Molecular Biological Effects of Weak Low-Frequency Magnetic Fields: Frequency–Amplitude Efficiency Windows and Possible Mechanisms
Abstract
1. Introduction
2. Resonance-like Biological Responses to LFMF
3. Early Biophysical Models of Resonance-like Biological Effects of LFMF
3.1. Ions as Primary Targets
3.2. Magnetic Moments as Primary Targets
3.3. Some Inconsistencies in Biophysical Models
4. Radical Pair Magnetoreception and Its Application to the Effects of Low-Frequency Magnetic Fields
5. Future Prospects
5.1. Incomprehension of the Molecular Pathways Forming Magnetobiological Effects
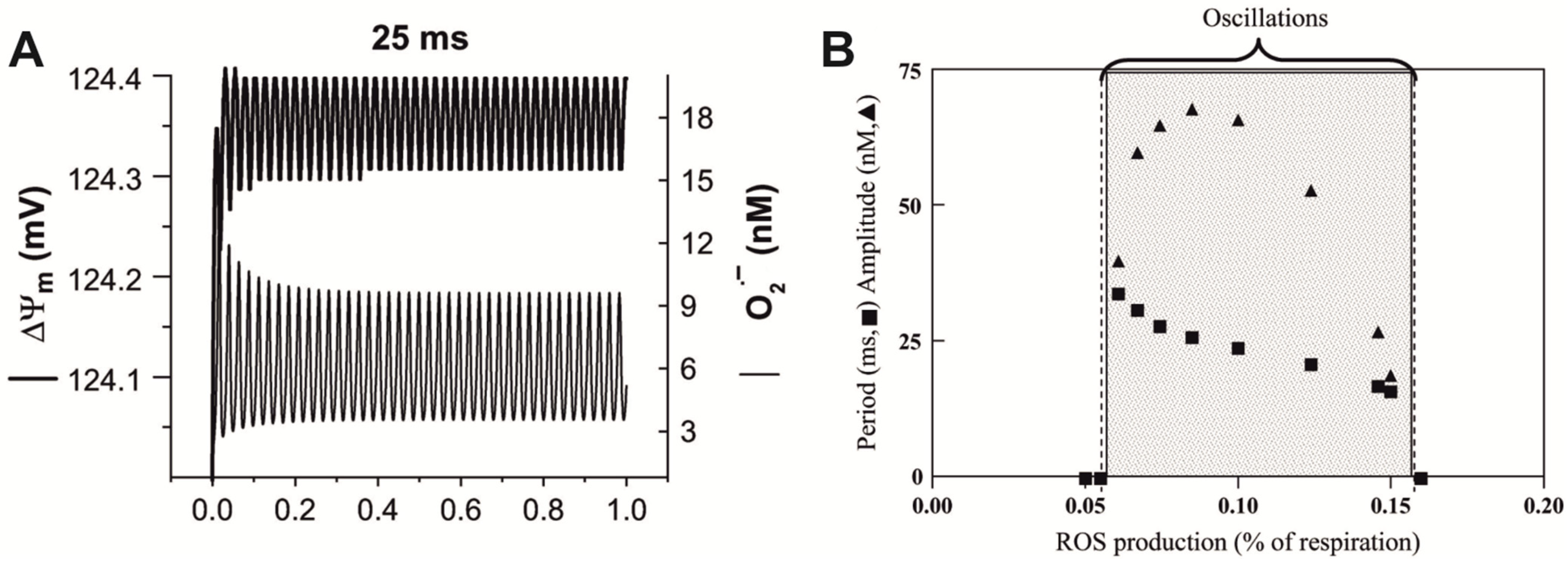
5.2. Oscillating Biochemical Processes as a Reason for Resonance-like Responses of Biological Systems to LFMF
- Radical pairs are the main target for LFMF’s influence on organisms. If radical pairs emerge in biochemical processes that oscillate in cells with the frequency f OSC, and this emerging occurs in a specific phase of the oscillations, then the manifestation of biological effects can depend on the frequency of LFMF.
- The altered or “signal” state of a given oscillating process depends on the ratio of the singlet and triplet yields of the radical-pair reaction included in it.
- If an external LFMF with frequency f = f OSC and amplitude BAC parallel to the static (geomagnetic) field (BDC) is applied to such an oscillating chemical process, then due to the negligible lifetime of radical pairs and depending on phase coincidence, some radical pairs of the biochemical oscillators will be located at the resulting magnetic field BDC + BAC throughout the whole LFMF exposure. The same part of the oscillators will generate radical pairs exposed to BDC − BAC throughout the whole LFMF exposure. Most of the radical pairs will be under a “quasistatic” magnetic field with the intensity from BDC − BAC to BDC + BAC throughout the exposure.
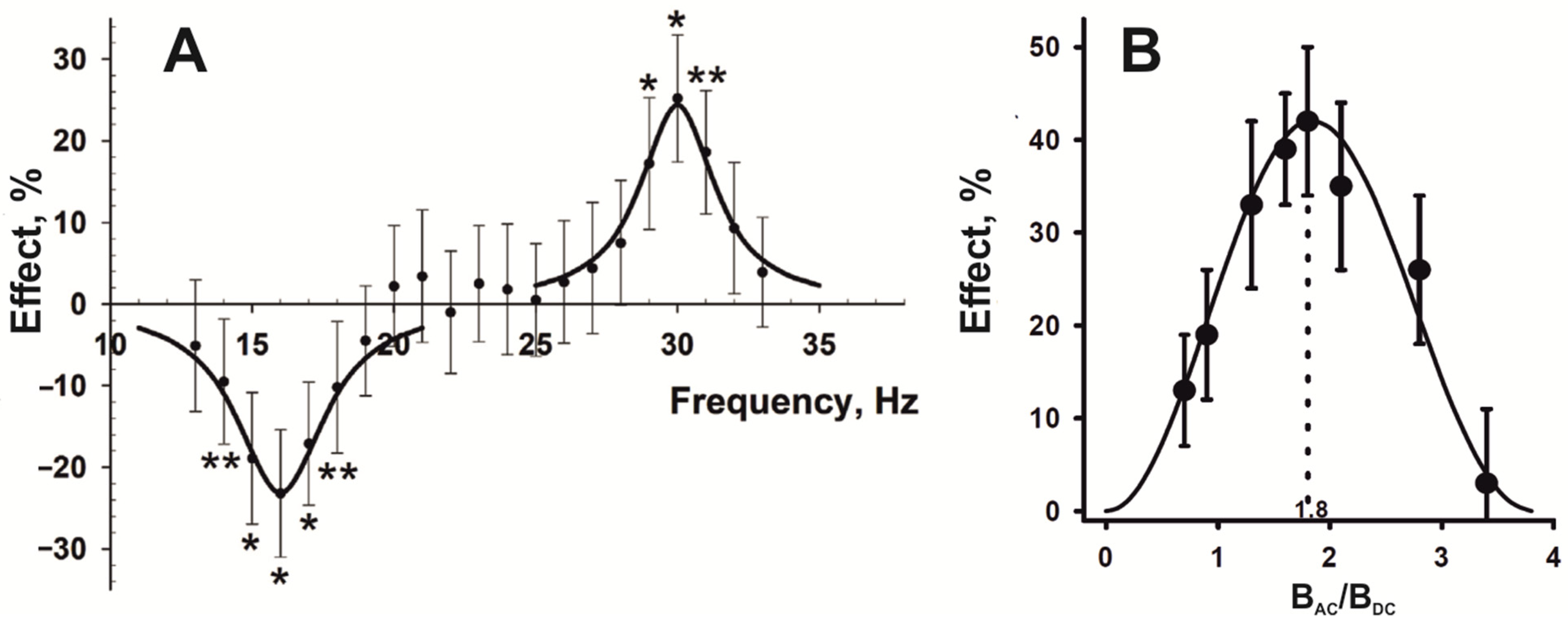
- According to Hore [83], changes in the ratio of singlet and triplet yields of a biradical reaction in response to LFMF occur if there is non-linear dependence between the singlet–triplet reaction yields and the magnetic field strength within limits from BDC − BAC to BDC + BAC (Figure 2B). Synchronization of LFMF frequency with the frequency of chemical oscillations provides a quasistatic “effective” magnetic field for radical pairs in a portion of chemical oscillators. The ratio of triplet and singlet yields for this portion of oscillators will differ from the state for the rest of the oscillators throughout the whole LFMF exposure due to the non-linear dependence between the triplet and singlet yields and magnetic field intensity as the “low field effect”. A notable change in LFMF frequency (f ≠ f OSC) leads to a condition where radical pairs regularly generated by a chemical oscillator will experience quasistatic magnetic fields of different intensities at different moments. The disappearance of the biological effect at a changed non-resonant LFMF frequency can be a consequence of the inability to maintain a “signal” state of the portion of the biochemical oscillators throughout the LFMF exposure. It ensures the appearance of frequency windows of magnetobiological effects.
- The “low field effect” [89] provides “non-linear dependence” conditions; therefore, the biologically effective amplitude of the LFMF exists for a specific radical-pair reaction. A change in this amplitude can shift the magnetic field intensity values to the area of linear dependence, which leads to the absence of a biological effect (Figure 2A). It explains the amplitude windows of the LFMF efficiency.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akasofu, S.I.; Chapman, S. Solar-Terrestrial Physics; Clarendon Press: Oxford, UK, 1972. [Google Scholar]
- Sarimov, R.; Binhi, V. Low-frequency magnetic fields in cars and office premises and the geomagnetic field variations. Bioelectromagnetics 2020, 41, 360–368. [Google Scholar] [CrossRef]
- Binhi, V.N.; Rubin, A.B. Magnetobiology: The kT paradox and possible solutions. Electromagn. Biol. Med. 2007, 26, 45–62. [Google Scholar] [CrossRef]
- Binhi, V.N. Magnetobiology: Underlying Physical Problems; Academic Press: London, UK, 2002. [Google Scholar]
- Ermakov, A.; Afanasyeva, V.; Ermakova, O.; Blagodatski, A.; Popov, A. Effect of weak alternating magnetic fields on planarian regeneration. Biochem. Biophys. Res. Commun. 2022, 592, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Belova, N.A.; Lednev, V.V. Dependence of gravitotropic reaction in segments of flax stems on frequency and amplitude of variable components of a weak combined magnetic field. Biofizika 2000, 45, 1108–1111. [Google Scholar] [PubMed]
- Blackman, C.F.; Benane, S.G.; Rabinowitz, J.R.; House, D.E.; Joines, W.T. A role for the magnetic field in the radiation-induced efflux of calcium ions from brain tissue in vitro. Bioelectromagnetics 1985, 6, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.R.; Schrot, J.; Liboff, A.R. Low-intensity magnetic fields alter operant behavior in rats. Bioelectromagnetics 1986, 7, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Liboff, A.R.; Rozek, R.J.; Sherman, M.L.; McLeod, B.R.; Smith, S.D. Ca2+-45 cyclotron resonance in human lymphocytes. J. Bioelectr. 1987, 6, 13–22. [Google Scholar]
- Smith, S.D.; McLeod, B.R.; Liboff, A.R.; Cooksey, K. Calcium cyclotron resonance and diatom mobility. Bioelectromagnetics 1987, 8, 215–227. [Google Scholar] [CrossRef]
- Lednev, V.V.; Srebnitskaya, L.K.; Il’Yasova, Y.N.; Rozhdestvenskaya, S.Y.; Klimov, A.A.; Belova, N.A.; Tiras, K.P. Magnetic parametric resonance in biosystems: Experimental verification of the theoretical predictions with the use of regenerating planarians Dugesia tigrina as a test-system. Biophysics 1996, 41, 815–825. [Google Scholar]
- Prato, F.S.; Carson, J.J.L.; Ossenkopp, K.P.; Kavaliers, M. Possible mechanisms by which extremely low frequency magnetic fields affect opioid function. FASEB J. 1995, 9, 807–814. [Google Scholar] [CrossRef]
- Blackman, C.F.; Blanchard, J.P.; Benane, S.G.; House, D.E. Empirical test of an ion parametric resonance model for magnetic field interactions with PC-12 cells. Bioelectromagnetics 1994, 15, 239–260. [Google Scholar] [CrossRef] [PubMed]
- Baureus Koch, C.L.M.; Sommarin, M.; Persson, B.R.R.; Salford, L.G.; Eberhardt, J.L. Interaction between weak low frequency magnetic fields and cell membranes. Bioelectromagnetics 2003, 24, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Sarimov, R.; Markova, E.; Johansson, F.; Jenssen, D.; Belyaev, I. Exposure to ELF magnetic field tuned to Zn inhibit growth of cancer cells. Bioelectromagnetics 2005, 26, 631–638. [Google Scholar] [CrossRef]
- Belova, N.A.; Potselueva, M.M.; Srebnitskaya, L.K.; Znobishcheva, A.V.; Lednev, V.V. The influence of weak magnetic fields on the production of the reactive oxygen species in peritoneal neutrophils of mice. Biophysics 2010, 55, 586–591. [Google Scholar] [CrossRef]
- Prato, F.S.; Kavaliers, M.; Thomas, A.W. Extremely low frequency magnetic fields can either increase or decrease analgaesia in the land snail depending on field and light conditions. Bioelectromagnetics 2000, 21, 287–301. [Google Scholar] [CrossRef]
- Blackman, C.F.; Blanchard, J.P.; Benane, S.G.; House, D.E. The ion parametric resonance model predicts magnetic field parameters that affect nerve cells. FASEB J. 1995, 9, 547–551. [Google Scholar] [CrossRef]
- Trillo, M.A.; Ubeda, A.; Blanchard, J.P.; House, D.E.; Blackman, C.F. Magnetic fields at resonant conditions for the hydrogen ion affect neurite outgrowth in PC-12 cells: A test of the ion parametric resonance model. Bioelectromagnetics 1996, 17, 10–20. [Google Scholar] [CrossRef]
- Belova, N.A.; Ermakov, A.M.; Znobishcheva, A.V.; Srebnitskaya, L.K.; Lednev, V.V. The influence of extremely weak alternating magnetic fields on the regeneration of planarians and the gravitropic response of plants. Biophysics 2010, 55, 623–627. [Google Scholar] [CrossRef]
- Belova, N.A.; Ermakova, O.N.; Ermakov, A.M.; Rojdestvenskaya, Z.Y.; Lednev, V.V. The bioeffects of extremely weak power-frequency alternating magnetic fields. Environmentalist 2007, 27, 411–416. [Google Scholar] [CrossRef]
- Lednev, V.V.; Belova, N.A.; Ermakov, A.M.; Akimov, E.B.; Tonevitsky, A.G. Modulation of cardiac rhythm in the humans exposed to extremely weak alternating magnetic fields. Biophysics 2008, 53, 648–654. [Google Scholar] [CrossRef]
- McLeod, B.R.; Smith, S.D.; Liboff, A.R. Calcium and potassium cyclotron resonance curves and harmonics in diatoms (A. coffeaeformis). J. Bioelectr. 1987, 6, 153–168. [Google Scholar] [CrossRef]
- Liboff, A.R. Geomagnetic cyclotron resonance in living cells. J. Biol. Phys. 1985, 13, 99–102. [Google Scholar] [CrossRef]
- Sandweiss, J. On the cyclotron resonance model of ion transport. Bioelectromagnetics 1990, 11, 203–205. [Google Scholar] [CrossRef] [PubMed]
- Halle, B. On the cyclotron resonance mechanism for magnetic field effects on transmembrane ion conductivity. Bioelectromagnetics 1988, 9, 381–385. [Google Scholar] [CrossRef]
- Kantserova, N.P.; Ushakova, N.V.; Krylov, V.V.; Lysenko, L.A.; Nemova, N.N. Modulation of Ca2+-dependent protease activity in fish and invertebrates by weak low-frequency magnetic fields. Russ. J. Bioorg. Chem. 2013, 39, 373–377. [Google Scholar] [CrossRef]
- Kuz’mina, V.V.; Ushakova, N.V.; Krylov, V.V. The effect of magnetic fields on the activity of proteinases and glycosidases in the intestine of the crucian carp Carassius carassius. Biol. Bull. 2015, 42, 61–66. [Google Scholar] [CrossRef]
- Tiras, H.P.; Petrova, O.N.; Myakisheva, S.N.; Popova, S.S.; Aslanidi, K.B. Effects of weak magnetic fields on different phases of planarian regeneration. Biophysics 2015, 60, 126–130. [Google Scholar] [CrossRef]
- Khokhlova, G.; Abashina, T.; Belova, N.; Panchelyuga, V.; Petrov, A.; Abreu, F.; Vainshtein, M. Effects of combined magnetic fields on bacteria Rhodospirillum rubrum VKM B-1621. Bioelectromagnetics 2018, 39, 485–490. [Google Scholar] [CrossRef]
- Krylov, V.V.; Papchenkova, G.A.; Golovanova, I.L. Influence of calcium resonance-tuned low-frequency magnetic fields on Daphnia magna. Int. J. Mol. Sci. 2022, 23, 15727. [Google Scholar] [CrossRef]
- Blanchard, J.P.; Blackman, C.F. Clarification and application of an ion parametric resonance model for magnetic field interactions with biological systems. Bioelectromagnetics 1994, 15, 217–238. [Google Scholar] [CrossRef]
- Lednev, V.V. Possible mechanism for the influence of weak magnetic fields on biological systems. Bioelectromagnetics 1991, 12, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Lednev, V.V. Bioeffects of weak static and alternating magnetic fields. Biofizika 1996, 41, 224–232. [Google Scholar] [PubMed]
- Helekar, S.A.; Hambarde, S.; Ijare, O.B.; Pichumani, K.; Baskin, D.S.; Sharpe, M.A. Selective induction of rapid cytotoxic effect in glioblastoma cells by oscillating magnetic fields. J. Cancer Res. Clin. Oncol. 2021, 147, 3577–3589. [Google Scholar] [CrossRef] [PubMed]
- Adair, R.K. Static and low-frequency magnetic field effects: Health risks and therapies. Rep. Prog. Phys. 2000, 63, 415–454. [Google Scholar] [CrossRef]
- Binhi, V.N.; Prato, F.S. A physical mechanism of magnetoreception: Extension and analysis. Bioelectromagnetics 2017, 38, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Ponomarev, V.O.; Novikov, V.V. Effect of low-frequency alternating magnetic fields on the rate of biochemical reactions proceeding with formation of reactive oxygen species. Biophysics 2009, 54, 163–168. [Google Scholar] [CrossRef]
- Lednev, V.V. Biological Effects of the Extremely Weak Alternating Magnetic Fields: The Identification of Primary Targets. In Modelling of Geophysical Processes; Sidorin, A., Ed.; Schmidt Institute of the Physics of the Earth: Moscow, Russia, 2003; pp. 130–136. [Google Scholar]
- Belova, N.A.; Panchelyuga, V.A. Lednev’s model: Theory and experiment. Biophysics 2010, 55, 661–674. [Google Scholar] [CrossRef]
- Binhi, V.N. Primary physical mechanism of the biological effects of weak magnetic fields. Biophysics 2016, 61, 170–176. [Google Scholar] [CrossRef]
- Binhi, V.N. Do naturally occurring magnetic nanoparticles in the human body mediate increased risk of childhood leukaemia with EMF exposure? Int. J. Radiat. Biol. 2008, 84, 569–579. [Google Scholar] [CrossRef]
- Binhi, V.N.; Savin, A.V. Molecular gyroscopes and biological effects of weak extremely low-frequency magnetic fields. Phys. Rev. E Stat. Nonlin. Soft. Matter. Phys. 2002, 65, 051912. [Google Scholar] [CrossRef]
- Haiech, J.; Klee, C.B.; Demaille, J.G. Effects of cations on affinity of calmodulin for calcium: Ordered binding of calcium ions allows the specific activation of calmodulin-stimulated enzymes. Biochemistry 1981, 20, 3890–3897. [Google Scholar] [CrossRef]
- Blackman, C.F.; Benane, S.G.; House, D.E.; Elliott, D.J. Importance of alignment between local DC magnetic field and an oscillating magnetic field in responses of brain tissue in vitro and in vivo. Bioelectromagnetics 1990, 11, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Blackman, C.F.; Blanchard, J.P.; Benane, S.G.; House, D.E. Effect of ac and dc magnetic field orientation on nerve cells. Biochem. Biophys. Res. Commun. 1996, 220, 807–811. [Google Scholar] [CrossRef] [PubMed]
- García-Sancho, J.; Montero, M.; Alvarez, J.; Fonteriz, R.I.; Sanchez, A. Effects of extremely-law-frequency electromagnetic fields on ion transport in several mammalian cells. Bioelectromagnetics 1994, 15, 579–588. [Google Scholar] [CrossRef]
- Picazo, M.L.; Vallejo, D.; Bardasano, J.L. An Introduction to the study of ELF magnetic field effects on white blood cells in mice. Electro-Magnetobiol. 1994, 13, 77–84. [Google Scholar] [CrossRef]
- Misakian, M.; Sheppard, A.R.; Krause, D.; Frazier, M.E.; Miller, D.L. Biological, physical, and electrical parameters for in-vitro studies with ELF magnetic and electric fields—A primer. Bioelectromagnetics 1993, 14, 1–73. [Google Scholar] [CrossRef]
- Valberg, P.A. Designing EMF experiments: What is requiredto characterize “exposure”? Bioelectromagnetics 1995, 16, 396–401. [Google Scholar] [CrossRef]
- Makinistian, L.; Muehsam, D.J.; Bersani, F.; Belyaev, I. Some recommendations for experimental work in magnetobiology, revisited. Bioelectromagnetics 2018, 39, 556–564. [Google Scholar] [CrossRef]
- Wiltschko, W.; Wiltschko, R. Disorientation of inexperienced young pigeons after transportation in total darkness. Nature 1981, 291, 433–434. [Google Scholar] [CrossRef]
- Wiltschko, W.; Wiltschko, R. Magnetic compass orientation in birds and its physiological basis. Naturwissenschaften 2002, 89, 445–452. [Google Scholar] [CrossRef]
- Wiltschko, W.; Wiltschko, R. The effect of yellow and blue light on magnetic compass orientation in European robins Erithacus rubecula. J. Comp. Physiol. A 1999, 184, 295–299. [Google Scholar] [CrossRef]
- Muheim, R.; Backman, J.; Akesson, S. Magnetic compass orientation in European robins is dependent on both wavelength and intensity of light. J. Exp. Biol. 2002, 205, 3845–3856. [Google Scholar] [CrossRef] [PubMed]
- Ritz, T.; Adem, S.; Schulten, K. A model for photoreceptor-based magnetoreception in birds. Biophys. J. 2000, 78, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Buchachenko, A.L.; Molin, Y.N.; Sagdeev, R.Z.; Salikhov, K.M.; Frankevich, E.L. Magneto-spin effects in chemical reactions. Sov. Phys. Usp. 1987, 30, 79–80. [Google Scholar] [CrossRef]
- Cashmore, A.; Jarillo, J.; Wu, Y.-J.; Liu, D. Cryptochromes: Blue light receptors for plants and animals. Science 1999, 284, 760–765. [Google Scholar] [CrossRef]
- Brautigam, C.A.; Smith, B.S.; Ma, Z.; Palnitkar, M.; Tomchick, D.R.; Machius, M.; Deisenhofer, J. Structure of the photolyase-like domain of cryptochrome 1 from Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2004, 101, 12142–12147. [Google Scholar] [CrossRef]
- Sancar, A. Structure and function of DNA photolyase and cryptochrome blue-light photoreceptors. Chem. Rev. 2003, 103, 2203–2237. [Google Scholar] [CrossRef]
- Maeda, K.; Robinson, A.J.; Henbest, K.B.; Hogben, H.J.; Biskup, T.; Ahmad, M.; Schleicher, E.; Weber, S.; Timmel, C.R.; Hore, P.J. Magnetically sensitive light-induced reactions in cryptochrome are consistent with its proposed role as a magnetoreceptor. Proc. Natl. Acad. Sci. USA 2012, 109, 4774–4779. [Google Scholar] [CrossRef]
- Hore, P.J.; Mouritsen, H. The radical-pair mechanism of magnetoreception. Annu. Rev. Biophys. 2016, 45, 299–344. [Google Scholar] [CrossRef]
- Solov’yov, I.A.; Chandler, D.E.; Schulten, K. Magnetic field effects in Arabidopsis thaliana cryptochrome-1. Biophys. J. 2007, 92, 2711–2726. [Google Scholar] [CrossRef]
- Ahmad, M.; Galland, P.; Ritz, T.; Wiltschko, R.; Wiltschko, W. Magnetic intensity affects cryptochrome-dependent responses in Arabidopsis thaliana. Planta 2006, 225, 615–624. [Google Scholar] [CrossRef]
- Sheppard, D.M.; Li, J.; Henbest, K.B.; Neil, S.R.; Maeda, K.; Storey, J.; Schleicher, E.; Biskup, T.; Rodriguez, R.; Weber, S.; et al. Millitesla magnetic field effects on the photocycle of an animal cryptochrome. Sci. Rep. 2017, 7, 42228. [Google Scholar] [CrossRef] [PubMed]
- Bradlaugh, A.A.; Fedele, G.; Munro, A.L.; Hansen, C.N.; Hares, J.M.; Patel, S.; Kyriacou, C.P.; Jones, A.R.; Rosato, E.; Baines, R.A. Essential elements of radical pair magnetosensitivity in Drosophila. Nature 2023, 615, 111–116. [Google Scholar] [CrossRef]
- Xu, J.; Jarocha, L.E.; Zollitsch, T.; Konowalczyk, M.; Henbest, K.B.; Richert, S.; Golesworthy, M.J.; Schmidt, J.; Dejean, V.; Sowood, D.J.C.; et al. Magnetic sensitivity of cryptochrome 4 from a migratory songbird. Nature 2021, 594, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Marley, R.; Giachello, C.N.G.; Scrutton, N.S.; Baines, R.A.; Jones, A.R. Cryptochrome-dependent magnetic field effect on seizure response in Drosophila larvae. Sci. Rep. 2014, 4, 5799. [Google Scholar] [CrossRef] [PubMed]
- Pooam, M.; Arthaut, L.D.; Burdick, D.; Link, J.; Martino, C.F.; Ahmad, M. Magnetic sensitivity mediated by the arabidopsis blue-light receptor cryptochrome occurs during flavin reoxidation in the dark. Planta 2018, 249, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Hammad, M.; Albaqami, M.; Pooam, M.; Kernevez, E.; Witczak, J.; Ritz, T.; Martino, C.; Ahmad, M. Cryptochrome mediated magnetic sensitivity in arabidopsis occurs independently of light-induced electron transfer to the flavin. Photochem. Photobiol. Sci. 2020, 19, 341–352. [Google Scholar] [CrossRef]
- Wan, G.; Hayden, A.N.; Iiams, S.E.; Merlin, C. Cryptochrome-1 mediates light-dependent inclination magnetosensing in monarch butterflies. Nat. Commun. 2021, 12, 771. [Google Scholar] [CrossRef]
- Gegear, R.J.; Casselman, A.; Waddell, S.; Reppert, S.M. Cryptochrome mediates light-dependent magnetosensitivity in Drosophila. Nature 2008, 454, 1014–1018. [Google Scholar] [CrossRef]
- Foley, L.E.; Gegear, R.J.; Reppert, S.M. Human cryptochrome exhibits light-dependent magnetosensitivity. Nat. Commun. 2011, 2, 356. [Google Scholar] [CrossRef]
- Kerpal, C.; Richert, S.; Storey, J.G.; Pillai, S.; Liddell, P.A.; Gust, D.; Mackenzie, S.R.; Hore, P.J.; Timmel, C.R. Chemical compass behaviour at microtesla magnetic fields strengthens the radical pair hypothesis of avian magnetoreception. Nat. Commun. 2019, 10, 3707. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.A.; Lau, J.C.; Hogben, H.J.; Biskup, T.; Kattnig, D.R.; Hore, P.J. Alternative radical pairs for cryptochrome-based magnetoreception. J. R. Soc. Interface 2014, 11, 20131063. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Ahmad, M. Light-activated cryptochrome reacts with molecular oxygen to form a flavin–superoxide radical pair consistent with magnetoreception. J. Biol. Chem. 2011, 286, 21033–21040. [Google Scholar] [CrossRef] [PubMed]
- Solov’yov, I.A.; Schulten, K. Magnetoreception through cryptochrome may involve superoxide. Biophys. J. 2009, 96, 4804–4813. [Google Scholar] [CrossRef]
- Hogben, H.J.; Efimova, O.; Wagner-Rundell, N.; Timmel, C.R.; Hore, P. Possible involvement of superoxide and dioxygen with cryptochrome in avian magnetoreception: Origin of Zeeman resonances observed by in vivo EPR spectroscopy. Chem. Phys. Lett. 2009, 480, 118–122. [Google Scholar] [CrossRef]
- Evans, E.W.; Kattnig, D.R.; Henbest, K.B.; Hore, P.J.; Mackenzie, S.R.; Timmel, C.R. Sub-millitesla magnetic field effects on the recombination reaction of flavin and ascorbic acid radicals. J. Chem. Phys. 2016, 145, 085101. [Google Scholar] [CrossRef]
- Moser, C.C.; Page, C.C.; Farid, R.; Dutton, P.L. Biological electron transfer. J. Bioenerg. Biomembr. 1995, 27, 263–274. [Google Scholar] [CrossRef]
- Zadeh-Haghighi, H.; Simon, C. Magnetic field effects in biology from the perspective of the radical pair mechanism. J. R. Soc. Interface 2022, 19, 20220325. [Google Scholar] [CrossRef]
- McLendon, G.; Hake, R. Interprotein electron transfer. Chem. Rev. 1992, 92, 481–490. [Google Scholar] [CrossRef]
- Hore, P. Upper bound on the biological effects of 50/60 Hz magnetic fields mediated by radical pairs. eLife 2019, 8, e44179. [Google Scholar] [CrossRef]
- Scaiano, J.C.; Mohtat, N.; Cozens, F.L.; McLean, J.; Thansandote, A. Application of the radical pair mechanism to free radicals in organized systems: Can the effects of 60 Hz be predicted from studies under static fields? Bioelectromagnetics 1994, 15, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Brocklehurst, B. Spin correlation in the geminate recombination of radical ions in hydrocarbons. Part 1. Theory of the magnetic field effect. J. Chem. Soc. Faraday Trans. 2 1976, 72, 1869–1884. [Google Scholar] [CrossRef]
- Eveson, R.W.; Timmel, C.R.; Brocklehurst, B.; Hore, P.J.; McLauchlan, K.A. The effects of weak magnetic fields on radical recombination reactions in micelles. Int. J. Radiat. Biol. 2000, 76, 1509–1522. [Google Scholar] [PubMed]
- Timmel, C.R.; Till, U.; Brocklehurst, B.; Mclauchlan, K.A.; Hore, P.J. Effects of weak magnetic fields on free radical recombination reactions. Mol. Phys. 1998, 95, 71–89. [Google Scholar] [CrossRef]
- Kattnig, D.R.; Evans, E.W.; Dejean, V.; Dodson, C.A.; Wallace, M.I.; Mackenzie, S.R.; Timmel, C.R.; Hore, P.J. Chemical amplification of magnetic field effects relevant to avian magnetoreception. Nat. Chem. 2016, 8, 384–391. [Google Scholar] [CrossRef]
- Lewis, A.M.; Fay, T.P.; Manolopoulos, D.E.; Kerpal, C.; Richert, S.; Timmel, C.R. On the low magnetic field effect in radical pair reactions. J. Chem. Phys. 2018, 149, 034103. [Google Scholar] [CrossRef]
- Blackman, C.F.; Benane, S.G.; House, D.E. Frequency-dependent interference by magnetic fields of nerve growth factor-induced neurite outgrowth in PC-12 cells. Bioelectromagnetics 1995, 16, 387–395. [Google Scholar] [CrossRef]
- Qin, S.; Yin, H.; Yang, C.; Dou, Y.; Liu, Z.; Zhang, P.; Yu, H.; Huang, Y.; Feng, J.; Hao, J.; et al. A magnetic protein biocompass. Nat. Mater. 2016, 15, 217–226. [Google Scholar] [CrossRef]
- Cortassa, S.; Aon, M.A.; Winslow, R.L.; O’Rourke, B. A mitochondrial oscillator dependent on reactive oxygen species. Biophys. J. 2004, 87, 2060–2073. [Google Scholar] [CrossRef]
- Aon, M.A.; Cortassa, S.; O’Rourke, B. The fundamental organization of cardiac mitochondria as a network of coupled oscillators. Biophys. J. 2006, 91, 4317–4327. [Google Scholar] [CrossRef]
- Sharpe, M.A.; Baskin, D.S.; Pichumani, K.; Ijare, O.B.; Helekar, S.A. Rotating magnetic fields inhibit mitochondrial respiration, promote oxidative stress and produce loss of mitochondrial integrity in cancer cells. Front. Oncol. 2021, 11, 768758. [Google Scholar] [CrossRef] [PubMed]
- Cortassa, S.; Aon, M.A.; Marbán, E.; Winslow, R.L.; O’Rourke, B. An integrated model of cardiac mitochondrial energy metabolism and calcium dynamics. Biophys. J. 2003, 84, 2734–2755. [Google Scholar] [CrossRef] [PubMed]
- Casani-Galdon, P.; Garcia-Ojalvo, J. Signaling oscillations: Molecular mechanisms and functional roles. Curr. Opin. Cell Biol. 2022, 78, 102130. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krylov, V.V.; Osipova, E.A. Molecular Biological Effects of Weak Low-Frequency Magnetic Fields: Frequency–Amplitude Efficiency Windows and Possible Mechanisms. Int. J. Mol. Sci. 2023, 24, 10989. https://doi.org/10.3390/ijms241310989
Krylov VV, Osipova EA. Molecular Biological Effects of Weak Low-Frequency Magnetic Fields: Frequency–Amplitude Efficiency Windows and Possible Mechanisms. International Journal of Molecular Sciences. 2023; 24(13):10989. https://doi.org/10.3390/ijms241310989
Chicago/Turabian StyleKrylov, Viacheslav V., and Elena A. Osipova. 2023. "Molecular Biological Effects of Weak Low-Frequency Magnetic Fields: Frequency–Amplitude Efficiency Windows and Possible Mechanisms" International Journal of Molecular Sciences 24, no. 13: 10989. https://doi.org/10.3390/ijms241310989
APA StyleKrylov, V. V., & Osipova, E. A. (2023). Molecular Biological Effects of Weak Low-Frequency Magnetic Fields: Frequency–Amplitude Efficiency Windows and Possible Mechanisms. International Journal of Molecular Sciences, 24(13), 10989. https://doi.org/10.3390/ijms241310989




