M. Role of SIRT1-FoxO1 Signaling in Dietary Saturated Fat-Dependent Upregulation of Liver Adiponectin Receptor 2 in Ethanol-Administered Mice. Antioxid. Redox Signal. 2011, 15, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.D.; Lee, S.R.; Kim, T.; Jang, S.-A.; Kang, S.C.; Koo, H.J.; Sohn, E.; Bak, J.P.; Namkoong, S.; Kim, H.K.; et al. Fucoidan from Fucus vesiculosus Protects against Alcohol-Induced Liver Damage by Modulating Inflammatory Mediators in Mice and HepG2 Cells. Mar. Drugs 2015, 13, 1051–1067. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, H.-I.; Lee, M.-H.; Jen, C.-L.; Hu, H.-H.; Lu, S.-N.; Wang, L.-Y.; You, S.-L.; Huang, Y.-T.; Chen, C.-J. Alcohol Drinking Mediates the Association between Polymorphisms of ADH1B and ALDH2 and Hepatitis B–Related Hepatocellular Carcinoma. Cancer Epidemiol. Biomark. Prev. 2016, 25, 693–699. [Google Scholar] [CrossRef]
- Liu, J. Ethanol and liver: Recent insights into the mechanisms of ethanol-induced fatty liver. World J. Gastroenterol. 2014, 20, 14672–14685. [Google Scholar] [CrossRef]
- Kruk, J.; Aboul-Enein, H.Y.; Kładna, A.; Bowser, J.E. Oxidative stress in biological systems and its relation with pathophysiological functions: The effect of physical activity on cellular redox homeostasis. Free Radic. Res. 2019, 53, 497–521. [Google Scholar] [CrossRef]
- Mandrekar, P.; Ambade, A.; Lim, A.; Szabo, G.; Catalano, D. An essential role for monocyte chemoattractant protein-1 in alcoholic liver injury: Regulation of proinflammatory cytokines and hepatic steatosis in mice. Hepatology 2011, 54, 2185–2197. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, A.; Gattolliat, C.-H.; Asselah, T. Mitochondrial Dysfunction and Signaling in Chronic Liver Diseases. Gastroenterology 2018, 155, 629–647. [Google Scholar] [CrossRef] [PubMed]
- Bergheim, I.; Guo, L.; Davis, M.A.; Lambert, J.C.; Beier, J.I.; Duveau, I.; Luyendyk, J.P.; Roth, R.A.; Arteel, G.E. Metformin Prevents Alcohol-Induced Liver Injury in the Mouse: Critical Role of Plasminogen Activator Inhibitor-1. Gastroenterology 2006, 130, 2099–2112. [Google Scholar] [CrossRef] [PubMed]
- Murohisa, G.; Kobayashi, Y.; Kawasaki, T.; Nakamura, S.; Nakamura, H. Involvement of platelet-activating factor in hepatic apoptosis and necrosis in chronic ethanol-fed rats given endotoxin. Liver Int. 2002, 22, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Petrasek, J.; Mandrekar, P.; Szabo, G. Toll-Like Receptors in the Pathogenesis of Alcoholic Liver Disease. Gastroenterol. Res. Pr. 2010, 2010, 710381. [Google Scholar] [CrossRef]
- Petrasek, J.; Dolganiuc, A.; Csak, T.; Nath, B.; Hritz, I.; Kodys, K.; Catalano, D.; Kurt-Jones, E.; Mandrekar, P.; Szabo, G. Interferon regulatory factor 3 and type I interferons are protective in alcoholic liver injury in mice by way of crosstalk of parenchymal and myeloid cells. Hepatology 2010, 53, 649–660. [Google Scholar] [CrossRef]
- Qin, C.-C.; Liu, Y.-N.; Hu, Y.; Yang, Y.; Chen, Z. Macrophage inflammatory protein-2 as mediator of inflammation in acute liver injury. World J. Gastroenterol. 2017, 23, 3043–3052. [Google Scholar] [CrossRef]
- Davis, R.L.; Syapin, P.J. Interactions of alcohol and nitric-oxide synthase in the brain. Brain Res. Rev. 2005, 49, 494–504. [Google Scholar] [CrossRef]
- Neuman, M.G.; Seitz, H.K.; Tuma, P.L.; Osna, N.A.; Casey, C.A.; Kharbanda, K.K.; Cohen, L.B.; Malnick, S.D.; Adhikari, R.; Mitra, R.; et al. Alcohol: Basic and translational research; 15th annual Charles Lieber &1st Samuel French satellite symposium. Exp. Mol. Pathol. 2022, 126, 104750. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Gordillo, K.; Shah, R.; Arellanes-Robledo, J.; Cheng, Y.; Ibrahim, J.; Tuma, P.L. Akt1 and Akt2 Isoforms Play Distinct Roles in Regulating the Development of Inflammation and Fibrosis Associated with Alcoholic Liver Disease. Cells 2019, 8, 1337. [Google Scholar] [CrossRef] [PubMed]
- Shafaghati, L.; Razaghi-Moghadam, Z.; Mohammadnejad, J. A Systems Biology Approach to Understanding Alcoholic Liver Disease Molecular Mechanism: The Development of Static and Dynamic Models. Bull. Math. Biol. 2017, 79, 2450–2473. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.C.; Huang, Z.Z.; Yang, H.; Mato, J.M.; Avila, M.A.; Tsukamoto, H. Changes in methionine adenosyltransferase and S-adenosyl methionine homeostasis in alcoholic rat liver. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 279, G178–G185. [Google Scholar] [CrossRef]
- Tan, W.; Bailey, A.P.; Shparago, M.; Busby, B.; Covington, J.; Johnson, J.W.; Young, E.; Gu, J.-W. Chronic alcohol consumption stimulates VEGF expression, Tumor angiogenesis and progression of melanoma in mice. Cancer Biol. Ther. 2007, 6, 1222–1228. [Google Scholar] [CrossRef]
- Tian, L.; Fan, F.; Zheng, S.; Tong, Q. Puerarin Exerts the Hepatoprotection from Chronic Alcohol-Induced Liver Injury via Inhibiting the Cyclooxygenase-2 and the 5-Lipoxygenase Pathway in Rats. Complement. Med. Res. 2020, 28, 104–113. [Google Scholar] [CrossRef]
- Lukkari, T.A.; Järveläinen, H.A.; Oinonen, T.; Kettunen, E.; Lindros, K.O. Short-term ethanol exposure increases the expression of Kupffer cell CD14 receptor and lipopolysaccharide binding protein in rat liver. Alcohol Alcohol. 1999, 34, 311–319. [Google Scholar] [CrossRef]
- Vrana, K.E.; Freeman, W.M.; Grant, K.A.; Gonzales, S. Compositions and Methods Relating to Monitoring Alcohol Consumption and Alcohol Abuse. U.S. Patent No. 8,647,825, 11 February 2014. [Google Scholar]
- Wang, F.; Yang, J.-L.; Yu, K.-K.; Xu, M.; Xu, Y.-Z.; Chen, L.; Lu, Y.-M.; Fang, H.-S.; Wang, X.-Y.; Hu, Z.-Q.; et al. Activation of the NF-κB pathway as a mechanism of alcohol enhanced progression and metastasis of human hepatocellular carcinoma. Mol. Cancer 2015, 14, 10. [Google Scholar] [CrossRef]
- Wang, Z.; Yao, T.; Song, Z. Chronic Alcohol Consumption Disrupted Cholesterol Homeostasis in Rats: Down-Regulation of Low-Density Lipoprotein Receptor and Enhancement of Cholesterol Biosynthesis Pathway in the Liver. Alcohol. Clin. Exp. Res. 2010, 34, 471–478. [Google Scholar] [CrossRef]
- Xu, T.; Zheng, L.; Xu, L.; Yin, L.; Qi, Y.; Xu, Y.; Han, X.; Peng, J. Protective effects of dioscin against alcohol-induced liver injury. Arch. Toxicol. 2013, 88, 739–753. [Google Scholar] [CrossRef]
- Yang, B.-Z.; Arias, A.J.; Feinn, R.; Krystal, J.H.; Gelernter, J.; Petrakis, I.L. GRIK1 and GABRA2 Variants Have Distinct Effects on the Dose-Related Subjective Response to Intravenous Alcohol in Healthy Social Drinkers. Alcohol. Clin. Exp. Res. 2017, 41, 2025–2032. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Si, X.; Wang, W. Overexpression of bcl-2 protects hepatoma cell line HCC-9204 from ethanol-induced apoptosis. Chin. Med. J. 2002, 115, 8–11. [Google Scholar] [PubMed]
- Lee, Y.J.; Yoo, M.-G.; Kim, H.-K.; Jang, H.B.; Park, K.J.; Lee, H.-J.; Kim, S.-G.; Park, S.I. The association between alcohol metabolism and genetic variants of ADH1A, SRPRB, and PGM1 in Korea. Alcohol 2019, 79, 137–145. [Google Scholar] [CrossRef] [PubMed]
- You, M.; Crabb, D.W. Molecular mechanisms of alcoholic fatty liver: Role of sterol regulatory element-binding proteins. Alcohol 2004, 34, 39–43. [Google Scholar] [CrossRef]
- You, M.; Jogasuria, A.; Taylor, C.; Wu, J. Sirtuin 1 signaling and alcoholic fatty liver disease. Hepatobiliary Surg. Nutr. 2015, 4, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.-X.; Wang, X.-Y.; Huang, Y.-J.; Fang, S.-H.; Wu, J.; Zhang, Y.-B.; Xiong, T.-Q.; Yang, C.; Shen, J.-G.; Sang, C.-L.; et al. Systems pharmacology-based investigation of Sanwei Ganjiang Prescription: Related mechanisms in liver injury. Chin. J. Nat. Med. 2018, 16, 756–765. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, C.; Wang, C.; Zhao, H.; Zhao, C.; Chen, Y.; Wang, Y.; McClain, C.; Feng, W. Enhanced AMPK phosphorylation contributes to the beneficial effects of Lactobacillus rhamnosus GG supernatant on chronic-alcohol-induced fatty liver disease. J. Nutr. Biochem. 2014, 26, 337–344. [Google Scholar] [CrossRef]
- Li, H.H.; Tyburski, J.B.; Wang, Y.W.; Strawn, S.; Moon, B.H.; Kallakury, B.V.; Gonzalez, F.J.; Fornace, A.J., Jr. Modulation of Fatty Acid and Bile Acid Metabolism By Peroxisome Proliferator-Activated Receptor α Protects Against Alcoholic Liver Disease. Alcohol. Clin. Exp. Res. 2014, 38, 1520–1531. [Google Scholar] [CrossRef]
- Wang, Z.; Yao, T.; Song, Z. Involvement and mechanism of DGAT2 upregulation in the pathogenesis of alcoholic fatty liver disease. J. Lipid Res. 2010, 51, 3158–3165. [Google Scholar] [CrossRef]
- Zhu, M.; Zhou, X.; Zhao, J. Quercetin prevents alcohol-induced liver injury through targeting of PI3K/Akt/nuclear factor-κB and STAT3 signaling pathway. Exp. Ther. Med. 2017, 14, 6169–6175. [Google Scholar] [CrossRef]
- Ahmed, M.M.E.; Al-Obosi, J.A.S.; Osman, H.M.; Shayoub, M.E. Overexpression of Aldose Reductase Render Mouse Hepatocytes More Sensitive to Acetaminophen Induced Oxidative Stress and Cell Death. Indian. J. Clin. Biochem. 2015, 31, 162–170. [Google Scholar] [CrossRef]
- DiGiovanni, K.; Hatstat, A.; Rote, J.; Cafiero, M. MP2//DFT calculations of interaction energies between acetaminophen and acetaminophen analogues and the aryl sulfotransferase active site. Comput. Theor. Chem. 2013, 1007, 41–47. [Google Scholar] [CrossRef]
- Dong, H.; Haining, R.L.; E Thummel, K.; E Rettie, A.; Nelson, S.D. Involvement of human cytochrome P450 2D6 in the bioactivation of acetaminophen. Drug Metab. Dispos. 2000, 28, 1397–1400. [Google Scholar]
- Gregory, M.; Adamson, A.; Harman, W. A role for the glutathione peroxidase/reductase enzyme system in the protection from paracetamol toxicity in isolated mouse hepatocytes. Biochem. Pharmacol. 1989, 38, 3323–3330. [Google Scholar]
- Gupta, S.; Rogers, L.K.; Taylor, S.K.; Smith, C.V. Inhibition of Carbamyl Phosphate Synthetase-I and Glutamine Synthetase by Hepatotoxic Doses of Acetaminophen in Mice. Toxicol. Appl. Pharmacol. 1997, 146, 317–327. [Google Scholar] [CrossRef]
- Hinson, J.A.; Roberts, D.W.; James, L.P. Mechanisms of acetaminophen-induced liver necrosis. Handb. Exp. Pharmacol. 2010, 2010, 369–405. [Google Scholar]
- Karthivashan, G.; Arulselvan, P.; Fakurazi, S. Pathways involved in acetaminophen hepatotoxicity with specific targets for inhibition/downregulation. RSC Adv. 2015, 5, 62040–62051. [Google Scholar] [CrossRef]
- Lee, Y.-P.; Liao, J.-T.; Cheng, Y.-W.; Wu, T.-L.; Lee, S.-L.; Liu, J.-K.; Yin, S.-J. Inhibition of human alcohol and aldehyde dehydrogenases by acetaminophen: Assessment of the effects on first-pass metabolism of ethanol. Alcohol 2013, 47, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Pu, S.; Ren, L.; Liu, Q. Loss of 5-lipoxygenase activity protects mice against paracetamol-induced liver toxicity. Br. J. Pharmacol. 2016, 173, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Su, G.L.; Gong, K.Q.; Fan, M.H.; Kelley, W.M.; Hsieh, J.; Sun, J.M.; Hemmila, M.R.; Arbabi, S.; Remick, D.G.; Wang, S.C. Lipopolysaccharide-binding protein modulates acetaminophen-induced liver injury in mice. Hepatology 2004, 41, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Tonge, R.P.; Kelly, E.J.; Bruschi, S.A.; Kalhorn, T.; Eaton, D.L.; Nebert, D.W.; Nelson, S.D. Role of CYP1A2 in the Hepatotoxicity of Acetaminophen: Investigations UsingCyp1a2Null Mice. Toxicol. Appl. Pharmacol. 1998, 153, 102–108. [Google Scholar] [CrossRef]
- Bhadauria, S.; Mishra, R.; Kanchan, R.; Tripathi, C.; Srivastava, A.; Tiwari, A.; Sharma, S. Isoniazid-induced apoptosis in HepG2 cells: Generation of oxidative stress and Bcl-2 down-regulation. Toxicol. Mech. Methods 2010, 20, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-H.; Nahid, P.; Eitzman, S.R. Hepatotoxicity in Children Receiving Isoniazid Therapy for Latent Tuberculosis Infection. J. Pediatr. Infect. Dis. Soc. 2014, 3, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ren, Q.; Zhu, L.; Li, Y.; Li, J.; Zhang, Y.; Zheng, G.; Han, T.; Sun, S.; Feng, F. Involvement of methylation of MicroRNA-122, -125b and -106b in regulation of Cyclin G1, CAT-1 and STAT3 target genes in isoniazid-induced liver injury. BMC Pharmacol. Toxicol. 2018, 19, 11. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.-L.; Cen, J.; Wang, J.-B.; Zhang, F.; Xia, Q.; Wang, X.; Chen, X.-Q.; Wang, R.-C.; Hsiao, C.-D.; Liu, K.-C.; et al. Mechanism of isoniazid-induced hepatotoxicity in zebrafish larvae: Activation of ROS-mediated ERS, apoptosis and the Nrf2 pathway. Chemosphere 2019, 227, 541–550. [Google Scholar] [CrossRef]
- Li, F.; Wang, P.; Liu, K.; Tarrago, M.G.; Lu, J.; Chini, E.N.; Ma, X. A High Dose of Isoniazid Disturbs Endobiotic Homeostasis in Mouse Liver. Drug Metab. Dispos. 2016, 44, 1742–1751. [Google Scholar] [CrossRef]
- Qu, X.; Zhang, Y.; Zhang, S.; Zhai, J.; Gao, H.; Tao, L.; Song, Y. Dysregulation of BSEP and MRP2 may play an important role in isoniazid-induced liver injury via the SIRT1/FXR pathway in rats and HepG2 cells. Biol. Pharm. Bulletin. 2018, 41, 1211–1218. [Google Scholar] [CrossRef]
- Wang, C.; Fan, R.-Q.; Zhang, Y.-X.; Nie, H.; Li, K. Naringenin protects against isoniazid- and rifampicin-induced apoptosis in hepatic injury. World J. Gastroenterol. 2016, 22, 9775–9783. [Google Scholar] [CrossRef]
- Wang, P.; Pradhan, K.; Zhong, X.-B.; Ma, X. Isoniazid metabolism and hepatotoxicity. Acta Pharm. Sin. B. 2016, 6, 384–392. [Google Scholar] [CrossRef]
- Zhang, T.; Ikejima, T.; Li, L.; Wu, R.; Yuan, X.; Zhao, J.; Wang, Y.; Peng, S. Impairment of Mitochondrial Biogenesis and Dynamics Involved in Isoniazid-Induced Apoptosis of HepG2 Cells Was Alleviated by p38 MAPK Pathway. Front. Pharmacol. 2017, 8, 753. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Li, J.; Li, B.; Chong, Y.; Zheng, G.; Sun, S.; Feng, F. SIRT1 alleviates isoniazid-induced hepatocyte injury by reducing histone acetylation in the IL-6 promoter region. Int. Immunopharmacol. 2018, 67, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, L.; Luo, N.; Wang, Y.-Q.; Gao, H.-M. Inhibition of PI3K/AKt/mTOR signaling pathway protects against d-galactosamine/lipopolysaccharide-induced acute liver failure by chaperone-mediated autophagy in rats. Biomed. Pharmacother. 2017, 92, 544–553. [Google Scholar] [CrossRef] [PubMed]
- El-Agamy, D.S.; Shebl, A.M.; Shaaban, A.A. Modulation of d-galactosamine/lipopolysacharride–induced fulminant hepatic failure by nilotinib. Hum. Exp. Toxicol. 2018, 37, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Hirono, S.; Nakama, T.; Tsubouchi, H. Molecular Mechanisms of D-Galactosamine/Lipopolysaccharide-Induced Fulminant Hepatic Failure in Mice and the Effects of Therapeutic Agents. In Trends in Gastroenterology and Hepatology; Springer: Berlin/Heidelberg, Germany, 2001; pp. 59–62. [Google Scholar] [CrossRef]
- Kemelo, M.; Wojnarová, L.; Canová, N.K.; Farghali, H. D-Galactosamine/Lipopolysaccharide-Induced Hepatotoxicity Downregulates Sirtuin 1 in Rat Liver: Role of Sirtuin 1 Modulation in Hepatoprotection. Physiol. Res. 2014, 615–623. [Google Scholar] [CrossRef]
- Kim, S.-J.; Lee, S.-M. NLRP3 inflammasome activation in d-galactosamine and lipopolysaccharide-induced acute liver failure: Role of heme oxygenase-1. Free Radic. Biol. Med. 2013, 65, 997–1004. [Google Scholar] [CrossRef]
- Leifeld, L.; Trautwein, C.; Dumoulin, F.L.; Manns, M.P.; Sauerbruch, T.; Spengler, U. Enhanced Expression of CD80 (B7-1), CD86 (B7-2), and CD40 and Their Ligands CD28 and CD154 in Fulminant Hepatic Failure. Am. J. Pathol. 1999, 154, 1711–1720. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.W.; Liu, R.; Wu, H.Y.; Zhang, W.; Xia, J.; Dong, M.N.; Yu, W.; Wang, Q.; Xie, F.M.; Wang, R.; et al. Protective effect of Xuebijing injection on D-galactosamine-and lipopolysaccharide-induced acute liver injury in rats through the regulation of p38 MAPK, MMP-9 and HO-1 expression by increasing TIPE2 expression. Int. J. Mol. Med. 2016, 38, 1419–1432. [Google Scholar] [CrossRef]
- Lv, H.; Qi, Z.; Wang, S.; Feng, H.; Deng, X.; Ci, X. Asiatic Acid Exhibits Anti-inflammatory and Antioxidant Activities against Lipopolysaccharide and d-Galactosamine-Induced Fulminant Hepatic Failure. Front. Immunol. 2017, 8, 785. [Google Scholar] [CrossRef]
- Ohta, Y.; Matsura, T.; Kitagawa, A.; Tokunaga, K.; Yamada, K. Xanthine oxidase-derived reactive oxygen species contribute to the development of D-galactosamine-induced liver injury in rats. Free Radic. Res. 2007, 41, 135–144. [Google Scholar] [CrossRef]
- Raj, P.V.; Nitesh, K.; Gang, S.S.; Jagani, V.H.; Chandrashekhar, H.R.; Rao, J.V.; Rao, C.M.; Udupa, N. Protective Role of Catechin on d-Galactosamine Induced Hepatotoxicity Through a p53 Dependent Pathway. Indian J. Clin. Biochem. 2010, 25, 349–356. [Google Scholar] [CrossRef]
- Raj, P.V.; Nitesh, K.; Prateek, J.; Sankhe, M.N.; Rao, J.V.; Rao, C.M.; Udupa, N. Effect of Lecithin on d-Galactosamine Induced Hepatotoxicity Through Mitochondrial Pathway Involving Bcl-2 and Bax. Indian J. Clin. Biochem. 2011, 26, 378–384. [Google Scholar] [CrossRef][Green Version]
- Tian, Y.; Li, Z.; Shen, B.; Wu, L.; Han, L.; Zhang, Q.; Feng, H. The protective effects of Shikonin on lipopolysaccharide/d-galactosamine-induced acute liver injury via inhibiting MAPK and NF-κB and activating Nrf2/HO-1 signaling pathways. RSC Adv. 2017, 7, 34846–34856. [Google Scholar] [CrossRef]
- Zhang, Z.; Tian, L.; Jiang, K. Propofol attenuates inflammatory response and apoptosis to protect d-galactosamine/lipopolysaccharide induced acute liver injury via regulating TLR4/NF-κB/NLRP3 pathway. Int. Immunopharmacol. 2019, 77, 105974. [Google Scholar] [CrossRef]
- Ajoolabady, A.; Aslkhodapasandhokmabad, H.; Zhou, Y.; Ren, J. Epigenetic modification in alcohol-related liver diseases. Med. Res. Rev. 2022, 42, 1463–1491. [Google Scholar] [CrossRef]
- Ambade, A.; Catalano, D.; Lim, A.; Mandrekar, P. Inhibition of heat shock protein (molecular weight 90 kDa) attenuates proinflammatory cytokines and prevents lipopolysaccharide-induced liver injury in mice. Hepatology 2011, 55, 1585–1595. [Google Scholar] [CrossRef]
- Bode, J.G.; Ehlting, C.; Häussinger, D. The macrophage response towards LPS and its control through the p38MAPK–STAT3 axis. Cell. Signal. 2012, 24, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Wang, W.; Tu, Z. Aberrantly DNA Methylated-Differentially Expressed Genes and Pathways in Hepatocellular Carcinoma. J. Cancer 2019, 10, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Campos, G.; Schmidt-Heck, W.; De Smedt, J.; Widera, A.; Ghallab, A.; Pütter, L.; González, D.; Edlund, K.; Cadenas, C.; Marchan, R.; et al. Inflammation-associated suppression of metabolic gene networks in acute and chronic liver disease. Arch. Toxicol. 2020, 94, 205–217. [Google Scholar] [CrossRef]
- Chi, F.; Zhang, G.; Ren, N.; Zhang, J.; Du, F.; Zheng, X.; Zhang, C.; Lin, Z.; Li, R.; Shi, X.; et al. The anti-alcoholism drug disulfiram effectively ameliorates ulcerative colitis through suppressing oxidative stresses-associated pyroptotic cell death and cellular inflammation in colonic cells. Int. Immunopharmacol. 2022, 111, 109117. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, P.; Chen, Z.-L.; Zhang, S.-J.; Wang, Y.-Q.; Cai, X.; Luo, L.; Zhou, X.; Zhao, L. Emodin Attenuates Lipopolysaccharide-Induced Acute Liver Injury via Inhibiting the TLR4 Signaling Pathway in vitro and in vivo. Front. Pharmacol. 2018, 9, 962. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Zhu, Y.; Zhang, Y.; Fan, Z.; Zhang, Z.; Fan, X.; Xu, Y. BRG1 Links TLR4 Trans-Activation to LPS-Induced SREBP1a Expression and Liver Injury. Front. Cell. Dev. Biol. 2021, 9, 617073. [Google Scholar] [CrossRef]
- Duan, Y.; An, W.; Wu, H.; Wu, Y. Salvianolic acid C attenuates LPS-induced inflammation and apoptosis in human periodontal ligament stem cells via toll-like receptors 4 (TLR4)/nuclear factor kappa B (NF-κB) pathway. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 9499. [Google Scholar] [CrossRef]
- Fang, W.Y.; Tseng, Y.T.; Lee, T.Y.; Fu, Y.C.; Chang, W.H.; Lo, W.W.; Lin, C.L.; Lo, Y.C. Triptolide prevents LPS-induced skeletal muscle atrophy via inhibiting NF-κB/TNF-α and regulating protein synthesis/degradation pathway. Br. J. Pharmacol. 2021, 178, 2998–3016. [Google Scholar] [CrossRef]
- Foley, J.F. Different Binding Properties, Different Responses. Sci. Signal. 2013, 6, ec209. [Google Scholar] [CrossRef]
- Karaa, A.; Thompson, K.J.; McKillop, I.H.; Clemens, M.G.; Schrum, L.W. S-adenosyl-l-methionine attenuates oxidative stress and hepatic stellate cell activation in an ethanol-lps-induced fibrotic rat model. Shock 2008, 30, 197–205. [Google Scholar] [CrossRef]
- Kondo, T.; Suda, T.; Fukuyama, H.; Adachi, M.; Nagata, S. Essential roles of the Fas ligand in the development of hepatitis. Nat. Med. 1997, 3, 409–413. [Google Scholar] [CrossRef]
- Kou, X.; Qi, S.; Dai, W.; Luo, L.; Yin, Z. Arctigenin inhibits lipopolysaccharide-induced iNOS expression in RAW264.7 cells through suppressing JAK-STAT signal pathway. Int. Immunopharmacol. 2011, 11, 1095–1102. [Google Scholar] [CrossRef]
- Lalazar, G.; Ilyas, G.; Malik, S.A.; Liu, K.; Zhao, E.; Amir, M.; Lin, Y.; Tanaka, K.E.; Czaja, M.J. Autophagy confers resistance to lipopolysaccharide-induced mouse hepatocyte injury. Am. J. Physiol. Liver Physiol. 2016, 311, G377–G386. [Google Scholar] [CrossRef] [PubMed]
- Lamlé, J.; Marhenke, S.; Borlak, J.; von Wasielewski, R.; Eriksson, C.P.; Geffers, R.; Manns, M.P.; Yamamoto, M.; Vogel, A. Nuclear Factor-Eythroid 2–Related Factor 2 Prevents Alcohol-Induced Fulminant Liver Injury. Gastroenterology 2008, 134, 1159–1168.e2. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-W.; Tseng, H.-P.; Chen, L.-C.; Chen, B.-K.; Chang, W.-C. Functional Cooperation of Simian Virus 40 Promoter Factor 1 and CCAAT/Enhancer-Binding Protein β and δ in Lipopolysaccharide-Induced Gene Activation of IL-10 in Mouse Macrophages. J. Immunol. 2003, 171, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Li, X.; Yu, J.; Li, Y.; Wang, C.; Zhang, L.; Wang, T.; Zhong, X. Curcumin Attenuates Lipopolysaccharide-Induced Hepatic Lipid Metabolism Disorder by Modification of m6 A RNA Methylation in Piglets. Lipids 2018, 53, 53–63. [Google Scholar] [CrossRef]
- Mita, S.; Shimizu, Y.; Notsu, T.; Imada, K.; Kyo, S. Dienogest inhibits Toll-like receptor 4 expression induced by costimulation of lipopolysaccharide and high-mobility group box 1 in endometrial epithelial cells. Fertil. Steril. 2011, 96, 1485–1489.e4. [Google Scholar] [CrossRef]
- Nehme, A.; Ghahramanpouri, M.; Ahmed, I.; Golsorkhi, M.; Thomas, N.; Munoz, K.; Abdipour, A.; Tang, X.; Wilson, S.M.; Wasnik, S.; et al. Combination therapy of insulin-like growth factor I and BTP-2 markedly improves lipopolysaccharide-induced liver injury in mice. FASEB J. 2022, 36, e22444. [Google Scholar] [CrossRef]
- Ondee, T.; Jaroonwitchawan, T.; Pisitkun, T.; Gillen, J.; Nita-Lazar, A.; Leelahavanichkul, A.; Somparn, P. Decreased Protein Kinase C-β Type II Associated with the Prominent Endotoxin Exhaustion in the Macrophage of FcGRIIb−/− Lupus Prone Mice is Revealed by Phosphoproteomic Analysis. Int. J. Mol. Sci. 2019, 20, 1354. [Google Scholar] [CrossRef]
- Ouyang, Y.; Guo, J.; Lin, C.; Lin, J.; Cao, Y.; Zhang, Y.; Wu, Y.; Chen, S.; Wang, J.; Chen, L.; et al. Transcriptomic analysis of the effects of Toll-like receptor 4 and its ligands on the gene expression network of hepatic stellate cells. Fibrogenesis Tissue Repair 2016, 9, 2. [Google Scholar] [CrossRef]
- Raeburn, C.D.; Dinarello, C.A.; Zimmerman, M.A.; Calkins, C.M.; Pomerantz, B.J.; McIntyre, R.C.; Harken, A.H.; Meng, X. Neutralization of IL-18 attenuates lipopolysaccharide-induced myocardial dysfunction. Am. J. Physiol. Circ. Physiol. 2002, 283, H650–H657. [Google Scholar] [CrossRef] [PubMed]
- Raish, M.; Ahmad, A.; Alkharfy, K.M.; Ahamad, S.R.; Mohsin, K.; Al-Jenoobi, F.I.; Al-Mohizea, A.M.; Ansari, M.A. Hepatoprotective activity of Lepidium sativum seeds against D-galactosamine/lipopolysaccharide induced hepatotoxicity in animal model. BMC Complement. Altern. Med. 2016, 16, 501. [Google Scholar] [CrossRef] [PubMed]
- Saad, B.; Frei, K.; Scholl, F.A.; Fontana, A.; Maier, P. Hepatocyte-Derived Interleukin-6 and Tumor-Necrosis Factor alpha Mediate the Lipopolysaccharide-Induced Acute-Phase Response and Nitric Oxide Release by Cultured Rat Hepatocytes. JBIC J. Biol. Inorg. Chem. 1995, 229, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Matsuura, M.; Hirai, Y. Regulation of Lipopolysaccharide-Induced Interleukin-12 Production by Activation of Repressor Element GA-12 through Hyperactivation of the ERK Pathway. Clin. Vaccine Immunol. 2006, 13, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Su, G.L.; Rahemtulla, A.; Thomas, P.; Klein, R.D.; Wang, S.C.; A Nanji, A. CD14 and lipopolysaccharide binding protein expression in a rat model of alcoholic liver disease. Am. J. Pathol. 1998, 152, 841–849. [Google Scholar]
- Tadic, S.D.; Elm, M.S.; Li, H.S.; Van Londen, G.J.; Subbotin, V.M.; Whitcomb, D.C.; Eagon, P.K. Sex differences in hepatic gene expression in a rat model of ethanol-induced liver injury. J. Appl. Physiology. 2002, 93, 1057–1068. [Google Scholar] [CrossRef]
- Tanaka, N.; Matsubara, T.; Krausz, K.W.; Patterson, A.D.; Gonzalez, F.J. Disruption of phospholipid and bile acid homeostasis in mice with nonalcoholic steatohepatitis. Hepatology 2012, 56, 118–129. [Google Scholar] [CrossRef]
- Velayudham, A.; Hritz, I.; Dolganiuc, A.; Mandrekar, P.; Kurt-Jones, E.; Szabo, G. Critical role of Toll-like receptors and the common TLR adaptor, MyD88, in induction of granulomas and liver injury. J. Hepatol. 2006, 45, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Waring, J.F.; Liguori, M.J.; Luyendyk, J.P.; Maddox, J.F.; Ganey, P.E.; Stachlewitz, R.F.; North, C.; Blomme, E.A.G.; Roth, R.A. Microarray Analysis of Lipopolysaccharide Potentiation of Trovafloxacin-Induced Liver Injury in Rats Suggests a Role for Proinflammatory Chemokines and Neutrophils. Experiment 2005, 316, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Han, M.; Li, J.; Yang, X.; Yang, D. Immunopathogenesis of HBV infection. Adv. Exp. Med. Biol. 2020, 1179, 71–107. [Google Scholar] [PubMed]
- Wu, Y.-R.; Li, L.; Sun, X.-C.; Wang, J.; Ma, C.-Y.; Zhang, Y.; Qu, H.-L.; Xu, R.-X.; Li, J.-J. Diallyl disulfide improves lipid metabolism by inhibiting PCSK9 expression and increasing LDL uptake via PI3K/Akt-SREBP2 pathway in HepG2 cells. Nutr. Metab. Cardiovasc. Dis. 2020, 31, 322–332. [Google Scholar] [CrossRef]
- Yin, X.; Gong, X.; Jiang, R.; Kuang, G.; Wang, B.; Zhang, L.; Xu, G.; Wan, J. Emodin ameliorated lipopolysaccharide-induced fulminant hepatic failure by blockade of TLR4/MD2 complex expression in D-galactosamine-sensitized mice. Int. Immunopharmacol. 2014, 23, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Meng, Z.; Qiu, S.; Xu, Y.; Yang, D.; Schlaak, J.F.; Roggendorf, M.; Lu, M. Lipopolysaccharide-induced innate immune responses in primary hepatocytes downregulates woodchuck hepatitis virus replication via interferon-independent pathways. Cell. Microbiol. 2009, 11, 1624–1637. [Google Scholar] [CrossRef]
- Zhao, D.; Wu, T.; Yi, D.; Wang, L.; Li, P.; Zhang, J.; Hou, Y.; Wu, G. Dietary Supplementation with Lactobacillus casei Alleviates Lipopolysaccharide-Induced Liver Injury in a Porcine Model. Int. J. Mol. Sci. 2017, 18, 2535. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, X.; Yi, K.; Liang, C.; Geng, S.; Zhu, J.; Xie, C.; Zhong, C. Magnesium isoglycyrrhizinate ameliorates lipopolysaccharide-induced liver injury by upregulating autophagy and inhibiting inflammation via IL-22 expression. Bioorganic Chem. 2022, 128, 106034. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, L.; Song, Z.; Saari, J.T.; McClain, C.J.; Kang, Y.J. Abrogation of nuclear factor-κB activation is involved in zinc inhibition of lipopolysaccharide-induced tumor necrosis factor-α production and liver injury. Am. J. Pathol. 2004, 164, 1547–1556. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Ma, X.; Rasmussen, T.P.; Zhong, X.-B. Genetic Variations Associated with Anti-Tuberculosis Drug-Induced Liver Injury. Curr. Pharmacol. Rep. 2018, 4, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Brancatella, A.; Cappellani, D.; Kaufmann, M.; Semeraro, A.; Borsari, S.; Sardella, C.; Baldinotti, F.; Caligo, M.A.; Jones, G.; Marcocci, C.; et al. Long-term Efficacy and Safety of Rifampin in the Treatment of a Patient Carrying a CYP24A1 Loss-of-Function Variant. J. Clin. Endocrinol. Metab. 2022, 107, e3159–e3166. [Google Scholar] [CrossRef] [PubMed]
- Budak, F.; Bal, S.H.; Tezcan, G.; Guvenc, F.; Akalin, E.H.; Goral, G.; Deniz, G.; Oral, H.B. MicroRNA Expression Patterns of CD8+ T Cells in Acute and Chronic Brucellosis. PLoS ONE 2016, 11, e0165138. [Google Scholar] [CrossRef] [PubMed]
- Chai, S.C.; Cherian, M.T.; Wang, Y.-M.; Chen, T. Small-molecule modulators of PXR and CAR. Biochim. Biophys. Acta BBA Gene Regul. Mech. 2016, 1859, 1141–1154. [Google Scholar] [CrossRef]
- Chiang, C.H.; Wu, W.W.; Li, H.Y.; Chien, Y.; Sun, C.C.; Peng, C.H.; Lin, A.T.; Huang, C.S.; Lai, Y.H.; Chiou, S.H.; et al. Enhanced antioxidant capacity of dental pulp-derived iPSC-differentiated hepatocytes and liver regeneration by injectable HGF-releasing hydrogel in fulminant hepatic failure. Cell Transplant. 2015, 24, 541–559. [Google Scholar] [CrossRef]
- Hakkola, J.; Rysä, J.; Hukkanen, J. Regulation of hepatic energy metabolism by the nuclear receptor PXR. Biochim. Biophys. Acta BBA Gene Regul. Mech. 2016, 1859, 1072–1082. [Google Scholar] [CrossRef]
- Hanafusa, H.; Morikawa, Y.; Uehara, T.; Kaneto, M.; Ono, A.; Yamada, H.; Ohno, Y.; Urushidani, T. Comparative gene and protein expression analyses of a panel of cytokines in acute and chronic drug-induced liver injury in rats. Toxicology 2014, 324, 43–54. [Google Scholar] [CrossRef]
- He, X.; Song, Y.; Wang, L.; Xu, J. Protective effect of pyrrolidine dithiocarbamate on isoniazid/rifampicin-induced liver injury in rats. Mol. Med. Rep. 2019, 21, 463–469. [Google Scholar] [CrossRef]
- Huang, J.-H.; Zhang, C.; Zhang, D.-G.; Li, L.; Chen, X.; Xu, D.-X. Rifampicin-Induced Hepatic Lipid Accumulation: Association with Up-Regulation of Peroxisome Proliferator-Activated Receptor γ in Mouse Liver. PLoS ONE 2016, 11, e0165787. [Google Scholar] [CrossRef]
- Hussain, Z.; Kar, P.; A Husain, S. Antituberculosis drug-induced hepatitis: Risk factors, prevention and management. Experiment 2003, 41, 1226–1232. [Google Scholar]
- Francis, P.; Navarro, V.J. Drug-Induced Hepatotoxicity. [Updated 2022 Nov 11]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557535/ (accessed on 4 June 2023).
- Javed, S.; Akmal, Z. Hepatic Adverse Effects of Anti-Mycobacterium Tuberculosis Drugs and Their Associations with Various Genetic Variants. Precis. Med. Commun. 2022, 2, 59–78. [Google Scholar] [CrossRef]
- Kamal, S.M. Advances in Treatment of Hepatitis C. 2017. Available online: https://doi.org/10.5772/66719 (accessed on 4 June 2023).
- Kowalec, K. The clinical and pharmacogenomic determinants of interferon beta induced liver injury in multiple sclerosis. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 2016. [Google Scholar]
- Li, D.; Mackowiak, B.; Brayman, T.G.; Mitchell, M.; Zhang, L.; Huang, S.-M.; Wang, H. Genome-wide analysis of human constitutive androstane receptor (CAR) transcriptome in wild-type and CAR-knockout HepaRG cells. Biochem. Pharmacol. 2015, 98, 190–202. [Google Scholar] [CrossRef]
- Lyu, M.; Zhou, J.; Chen, H.; Bai, H.; Song, J.; Liu, T.; Cheng, Y.; Ying, B. The genetic variants in calcium signaling related genes influence anti-tuberculosis drug induced liver injury. Medicine 2019, 98, e17821. [Google Scholar] [CrossRef]
- Miyata, S.; Saku, N.; Akiyama, S.; Javaregowda, P.K.; Ite, K.; Takashima, N.; Toyoda, M.; Yura, K.; Kimura, T.; Nishina, H.; et al. Puromycin-based purification of cells with high expression of the cytochrome P450 CYP3A4 gene from a patient with drug-induced liver injury (DILI). Stem Cell. Res. Ther. 2022, 13, 6. [Google Scholar] [CrossRef] [PubMed]
- More, A.N.; Shah, T.K.; Parab, P.B.; Apte, K.G. Oroxylum indicum (Linn.) whole stem extract regulates expression of TNFα, IL6, NFkB, P38 MAPK and oxidative status in antitubercular therapy induced hepatotoxicity in Wistar rats. Matters 2017, 3. [Google Scholar] [CrossRef]
- Perea-Jacobo, R.; Muñiz-Salazar, R.; Laniado-Laborín, R.; Zenteno-Cuevas, R.; Cabello-Pasini, A.; Ochoa-Terán, A.; Radilla-Chávez, P. SLCO1B1 and SLC10A1 polymorphism and plasma rifampin concentrations in patients with co-morbidity tuberculosis-diabetes mellitus in Baja California, Mexico. Tuberculosis 2022, 136. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Mahadevan, A.; Lokesh, L.; Ashraf, V.; Sagar, B.K.C.; Taly, A.B.; Shankar, S.K. Tangier disease--a diagnostic challenge in countries endemic for leprosy. J. Neurol. Neurosurg. Psychiatry 2004, 75, 301–304. [Google Scholar] [CrossRef]
- Tan, W.; Zhao, K.; Xiang, J.; Zhou, X.; Cao, F.; Song, W.; Liu, Q.; Zhang, X.; Li, X.; Tan, Z. Pyrazinamide alleviates rifampin-induced steatohepatitis in mice by regulating the activities of cholesterol-activated 7α-hydroxylase and lipoprotein lipase. Eur. J. Pharm. Sci. 2020, 151, 105402. [Google Scholar] [CrossRef]
- Wang, P.; Yang, Y.; Pang, G.; Zhang, C.; Wei, C.; Tao, X.; Liu, J.; Xu, J.; Zhang, W.; Shen, Y. Hepatocyte-derived MANF is protective for rifampicin-induced cholestatic hepatic injury via inhibiting ATF4-CHOP signal activation. Free Radic. Biol. Med. 2020, 162, 283–297. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.; Wang, L.-K.; Wei, N. Pyrrolidine dithiocarbamate alleviates the anti-tuberculosis drug-induced liver injury through JAK2/STAT3 signaling pathway: An experimental study. Asian Pac. J. Trop. Med. 2017, 10, 520–523. [Google Scholar] [CrossRef]
- Zhang, J.; Jiao, L.; Song, J.; Wu, T.; Bai, H.; Liu, T.; Zhao, Z.; Hu, X.; Ying, B. Genetic and Functional Evaluation of the Role of FOXO1 in Antituberculosis Drug-Induced Hepatotoxicity. Evid. Based Complement. Altern. Med. 2021, 2021, 3185874. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, L.; Shen, Y.; Xu, J. Rifampicin-induced injury in L02 cells is alleviated by 4-PBA via inhibition of the PERK-ATF4-CHOP pathway. Toxicol. Vitr. 2016, 36, 186–196. [Google Scholar] [CrossRef]
- Zucchini, N.; de Sousa, G.; Bailly-Maitre, B.; Gugenheim, J.; Bars, R.; Lemaire, G.; Rahmani, R. Regulation of Bcl-2 and Bcl-xL anti-apoptotic protein expression by nuclear receptor PXR in primary cultures of human and rat hepatocytes. Biochim. Biophys. Acta BBA Mol. Cell. Res. 2005, 1745, 48–58. [Google Scholar] [CrossRef]
- Mi, X.-J.; Hou, J.-G.; Jiang, S.; Liu, Z.; Tang, S.; Liu, X.-X.; Wang, Y.-P.; Chen, C.; Wang, Z.; Li, W. Maltol Mitigates Thioacetamide-induced Liver Fibrosis through TGF-β1-mediated Activation of PI3K/Akt Signaling Pathway. J. Agric. Food Chem. 2019, 67, 1392–1401. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, R.S.; El-Tanbouly, G.S. Protocatechuic acid protects against thioacetamide-induced chronic liver injury and encephalopathy in mice via modulating mTOR, p53 and the IL-6/IL-17/IL-23 immunoinflammatory pathway. Toxicol. Appl. Pharmacol. 2022, 440, 115931. [Google Scholar] [CrossRef]
- Ali, S.O.; Darwish, H.A.E.-M.; Ismail, N.A.E.-F. Modulatory effects of curcumin, silybin-phytosome and alpha-R-lipoic acid against thioacetamide-induced liver cirrhosis in rats. Chem. Interact. 2014, 216, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.-S.; Chen, Y.-C.; Chou, C.-H.; Chuang, R.-F.; Sheen, L.-Y.; Chiu, C.-H. Hepatoprotection of silymarin against thioacetamide-induced chronic liver fibrosis. J. Sci. Food Agric. 2011, 92, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.C.; Yang, W.Y.; Hsiao, M.C.; Lin, K.H.; Lee, H.W.; Yuh, C.H. Transcriptomically revealed oligo-fucoidan enhances the immune system and protects hepatocytes via the ASGPR/STAT3/HNF4A axis. Biomolecules 2020, 10, 898. [Google Scholar] [CrossRef]
- de David, C.; Rodrigues, G.; Bona, S.; Meurer, L.; González-Gallego, J.; Tuñón, M.J.; Marroni, N.P. Role of Quercetin in Preventing Thioacetamide-Induced Liver Injury in Rats. Toxicol. Pathol. 2011, 39, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Dewhurst, M.R.; Ow, J.R.; Zafer, G.; Van Hul, N.K.M.; Wollmann, H.; Bisteau, X.; Brough, D.; Choi, H.; Kaldis, P. Loss of hepatocyte cell division leads to liver inflammation and fibrosis. PLoS Genet. 2020, 16, e1009084. [Google Scholar] [CrossRef] [PubMed]
- El-Mihi, K.A.; Kenawy, H.I.; El-Karef, A.; Elsherbiny, N.M.; Eissa, L.A. Naringin attenuates thioacetamide-induced liver fibrosis in rats through modulation of the PI3K/Akt pathway. Life Sci. 2017, 187, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Weng, C.F. Co-administration of cyclosporine A alleviates thioacetamide-induced liver injury. WJG 2005, 11, 1411. [Google Scholar] [CrossRef]
- Fazal, Y.; Fatima, S.N.; Shahid, S.M.; Mahboob, T. Effects of curcumin on angiotensin-converting enzyme gene expression, oxidative stress and anti-oxidant status in thioacetamide-induced hepatotoxicity. J. Renin-Angiotensin-Aldosterone Syst. 2014, 16, 1046–1051. [Google Scholar] [CrossRef]
- Feng, Y.; Ying, H.-Y.; Qu, Y.; Cai, X.-B.; Xu, M.-Y.; Lu, L.-G. Novel matrine derivative MD-1 attenuates hepatic fibrosis by inhibiting EGFR activation of hepatic stellate cells. Protein Cell. 2016, 7, 662–672. [Google Scholar] [CrossRef]
- Ghanim, A.M.; Younis, N.S.; Metwaly, H.A. Vanillin augments liver regeneration effectively in Thioacetamide induced liver fibrosis rat model. Life Sci. 2021, 286, 120036. [Google Scholar] [CrossRef]
- Low, T.Y.; Leow, C.K.; Salto-Tellez, M.; Chung, M.C.M. A proteomic analysis of thioacetamide-induced hepatotoxicity and cirrhosis in rat livers. Proteomics 2004, 4, 3960–3974. [Google Scholar] [CrossRef]
- Marchyshak, T.; Yakovenko, T.; Shmarakov, I.; Tkachuk, Z. The Potential Protective Effect of Oligoribonucleotides-d-Mannitol Complexes against Thioacetamide-Induced Hepatotoxicity in Mice. Pharmaceuticals 2018, 11, 77. [Google Scholar] [CrossRef]
- Metwaly, H.A.; El-Gayar, A.M.; El-Shishtawy, M.M. Inhibition of the signaling pathway of syndecan-1 by synstatin: A promising anti-integrin inhibitor of angiogenesis and proliferation in HCC in rats. Arch. Biochem. Biophys. 2018, 652, 50–58. [Google Scholar] [CrossRef]
- Park, J.-H.; Kum, Y.-S.; Lee, T.-I.; Kim, S.-J.; Lee, W.-R.; Kim, B.-I.; Kim, H.-S.; Kim, K.-H.; Park, K.-K. Melittin attenuates liver injury in thioacetamide-treated mice through modulating inflammation and fibrogenesis. Exp. Biol. Med. 2011, 236, 1306–1313. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Shin, H.W.; Lee, K.B.; Lee, M.-J.; Jang, J.-J. Differential Expression of Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases in Thioacetamide-Induced Chronic Liver Injury. J. Korean Med. Sci. 2010, 25, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Said, A.M.; Waheed, R.M.; Khalifa, O.A. Protective role of rosemary ethanolic extract on thioacetamide induced hepatic encephalopathy: Biochemical and molecular studies. AJBAS 2019, 13, 1–6. [Google Scholar]
- Traber, P.G.; Chou, H.; Zomer, E.; Hong, F.; Klyosov, A.; Fiel, M.-I.; Friedman, S.L. Regression of Fibrosis and Reversal of Cirrhosis in Rats by Galectin Inhibitors in Thioacetamide-Induced Liver Disease. PLoS ONE 2013, 8, e75361. [Google Scholar] [CrossRef] [PubMed]
- Weiskirchen, R. Special Issue on “Cellular and Molecular Mechanisms Underlying the Pathogenesis of Hepatic Fibrosis”. Cells 2020, 9, 1105. [Google Scholar] [CrossRef] [PubMed]
- Yahya, S.; Shalaby, R.K.; Mannaa, F.A.; Abdel-Wahhab, K.G.; Mohamed, N.R.; Shabana, M.S.; Elwakeel, S.H. Hepatoprotective effects of chitosan on thioacetamide induced liver toxicity in male albino rats. Biointerface Res. Appl. Chem. 2021, 11, 14490–14505. [Google Scholar]
- Zhang, C.; Huang, J.; An, W. Hepatic stimulator substance resists hepatic ischemia/reperfusion injury by regulating Drp1 translocation and activation. Hepatology 2017, 66, 1989–2001. [Google Scholar] [CrossRef]
- Sa-Nguanmoo, P.; Rianthavorn, P.; Amornsawadwattana, S.; Poovorawan, Y. Hepatitis B Virus Infection in Non-Human Primates. Acta Virol. 2009, 53, 73–82. [Google Scholar] [CrossRef]
- Wieland, S.F. The Chimpanzee Model for Hepatitis B Virus Infection. Cold Spring Harb. Perspect. Med. 2015, 5, a021469. [Google Scholar] [CrossRef]
- Ortega-Prieto, A.M.; Cherry, C.; Gunn, H.; Dorner, M. In Vivo Model Systems for Hepatitis Virus Research. ACS Infect. Dis. 2019, 5, 688. [Google Scholar] [CrossRef]
- Tan, A.; Koh, S.; Bertoletti, A. Immune Response in Hepatitis B Virus Infection. Cold Spring Harb. Perspect. Med. 2015, 5, a021428. [Google Scholar] [CrossRef]
- Xu, R.; Hu, P.; Li, Y.; Tian, A.; Li, J.; Zhu, C. Advances in HBV infection and replication systems in vitro. Virol. J. 2021, 18, 105. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.H.; Hu, S.Q.; Liu, J.; Cao, H.C.; Xu, W.; Li, Y.J.; Li, L.J. Nature and mechanisms of hepatocyte apoptosis induced by D-galactosamine/lipopolysaccharide challenge in mice. Int. J. Mol. Med. 2014, 33, 1498–1506. [Google Scholar] [CrossRef] [PubMed]
- Wills, P.J.; Asha, V.V. Protective effect of Lygodium flexuosum (L.) Sw. (Lygodiaceae) against D-galactosamine induced liver injury in rats. J. Ethnopharmacol. 2006, 108, 116–123. [Google Scholar] [CrossRef]
- Mondal, M.; Hossain, M.; Hasan, R.; Tarun, T.I.; Islam, A.F.; Choudhuri, M.S.K.; Islam, M.T.; Mubarak, M.S. Hepatoprotective and antioxidant capacity of Mallotus repandus ethyl acetate stem extract against D-galactosamine-induced hepatotoxicity in rats. ACS Omega 2020, 5, 6523–6531. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Guo, J.; Li, W.; Xia, H.; Lu, Y.; Dong, X.; Chen, Y.; Xie, X.; Fu, S.; Li, M. HBx Induced AFP Receptor Expressed to Activate PI3K/AKT Signal to Promote Expression of Src in Liver Cells and Hepatoma Cells. BMC Cancer 2015, 15, 362. [Google Scholar] [CrossRef]
- Cheng, Q.; Li, Y.W.; Yang, C.F.; Zhong, Y.J.; Li, L. Ethanol-Induced Hepatic Insulin Resistance Is Ameliorated by Methyl Ferulic Acid through the PI3K/Akt Signaling Pathway. Front. Pharmacol. 2019, 10, 949. [Google Scholar] [CrossRef]
- Cho, Y.-A.; Ko, I.-G.; Jin, J.-J.; Hwang, L.; Kim, S.-H.; Jeon, J.W.; Yang, M.J.; Kim, C.-J. Polydeoxyribonucleotide Ameliorates Alcoholic Liver Injury Though Suppressing Phosphatidylinositol 3-Kinase/Protein Kinase B Signaling Pathway in Mice. J. Exerc. Rehabil. 2022, 18, 350–355. [Google Scholar] [CrossRef]
- Jung, J.S.; Choi, M.J.; Lee, Y.Y.; Moon, B.I.; Park, J.S.; Kim, H.S. Suppression of Lipopolysaccharide-Induced Neuroinflammation by Morin via MAPK, PI3K/Akt, and PKA/HO-1 Signaling Pathway Modulation. J. Agric. Food Chem. 2017, 65, 373–382. [Google Scholar] [CrossRef]
- Tan, W.; Luo, X.; Li, W.; Zhong, J.; Cao, J.; Zhu, S.; Chen, X.; Zhou, R.; Shang, C.; Chen, Y. TNF-α Is a Potential Therapeutic Target to Overcome Sorafenib Resistance in Hepatocellular Carcinoma. EBioMedicine 2019, 40, 446. [Google Scholar] [CrossRef]
- Shukla, R.; Yue, J.; Siouda, M.; Gheit, T.; Hantz, O.; Merle, P.; Zoulim, F.; Krutovskikh, V.; Tommasino, M.; Sylla, B.S. Proinflammatory Cytokine TNF-α Increases the Stability of Hepatitis B Virus X Protein through NF-ΚB Signaling. Carcinogenesis 2011, 32, 978–985. [Google Scholar] [CrossRef]
- Nowak, A.J.; Relja, B. The Impact of Acute or Chronic Alcohol Intake on the NF-κB Signaling Pathway in Alcohol-Related Liver Disease. Int. J. Mol. Sci. 2020, 21, 9407. [Google Scholar] [CrossRef]
- Oeckinghaus, A.; Ghosh, S. The NF-κB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef] [PubMed]
- Mandrekar, P.; Szabo, G. Signalling Pathways in Alcohol-Induced Liver Inflammation. J. Hepatol. 2009, 50, 1258–1266. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, Y.; Ma, H.; Wang, B.; Xu, L.; Zhang, H.; Song, X.; Gao, L.; Liang, X.; Ma, C. Hepatitis B Virus X Protein Amplifies TGF-β Promotion on HCC Motility through down-Regulating PPM1a. Oncotarget 2016, 7, 33125. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Zhang, Y.-J. Interference of Apoptosis by Hepatitis B Virus. Viruses 2017, 9, 230. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; De Lisle, R.C.; Huang, H.; Ding, W.-X. A Mechanistic Review of Cell Death in Alcohol-Induced Liver Injury. Alcohol Clin. Exp. Res. 2016, 40, 1215–1223. [Google Scholar] [CrossRef]
- Bannerman, D.D.; Goldblum, S.E. Mechanisms of Bacterial Lipopolysaccharide-Induced Endothelial Apoptosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003, 284, L899–L914. [Google Scholar] [CrossRef]
- You, H.; Qin, S.; Zhang, F.; Hu, W.; Li, X.; Liu, D.; Kong, F.; Pan, X.; Zheng, K.; Tang, R. Regulation of Pattern-Recognition Receptor Signaling by HBX During Hepatitis B Virus Infection. Front. Immunol. 2022, 13, 438. [Google Scholar] [CrossRef]
- Karatayli, E.; Hall, R.A.; Weber, S.N.; Dooley, S.; Lammert, F. Effect of alcohol on the interleukin 6-mediated inflammatory response in a new mouse model of acute-on-chronic liver injury. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 298–307. [Google Scholar] [CrossRef]
- Hamesch, K.; Borkham-Kamphorst, E.; Strnad, P.; Weiskirchen, R. Lipopolysaccharide-induced inflammatory liver injury in mice. Lab. Anim. 2015, 49, 37–46. [Google Scholar] [CrossRef]
- Miller, A.M.; Wang, H.; Park, O.; Horiguchi, N.; Lafdil, F.; Mukhopadhyay, P.; Moh, A.; Fu, X.Y.; Kunos, G.; Pacher, P.; et al. Anti-Inflammatory and Anti-Apoptotic Roles of Endothelial Cell STAT3 in Alcoholic Liver Injury. Alcohol Clin. Exp. Res. 2010, 34, 719–725. [Google Scholar] [CrossRef]
- Oliveros, J.C. Venny. An Interactive Tool for Comparing Lists with Venn Diagrams. 2017. Available online: http://bioinfogp.cnb.csic.es/tools/venny/index.html (accessed on 29 March 2023).
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING Database in 2021: Customizable Protein–Protein Networks, and Functional Characterization of User-Uploaded Gene/Measurement Sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: New Perspectives on Genomes, Pathways, Diseases and Drugs. Nucleic Acids Res. 2017, 45, D353–D361. [Google Scholar] [CrossRef]
- Kumari, S.; Kumar, P. Design and Computational Analysis of an MMP9 Inhibitor in Hypoxia-Induced Glioblastoma Multiforme. ACS Omega 2023, 8, 10565–10590. [Google Scholar] [CrossRef]
- Ma, H.; Fu, W.; Yu, H.; Xu, Y.; Xiao, L.; Zhang, Y.; Wu, Y.; Liu, X.; Chen, Y.; Xu, T. Exploration of the anti-inflammatory mechanism of Lanqin oral solution based on the network pharmacology analysis optimized by Q-markers selection. Comput. Biol. Med. 2023, 154, 106607. [Google Scholar] [CrossRef]
- Franz, M.; Lopes, C.T.; Fong, D.; Kucera, M.; Cheung, M.; Siper, M.C.; Huck, G.; Dong, Y.; Sumer, O.; Bader, G.D. Cytoscape.Js 2023 Update: A Graph Theory Library for Visualization and Analysis. Bioinformatics 2023, 39, btad031. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Zeng, Z.; Jiang, J.; Xu, A.; Li, S.; Li, Y.; Chen, Z.; Chen, W.; Zhang, J.; Bi, X. Network pharmacology analysis and experimental validation to explore the mechanism of Bushao Tiaozhi capsule (BSTZC) on hyperlipidemia. Sci. Rep. 2022, 12, 6992. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Lou, G.; Ren, Y.; Li, T.; Zhang, X.; Wang, J.; Huang, Q. Network pharmacology-based analysis of Zukamu granules for the treatment of COVID-19. Eur. J. Integr. Med. 2021, 42, 101282. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).