Abstract
Many organisms can sense and respond to magnetic fields (MFs), with migratory species in particular utilizing geomagnetic field information for long-distance migration. Cryptochrome proteins (Crys) along with a highly conserved Iron-sulfur cluster assembly protein (i.e., MagR) have garnered significant attention for their involvement in magnetoresponse (including magnetoreception). However, in vivo investigations of potential transcriptional crosstalk between Crys and MagR genes have been limited. The brown planthopper, Nilaparvata lugens, is a major migratory pest insect and an emerging model for studying MF intensity-related magnetoresponse. Here, we explored in vivo transcriptional crosstalk between Crys (Cry1 and Cry2) and MagR in N. lugens. The expression of Crys and MagR were found to be sensitive to MF intensity changes as small as several micro-teslas. Knocking down MagR expression led to a significant downregulation of Cry1, but not Cry2. The knockdown of either Cry1 or Cry2 individually did not significantly affect MagR expression. However, their double knockdown resulted in significant upregulation of MagR. Our findings clearly indicate transcriptional crosstalk between MagR and Crys known to be involved in magnetoresponse. This work advances the understanding of magnetoresponse signaling and represents a key initial step towards elucidating the functional consequences of these novel in vivo interactions.
1. Introduction
Many organisms, ranging from microbes to vertebrates, possess the ability to sense and respond to magnetic fields (MFs) [1,2,3], with migratory species in particular utilizing geomagnetic field (GMF) information for navigation or orientation [4,5,6]. The conservation of magnetoresponse across taxa underscores its biological significance, and studies on the topic have experienced substantial growth over the past two decades [6,7,8,9,10,11,12]. With respect to elucidating the mechanisms underlying magnetoresponse, magnetite-based mechanisms [1,13], the radical-pair quantum model [11,14], and, more recently, iron-sulfur cluster assembly (IscA, i.e., MagR)/Cryptochrome (Cry) complex magnetosensing [15,16,17] have been the focus of attention, particularly with regards to the latter two.
In animals, Crys play crucial roles in the circadian function and can be divided into three categories: Drosophila-like type 1 Crys, mammalian-like type 2 Crys, and bird-like type 4 Crys (Cry4s) [18]. Type 1 Crys (Cry1s) are UV-A/blue-light photoreceptors responsible for the synchronization of the circadian clock to the daily light-dark cycle [19,20] in most insects, but are absent in vertebrates. Type 4 Crys are light-sensitive, but currently have no known roles in clock function [21]. Type 2 Crys (Cry2s) are light-insensitive and function as circadian transcriptional repressors. They are present not only in mammals but also in most insects, except for flies in the brachyceran lineage [20,22,23]. The canonical radical-pair model proposes that magnetoreception is achieved through quantum effects of magnetically-sensitive flavin-tryptophan radical pairs formed by photosensitive Cry1 and Cry4 [6,8,11,14], although Cry2 has also been found critical in magnetoresponses of several insect species recently [24,25,26]. As technology continues to advance and magnetoresponse research deepens, the canonical biophysical model is evolving. This mainly includes a shift from three to four successive flavin-tryptophan radical pairs [8] and a possible transition from Cry-centric radical-pair mechanisms towards a non-Cry-dependent one [7,27].
Iron-sulfur proteins are widely recognized for their crucial roles in many fundamental physiological processes including cellular respiration, nitrogen fixation, photosynthesis, DNA replication, and repair [28,29,30,31,32]. As a highly conserved A-type iron-sulfur protein, IscA has been proposed as a magnetoreceptor renamed MagR [15,33], which has also been suggested to influence circadian rhythms in Drosophila [34]. Research into MagR’s role in magnetoresponse and its potential applications is expanding [16,25,26,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49]. Although no protein interactions between MagR and Cry were observed in the European robin, Erithacus rubecula [50], a well-known migrant bird species, such interactions have been identified in other vertebrates and invertebrates. These include pigeon Columba livia, human Homo sapiens, the brown planthopper, Nilaparvata lugens [39], D. melanogaster flies, and Danaus plexippus butterflies [15], indicating a remarkable degree of conservation. Increasing transcriptional evidence from microorganisms [51], plants [45], and insects [25,36,40,48,49,52] also support MagR’s function in magnetoresponse signaling. Notably, research on migratory insects has provided unique insights, suggesting that MagR may play a role in regulating migration through magnetoreception-related processes [26,40,49]. However, some fundamental questions remain and require further investigation, such as details regarding the origin of MagR’s magnetism [16] and its interaction with Cry.
In recent years, insects have increasingly been utilized in magnetoresponse studies due to their short generation time and the availability of a simple yet powerful genetic toolbox. For example, D. melanogaster has been frequently employed to verify the Cry-involved radical-pair quantum mechanism through various assays including food rewarding-based assay [53,54], geotaxis assay [55,56], circadian magnetoresponse assay [7,57,58], and electrophysiology assay [7]. The brown planthopper, N. lugens, is a major migratory pest of rice that employs a partial seasonal migration strategy [59]. Its northward migration comprises multiple waves from spring to almost the end of summer, each lasting for one to several days. Upon arrival in new areas, it reproduces, and its migratory offspring continue the next wave of migration. As N. lugens is unable to survive winter in temperate East Asia, it undertakes a southward return migration along similar routes during autumn. A series of studies has been conducted to explore the magnetoresponses of N. lugens to variations in GMF intensity [38,40,60,61,62,63,64]. In addition, magnetite-based magnetoreception [65] and putative key magnetoresponse genes including Cry1, Cry2, and MagR in N. lugens have been investigated [38,40,61,62,66], establishing it as an emerging model for magnetoresponse research.
Recently, there have been increasing reports of in vivo reverse-genetic magnetoresponse studies targeting potential magnetoreceptor genes across taxa [6,7,24,25,26,67]. However, the potential crosstalk between MagR and Cry(s) has not been fully considered, which may introduce bias in functional identification. Here, we investigate the transcriptional interactions between Crys (Cry1 and Cry2) and MagR in magnetoresponse using an RNAi assay with the migratory N. lugens. We show that transcript expression of all three putative magnetoreceptor genes can respond to changes in MF intensity. Notably, there exists a transcriptional interplay between them, indicating that they sense or transduce magnetoresponse in the same signaling pathway.
2. Results
2.1. Transcriptional Responses of the Putative Magnetosensing Genes to Magnetic Field Intensity Changes
Nilaparvata lugens typically undergoes at least one generation of breeding before migrating again to a new site, and unmated 2-day-old adults begin to take off for a nocturnal migration [68,69]. Furthermore, considering the absence of identified magnetosensory organs in this species, this study began by examining the transcriptional responses of potential magnetoreceptor genes (Cry1, Cry2, and MagR) in whole unmated female adults aged 1–3 days old. These individuals were exposed to either a physiological geomagnetic field of 45 μT (GMF45μT) or a non-physiological near-zero magnetic field (NZMF) for one generation, compared to the local GMF intensity of 50 μT (GMF50μT), before transcript expression analyses.
Transcript expression levels of three putative magnetosensing genes in adult female N. lugens were found to be upregulated when the insects were exposed to GMF45μT along their migratory route, as compared to the local GMF50μT, for one generation. Significant upregulation of Cry1 expression was observed in 1-day- (+45.0%, p = 0.049), 2-day- (+74.2%, p = 0.029), and 3-day-old (+74.1%, p = 0.009; Figure 1A) female adults. Transcript expression of Cry2 was significantly upregulated in 2-day- (+99.5%, p = 0.044) and 3-day-old (+68.2%, p = 0.039; Figure 1B) female adults, while significantly upregulated MagR was only found in 3-day-old (+90.0%, p = 0.029; Figure 1C) female adults. Only a marginal upregulation of MagR was found in 2-day-old (+52.8%, p = 0.092; Figure 1C) female adults.
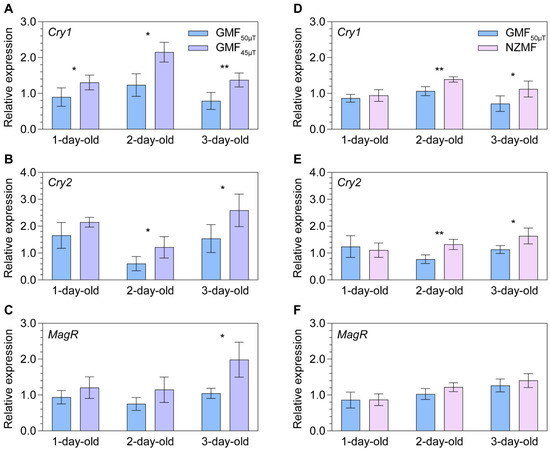
Figure 1.
The relative transcript abundance of putative key genes Cryptochromes (Cry1 and Cry2) and Iron-sulfur Cluster Assembly 1 (i.e., MagR) in magnetoresponse signaling of one- to three-day-old female adult Nilaparvata lugens under the GMF50μT vs. GMF45μT (panels (A–C)) and the GMF50μT vs. near-zero magnetic field vs. (NZMF) (panels (D–F)). Statistical significance of expression differences between magnetic fields is tested using one-way ANOVA or two-tailed Mann–Whitney U test at p < 0.05 (*) and p < 0.01 (**).
When N. lugens was exposed to NZMF vs. GMF50μT, significant upregulation of putative magnetosensing genes in both 2-day- and 3-day-old female adults was found for Cry1 (+30.5%, p = 0.004; +57.3%, p = 0.039; Figure 1D) and Cry2 (+71.0%, p = 0.004; +44.6%, p = 0.022; Figure 1E), but not for MagR (Figure 1F).
2.2. Transcript Expression of Cryptochromes after Knocking down the Putative Magnetoreceptor Iron-sulfur Cluster Assembly 1 (MagR)
The RNAi assay was conducted with 2-day- and 3-day-old individuals under a local GMF50μT since the significant differences between magnetic field groups were mainly found in female adults of that age range. The effectiveness of RNA interference targeting individual genes (Cry1, Cry2, and MagR) and double knockdown of Cry1 and Cry2 (Cry1/2) was validated before investigating transcriptional interplay, as demonstrated in Figure S1.
There was a significant downregulation of Cry1 relative to the control dsGFP treatment in both 2-day- (−30.6%, p = 0.028) and 3-day-old (+34.7%, p = 0.029) females after the knockdown of MagR (Figure 2A). However, only a marginal downregulation was observed for Cry2 in 2-day-old females (−27.8%, p = 0.077; Figure 2B) and the difference was negligible in 3-day-old females (Figure 2B).
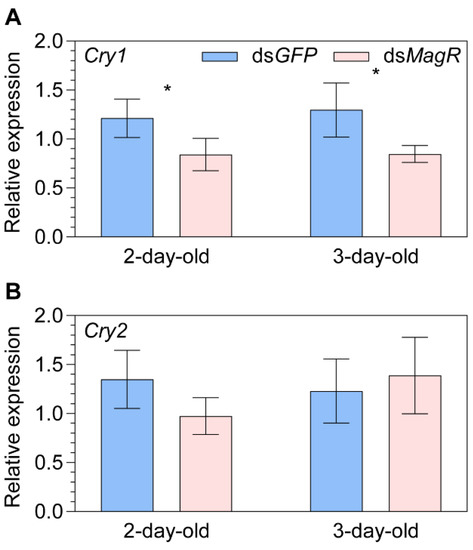
Figure 2.
The relative transcript abundance of Cryptochromes (A) Cry1 and (B) Cry2 in two- and three-day-old female adult Nilaparvata lugens after knocking down of Iron-sulfur Cluster Assembly 1 (i.e., MagR) by dsMagR injection. Statistical significance of expression differences between dsMagR and dsGFP control treatment groups is tested using one-way ANOVA or two-tailed Mann–Whitney U test at p < 0.05 (*).
2.3. Transcript Expression of the Putative Magnetoreceptor Iron-sulfur Cluster Assembly 1 after Knocking down Cryptochrome 1 or Cryptochrome 2
Cry1 and Cry2 were individually knocked down to explore the effects on the transcript expression of MagR. However, no significant difference in MagR expression compared to the dsGFP control group was found for either gene (dsCry1 vs. dsGFP; dsCry2 vs. dsGFP; p ≥ 0.169; Figure 3).
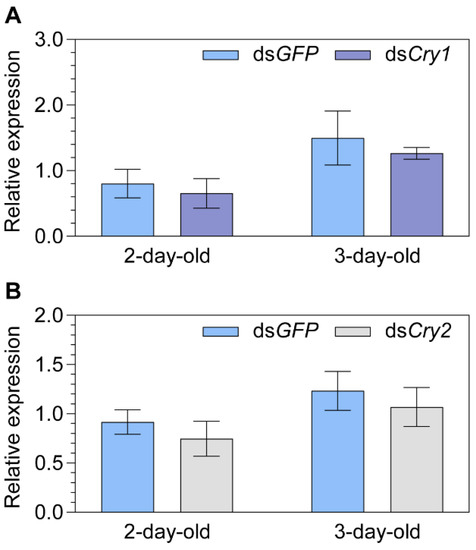
Figure 3.
The relative transcript abundance of Iron-sulfur Cluster Assembly 1 (i.e., MagR) in two- and three-day-old female adult Nilaparvata lugens after injection of either (A) dsCry1 or (B) dsCry2. Statistical significance between the target gene and dsGFP control group expression of MagR is tested using one-way ANOVA or two-tailed Mann–Whitney U test at p < 0.05. No significance was found.
2.4. Potential Transcriptional Interactions between Cryptochrome 1 and Cryptochrome 2
To address potential transcriptional interactions between Cry1 and Cry2 that could mask the effect of Crys’ knockdown on the transcript expression of MagR, we evaluated the transcript expressions of both Crys individually by knocking down the other Cry.
A significant downregulation of Cry1 was found for 3-day-old females (−31.3%, p = 0.029; Figure 4A) in the dsCry2 vs. dsGFP group, while a significant upregulation of Cry2 was found for 2-day-old females (+48.6%, p = 0.044; Figure 4B) in dsCry1 vs. dsGFP group. Moreover, a marginal upregulation of Cry1 was found for 2-day-old females (+32.1%, p = 0.093; Figure 4A) in the dsCry2 vs. dsGFP group. No significance was found between dsCry1 vs. dsGFP group for the 3-day-old females (p = 0.110; Figure 4B).
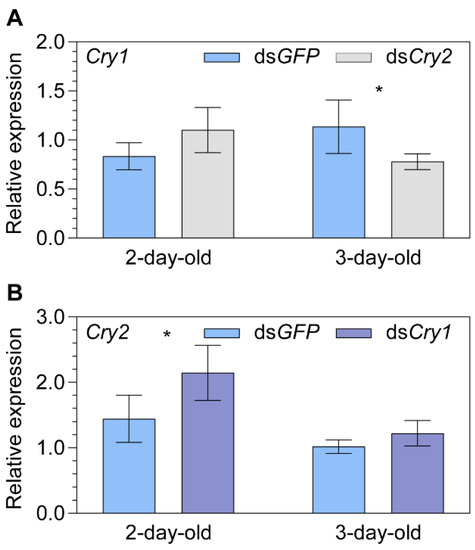
Figure 4.
The relationship between Cryptochrome 1 (Cry1) and 2 (Cry2) expression levels explored by dsRNA injections. (A) The relative transcript expression of Cry1 in two- and three-day-old female adult Nilaparvata lugens after injection of dsCry2. (B) The relative transcript expression of Cry2 in two- and three-day-old female adult Nilaparvata lugens after injection of dsCry1. Statistical significance between the target gene and dsGFP control group expression levels is tested using one-way ANOVA or two-tailed Mann–Whitney U test at p < 0.05 (*).
2.5. Transcript Expression of the Putative Magnetoreceptor Iron-sulfur Cluster Assembly 1 after Knocking down Both Cryptochrome 1 and Cryptochrome 2
To ensure that MagR expression was not masked by the indirect effects of knocking down one Cry on the expression of the other, a double knockdown of Cry1/2 was executed.
A significant upregulation of MagR was found for 2-day-old females (+115.0%, p = 0.026) in the dsCry1/2 vs. dsGFP group, while the difference was negligible in 3-day-old females (Figure 5).
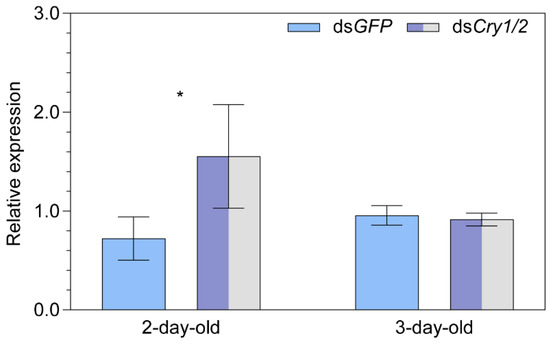
Figure 5.
The relative transcript abundance of Iron-sulfur Cluster Assembly 1 (i.e., MagR) in two- and three-day-old female adult Nilaparvata lugens after injection of both dsCry1 and dsCry2. Statistical significance of differences in MagR expression between dsCry1/2 treatment and dsGFP control groups is tested using one-way ANOVA at p < 0.05 (*).
3. Discussion
The enigmatic mechanisms underlying magnetoresponse, especially radical-pair-based magnetoreception or magnetosensitivity, have been extensively explored across various taxa and serve as a major area of study in quantum biology [4,6,7,9,11,70]. Recently, MagR (originally named IscA1)/Cry complex magnetosensing [15,16,17] has expanded the canonical Cry-involved radical-pair magnetoreception model, and is supported by increasing evidence [16,25,26,36,39,45,48,49,52]. Using a reverse genetic assay, we show for the first time in vivo transcriptional crosstalk between Crys and MagR in the migratory brown planthopper, N. lugens, genes that may enable its magnetoresponse to both physiological and non-physiological changes in MF intensity. The magnetoreception and orientational abilities of migrating birds are well known, and birds most likely possess both cryptochrome-based and magnetite-based sensing systems [70]. It is intriguing to note that a seemingly simple invertebrate like N. lugens studied here has also seemingly developed such a complex magnetoresponse, likely as a result of long-term evolution driven by selection for traits that confer a migratory advantage.
We replicated the findings of our previous study [61], which demonstrated an increase in both Cry1 and Cry2 expression in N. lugens when exposed to the GMF45μT vs. GMF50μT. Consistent with the upregulation of Cry1 in nymphs under the NZMF vs. GMF50μT [62], the transcript expression of Cry1 in female adults was also found upregulated in the present study. However, the comparison of Cry2 magnetoresponse between nymphs in [62] and adults in this study under NZMF vs. GMF50μT suggests a temporal-specific transcriptional magnetoresponse consistent with its multifunctional role as a circadian repressor, exhibiting rhythmic expression [66]. The involvement of Crys in the magnetoresponse of N. lugens, as implicated here, is consistent with prior transgenic research on the magnetoreception system of Drosophila [54].
In contrast to Crys expression patterns, the N. lugens treatment group exposed to physiological magnetic fields showed a general increase in MagR transcript levels, while no such increase was observed in the NZMF vs. GMF50μT group, implying a narrower threshold for magnetoresponsiveness of MagR. It is interesting to note that Cry1 appears to hold greater importance or at least sensitivity as a magnetoreceptor or magnetotransducer, given that significant magnetoresponses were observed in all 1- to 3-day-old adults under physiological changes in magnetic field intensity while changes in the expression of the other two genes examined were less extensive. Notably, all three putative magnetosensing genes exhibited a greater overall relative increase in expression levels in the physiological MF group compared to the non-physiological one, consistent with their potential roles in magnetoreception during normal insect migration.
In vitro studies across taxa have shown that Cry and MagR proteins interact to form a rod-like polymeric complex with an intrinsic magnetic moment [15]. However, limited in vivo information is available regarding their potential interplay. Knockdown of MagR in Drosophila has been reported to disrupt circadian behavior, indicating its involvement in the circadian pacemaker [34]. Consistent with the findings in Drosophila, our study has observed that knocking down MagR affects the expression of multifunctional Cry1, which not only acts as a putative magnetoreceptor but also plays a role as the photoreceptor responsible for synchronizing the circadian clock [6,19,20,66]. Our single gene knockdown assay did not reveal any transcriptional interactions between Cry2 and MagR, which is consistent with the results of our protein interaction investigations using a yeast two-hybrid assay in N. lugens [39]. This finding also supports the suggested Cry/MagR complex magnetoreception model [15], which proposes that the Cry should be photosensitive like Cry1 here.
The interplay between Cry1 and Cry2 has been insufficiently explored to date across taxa. In our study of N. lugens here, reciprocal knockdown experiments resulted in significant changes in both Cry1 and Cry2 in 2- and 3-day-old adults. This can be attributed to their involvement in the circadian pathway, where Cry1 functions as a blue-light circadian photoreceptor that resets the molecular clock upon light exposure, while Cry2 acts as a transcriptional repressor in the clockwork [71]. Interestingly, while the individual knockdown of Cry1 or Cry2 did not affect the transcriptional expression of MagR, the double knockdown of both significantly upregulated MagR expression in 2-day-old adults, which could be due to a complementary effect between Cry1 and Cry2. Thus, Cry1 and Cry2 seemingly act in concert as the repressor of MagR expression, although the involvement of unknown gene (s) such as opsins [50,72] cannot be excluded. As 2- and 3-day-old adults correspond to the primary age for long-distance migration takeoff and termination [68], both Crys and MagR may have potential signaling roles in initiating and terminating migration. However, further investigation is required to elucidate the specific mechanism and functional significance for migration.
Compared to non-migratory insects [7,56,57,73], insect migrants appear to possess a higher degree of magnetosensitivity in response to changes in MFs, which is consistent with their potential utilization of MF information during their long-distance movements [61]. Therefore, they hold great promise for providing unique insights into magnetoresponse mechanisms. A recent study has demonstrated that free FAD itself can mediate a magnetoresponse in vivo, albeit at high levels that are non-physiological, suggesting a possible shift from Cry-centric radical-pair mechanism to a non-Cry-dependent model [7,27]. Considering this, Cry1, Cry2 and MagR of the migratory N. lugens may act in concert (as proposed in Figure 6) as the magnetotransducer due to their reported involvement in animal migration or magnetoresponses [6,7,24,25,26,67]. An improved understanding of these interactions may also provide useful in the optimization of bionic magnetosensors based on the MagR/Cry complex [46,47]. Lastly, as a note of caution, given that our work has clearly depicted a transcriptional interplay among three genes involved in magnetoresponse, particularly between Cry1 and MagR, careful thought should be used when conducting functional genetic analyses of magnetoresponse to avoid potential bias resulting from their crosstalk.
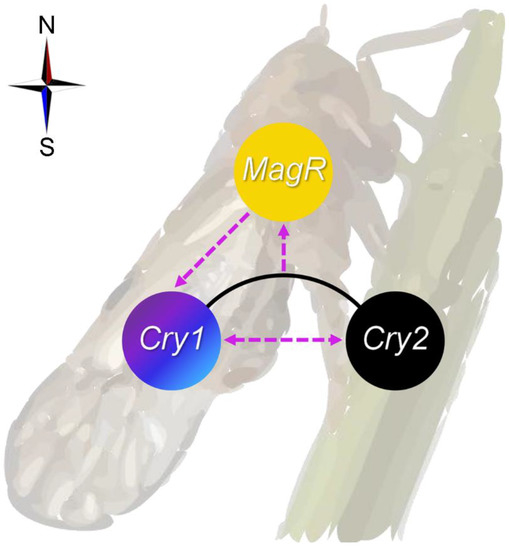
Figure 6.
Proposed transcriptional crosstalk between Cryptochromes (Cry1 and Cry2) and Iron-sulfur Cluster Assembly 1 (MagR) in magnetoresponse of the migratory brown planthopper, Nilaparvata lugens. The potential transcriptional interactions, whether activating or inhibiting, are indicated by dashed lines with an arrow. The compass represents that the crosstalk is proposed within the context of magnetoresponse.
4. Materials and Methods
4.1. Insects
The brown planthopper N. lugens were initially gathered from paddy fields located in Nanjing, Jiangsu province of China, in summer. They were kept indoors and fed on Taichung Native 1 rice seedlings, with a 14-h light and 10-h dark cycle, at a temperature of 26 °C and relative humidity between 70% and 80%. All subsequent experiments were conducted under these same environmental conditions, with the exception of the magnetic field variations. Before being assigned to the magnetic field experimental groups, the colony was maintained under the local geomagnetic field conditions (~50 μT).
4.2. Exposure of Insects to Magnetic Fields
In this study, we used two three-axis DC-type Helmholtz coil systems with an external diameter of 1200 mm to mimic the local geomagnetic field (50,000 ± 307 nT; i.e., GMF50μT) at Nanjing city (32°3′42″ N, 118°46′40″ E) vs. the near-zero magnetic field (NZMF) (519 ± 32 nT) and GMF50μT vs. the GMF intensity of another point on the migration route of N. lugens, Zhanjiang city (21°12′29″ N, 110°21′11″ E; mimic intensity: 45,000 ± 233 nT; i.e., GMF45μT), at a similar inclination and declination within the effective homogeneous areas of 300 × 300 × 300 mm (<2% heterogeneity), as described before [61,64]. A Faraday cage was placed inside each coil to shield the insects from potential anthropogenic electromagnetic noise. We measured and adjusted the magnetic field parameters daily using a fluxgate magnetometer (Model 191A, HONOR TOP Magnetoelectric Technology Co., Ltd., Qingdao, China) and ensured that both groups were in the same room with uniform environmental factors except for magnetic fields.
During the study, we exposed N. lugens raised separately in glass tubes (diameter: height = 3.0 cm: 15 cm) to two distinct treatments: GMF50μT vs. GMF45μT and GMF50μT vs. NZMF. This exposure began from mated F0 females up to unmated 1- to 3-day-old F1 adults, following the protocol outlined in our previous study [74], with the addition of a quick dsRNA injection. After sampling at the same time point under corresponding magnetic conditions, individuals were quickly euthanized in liquid nitrogen for total RNA isolation.
4.3. RNA Isolation and cDNA Synthesis
Four biologically independent pools were used, each containing five female adults for each group based on their developmental stage, dsRNA injection, and magnetic field intensity. Total RNA was extracted from these pooled samples using TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The extracted RNA samples were individually analyzed for quality and quantity using a NanoDrop2000 (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Prior to reverse transcription, the integrity of each total RNA sample was assessed by electrophoresis in 1% agarose gels. Subsequently, cDNA was synthesized from 500 ng of total RNA in a 20 μL reaction, utilizing the PrimeScript RT reagent kit supplemented with a gDNA Eraser (Takara Bio Inc., Dalian, China).
4.4. Double-Stranded RNA Preparation
The gene sequences of potential key magnetoreception genes Cry1, Cry2, and MagR in N. lugens were obtained from our previous studies [38,66]. To minimize the occurrence of off-target RNA interference (RNAi), the specific regions of the target genes selected for double-stranded RNA (dsRNA) synthesis were carefully verified to ensure that there were no other matches in the transcriptome and genome databases of N. lugens. A green fluorescent protein (GFP) gene (GenBank: MF169984.1), which is an exogenous gene to N. lugens, was utilized as the control (dsGFP) [75]. Their specific primers used to synthesize the dsRNA containing the T7 promoter sequence were designed using Oligo 7 software (Molecular Biology Insights, Inc., Cascade, CO, USA) (Table S1, underlined sequence indicates T7 promoter). The dsRNA was synthesized using the T7 RNAi Transcription Kit (Vazyme Biotech Co., Ltd., Nanjing, China) according to the manufacturer’s instructions. To purify the dsRNAs, 50 μL of 95% ethanol and 2 μL of 3 M sodium acetate (pH 5.2) were added, followed by washing with 70% ethanol. After drying, the dsRNA was resuspended in an appropriate amount of nuclease-free water. The concentration and quality of dsRNAs were determined by NanoDrop 2000 (Thermo, Waltham, MA, USA) and gel electrophoresis analysis (1%), respectively. All dsRNAs were then adjusted to a concentration of 4500 ng/μL and stored at −80 °C for future use.
4.5. Microinjection of dsRNA
Fourth-instar N. lugens nymphs were subjected to low-temperature anesthesia, and the immobilized nymphs were gently transferred onto an injection plate using a soft brush. Using a Micro4 microinjection apparatus, 40 nL of purified dsRNA with a concentration of 4500 ng/μL was slowly injected into the outer epidermis of the thoracic and hind legs of N. lugens. After injection, the nymphs were placed back and maintained separately in glass tubes.
4.6. Transcript Expression Analysis
The potential key genes in magnetoreception signaling including Cryptochrome 1 (Cry1), Cryptochrome 2 (Cry2) and Iron-sulfur Cluster Assembly 1 (i.e., MagR) were selected for transcript expression analysis using quantitative real-time polymerase chain reaction (qRT-PCR) assay. The qRT-PCR was conducted on an Applied Biosystems® QuantStudio™ 5 Flex Real-Time PCR System (Thermo Fisher Scientific, Inc., Waltham, MA, USA) using SYBR Premix Ex Taq (Tli RNaseH Plus; Takara Bio Inc., Dalian, China). The reactions were conducted in a final volume of 20 μL (including 2 μL of a 1/20 dilution of the cDNA template and primers in a final concentration of 200 nM) with the following conditions: an initial 30 s step of 95 °C followed by 40 denaturation cycles at 95 °C for 5 s and primer annealing at 60 °C for 34 s. The EF1-α and RPL5 were used as the reference genes [64], and the 2−∆∆Ct method (Ct, cycle threshold) was applied to evaluate the relative expression levels [76]. Four biological replicates were used for statistical comparison between groups.
4.7. Statistical Analysis
All data were analyzed using SPSS 26 (IBM Inc., Armonk, NY, USA). The Shapiro–Wilk test was used to test for normality (p > 0.05) and Levene’s test for the homogeneity of variances (p > 0.05), before an analysis of variance (ANOVA). The one-way ANOVA or a two-tailed nonparametric Mann–Whitney U test (if p ≤ 0.05 for normality or homogeneity of variances test) was used to test for the effect of magnetic field intensity or gene knockdown on the transcript relative expression levels of Cry1, Cry2 and MagR at α = 0.05.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms241311101/s1.
Author Contributions
Conceptualization, G.W., F.C. and W.P.; methodology, J.H., Y.Z. (Ying Zhang), J.Z. and Y.Z. (Yuning Zhang); validation, Y.Z. (Yuning Zhang), Y.Z. (Ying Zhang), J.Z. and J.H.; formal analysis, G.W., Y.Z. (Yuning Zhang), J.Z., Y.Z. (Ying Zhang), J.H. and Z.X.; writing—original draft preparation, G.W., Y.Z. (Yuning Zhang), Y.Z. (Ying Zhang) and J.Z.; writing—review and editing, G.A.S., G.W., W.P. and F.C.; supervision, G.W. and F.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (grant nos. 32172414 and 32072413), the Natural Science Foundation of Jiangsu Province (BK20221510), the National Key Research and Development Program of China (2021YFD1401100), and the Young Scientific and Technological Talents of Jiangsu Association for Science and Technology (TJ-2021-003).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Luying Zeng and Ming Zhang for their help in the preliminary experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Monteil, C.L.; Lefevre, C.T. Magnetoreception in microorganisms. Trends Microbiol. 2020, 28, 266–275. [Google Scholar] [CrossRef]
- Zhang, X. Biological Effects of Static Magnetic Fields, 2nd ed.; Springer: Singapore, 2023. [Google Scholar]
- Wang, C.X.; Hilburn, I.A.; Wu, D.A.; Mizuhara, Y.; Couste, C.P.; Abrahams, J.N.H.; Bernstein, S.E.; Matani, A.; Shimojo, S.; Kirschvink, J.L. Transduction of the geomagnetic field as evidenced from alpha-band activity in the human brain. eNeuro 2019, 6, ENEURO.0483-18.2019. [Google Scholar] [CrossRef] [PubMed]
- Mouritsen, H. Long-distance navigation and magnetoreception in migratory animals. Nature 2018, 558, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Wynn, J.; Padget, O.; Mouritsen, H.; Morford, J.; Jaggers, P.; Guilford, T. Magnetic stop signs signal a European songbird’s arrival at the breeding site after migration. Science 2022, 375, 446–449. [Google Scholar] [CrossRef] [PubMed]
- Wan, G.; Hayden, A.N.; Iiams, S.E.; Merlin, C. Cryptochrome 1 mediates light-dependent inclination magnetosensing in monarch butterflies. Nat. Commun. 2021, 12, 771. [Google Scholar] [CrossRef]
- Bradlaugh, A.A.; Fedele, G.; Munro, A.L.; Hansen, C.N.; Hares, J.M.; Patel, S.; Kyriacou, C.P.; Jones, A.R.; Rosato, E.; Baines, R.A. Essential elements of radical pair magnetosensitivity in Drosophila. Nature 2023, 615, 111–116. [Google Scholar] [CrossRef]
- Xu, J.; Jarocha, L.E.; Zollitsch, T.; Konowalczyk, M.; Henbest, K.B.; Richert, S.; Golesworthy, M.J.; Schmidt, J.; Déjean, V.; Sowood, D.J.C.; et al. Magnetic sensitivity of cryptochrome 4 from a migratory songbird. Nature 2021, 594, 535–540. [Google Scholar] [CrossRef]
- Binhi, V.N.; Rubin, A.B. Theoretical concepts in magnetobiology after 40 years of research. Cells 2022, 11, 274. [Google Scholar] [CrossRef] [PubMed]
- Clites, B.L.; Pierce, J.T. Identifying cellular and molecular mechanisms for magnetosensation. Annu. Rev. Neurosci. 2017, 40, 231–250. [Google Scholar] [CrossRef]
- Hore, P.J.; Mouritsen, H. The radical-pair mechanism of magnetoreception. Annu. Rev. Biophys. 2016, 45, 299–344. [Google Scholar] [CrossRef]
- Ritz, T.; Thalau, P.; Phillips, J.B.; Wiltschko, R.; Wiltschko, W. Resonance effects indicate a radical-pair mechanism for avian magnetic compass. Nature 2004, 429, 177–180. [Google Scholar] [CrossRef]
- Kirschvink, J.L.; Walker, M.M.; Diebel, C.E. Magnetite-based magnetoreception. Curr. Opin. Neurobiol. 2001, 11, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Ritz, T.; Adem, S.; Schulten, K. A model for photoreceptor-based magnetoreception in birds. Biophys. J. 2000, 78, 707–718. [Google Scholar] [CrossRef]
- Qin, S.; Yin, H.; Yang, C.; Dou, Y.; Liu, Z.; Zhang, P.; Yu, H.; Huang, Y.; Feng, J.; Hao, J.; et al. A magnetic protein biocompass. Nat. Mater. 2016, 15, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tong, T.; Wei, M.; Zhang, P.; Fei, F.; Zhou, X.; Guo, Z.; Zhang, J.; Xu, H.; Zhang, L.; et al. Towards magnetism in pigeon MagR: Iron- and iron-sulfur binding work indispensably and synergistically. Zool. Res. 2023, 44, 142–152. [Google Scholar] [CrossRef]
- Xie, C. Searching for unity in diversity of animal magnetoreception: From biology to quantum mechanics and back. Innovation 2022, 3, 100229. [Google Scholar] [CrossRef]
- Ozturk, N. Light-dependent reactions of animal circadian photoreceptor cryptochrome. FEBS J. 2022, 289, 6622–6639. [Google Scholar] [CrossRef] [PubMed]
- Emery, P.; Stanewsky, R.; Helfrich-Förster, C.; Emery-Le, M.; Hall, J.C.; Rosbash, M. Drosophila CRY is a deep brain circadian photoreceptor. Neuron 2000, 26, 493–504. [Google Scholar] [CrossRef]
- Zhu, H.; Yuan, Q.; Briscoe, A.D.; Froy, O.; Casselman, A.; Reppert, S.M. The two CRYs of the butterfly. Curr. Biol. 2005, 15, R953–R954. [Google Scholar] [CrossRef]
- Zoltowski, B.D.; Chelliah, Y.; Wickramaratne, A.; Jarocha, L.; Karki, N.; Xu, W.; Mouritsen, H.; Hore, P.J.; Hibbs, R.E.; Green, C.B.; et al. Chemical and structural analysis of a photoactive vertebrate cryptochrome from pigeon. Proc. Natl. Acad. Sci. USA 2019, 116, 19449–19457. [Google Scholar] [CrossRef]
- Yuan, Q.; Metterville, D.; Briscoe, A.D.; Reppert, S.M. Insect cryptochromes: Gene duplication and loss define diverse ways to construct insect circadian clocks. Mol. Biol. Evol. 2007, 24, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Markert, M.J.; Groves, S.C.; Hardin, P.E.; Merlin, C. Vertebrate-like CRYPTOCHROME 2 from monarch regulates circadian transcription via independent repression of CLOCK and BMAL1 activity. Proc. Natl. Acad. Sci. USA 2017, 114, E7516–E7525. [Google Scholar] [CrossRef]
- Netusil, R.; Tomanova, K.; Chodakova, L.; Chvalova, D.; Dolezel, D.; Ritz, T.; Vacha, M. Cryptochrome-dependent magnetoreception in a heteropteran insect continues even after 24 h in darkness. J. Exp. Biol. 2021, 224, jeb243000. [Google Scholar] [CrossRef]
- Gao, Y.; Wen, P.; Carde, R.T.; Xu, H.; Huang, Q. In addition to cryptochrome 2, magnetic particles with olfactory co-receptor are important for magnetic orientation in termites. Commun. Biol. 2021, 4, 1121. [Google Scholar] [CrossRef] [PubMed]
- Tong, D.; Zhang, L.; Wu, N.; Xie, D.; Fang, G.; Coates, B.S.; Sappington, T.W.; Liu, Y.; Cheng, Y.; Xia, J.; et al. The oriental armyworm genome yields insights into the long-distance migration of noctuid moths. Cell Rep. 2022, 41, 111843. [Google Scholar] [CrossRef]
- Merlin, C. Insect magnetoreception: A Cry for mechanistic insights. J. Comp. Physiol. A-Neuroethol. Sens. Neural Behav. Physiol. 2023. [Google Scholar] [CrossRef]
- Fontecave, M. Iron-sulfur clusters: Ever-expanding roles. Nat. Chem. Biol. 2006, 2, 171–174. [Google Scholar] [CrossRef]
- Johnson, D.C.; Dean, D.R.; Smith, A.D.; Johnson, M.K. Structure, function, and formation of biological iron-sulfur clusters. Annu. Rev. Biochem. 2005, 74, 247–281. [Google Scholar] [CrossRef]
- Kiley, P.J.; Beinert, H. The role of Fe-S proteins in sensing and regulation in bacteria. Curr. Opin. Microbiol. 2003, 6, 181–185. [Google Scholar] [CrossRef]
- Mettert, E.L.; Kiley, P.J. How is Fe-S cluster formation regulated? Annu. Rev. Microbiol. 2015, 69, 505–526. [Google Scholar] [CrossRef] [PubMed]
- Rouault, T.A. Mammalian iron-sulphur proteins: Novel insights into biogenesis and function. Nat. Rev. Mol. Cell Biol. 2015, 16, 45–55. [Google Scholar] [CrossRef]
- Ollagnier-de-Choudens, S.; Mattioli, T.; Takahashi, Y.; Fontecave, M. Iron-sulfur cluster assembly: Characterization of IscA and evidence for a specific and functional complex with ferredoxin. J. Biol. Chem. 2001, 276, 22604–22607. [Google Scholar] [CrossRef] [PubMed]
- Mandilaras, K.; Missirlis, F. Genes for iron metabolism influence circadian rhythms in Drosophila melanogaster. Metallomics 2012, 4, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Xu, S.; Chen, X.; Wang, C.; Yang, P.; Qin, S.; Zhao, C.; Fei, F.; Zhao, X.; Tan, P.H.; et al. Modulation of MagR magnetic properties via iron-sulfur cluster binding. Sci. Rep. 2021, 11, 23941. [Google Scholar] [CrossRef] [PubMed]
- Mannino, G.; Casacci, L.P.; Bianco Dolino, G.; Badolato, G.; Maffei, M.E.; Barbero, F. The geomagnetic field (GMF) is necessary for black garden ant (Lasius niger L.) foraging and modulates orientation potentially through aminergic regulation and MagR expression. Int. J. Mol. Sci. 2023, 24, 4387. [Google Scholar] [CrossRef]
- Yang, P.; Cai, T.; Zhang, L.; Yu, D.; Guo, Z.; Zhang, Y.; Li, G.; Zhang, X.; Xie, C. A rationally designed building block of the putative magnetoreceptor MagR. Bioelectromagnetics 2022, 43, 317–326. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Wu, J.; Wang, W.; Li, Y.; Wan, G.; Chen, F.; Sword, G.A.; Pan, W. Molecular characterization, spatial-temporal expression and magnetic response patterns of iron-sulfur cluster assembly1 (IscA1) in the rice planthopper, Nilaparvata lugens. Insect Sci. 2019, 26, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zeng, L.; Wan, G.; Jiang, X.; Chen, F.; Pan, W. Interaction between cryptochrome and iron-sulfur cluster assembly 1 protein and their roles in the development and reproduction of the brown planthopper, Nilaparvata lugens. Chin. J. Appl. Entomol. 2021, 58, 74–82. [Google Scholar]
- Zhang, Y.; Pan, W. Removal or component reversal of local geomagnetic field affects foraging orientation preference in migratory insect brown planthopper Nilaparvata lugens. PeerJ 2021, 9, e12351. [Google Scholar] [CrossRef]
- Parmagnani, A.S.; D’Alessandro, S.; Maffei, M.E. Iron-sulfur complex assembly: Potential players of magnetic induction in plants. Plant Sci. 2022, 325, 111483. [Google Scholar] [CrossRef]
- Cao, Y.; Yan, P. Role of atomic spin-mechanical coupling in the problem of a magnetic biocompass. Phys. Rev. E 2018, 97, 042409. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Hu, W.; Cai, Y.; Zhao, N. Magnetic noise enabled biocompass. Phys. Rev. Lett. 2020, 124, 128101. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, H.; Lu, L.; Li, Y. A mechanism of compass-free migratory navigation. J. Phys. D Appl. Phys. 2022, 55, 245004. [Google Scholar] [CrossRef]
- Parmagnani, A.S.; Betterle, N.; Mannino, G.; D’Alessandro, S.; Nocito, F.F.; Ljumovic, K.; Vigani, G.; Ballottari, M.; Maffei, M.E. The geomagnetic field (GMF) is required for lima bean photosynthesis and reactive oxygen species production. Int. J. Mol. Sci. 2023, 24, 2896. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Sun, J.; Ge, Y.; Xue, L.; Mao, H.; Zhou, L.; Zhao, J. Bionic magnetic sensor based on the MagR/Cry4 Complex-Configured graphene transistor with an integrated on-chip gate. ACS Sens. 2023, 8, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Hu, T.; Guo, Z.; Yang, C.; Wang, Z.; Qin, S.; Yang, P.; Xie, C.; Xu, J.; Li, N.; et al. A novel biomimetic magnetosensor based on magneto-optically involved conformational variation of MagR/Cry4 Complex. Adv. Electron. Mater. 2020, 6, 1901168. [Google Scholar] [CrossRef]
- Chang, H.; Guo, J.; Fu, X.; Hou, Y.; Wu, K. Orientation behavior and regulatory gene expression profiles in migratory Agrotis ipsilon (Lepidoptera: Noctuidae). J. Insect Behav. 2019, 32, 59–67. [Google Scholar] [CrossRef]
- Jin, M.; Liu, B.; Zheng, W.; Liu, C.; Liu, Z.; He, Y.; Li, X.; Wu, C.; Wang, P.; Liu, K.; et al. Chromosome-level genome of black cutworm provides novel insights into polyphagy and seasonal migration in insects. BMC Biol. 2023, 21, 2. [Google Scholar] [CrossRef]
- Wu, H.; Scholten, A.; Einwich, A.; Mouritsen, H.; Koch, K.W. Protein-protein interaction of the putative magnetoreceptor cryptochrome 4 expressed in the avian retina. Sci. Rep. 2020, 10, 7364. [Google Scholar] [CrossRef]
- Xiong, X.; Liu, Y.; Zhang, J.; Wang, S.; Li, L.; Gao, M. Mutational analysis of MpPhy reveals magnetoreception and photosensitivity involvement in secondary metabolites biosynthesis in Monascus purpureus. J. Photochem. Photobiol. B-Biol. 2021, 217, 112164. [Google Scholar] [CrossRef]
- Chang, H.; Guo, J.; Fu, X.; Shen, X.; Hou, Y.; Wu, K. Molecular characterization and expression profiles of IscA1 gene in a long-distance migrant, Agrotis segetum. J. Asia Pac. Econ. 2018, 21, 1299–1306. [Google Scholar] [CrossRef]
- Gegear, R.J.; Casselman, A.; Waddell, S.; Reppert, S.M. Cryptochrome mediates light-dependent magnetosensitivity in Drosophila. Nature 2008, 454, 1014–1018. [Google Scholar] [CrossRef]
- Gegear, R.J.; Foley, L.E.; Casselman, A.; Reppert, S.M. Animal cryptochromes mediate magnetoreception by an unconventional photochemical mechanism. Nature 2010, 463, 804–807. [Google Scholar] [CrossRef]
- Oh, I.T.; Kwon, H.J.; Kim, S.C.; Kim, H.J.; Lohmann, K.J.; Chae, K.S. Behavioral evidence for geomagnetic imprinting and transgenerational inheritance in fruit flies. Proc. Natl. Acad. Sci. USA 2019, 117, 1216–1222. [Google Scholar] [CrossRef] [PubMed]
- Fedele, G.; Green, E.W.; Rosato, E.; Kyriacou, C.P. An electromagnetic field disrupts negative geotaxis in Drosophila via a CRY-dependent pathway. Nat. Commun. 2014, 5, 4391. [Google Scholar] [CrossRef]
- Fedele, G.; Edwards, M.D.; Bhutani, S.; Hares, J.M.; Murbach, M.; Green, E.W.; Dissel, S.; Hastings, M.H.; Rosato, E.; Kyriacou, C.P. Genetic analysis of circadian responses to low frequency electromagnetic fields in Drosophila melanogaster. PLoS Genet. 2014, 10, e1004804. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, T.; Ahmad, M.; Helfrich-Forster, C. Cryptochrome mediates light-dependent magnetosensitivity of Drosophila’s circadian clock. PLoS Biol. 2009, 7, e1000086. [Google Scholar] [CrossRef]
- Menz, M.H.M.; Reynolds, D.R.; Gao, B.; Hu, G.; Chapman, J.W.; Wotton, K.R. Mechanisms and consequences of partial migration in insects. Front. Ecol. Evol. 2019, 7, 403. [Google Scholar] [CrossRef]
- Wan, G.; Jiang, S.; Zhao, Z.; Xu, J.; Tao, X.; Sword, G.A.; Gao, Y.; Pan, W.; Chen, F. Bio-effects of near-zero magnetic fields on the growth, development and reproduction of small brown planthopper, Laodelphax striatellus and brown planthopper, Nilaparvata lugens. J. Insect Physiol. 2014, 68, 7–15. [Google Scholar] [CrossRef]
- Wan, G.; Liu, R.; Li, C.; He, J.; Pan, W.; Sword, G.A.; Hu, G.; Chen, F. Change in geomagnetic field intensity alters migration-associated traits in a migratory insect. Biol. Lett. 2020, 16, 20190940. [Google Scholar] [CrossRef]
- Wan, G.; Jiang, S.; Zhang, M.; Zhao, J.; Zhang, Y.; Pan, W.; Sword, G.A.; Chen, F. Geomagnetic field absence reduces adult body weight of a migratory insect by disrupting feeding behavior and appetite regulation. Insect Sci. 2020, 28, 251–260. [Google Scholar] [CrossRef]
- Zhang, Y.; Wan, G.; Wang, W.; Li, Y.; Yu, Y.; Zhang, Y.; Chen, F.; Pan, W. Enhancement of the geomagnetic field reduces the phototaxis of rice brown planthopper Nilaparvata lugens associated with frataxin down-regulation. Insect Sci. 2020, 27, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zeng, L.; Wei, Y.; Zhang, M.; Pan, W.; Sword, G.A.; Yang, F.; Chen, F.; Wan, G. Reliable reference genes for gene expression analyses under the hypomagnetic field in a migratory insect. Front. Physiol. 2022, 13, 954228. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Wan, G.; Xu, J.; Li, X.; Liu, Y.; Qi, L.; Chen, F. Evidence for the presence of biogenic magnetic particles in the nocturnal migratory brown planthopper, Nilaparvata lugens. Sci. Rep. 2016, 6, 18771. [Google Scholar] [CrossRef]
- Xu, J.; Wan, G.; Hu, D.; He, J.; Chen, F.; Wang, X.; Hua, H.; Pan, W. Molecular characterization, tissue and developmental expression profiles of cryptochrome genes in wing dimorphic brown planthoppers, Nilaparvata lugens. Insect Sci. 2016, 23, 805–818. [Google Scholar] [CrossRef] [PubMed]
- Bazalova, O.; Kvicalova, M.; Valkova, T.; Slaby, P.; Bartos, P.; Netusil, R.; Tomanova, K.; Braeunig, P.; Lee, H.J.; Sauman, I.; et al. Cryptochrome 2 mediates directional magnetoreception in cockroaches. Proc. Natl. Acad. Sci. USA 2016, 113, 1660–1665. [Google Scholar] [CrossRef]
- Cheng, X.; WU, J.; Ma, F. Brown Planthopper: Occurrence and Control; China Agricultural Press: Beijing, China, 2003. [Google Scholar]
- Zheng, D.; Hu, G.; Yang, F.; Du, X.; Yang, H.; Zhang, G.; Qi, G.; Liang, Z.; Zhang, X.; Cheng, X.; et al. Ovarian development status and population characteristics of Sogatella furcifera (Horváth) and Nilaparvata lugens (Stål): Implications for pest forecasting. J. Appl. Entomol. 2014, 138, 67–77. [Google Scholar] [CrossRef]
- Wiltschko, R.; Niessner, C.; Wiltschko, W. The magnetic compass of birds: The role of cryptochrome. Front. Physiol. 2021, 12, 667000. [Google Scholar] [CrossRef]
- Merlin, C.; Iiams, S.E.; Lugena, A.B. Monarch butterfly migration moving into the genetic era. Trends Genet. 2020, 36, 689–701. [Google Scholar] [CrossRef]
- Filiba, O.; Borin, V.A.; Schapiro, I. The involvement of triplet states in the isomerization of retinaloids. Phys. Chem. Chem. Phys. 2022, 24, 26223–26231. [Google Scholar] [CrossRef]
- Bartos, P.; Netusil, R.; Slaby, P.; Dolezel, D.; Ritz, T.; Vacha, M. Weak radiofrequency fields affect the insect circadian clock. J. R. Soc. Interface 2019, 16, 20190285. [Google Scholar] [CrossRef] [PubMed]
- Wan, G.; Yuan, R.; Wang, W.; Fu, K.; Zhao, J.; Jiang, S.; Pan, W.; Sword, G.A.; Chen, F. Reduced geomagnetic field may affect positive phototaxis and flight capacity of a migratory rice planthopper. Anim. Behav. 2016, 121, 107–116. [Google Scholar] [CrossRef]
- Chen, T.; Jiao, Q.; Ye, C.; Wu, J.; Zheng, Y.; Sun, C.; Hao, P.; Yu, X. A novel cuticular protein-like Cpr21L is essential for nymph survival and male fecundity in the brown planthopper. Int. J. Mol. Sci. 2023, 24, 2163. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).